Abstract
Commensal oral streptococci play critical roles in oral biofilm formation and promote dental health by competing with, and antagonizing the growth of, pathogenic organisms, such as Streptococcus mutans. Efficient utilization of the spectrum of carbohydrates in the oral cavity by commensal streptococci is essential for their persistence, and yet very little is known about the regulation of carbohydrate catabolism by these organisms. Carbohydrate catabolite repression (CCR) in the abundant oral commensal Streptococcus gordonii strain DL-1 was investigated using the exo-β-d-fructosidase gene (fruA) and a fructose/mannose sugar:phosphotransferase (PTS) enzyme II operon (levDEFG) as model systems. Functional studies confirmed the predicted roles of FruA and LevD in S. gordonii. ManL, the AB domain of a fructose/mannose-type enzyme II PTS permease, contributed to utilization of glucose, mannose, galactose, and fructose and exerted primary control over CCR of the fruA and levD operons. Unlike in S. mutans, ManL-dependent CCR was not sugar specific, and galactose was very effective at eliciting CCR in S. gordonii. Inactivation of the apparent ccpA homologue of S. gordonii actually enhanced CCR of fruA and levD, an effect likely due to its demonstrated role in repression of manL expression. Thus, there are some similarities and fundamental differences in CCR control mechanisms between the oral pathogen S. mutans and the oral commensal S. gordonii that may eventually be exploited to enhance the competitiveness of health-associated commensals in oral biofilms.
The human oral cavity provides a complex and substantial source of nutrients to support the growth of a diverse population of microorganisms, with more than 750 bacterial phylotypes already detected in the oral cavity (1, 17, 36). However, a comparatively small subset of genera account for the majority of the population, with the oral streptococci being among the earliest colonizers and the most abundant inhabitants of the human mouth (42). Oral commensal streptococci include members of the sanguinis, salivarius, mitis, and anginosus groups of streptococci, many of which have been associated with dental and periodontal health. In contrast, Streptococcus mutans, the major etiologic agent for human dental caries, is not generally found in significant proportions on healthy dental tissues. Instead, this organism thrives when the diet of the host is excessively rich in carbohydrates, due in large part to its enhanced acidogenic and aciduric capacities compared to many commensal streptococci (6, 31, 32). Notably, a number of commensal oral streptococci can inhibit S. mutans growth by producing hydrogen peroxide and other inhibitory substances (21, 25, 44, 49, 50). Since it is generally recognized that dental caries has an ecological basis (34, 44), developing a complete picture of the mechanisms that commensals use to gain a selective advantage over cariogenic microbes will help to facilitate the rational design of technologies that discourage colonization and pathogenesis by undesirable organisms.
Carbohydrates are the principal source of energy for growth of Streptococcus spp., which lack a complete respiratory chain. Although there are some examples of carbohydrates that are internalized by streptococci through ABC transporters (4, 5, 43) or symport systems (14), the dominant high-affinity, high-capacity pathway for uptake of carbohydrates is the phosphoenolpyruvate (PEP)-dependent sugar:phosphotransferase system (PTS) (5, 51). The PTS consists of the general components enzyme I (EI) and histidine phosphocarrier protein (HPr), and an array of substrate-specific enzyme II (EII) complexes (37). EI-catalyzed phosphorylation of His15 of HPr by PEP is followed by transfer of the phosphate group to the EIIA and then the EIIB domains of PTS permeases, followed by internalization and concurrent phosphorylation of mono- or disaccharides mediated by a membrane-associated EIIC domain and, in some cases for certain permeases, an EIID domain (37). As with most bacteria, when rapidly metabolizable carbohydrates are present, the genes for utilization of nonpreferred carbohydrates are repressed in streptococci. Thus, carbon catabolite repression (CCR) is common in these bacteria (11, 12), allowing the organisms to optimize energy generation (18).
The primary route for CCR in most low-G+C Gram-positive bacteria involves HPr of the PTS and catabolite control protein A (CcpA), which binds to catabolite response elements (CRE) found in promoter regions of CCR-sensitive genes and regulates their transcription (12). The DNA-binding activity of CcpA is enhanced by interactions with the Ser46-phosphorylated form of HPr (HPr-Ser-P) and in some cases can be stimulated by the presence of certain glycolytic intermediates, including fructose-1,6-bisphosphate (12). Although a CcpA homologue in S. mutans can affect the regulation of central carbon metabolism (3), CcpA does not play a dominant role in CCR in S. mutans (19, 47, 53). Instead, HPr-Ser-P and three EII complexes (EIIABMan, FruI, and EIILev) exert primary control over the transport and catabolism of nonpreferred carbohydrates (2, 54, 55) at the transcriptional and posttranscriptional levels.
The metabolic pathways, nutrient requirements, and genomic structure of commensal oral streptococci and S. mutans are similar, so they likely compete for common nutrients in the oral cavity. As one of the most abundant oral commensals and an early colonizer of the oral cavity, Streptococcus gordonii interacts with various oral bacteria during the formation of oral biofilms and is able to antagonize the growth of S. mutans (21, 24, 26, 27). Notably, some differences in regulation of CCR between S. mutans and S. gordonii are already apparent. In particular, whereas CcpA does not play dominant roles in CCR in S. mutans (2, 47, 53-55), a ccpA mutant of S. gordonii displayed a loss of CCR of the arginine deiminase pathway (15), aberrant expression of amylase-binding protein (41), and loss of penicillin tolerance (7). If, in fact, oral commensals and S. mutans manage CCR in fundamentally different ways, it may be possible to design therapeutics that disrupt the ability of S. mutans to persist without interfering with the growth of commensals. Using two CCR-sensitive model systems that we show to have similar functions in S. mutans and S. gordonii, we demonstrate that there are core similarities and notable differences in the global regulation of carbohydrate catabolism by these competing microorganisms.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The wild-type S. gordonii strain DL1 and its derivatives used in the present study (see Table S1 in the supplemental material) were maintained in brain heart infusion broth (BHI; Difco Laboratories, Detroit, MI) at 37°C in 5% CO2 and 95% air with antibiotics added at the following concentrations: kanamycin (1.0 mg ml−1), erythromycin (10 μg ml−1), and spectinomycin (500 μg ml−1), when necessary. S. gordonii cultures for chloramphenicol acetyltransferase (CAT) assays and for measuring growth rates were prepared in TV-base medium (9) containing the specified amounts of glucose, fructose, mannose, galactose, or inulin. Cells for PTS assays were cultured in an anaerobic chamber maintained with 85% N2, 10% H2, and 5% CO2. All chemical reagents and antibiotics were obtained from Sigma Chemical (St. Louis, MO). Growth curves of S. gordonii strains were generated by using a Bioscreen C monitor (OyGrowth Curves AB, Helsinki, Finland) with readings taken every 30 min. Escherichia coli strains were grown in Luria-Bertani medium supplemented with antibiotics, when needed, at the following concentrations: kanamycin (40 μg ml−1), erythromycin (300 μg ml−1), and spectinomycin (50 μg ml−1).
DNA manipulation.
