Abstract
Platelets are crucial elements for maintenance of hemostasis. Other functions attributable to platelets are now being appreciated, such as their role in inflammatory reactions and host defense. Platelets have been reported to bind immunological stimuli like IgG complexes, and for nearly 50 years it has been speculated that platelets may participate in immunological reactions. Platelets have been reported to bind and internalize various substances, similar to other leukocytes, such as macrophages and dendritic cells. In the present study, we tested the hypothesis that human platelets can bind and internalize IgG-coated particles, similar to leukocytes. To this end, we observed that interaction with IgG-coated beads resulted in platelet activation (as measured by CD62P expression), internalization of targets, and significant soluble CD40 ligand (sCD40L) and RANTES (regulated upon activation, normal T cell expresses and secreted) secretion. Blocking FcγRIIA with monoclonal antibody (MAb) IV.3 or inhibiting actin remodeling with cytochalasin D inhibited platelet activation, internalization, and cytokine production. These data suggest that platelets are capable of mediating internalization of IgG-coated particles, resulting in platelet activation and release of both sCD40L and RANTES.
Platelets are most commonly known for their important role in maintenance of vascular integrity and hemostasis. Subsequent to vascular injury, various platelet agonists and intrinsic factors are exposed to the blood, resulting in platelet activation and clot formation. Platelets can be activated by an array of agents, including collagen and thrombin. Platelets have also been reported to interact with various pathogens, including bacteria and viruses (12, 19, 35, 43), and platelet responses to other stimuli have been well characterized (7, 33, 37).
Consistent with these observations, sepsis patients often experience thrombocytopenia, indicating that platelets may participate in responding to infections (14, 24). Most studies of platelet-bacterium interactions have included platelet aggregation assays and measurement of the expression of activation markers. However, recent evidence suggests that platelets can respond to stimuli independent of traditional activation mechanisms resulting in production of cytokines and other inflammatory molecules (1).
Interaction of platelets with various particles has been reported to result in phagocytosis. For example, human platelets have been observed to phagocytose liposomes, erythrocyte fragments, some bacteria, viruses, and even latex beads (26, 28, 36, 43). Although the mechanism(s) for phagocytosis and the fate of internalized particles are disputed, nonetheless, platelets can sequester various targets (38, 39).
The ability of platelets to interact with bacteria has been suggested to be mediated via plasma proteins (e.g., fibrinogen and von Willebrand factor) or by specific antibody recognized by platelet Fcγ receptor (FcγR). Platelets express FcγRIIA, a receptor for IgG, which mediates the recognition of immune complexes and IgG-coated targets (18, 21, 22, 31). In contrast to leukocytes that express a variety of FcγRs, platelets express only FcγRIIA. Although FcγRIIA is expressed by leukocytes, such as monocytes, neutrophils, and dendritic cells, due to the vast number of platelets in the circulation, nearly 90% of FcγRIIA in blood is present on platelets.
Platelet FcγRIIA has been shown to bind and endocytose IgG complexes (1, 32, 40). Furthermore, platelets have been shown to interact with IgG-coated beads and IgG has been reported to be the factor by which many platelets interact with bacteria (6, 12, 13, 25, 29). Therefore, we sought to determine the functional response of platelets to IgG-coated targets. Using IgG-coated polystyrene beads, our observations were limited to FcγRIIA-mediated activity without complication due to other receptors (e.g., Toll-like receptor [TLR], CD36, mannose receptor, and scavenger receptor).
To examine the functional response of platelets to IgG-coated targets, a variety of parameters were measured. Upon binding, platelet activation was measured by CD62P (P-selectin) expression. CD62P is found in α-granules and is rapidly exposed on the platelet surface upon activation by fusion of α-granules with the plasma membrane. We next measured the capacity of platelets to internalize the IgG-coated targets by fluorescence microscopy and flow cytometry, established techniques that can discriminate between bound and internalized targets and which are used to monitor leukocyte phagocytosis (41, 42). Finally, since platelets secrete agents such as soluble CD40 ligand (sCD40L) (CD154) and RANTES (regulated upon activation, normal T cell expresses and secreted) in response to coagulation factors and IgG complexes, we investigated the relationship between platelet activation and cytokine secretion stimulated by IgG-coated targets (2, 4, 5, 9, 11, 15, 16, 20, 23, 30, 34). We report here that stimulation of platelets by IgG-coated targets results in substantial CD62P expression, internalization of the targets, and release of significant amounts of sCD40L and RANTES, further supporting a role for platelets in inflammatory and host defense responses.