Standard recombinant DNA techniques were performed to engineer plasmids. All restriction and modifying enzymes were purchased from Invitrogen (Carlsbad, CA) or New England Biolabs (Beverly, MA) and used as recommended by the supplier. DNA purification was carried out using QiaQuick DNA purification kits purchased from Qiagen (Valencia, CA). All primers (see Table S2 in the supplemental material) were synthesized by Integrated DNA Technologies (Coralville, IA). PCR-ligation mutagenesis was used to replace the coding sequences of fruA, levD, and manL with a nonpolar erythromycin resistance marker (57), as detailed elsewhere (29). To construct a manL ccpA double mutant, a 0.9-kbp internal fragment of ccpA was PCR amplified and cloned onto pUC19. A nonpolar spectinomycin resistance cassette was subsequently inserted into the ccpA gene at an EcoRV site, and the mutation was introduced by competent transformation into a manL mutant. All engineered strains were verified by PCR and DNA sequencing, including determining the sequence of the chromosomal content of the arms used for recombination to ensure that no undesired mutations were introduced outside of the gene of interest.
CAT reporter gene fusions to the promoters for fruA (PfruA-cat), levD (PlevD-cat), and manL (PmanL-cat) were constructed using plasmid pJL84 (54) by inserting a promoter-containing DNA fragment, including the cognate ribosome-binding site, in front of a promoterless cat gene from Staphylococcus aureus that lacked a ribosome-binding site (58). The promoter-cat fusion was released by restriction digestion with SphI and SacI and subcloned into the integration vector pMJB8, which allows for delivery in single-copy of DNA inserts into the gtfG gene on the chromosome of S. gordonii (10). All gene fusions were confirmed by DNA sequencing prior to transforming S. gordonii and the correct conformation of the integration cassette in the resultant isolates was confirmed by PCR.
Biochemical assays.
Bacterial cultures were grown to mid-exponential phase in 30 ml of TV-base medium supplemented with various carbohydrates, and the cells were collected by centrifugation, washed once with the same volume of 10 mM Tris buffer (pH 7.8), resuspended in 750 μl of the same buffer, and then homogenized in a Bead Beater (Biospec Products, Bartlesville, OK) for 30 s, twice, with a 2-min interval on ice. After centrifugation at 18,000 × g for 10 min at 4°C, the supernatant fluid was recovered and used for measuring the CAT activity by the method of Shaw (45). The protein concentration of the lysates was determined by the Bradford (Bio-Rad) or bicinchoninic acid (BCA; Thermo Scientific) assay. CAT activity was expressed as nmol of chloramphenicol acetylated mg of protein−1 min−1.
PEP-dependent PTS assays were carried out according to protocols described elsewhere (30) with minor modifications. Briefly, 30 ml of a culture growing anaerobically in TV-base medium with 0.5% of the specified carbohydrate was harvested in late exponential phase (optical density at 600 nm [OD600] = 0.5 to 0.6), washed twice with 30 ml of 100 mM Na-K-PO4 buffer (pH 7.2) containing 5 mM MgCl2, resuspended in 1 ml of the same buffer, and then permeabilized by vortexing with 50 μl of toluene-acetone (1:9, vol/vol) for 2 min, twice, with a 2-min interval on ice. Anaerobically grown cells were utilized in these assays because cells that were grown in the presence of oxygen had high levels of spontaneous oxidization of NADH that interfered with the assay, apparently associated with induction of NADH oxidases. Permeabilized cells were then subjected to PTS assays (30). A BCA assay was used to measure protein concentrations of the samples, and the PTS activity was expressed as nmol of NADH oxidized mg of protein−1 min−1 in a PEP-dependent manner.
RESULTS
Identification of fruA, levD, and manL homologues in S. gordonii.
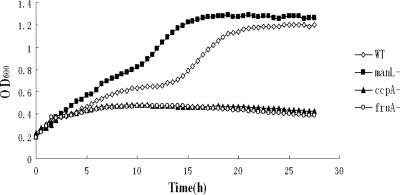
To find a homologous exo-β-d-fructosidase (fruA) gene in the genome of S. gordonii strain DL1, the protein sequence of fruA from S. mutans (SMU.78; GenBank locus tag) was used in a BLAST search against the S. gordonii Challis genome sequence at http://www.oralgen.lanl.gov. A homologous open reading frame (ORF) SGO_0385 (Oralgen gene ID), annotated also as an exo-β-d-fructosidase, was identified. The S. gordonii fruA gene was predicted to encode a polypeptide of 1,408 amino acid residues with 95, 58, and 56% identity to β-fructosidases from Streptococcus sanguinis, S. mutans, and Streptococcus salivarius, respectively. The second most closely related gene product identified during the BLAST search was a sucrose-6-phosphate hydrolase (SGO_1302). To verify the function of fruA as a fructosidase, a fruA mutant was constructed by allelic exchange with a nonpolar erythromycin resistance determinant (em) and tested for growth in TV medium containing 0.05% glucose and 0.5% of the β2,1-linked fructose homopolymer inulin. Whereas the wild-type strain grew normally, presenting with a typical diauxic growth curve, the fruA mutant stopped growing after the glucose was exhausted (Fig. 1). Therefore, the phenomenon provided an indication that the fruA gene of S. gordonii encodes a functional fructan hydrolase. Additional tests confirmed that the fruA mutant had no defect in the utilization of glucose and could not grow at all if inulin was the only carbohydrate source in the medium (data not shown).
FIG. 1.
Growth of S. gordonii wild-type strain DL1 (⋄) and manL (▪), ccpA (▴), and fruA (○) mutants in TV supplemented with 0.05% glucose and 0.5% inulin.
Similarly, the protein sequence of a fructose/mannose-specific enzyme IIA component from S. mutans (levD, SMU.1961c) was used in a BLAST search against the S. gordonii genome sequence. An ORF, SGO_1893, annotated as a fructose (mannose)-specific IIA component of the PTS system was identified as the first gene in a four-gene operon encoding the IIA component (levD) and the IIB, IIC, and IID domains of a fructose/mannose-type permease, encoded by levE, levF, and levG, respectively. The levD gene was predicted to encode a polypeptide of 145 amino acids with 80, 68, and 53% identities to fructose PTS IIA components of S. sanguinis, S. mutans, and Streptococcus uberis, respectively. Also identified was a glucose/mannose-specific PTS IIAB component (SGO_1679) that was designated manL. The manL gene of S. gordonii was predicted to encode a polypeptide of 329 amino acid residues with 94, 91, 87, and 83% identities to the gene for IIABMan of Streptococcus pneumoniae, S. sanguinis, S. salivarius, and S. mutans, respectively, and was part of an apparent operon encoding the IIC and IID components of the EIIMan permease.
Characterization of manL and levD mutants of S. gordonii.
To assess the function of manL and levD in S. gordonii, allelic replacement mutants were constructed in the background of strain DL1 using a nonpolar em cassette. To determine the role of manL and levD in carbohydrate transport, growth of the wild-type strain and the manL and levD mutants in TV medium supplemented with 0.2% of glucose, fructose, mannose, or galactose was monitored using a Bioscreen C. Compared to the wild-type strain, the growth rate of the manL mutant was slower in glucose and mannose, dramatically slower in galactose, but only modestly slower on fructose (Table 1), suggesting that ManL may participate in the uptake of glucose, mannose, and galactose by S. gordonii. In contrast, the levD mutant grew slower than DL1 in the presence of fructose or mannose, which is indicative of a role of LevDEFG in the transport of these hexoses. There were no significant differences in doubling times between the wild-type strain and the levD mutant when grown in glucose.
TABLE 1.
Doubling times and maximal OD600 values for wild-type strain DL1 and manL and levD mutants growing in TV medium supplemented with 0.2% glucose, fructose, mannose, or galactose as the carbohydrate source
| Strain |
Td (h) and OD600 (SD)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| Glucose |
Fructose |
Mannose |
Galactose |
|||||
| Td | OD600 | Td | OD600 | Td | OD600 | Td | OD600 | |
| DL1 | 2.03 (0.02) | 0.51 (0.01) | 2.30 (0.01) | 0.49 (0.01) | 2.29 (0.02) | 0.55 (0.01) | 5.92 (0.02) | 0.79 (0.03) |
| manL mutant | 2.70 (0.01)* | 0.54 (0.01) | 2.67 (0.02)* | 0.46 (0.02) | 3.98 (0.01)* | 0.59 (0.01) | 25.57 (0.02)* | 0.43 (0.02)* |
| levD mutant | 2.01 (0.01) | 0.53 (0.01) | 2.59 (0.01)* | 0.47 (0.01) | 2.67 (0.01)* | 0.50 (0.01) | 5.76 (0.01)* | 0.78 (0.02) |
Td, doubling time; *, data significantly different from that in the DL1 background in corresponding carbohydrates, as verified by the Student t test (P < 0.01).
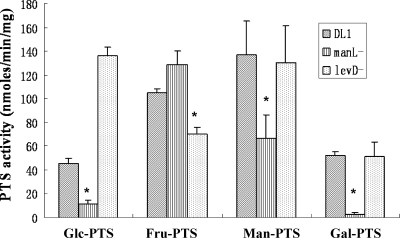
To verify the role in sugar transport by ManL and LevD, DL1 and the manL and levD mutants were cultured anaerobically in TV with 0.5% glucose, fructose, mannose, or galactose. Cells were collected in mid-exponential phase, and the PTS specific activities were measured by using glucose, fructose, mannose, or galactose as substrates. Compared to strain DL1, the manL mutant had significantly decreased PTS activities for glucose, mannose, and galactose but slightly increased activity for fructose. The levD mutant showed significantly lower PTS activity for fructose, significantly higher activity for glucose, and little change in mannose and galactose PTS activity (Fig. 2). These results further support that ManL participates in the transport of glucose, galactose, and mannose and may also be involved in the utilization of fructose, whereas LevD appears to be required primarily for the internalization of fructose.
FIG. 2.
PEP-dependent PTS activities of S. gordonii DL1 and manL and levD mutants grown anaerobically in 0.5% of glucose, fructose, mannose, or galactose were measured using 10 mM glucose, fructose, mannose, or galactose, respectively, as the substrate. An asterisk represents a P value of <0.05 (Student t test) compared to DL1. Glc, glucose; Fru, fructose; Man, mannose; Gal, galactose.
CCR of the fruA and levD genes of S. gordonii.
We examined how the expression of fruA and levD was regulated at the transcriptional level in response to different carbohydrates. A PfruA-cat and a PlevD-cat fusion were constructed by fusing a DNA fragment containing the fruA or levD promoters to a promoterless cat gene on an integration vector and establishing the promoter fusion in single copy at a remote site in the S. gordonii chromosome. Strain DL1 was transformed with the gene fusion constructs, and the resultant strains were cultured in TV medium containing 0.5% each of glucose, fructose, galactose, or inulin or a combination of inulin and glucose, fructose, or galactose and then subjected to CAT assays. As shown in Table 2, the activities of the fruA and levD promoters were optimal when cells were growing on inulin but were lower when cells were growing on the preferred carbohydrates glucose or fructose. Expression from these promoter fusions was also repressed in cells growing on the combination of inulin and glucose, galactose, or fructose. Therefore, similar to the homologous systems in S. mutans, transcription of fruA and levD is inducible by the presence of a fructan polymer and repressed when preferred carbohydrates are present. Notably, CCR was most evident in cultures containing fructose, whereas CCR of fruA in S. mutans is elicited most effectively by glucose. Also of note, galactose caused apparent repression of the expression of fruA, but galactose is essentially a nonrepressing carbohydrate in S. mutans.
TABLE 2.
CAT specific activities representing the expression levels of PfruA-cat and PlevD-cat fusions in the wild-type background growing on various carbohydrates
| Fusion | CAT activitya (SD) in cells grown on the indicated carbohydrate |
||||||
|---|---|---|---|---|---|---|---|
| Glc | Fru | Gal | Inulin | Glc/inulin | Fru/inulin | Gal/inulin | |
| PfruA-cat | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 24.8 (1.6) | 7.5 (1.0) | 0.0 (0.0) | 9.5 (0.4) |
| PlevD-cat | 14.9 (1.9) | 8.9 (2.4) | 21.0 (5.7) | 630.1 (27) | 235.6 (19) | 12.9 (2.4) | 338.7 (49) |
CAT activity is expressed as nmol of chloramphenicol acetylated mg of protein−1 min−1, followed by the standard deviation in parentheses. The data are averages from at least three independent cultures, and all assays were performed in triplicate. The carbohydrate concentration in the growth medium was 0.5% (wt/vol). Glc, glucose; Fru, fructose; Gal, galactose.
CCR is not abolished by inactivating ccpA.
To determine the underlying basis for the sensitivity of fruA to CCR, S. gordonii DL1 and a ccpA mutant derived from DL1 (15) were grown in TV medium supplemented with 0.05% of glucose and 0.5% of inulin (Fig. 1). DL1 displayed classic diauxic growth, whereas the ccpA mutant achieved a level of growth comparable to that observed for the fruA mutant. The ccpA mutant of S. gordonii grows nearly as well as the parental strain on glucose alone (15), so it appears that the ccpA mutant lost its capacity to utilize inulin. To investigate the role of CcpA in CCR of the fruA and levDEFG genes in S. gordonii, PfruA-cat and PlevD-cat fusions were introduced into the ccpA mutant, and the CAT activity was measured in various growth carbohydrates (Tables 3 and 4). Consistent with the growth phenotype of the ccpA mutant on the combination of glucose and inulin, the expression of fruA was ∼120-fold lower in the ccpA mutant when cells were grown on inulin. Expression of levD was lower in the ccpA mutant across all five carbohydrates tested. Thus, consistent with the poor growth of the ccpA mutant of S. gordonii on inulin (Fig. 1), inactivation of CcpA might have lead to strong inhibition of inulin-induced fruA and levD expression. Since it is known that CcpA can function as a transcriptional activator for certain genes and operons (12), it might be possible that loss of CcpA leads to lack of activation of fruA and levD expression.