MATERIALS AND METHODS
Platelet isolation.
Human platelets were isolated from the blood of healthy donors obtained by venipuncture in accordance with the University of Toledo Biomedical IRB. Blood was drawn into Vacutainers containing 10% ACD solution (B-D, Franklin Lakes, NJ): 56 mM sodium citrate, 65 mM citric acid, and 100 mM glucose. The blood was centrifuged at 200 × g for 10 min to obtain platelet-rich plasma (PRP). The PRP fraction was collected and washed in pH 6.5 buffer containing: 2.75 g/liter sodium citrate, 1.0 g/liter citric acid, 3.2 g/liter glucose, and 8.5 g/liter sodium chloride and mixed well, and the pH was adjusted to 6.5. After being washed, platelets were kept in the buffer and stored for no more than 30 min before use. For experiments, platelets were resuspended in pH 7.4 buffer containing 8.0 g/liter sodium chloride, 0.2 g/liter potassium chloride, 0.2 g/liter magnesium chloride, 0.45 g/liter sodium phosphate dibasic, 0.9 g/liter HEPES, 3.5 g/liter bovine serum albumin, and 1.0 g/liter glucose and adjusted to pH 7.4. The platelets were then placed in a 37°C water bath for stimulation.
Polystyrene bead opsonization.
Polystyrene beads, 0.5 or 1.5 μm in diameter (Polysciences, Warrington, PA), were opsonized for 90 min at 37°C in a 5-mg/ml solution containing either bovine serum albumin (BSA) as a control or a mixture of 0.5 mg/ml fluorescein isothiocyanate (FITC)-labeled human IgG (SigmaAldrich, St. Louis, MO) and 9.5 mg/ml unlabeled human IgG (Sigma, St. Louis, MO). After being washed three times in phosphate-buffered saline (PBS), small aliquots were reacted with phycoerythrin (PE)-conjugated anti-human IgG to confirm opsonization and observed with a Zeiss Axiovert 200 fluorescence microscope (Carl Zeiss, Thronwood, NY), using bandpass filters for FITC (excitation, 480DF22; and emission, 530DF30) and PE (excitation, 530DF25; and emission, 560LP) (Chroma Technologies, Bellows Falls, VT).
Platelet activation.
Platelets were stimulated with 0.5-μm or 1.5-μm beads opsonized with bovine serum albumin (BSA) or IgG in the presence or absence of 2 U/ml thrombin (Sigma-Aldrich, St. Louis, MO) as a positive control for platelet activation (target/effector ratio of 10:1). Platelets and beads were incubated for 30 min at 4°C and then placed in a 37°C water bath for 30 min. Samples were returned to ice, fixed for 1 h in 2% paraformaldehyde, washed, and then labeled with PE-Cy5-conjugated anti-CD62P (BD Biosciences, San Jose, CA) and allophycocyanin (APC)-conjugated anti-CD42b (BD Biosciences) for 30 min on ice. Platelet samples were then washed twice in PBS and analyzed with a FACSCalibur (BD Biosciences) flow cytometer. Data were analyzed with Cell Quest (BD Biosciences) and FloJo (Tree Star, Inc., Ashland, OR) software.
IgG-coated bead internalization.
Platelets in pH 7.4 buffer were exposed to IgG-opsonized beads at a target/effector cell ratio of 10:1. The platelets were allowed to bind IgG-coated targets on ice for 30 min and then placed in a 37°C water bath for 30 min to allow for phagocytosis. Following the incubation, aliquots were placed on ice and reacted with APC-conjugated anti-CD42b (BD Biosciences, San Jose, CA) and PE-conjugated anti-human IgG (Rockland Immunochemicals, Gilbertsville, PA) for 30 min on ice. Platelets were then fixed for 1 h using 2% paraformaldehyde and washed in pH 6.5 buffer.