TABLE 3.
PfruA-cat specific activities in the wild-type background and in manL, ccpA, and manL ccpA mutants growing on various carbohydratesa
| Strain | CAT activity (SD) in cells grown on the indicated carbohydrate |
||||
|---|---|---|---|---|---|
| Glucose | Fructose | Galactose | Inulin | Mannose | |
| DL1 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 24.8 (1.6)A | 0.1 (0.1) |
| manL mutantB | 1.9 (0.5) | 1.1 (0.2) | 2.1 (0.6) | 42.5 (4.7) | 50.0 (3.0) |
| ccpA mutant | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.2 (0.2)C | 0.0 (0.0) |
| manL ccpA mutantD | 1.2 (0.4) | 0.8 (0.0) | 0.9 (0.1)E | 49.7 (12.1) | 31.8 (2.6)E |
CAT activity is expressed as nmol of chloramphenicol acetylated mg of protein−1 min−1, followed by the standard deviation in parentheses. The data are averages from at least three independent cultures, and all assays were performed in triplicate. The carbohydrate concentration in the growth medium was 0.5% (wt/vol). Superscript capital letters indicate significance as follows. Superscript A, data statistically different from that in other sugars in the DL1 background, as verified by the Student t test (P < 0.01). Superscripts B, C, and D, data statistically different from that in the DL1 background in the corresponding sugars, as verified by the Student t test (P < 0.05). Superscript E, data statistically different from that in the manL mutant, as verified by the Student t test (P < 0.05).
TABLE 4.
PlevD-cat activities in the wild-type background and manL, ccpA, and manL ccpA mutants growing in various carbohydratesa
| Strain | CAT activity (SD) in cells grown on the indicated carbohydrate |
||||
|---|---|---|---|---|---|
| Glucose | Fructose | Galactose | Inulin | Mannose | |
| DL1 | 14.9 (1.9) | 8.9 (2.7) | 21.0 (5.7) | 630.1 (27.1) | 23.8 (2.7) |
| manL mutantA | 44.2 (2.0) | 19.4 (1.7) | 108.6 (11.3) | 1193 (243) | 1340 (46) |
| ccpA mutant | 0.0 (0.0) | 3.6 (1.7) | 0.1 (0.1) | 0.7 (0.2) | 0.02 (0.0) |
| manL ccpA mutantB | 38.4 (7.7) | 36.4 (5.9)C | 26.1 (1.5)C | 1055.0 (60) | 966.3 (93)C |
CAT activity is expressed as nmol of chloramphenicol acetylated mg of protein−1 min−1, followed by the standard deviation in parentheses. The data are averages from at least three independent cultures, and all assays were performed in triplicate. The carbohydrate concentration in the growth medium was 0.5% (wt/vol). Superscript capital letters indicate significance as follows. Superscript A and B, data are significantly different from that in the DL1 background in the corresponding carbohydrates, as verified by the Student t test (P < 0.05). Superscript C, data significantly different from that in the manL mutant, as verified by the Student t test (P < 0.05).
A repressive effect of CcpA on expression of fruA, but not levD, can be observed.
In S. mutans, the expression of the fruA and levD operons is controlled by a four-component regulatory system, LevQRST, which senses the presence of the inducing substrates fructose or mannose (54, 58). Due to relatively dominant regulation by CcpA-independent CCR mechanisms, CCR of fruA via CcpA in S. mutans was only apparent when expression was monitored in a fructose-pulsing experiment (3). That is, evidence of CCR by CcpA could only be observed when cells were grown to mid-exponential phase in a noninducing carbohydrate and then the cultures were pulsed with inducer for 1 to 3 h. In a similar experiment, S. gordonii carrying the PfruA-cat or PlevD-cat fusion was incubated in TV with 0.5% glucose to early exponential phase (OD600 ≅ 0.2) and then treated for 3 h with various concentrations of fructose or inulin before being harvested for CAT assays. As shown in Table 5, there was an ∼5-fold increase in fruA expression when the fructose concentration increased from 0.05 to 7.5 mM, with a clear dose-dependent response to the presence of fructose by the PlevD-cat strain (Table 5). Similarly, when inulin was used to pulse these strains, there was a concentration-dependent increase in the expression of levD in response to the addition of inulin.
TABLE 5.
PfruA-cat and PlevD-cat activities measured in wild-type and ccpA and fruA mutant backgrounds under fructose or inulin pulsinga
| Strain and fusion | CAT activity (SD) in cells pulsed with the indicated concentration of fructose or inulin |
||||
|---|---|---|---|---|---|
| 0 mM | 0.05 mM | 3 mM | 5 mM | 7.5 mM | |
| Pulsed with fructose | |||||
| DL1/PfruA-cat | 0.00 (0.00) | 0.03 (0.02) | 0.03 (0.01) | 0.04 (0.02) | 0.16 (0.10) |
| ccpA mutant/PfruA-cat | 0.00 (0.00) | 0.08 (0.09) | 0.16 (0.13) | 0.15 (0.10) | 0.49 (0.08)* |
| DL1/PlevD-cat | 6.13 (1.92) | 7.46 (1.47) | 19.52 (5.38) | 23.07 (2.40) | 34.98 (0.56) |
| ccpA mutant/PlevD-cat | 0.62 (0.12) | 1.03 (0.17) | 17.84 (3.31) | 21.23 (1.19) | 24.84 (0.78) |
| fruA mutant/PlevD-cat | 6.73 (0.19) | 7.40 (0.48) | 26.46 (3.37) | 38.80 (5.20) | 17.45 (2.69) |
| Pulsed with inulin | |||||
| DL1/PfruA-cat | 0.00 (0.00) | 0.33 (0.02) | 0.10 (0.01) | 0.14 (0.02) | |
| DL1/PlevD-cat | 5.28 (2.80) | 5.77 (1.41) | 8.28 (0.44) | 15.23 (2.64) | |
| fruA mutant/PlevD-cat | 6.73 (0.19) | 7.02 (0.85) | 6.65 (0.23) | 6.68 (0.46) | |
The cells were cultured in TV containing 0.5% glucose to an OD600 of 0.2, and then different concentrations of fructose or inulin were added. Cells were incubated for 3 h and harvested for CAT assays as detailed in Materials and Methods. CAT activity is expressed as nmol of chloramphenicol acetylated mg of protein−1 min−1, followed by the standard deviation in parentheses. The data are averages from at least three independent cultures, and all assays were performed in triplicate. The carbohydrate concentration in the growth medium was 0.5% (wt/vol). *, data statistically different from that in the DL1 background, as verified by the Student t test (P < 0.05).
Since inulin is a polymer of fructose, the induction effect on fruA and levD likely results from degradation of inulin by FruA and release of free fructose, which serves as the inducing signal. A fruA knockout mutant was constructed, and the ability to induce PlevD::cat expression by inulin was tested in the mutant. As shown in Table 5, no induction above the level seen in the negative control (no inulin) was evident with inulin, although the strain remained responsive to induction by fructose (Table 5). Therefore, as in S. mutans, inulin itself does not appear to be an inducing signal for the expression of levD, and instead the fructose released from hydrolysis of inulin appears to function as the most effective inducing signal (Table 2). Notably, in both organisms, the steady-state levels of fructose liberated from inulin by FruA remain below those needed to elicit substantial CCR (9).
When the expression from the PfruA-cat and PlevD-cat fusions in response to pulsing by fructose was assessed there was a 2- to 5-fold derepression for fruA in the ccpA mutant (Table 5). However, expression of levD in the ccpA mutant under the same conditions was always lower than that measured in the wild-type background. Notably, no apparent CRE sequences could be detected near the promoter regions of either fruA or levD.
Disruption of enzyme IIMan (manL) eliminates CCR of fruA and levD.
Given the importance of ManL in CCR of S. mutans, a manL deletion in S. gordonii was engineered, and its impact on CCR was investigated. When the mutant was tested for diauxic growth in TV medium containing 0.05% glucose and 0.5% inulin, diauxic growth was nearly eliminated (Fig. 1). When fruA and levD gene expression was measured using the PfruA-cat and PlevD-cat fusions in the manL mutant background (Tables 3 and 4), both genes were derepressed relative to the expression levels in the wild-type background in all of the carbohydrates tested. The greatest level of derepression, at least according to data from levD expression, was seen in mannose cultures. Although a similar fold change in expression was seen with glucose and galactose, the amount of derepression seen in the ManL-deficient strain was lowest when fructose was the growth carbohydrate. When the cells were grown in TV-inulin, an ∼3-fold derepression for both genes was observed in the manL mutant strain compared to the wild-type background.
CcpA negatively regulates manL expression.
Since we have shown that disruption of CcpA results in decreased expression of fruA and levD under most conditions (Tables 3 and 4) and that loss of ManL generally leads to relieved CCR, it is possible that CcpA could indirectly influence CCR by regulating manL, as appears to be the case for S. mutans (54). A ccpA deletion was introduced into the manL mutant strain and expression of PfruA- and PlevD-cat fusions in the double mutant background was monitored. When grown in TV supplemented with 0.5% of glucose, fructose, galactose, inulin, or mannose, the ccpA manL double mutant produced CAT activities at significantly higher levels than in the wild-type strain or the ccpA mutant (Tables 3 and 4). Compared to the strain carrying the manL mutation alone, the level of derepression seen in the ccpA manL double mutant was similar. However, the double mutant displayed lower activity when galactose or mannose was used to culture the cells (Tables 3 and 4). These results provide support for the idea that CcpA could influence expression of fruA and levD by affecting the amount of ManL in the cells.
To further test whether CcpA regulated manL expression, a PmanL-cat fusion was constructed and introduced into the wild-type and ccpA mutant strains. Upon growth in glucose, fructose, or galactose, expression of the PmanL-cat fusion was significantly higher in the ccpA mutant relative to that in the wild-type background (Table 6), a finding indicative of negative regulation of manL by CcpA. Also of note, manL expression was higher in galactose than in glucose or fructose in the wild-type background, possibly due to less efficient induction of CCR through CcpA by galactose, compared to glucose or fructose. Galactose should flow through the tagatose pathway in S. gordonii, yielding less glucose-6-phosphate (G-6-P) and fructose-1,6-bisphosphate (F-1,6-bP) than would growth on glucose or fructose. Since G-6-P and F-1,6-bP are activators of HPr kinase (12), CcpA-dependent repression of manL would then be less severe in cells growing on galactose. Consistent with this notion, there was little difference in the expression levels of manL in response to the various sugars tested in a strain lacking CcpA (Table 6).
TABLE 6.
PmanL-cat activities measured in wild-type and ccpA, manL, and manL ccpA mutant backgrounds growing on various carbohydratesa
| Strain | CAT activity (SD) in cells grown on the indicated carbohydrate |
||
|---|---|---|---|
| Glucose | Fructose | Galactose | |
| DL1 | 16.1 (0.8) | 12.6 (1.4) | 51.6 (2.5) |
| ccpA mutant | 48.9 (2.9) | 58.3 (1.1) | 67.9 (6.5) |
| manL mutant | 22.0 (6.5) | 21.5 (1.5) | 131.5 (67.8) |
| ccpA manL mutant | 416.4 (51.3) | 375.1 (70.9) | 449.4 (86.3) |
CAT activity is expressed as nmol of chloramphenicol acetylated mg of protein−1 min−1, followed by the standard deviation in parentheses. The data are averages from at least three independent cultures, and all assays were performed in triplicate. The carbohydrate concentration in the growth medium was 0.5% (wt/vol).
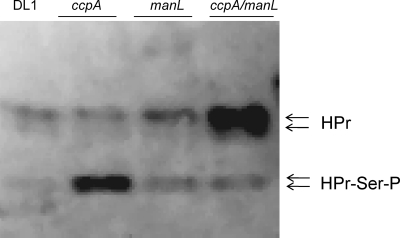
Another possible cause for decreased activities of the fruA and levD promoters in the ccpA mutant is a potential shift in the phosphorylation status of the phosphocarrier protein HPr, since enhanced phosphorylation of HPr on Ser46 has been reported in a ccpA mutant of Bacillus subtilis (33). Also, increased seryl-phosphorylation of HPr in S. mutans negatively affected the expression of both fruA and levD independently of CcpA (55). Therefore, an anti-HPr antiserum was used in Western blots to detect the levels of seryl-phosphorylated and unphosphorylated HPr in the wild-type and various mutant backgrounds. Lysates were prepared from cells growing exponentially in BHI and separated by nondenaturing polyacrylamide gel after treatment at 100°C for 5 min to remove any phosphate from His15, as described elsewhere (16, 48, 55). Significantly higher levels of HPr-Ser-P were produced in the strain lacking CcpA (Fig. 3) than in the wild-type strain. The increases in Ser46-phosphorylated HPr were less pronounced in the ccpA manL double mutant background, despite a clear increase in the overall abundance of the HPr protein. This observation correlates well with the observed elevated CCR of the fruA and levD genes in the ccpA mutant background and the alleviation of CCR when manL was deleted along with ccpA (Tables 3 and 4). Importantly, since little change in HPr-Ser-P levels was seen in the strain lacking only manL, loss of CCR in the manL ccpA double mutant cannot be attributed solely to the change in HPr phosphorylation status.
FIG. 3.
Western blot analysis of HPr of S. gordonii DL1 and ccpA, manL, and ccpA manL mutants. Cells were cultured in BHI broth and cell lysates were prepared as described in the text. All samples were treated at 100°C for 5 min to eliminate His15-phosphorylated HPr prior to resolving the proteins on a 12.5% nondenaturing polyacrylamide gel. The doublets of both HPr (top) and HPr-Ser-P (bottom) represent the full-length protein and HPr without the N-terminal methionine (16, 48). The antisera, kindly provided by Christian Vadeboncouer, was raised against purified HPr from Streptococcus salivarius and used as previously described (55).
Autoregulation of manL.