Samples were prepared for flow cytometry and fluorescence microscopy. For fluorescence microscopy, aliquots of the platelet-bead suspension were placed onto glass coverslips (no. 1, 25 mm in diameter; Corning Life Sciences, Lowell, MA), mounted on glass microscope slides (Corning Life Sciences), and visualized with a Zeiss Axiovert 200 fluorescence microscope (Carl Zeiss, Thornwood, NY), using mercury illumination. Cells were visualized by differential interference contrast (DIC) or fluorescence, using the filter sets described above. Images were captured using an Orca ER-AG (Hamamatsu, Japan) charge-coupled device (CCD) camera connected to a Dell Optiplex 620 workstation (Dell, Round Rock, TX). Metamorph software (Molecular Devices, Downingtown, PA) was used to acquire and process images. For flow cytometry, platelets with associated beads were gated on FITC (CD42b+ FITC+) and analyzed for internal/external by the absence/presence of PE expression on a FACSCalibur (B-D Biosciences). Samples reacted with individual fluorophores and isotype-matched control antibodies were used for instrument and compensation settings. Data were analyzed using Cell Quest (B-D Biosciences) and FloJo (Tree Star, Inc., Ashland, OR) software.
Platelet secretion.
Following activation, platelet supernatants were isolated by a modified approach based on those previously described (8). Briefly, following activation, platelets were centrifuged at 1,000 × g for 10 min and the supernatant was harvested. Subsequently, samples were centrifuged at 5,000 × g and supernatants were harvested. Samples were stored at −80°C for up to 2 weeks before analysis of released products. Enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) was performed per the manufacturer's recommendation to measure the amounts of sCD40L and RANTES released from platelets stimulated with 2 U of thrombin, BSA-coated beads, or IgG-coated beads.
RESULTS
FcγRIIA is responsible for platelet interaction with IgG-coated targets.
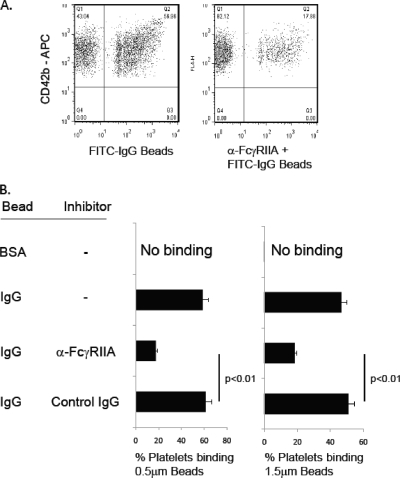
Platelets have many membrane receptors, including scavenger receptors (e.g., CD36, SR-A, and SR-B) and TLRs, which can mediate interaction with particles containing an array of ligands. To limit our study to the activity of FcγRIIA, we employed polystyrene beads (0.5 and 1.5 μm) coated with FITC-conjugated human IgG. Previous studies analyzing FcγRIIA function have reported significantly different signaling mechanisms required to internalize IgG complexes (∼50 nm) or IgG-coated beads (2 μm). Therefore, we utilized beads of two different sizes (0.5 and 1.5 μm) to determine if platelets respond differently to large and small targets (3, 17, 27). Platelets interacting with beads can be measured by both fluorescence microscopy and flow cytometry. In initial experiments, the number of platelets interacting with FITC-IgG-coated beads was determined by flow cytometry. As shown in Fig. 1, ∼58% of platelets (identified by CD42b staining) were found to associate with FITC-IgG-coated beads (upper right quadrant in Fig. 1A). This interaction can be significantly inhibited (∼70% inhibition) by addition of a monoclonal antibody (MAb), IV.3, that blocks FcγRIIA (Fig. 1A and B) but not an isotype-matched control IgG. Furthermore, beads coated with FITC-BSA do not interact with platelets (Fig. 1B).
FIG. 1.
Platelet FcγRIIA mediates binding of IgG-coated targets. (A) The 1.5-μm polystyrene beads coated with FITC-IgG were added to platelets for 30 min at 4°C in the absence (left) or presence (right) of 5 μg/ml anti-FcγRIIA monoclonal antibody IV.3. The platelets were then labeled with anti-CD42b-APC, and CD42+ events were measured for the presence of FITC-labeled beads. (B) Platelets incubated with 0.5- or 1.5-μm polystyrene beads coated with BSA or IgG. In some samples, 5 μg/ml anti-FcγRIIA MAb or an isotype-matched control IgG (5 μg/ml) was added to inhibit interaction. Data are means ± standard deviations (SD) representative of one experiment performed in triplicate. Experiments were repeated on 3 different days with platelets from three healthy volunteers with similar results.
IgG-coated beads induce platelet activation.
In response to various stimuli, platelets have been shown to express defined surface markers indicative of activation. The most common markers include surface expression of CD62P (P-selectin), annexin V binding (phosphatidylserine), and the active conformation of αIIbβ3. CD62P is a component of platelet α-granules that becomes surface exposed after platelet activation when α-granules are released. We employed monoclonal antibodies (MAbs) to CD62P to determine the activation status of platelets stimulated with thrombin or polystyrene beads coated with BSA or IgG.