To investigate whether ManL of S. gordonii could influence manL expression, PmanL-cat fusion activity was measured in the manL mutant. The results (Table 6) showed that expression from the manL promoter was derepressed in the manL mutant relative to the wild-type strain when growing in galactose, although much smaller changes were seen when cells were growing in glucose or fructose. Again, this difference could have arisen from the variation in the efficiency of induction of CCR by these sugars. Indeed, when manL expression levels were determined in a manL ccpA double mutant, expression was highly derepressed in all of the sugars tested, indicating that the differential expression in the various sugars was related to more efficient CcpA-dependent CCR of manL in glucose and fructose, compared to galactose. A potential catabolite response element (CRE; TGAAAACGTTTTAT) was also identified in the promoter region of manL, which differs only slightly from the consensus CRE sequence (TGWNANCGNTNWCA) in Gram-positive bacteria (23). Notably, members of our group have recently made the observation that ManL in S. mutans can affect expression of the manL operon (L. Zeng and R. A. Burne, unpublished data).
DISCUSSION
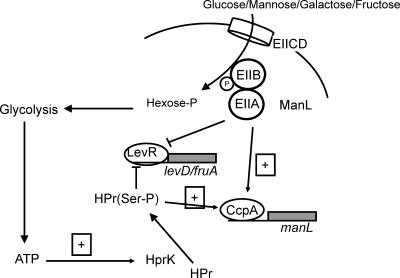
Carbohydrate utilization and the global regulatory pathways modulating sugar catabolism are intimately related to persistence and bacterial virulence in a number of important pathogens, including S. mutans, Streptococcus pyogenes, and S. pneumoniae (2, 3, 20, 46). The microbial communities of the oral cavity harbor hundreds of bacterial phylotypes and intense interspecies competition exists between bacteria that depend on the limited nutrients available in these biofilms (8, 35, 38, 52). The interactions between oral commensals and oral pathogens are critical in determining the “healthy” or “pathogenic” status of dental plaque (22, 24, 28, 39). Whereas in most low G+C Gram-positive bacteria, CcpA and its corepressor HPr-Ser-P are primary effectors of CCR, CcpA-independent CCR dominates the regulation of a variety of catabolic pathways in S. mutans, including fruAB, levDEFG, and the cellobiose and lactose operons (54, 55, 58). These observations with S. mutans raised the question of whether the evolution of CCR in this pathogenic organism was unique to S. mutans or perhaps reflected a general divergence in CCR control in organisms that have as their natural habitat the tissues of the mouth. From the present study, it appears that at least one abundant commensal governs the expression of two homologous CCR-sensitive operons in similar ways to S. mutans but that there are some notable differences in the roles of ManL and CcpA in regulation of carbohydrate utilization (Fig. 4).
FIG. 4.
Model depicting carbohydrate catabolite repression of the levD, fruA, and manL genes in S. gordonii. Binding of the transcriptional activator LevR to the levD (or fruA) promoter is negatively impacted by HPr(Ser-P) and underphosphorylated ManL. Conversely, negative regulation of manL by CcpA could be augmented by ManL and HPr(Ser-P). Similar to the findings in S. mutans, ManL in S. gordonii is capable of repressing the expression of levD and fruA, regardless of whether CcpA is present. Whereas CcpA-independent CCR in S. mutans is strongly elicited by glucose or mannose and but not particularly effective when fructose or galactose is the growth carbohydrate, ManL-dependent CCR in S. gordonii does not show such sugar specificity.
Characterization of various mutants constructed in the present study demonstrated strong conservation in function and regulation of apparent homologues of fruA, levDEFG, and manL between S. mutans and S. gordonii. Similar to what has been found in S. mutans, FruA is required for utilization of fructose polymers by S. gordonii, and the levDEFG operon is primarily responsible for internalization of fructose. Also similar to S. mutans, the substrates of EIIMan appear to include glucose, mannose, galactose, and even fructose. Importantly, the LevQRST system required for the expression of both the fruA and the levDEFG operons in S. mutans is conserved in sequence and function in S. gordonii. Specifically, inactivation of the levR gene, encoding the response regulator of this four-component signal transduction system led to a complete loss of activation of both operons by inducing substrates (data not shown). Consequently, the high degree of conservation in these model genes and their regulatory elements allowed for a reliable contrast of the CCR regulatory pathways between the two organisms.
A number of studies support a role for CcpA in catabolite repression and carbohydrate-dependent regulation of gene expression in S. gordonii, and it has been shown that CcpA can bind to CRE sequences in this organism to effect changes in transcription of target genes. These include the CCR-sensitive arginine deiminase (AD) genes, which are tightly repressed in the presence of glucose (15). Inactivation of CcpA resulted in a profound loss of glucose sensitivity for AD expression (15), as did deletion of the CRE sequence 5′ to the arcA gene (57), supporting that the classic CcpA-dependent pathway for gene expression is functional in S. gordonii. Other examples of glucose-dependent repression of gene expression by CcpA in S. gordonii include the gene for amylase-binding protein A gene (abpA [41]). In contrast, although loss of CcpA has a global impact on gene expression in S. mutans (3), only a minor impact on CCR of genes in a ccpA mutant have been demonstrated and only under very specific in vitro conditions (3). Otherwise, there is no evidence for alleviation of CCR in a ccpA mutant during growth in the presence of combinations of inducing and repressing substrates, either at the gene expression level or by assessing diauxic growth of the mutant strains. This statement also applies to the agmatine deiminase (AgD) pathway of S. mutans. The AgD system is highly similar to the AD pathway of S. gordonii in biochemical terms and in regulation of expression, with both requiring substrate for induction and having sensitivity to CCR. However, unlike the AD gene in S. gordonii, inactivation of CcpA in S. mutans did not relieve CCR of AgD gene expression (19).
Although we were able to create conditions in which CcpA could be shown to have an impact on fruA and levD expression, the lack of a CRE sequence near the promoter regions of these genes in S. gordonii implies that the observed minor impact of CcpA on repression of these operons is indirect. In contrast, expression of fruA in S. mutans can be controlled by CcpA binding to two CREs near the fruA promoter (3). The work presented here provides the first evidence to demonstrate that CcpA-independent CCR plays major roles in regulating genes in response to carbohydrates in an oral commensal. Specifically, deletion of manL had the most profound effect on diauxic growth and resulted in high-level expression of fruA and levD in all carbohydrates tested. Notably, inactivation of ccpA in S. gordonii caused a lower level expression of the fruA and levD operons. Our data demonstrating that the effect of CcpA on repression could be eliminated in a ManL-deficient strain and the use of mRNA measurements and gene fusions to show that CcpA repressed manL expression demonstrated that the effects of CcpA on levD and fruA transcription were almost entirely attributable to the role of CcpA in the negative regulation of manL. The mechanism by which CCR is effected by ManL has not been definitively determined, but available evidence suggests that allosteric interactions with regulatory proteins or phosphorelay involving ManL and possibly other PTS components could be underlying mechanisms for ManL-dependent CCR (54, 55).
Another significant finding in the present study was that a dramatic increase in the amount of the Ser46-phosphorylated HPr protein was observed in the ccpA mutant. Introduction of the manL mutation into the ccpA mutant strain reversed this effect, with the double mutant having lower levels of Ser-46-HPr, but also the double mutant had higher overall levels of HPr protein (Fig. 3) than the wild-type strain. Since CCR of the fruA and levD genes was enhanced in the ccpA mutant and abolished in the ccpA manL background, this finding raises the possibility that HPr-Ser-P is also an effector of CcpA-independent CCR of these model genes in S. gordonii, similar to what we recently reported for S. mutans (55). We propose that both ManL and HPr-Ser-P participate in CCR in S. gordonii, since deletion of manL alone did not elicit any changes in the levels of HPr-Ser-P (Fig. 3). The potential for ManL to influence CCR by modulating the expression and activities of the gene for HPr, ptsH, cannot be excluded at this time. However, our laboratory previously reported that altered expression of the ptsH and ptsI (EI) genes could be observed in a manL mutant of S. mutans (2).
Another important difference in the regulation of CCR between S. mutans and S. gordonii has also been disclosed by the present study. In S. mutans, CCR exerted through PTS permeases displays a clear sugar specificity, with greater effects on CCR being evident when the cognate or preferred carbohydrates for individual permeases are present. For example, EIIABMan confers CCR mainly in the presence of glucose and mannose (2), which is consistent with its role in glucose and mannose uptake, whereas FruI and EIILev only exert CCR efficiently in the presence of their preferred substrate, fructose (54). It has therefore been proposed that underphosphorylated EII proteins, which are present when EIIs are engaged in phosphotransfer to incoming carbohydrates, regulate the LevQRST signal transduction system through allosteric or other mechanisms. In contrast to S. mutans, significantly elevated expression of fruA and levD was seen in the manL mutant of S. gordonii growing in glucose, fructose, galactose, mannose, and even inulin (Tables 3 and 4). We propose that this observation arises from a broader substrate specificity of the EIIMan complex in S. gordonii than in S. mutans. In particular, PTS assays (Fig. 2) indicate that ManL could be involved in transport of glucose, mannose, and galactose, and there was a slight increase in fructose-PTS activity in the manL mutant, likely arising from elevated expression of the LevDEFG complex that transports fructose. However, since the manL mutant also grows slower on fructose, it is possible that ManL can also internalize fructose. Accordingly, expression of fruA and levD was enhanced in the manL mutant growing on inulin, which must be hydrolyzed into fructose before being used by the bacterium. Therefore, the comparative independence of CCR on the particular sugar source in S. gordonii may be associated with the capacity of ManL to efficiently internalize more substrates in S. gordonii than in S. mutans. It is notable that expression of the fruA and levD genes in the manL mutant growing on mannose was almost on the same level as inulin, which is probably induced by mannose transported via the fructose/mannose specific enzyme II LevDEFG complex in the manL mutant. It should be emphasized, though, that loss of ManL might also lead to a reduced ability to utilize multiple preferred sugars, which could result in a reduction in glycolytic intermediates that in turn lead to lower levels of HPr-Ser-P (40). Although this does not appear to be the case (Fig. 3), it cannot be excluded that the impact of ManL deficiency arises in part from alterations in the amount or phosphorylation state of HPr.
Yet another dimension of complexity in CCR in oral streptococci disclosed in the present study was the ability of ManL to influence expression of its own operon. Thus, repression of the manL operon, encoding the EIIMan permease, is governed by both CcpA and ManL. Based on experiments described here and our studies with S. mutans, it seems that CcpA may repress manL, and other genes, when the concentration of carbohydrates is fairly high (3 to 5 mM). In this case, the classical CcpA model would apply in which cells monitor the flow of carbohydrate through the glycolytic pathway, regulating the amount of HPr-Ser-P through an ATP-dependent HPr kinase (13, 33). At lower carbohydrate concentrations, ManL could regulate its own production, and the levels of other permeases (2, 54, 56, 58), in response to the availability of its substrates outside of the cells, saving energy by not overproducing transport proteins. This two-tiered regulatory system (Fig. 4) is one that may have evolved in, and should be well suited to, oral bacteria, which confront extended periods of existing on low concentrations of the spectrum of carbohydrates released from glycoproteins, interspersed with high concentrations of preferred carbohydrates that are common in the modern diet. We propose that these subtle differences in the way in which the oral commensal S. gordonii and the oral pathogen S. mutans regulate catabolism of preferred carbohydrates reflects a niche adaptation that may give the commensal a competitive advantage in conditions conducive to dental health, whereas S. mutans optimizes sugar utilization more effectively under conditions that promote the emergence of a cariogenic flora and dental caries. Future studies will examine this hypothesis in more detail.
Supplementary Material
Acknowledgments
This research was supported by grants DE12236 and DE19106 to R.A.B. from the National Institute for Dental and Craniofacial Research.
Footnotes
Published ahead of print on 14 January 2011.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abranches, J., M. M. Candella, Z. T. Wen, H. V. Baker, and R. A. Burne. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J. Bacteriol. 188:3748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abranches, J., et al. 2008. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. J. Bacteriol. 190:2340-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajdic, D., et al. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. U. S. A. 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajdic, D., and V. T. Pham. 2007. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. J. Bacteriol. 189:5049-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender, G. R., S. V. Sutton, and R. E. Marquis. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 53:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bizzini, A., J. M. Entenza, and P. Moreillon. 2007. Loss of penicillin tolerance by inactivating the carbon catabolite repression determinant CcpA in Streptococcus gordonii. J. Antimicrob. Chemother. 59:607-615. [DOI] [PubMed] [Google Scholar]
- 8.Bowden, G. H., and Y. H. Li. 1997. Nutritional influences on biofilm development. Adv. Dent. Res. 11:81-99. [DOI] [PubMed] [Google Scholar]
- 9.Burne, R. A., Z. T. Wen, Y. Y. Chen, and J. E. Penders. 1999. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J. Bacteriol. 181:2863-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y. Y., M. J. Betzenhauser, and R. A. Burne. 2002. cis-Acting elements that regulate the low-pH-inducible urease operon of Streptococcus salivarius. Microbiology 148:3599-3608. [DOI] [PubMed] [Google Scholar]
- 11.Deutscher, J. 2008. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 11:87-93. [DOI] [PubMed] [Google Scholar]
- 12.Deutscher, J., C. Francke, and P. W. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70:939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutscher, J., and M. H. Saier, Jr. 1983. ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc. Natl. Acad. Sci. U. S. A. 80:6790-6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vos, W. M., and E. E. Vaughan. 1994. Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol. Rev. 15:217-237. [DOI] [PubMed] [Google Scholar]
- 15.Dong, Y., Y. Y. Chen, and R. A. Burne. 2004. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J. Bacteriol. 186:2511-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubreuil, J. D., M. Jacques, D. Brochu, M. Frenette, and C. Vadeboncoeur. 1996. Surface location of HPr, a phosphocarrier of the phosphoenolpyruvate: sugar phosphotransferase system in Streptococcus suis. Microbiology 142(Pt. 4):837-843. [DOI] [PubMed] [Google Scholar]
- 17.Faveri, M., et al. 2008. Microbiological diversity of generalized aggressive periodontitis by 16S rRNA clonal analysis. Oral Microbiol. Immunol. 23:112-118. [DOI] [PubMed] [Google Scholar]
- 18.Gorke, B., and J. Stulke. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6:613-624. [DOI] [PubMed] [Google Scholar]
- 19.Griswold, A. R., M. Jameson-Lee, and R. A. Burne. 2006. Regulation and physiologic significance of the agmatine deiminase system of Streptococcus mutans UA159. J. Bacteriol. 188:834-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389-1406. [PMC free article] [PubMed] [Google Scholar]
- 21.Heng, N. C., J. R. Tagg, and G. R. Tompkins. 2007. Competence-dependent bacteriocin production by Streptococcus gordonii DL1 (Challis). J. Bacteriol. 189:1468-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hojo, K., S. Nagaoka, T. Ohshima, and N. Maeda. 2009. Bacterial interactions in dental biofilm development. J. Dent. Res. 88:982-990. [DOI] [PubMed] [Google Scholar]
- 23.Hueck, C. J., W. Hillen, and M. H. Saier, Jr. 1994. Analysis of a cis-active sequence mediating catabolite repression in gram-positive bacteria. Res. Microbiol. 145:503-518. [DOI] [PubMed] [Google Scholar]
- 24.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighboring species. Mol. Microbiol. 57:392-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 187:7193-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuboniwa, M., et al. 2009. Proteomics of Porphyromonas gingivalis within a model oral microbial community. BMC Microbiol. 9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuboniwa, M., et al. 2006. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol. Microbiol. 60:121-139. [DOI] [PubMed] [Google Scholar]
- 28.Kuramitsu, H. K., X. He, R. Lux, M. H. Anderson, and W. Shi. 2007. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 71:653-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau, P. C., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193-205. [DOI] [PubMed] [Google Scholar]
- 30.LeBlanc, D. J., V. L. Crow, L. N. Lee, and C. F. Garon. 1979. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J. Bacteriol. 137:878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemos, J. A., J. Abranches, and R. A. Burne. 2005. Responses of cariogenic streptococci to environmental stresses. Curr. Issues Mol. Biol. 7:95-107. [PubMed] [Google Scholar]
- 32.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludwig, H., N. Rebhan, H. M. Blencke, M. Merzbacher, and J. Stulke. 2002. Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol. Microbiol. 45:543-553. [DOI] [PubMed] [Google Scholar]
- 34.Marsh, P. D. 1994. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 8:263-271. [DOI] [PubMed] [Google Scholar]
- 35.Nobbs, A. H., Y. Zhang, A. Khammanivong, and M. C. Herzberg. 2007. Streptococcus gordonii Hsa environmentally constrains competitive binding by Streptococcus sanguinis to saliva-coated hydroxyapatite. J. Bacteriol. 189:3106-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paster, B. J., I. Olsen, J. A. Aas, and F. E. Dewhirst. 2006. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol. 2000 42:80-87. [DOI] [PubMed] [Google Scholar]
- 37.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi, F., P. Chen, and P. W. Caufield. 2001. The group I strain of Streptococcus mutans, UA140, produces both the lantibiotic mutacin I and a nonlantibiotic bacteriocin, mutacin IV. Appl. Environ. Microbiol. 67:15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi, F., J. Merritt, R. Lux, and W. Shi. 2004. Inactivation of the ciaH gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect. Immun. 72:4895-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reizer, J., et al. 1989. Mechanistic and physiological consequences of HPr(ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J. 8:2111-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers, J. D., and F. A. Scannapieco. 2001. RegG, a CcpA homolog, participates in regulation of amylase-binding protein A gene (abpA) expression in Streptococcus gordonii. J. Bacteriol. 183:3521-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosan, B., and R. J. Lamont. 2000. Dental plaque formation. Microbes Infect. 2:1599-1607. [DOI] [PubMed] [Google Scholar]
- 43.Russell, R. R., J. Aduse-Opoku, I. C. Sutcliffe, L. Tao, and J. J. Ferretti. 1992. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J. Biol. Chem. 267:4631-4637. [PubMed] [Google Scholar]
- 44.Ryan, C. S., and I. Kleinberg. 1995. Bacteria in human mouths involved in the production and utilization of hydrogen peroxide. Arch. Oral Biol. 40:753-763. [DOI] [PubMed] [Google Scholar]
- 45.Shaw, W. V. 1975. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 43:737-755. [DOI] [PubMed] [Google Scholar]
- 46.Shelburne, S. A., M. T. Davenport, D. B. Keith, and J. M. Musser. 2008. The role of complex carbohydrate catabolism in the pathogenesis of invasive streptococci. Trends Microbiol. 16:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson, C. L., and R. R. Russell. 1998. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect. Immun. 66:2085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutcliffe, I. C., S. D. Hogg, and R. R. Russell. 1993. Identification of Streptococcus mutans antigen D as the HPr component of the sugar-phosphotransferase transport system. FEMS Microbiol. Lett. 107:67-70. [DOI] [PubMed] [Google Scholar]
- 49.Tong, H., et al. 2007. Streptococcus oligofermentans inhibits Streptococcus mutans through conversion of lactic acid into inhibitory H2O2: a possible counteroffensive strategy for interspecies competition. Mol. Microbiol. 63:872-880. [DOI] [PubMed] [Google Scholar]
- 50.Tong, H., W. Chen, W. Shi, F. Qi, and X. Dong. 2008. SO-LAAO, a novel L-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone. J. Bacteriol. 190:4716-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vadeboncoeur, C., and M. Pelletier. 1997. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19:187-207. [DOI] [PubMed] [Google Scholar]
- 52.Van Hoogmoed, C. G., et al. 2008. Reduction of periodontal pathogens adhesion by antagonistic strains. Oral Microbiol. Immunol. 23:43-48. [DOI] [PubMed] [Google Scholar]
- 53.Wen, Z. T., and R. A. Burne. 2002. Analysis of cis- and trans-acting factors involved in regulation of the Streptococcus mutans fructanase gene (fruA). J. Bacteriol. 184:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng, L., and R. A. Burne. 2008. Multiple sugar: phosphotransferase system permeases participate in catabolite modification of gene expression in Streptococcus mutans. Mol. Microbiol. 70:197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng, L., and R. A. Burne. 2010. Seryl-phosphorylated HPr Regulates CcpA-Independent Carbon Catabolite Repression in Conjunction with PTS Permeases in Streptococcus mutans. Mol. Microbiol. 75:1145-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng, L., S. Das, and R. A. Burne. 2010. Utilization of lactose and galactose by Streptococcus mutans: transport, toxicity, and carbon catabolite repression. J. Bacteriol. 192:2434-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng, L., Y. Dong, and R. A. Burne. 2006. Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J. Bacteriol. 188:941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng, L., Z. T. Wen, and R. A. Burne. 2006. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans. Mol. Microbiol. 62:187-200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.