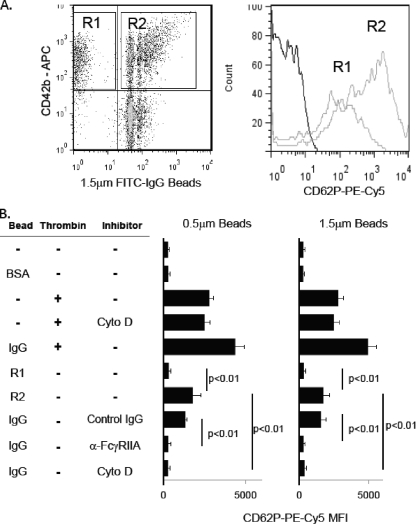
As shown in Fig. 2 A and B, platelets exposed to IgG-coated polystyrene beads showed a significant increase in CD62P surface exposure, as measured by binding of PE-Cy5-tagged anti-CD62P. Platelets interacting with IgG-coated beads (Fig. 2A, region R2; CD42+ FITC+) exhibited significantly more CD62P expression than platelets from the same tube that have not interacted with beads (Fig. 2A, region R1; CD42b+ FITC−). The amount of activation measured by the mean fluorescence intensity of anti-CD62P binding in R2 is less than that observed with 2 U/ml of thrombin (Fig. 2B) or by platelets stimulated with aggregated IgG (1). Interestingly, exposure of platelets to thrombin and IgG-coated beads simultaneously enhanced expression of CD62P compared to either alone, but the enhancement was not synergistic or additive at the indicated concentration (Fig. 2B) or other concentrations (not shown). Pretreatment of platelets with either anti-FcγRIIA MAb IV.3, but not isotype-matched control IgG, inhibited binding of IgG-coated beads (Fig. 1), thus resulting in low levels of activation. In other samples, platelets were allowed to bind beads and then treated with 20 μM cytochalasin D, which prevents actin rearrangement. Of note, cytochalasin D did not prevent thrombin-induced activation (not shown) but did significantly inhibit IgG-coated-bead-induced activation. These data suggest platelet activation, as measured by CD62P expression, may be dependent on internalization of targets rather than simply binding targets, although further investigation of this observation is necessary.
FIG. 2.
IgG-coated targets activate platelets. (A) Platelets were exposed to IgG-coated 1.5-μm polystyrene beads, and CD42b-expressing platelets were assessed for activation by measuring CD62P surface expression (gray lines) or isotype-matched control IgG (black line) on platelets not in contact with beads (region R1) or in contact with beads (region R2). (B) Quantitative analysis of CD62P expression of CD42b-expressing platelets incubated with 0.5- or 1.5-μm polystyrene beads coated with BSA or IgG. Platelets incubated with 2 U/ml thrombin served as a positive control for platelet activation. Some samples were pretreated with either 5 μg/ml anti-FcγRIIA MAb IV.3 or 5 μg/ml isotype-matched control IgG to assess the dependence on FcγRIIA. Other samples were allowed to bind beads and then incubated with 20 μM cytochalasin D (Cyto D) to determine the role of actin rearrangement on activation. Data are means ± SD representative of one experiment performed in triplicate. Experiments were repeated on 3 different days with platelets from three healthy volunteers with similar results. MFI, mean fluorescence intensity.
Platelets internalize IgG-coated beads.
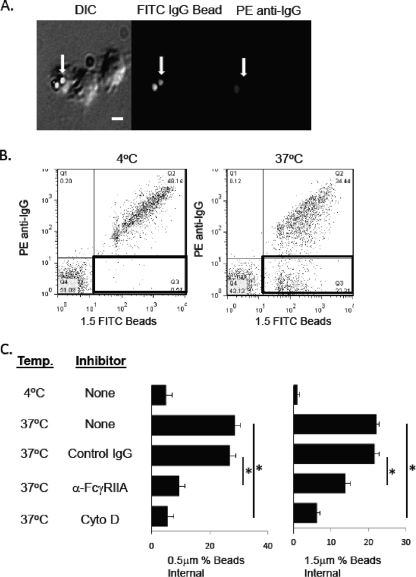
Platelets exposed to IgG-coated beads of either 0.5 μm or 1.5 μm were examined to determine whether platelets have the ability to internalize the beads. To do this, platelets were mixed with IgG-coated beads and then visualized by fluorescence microscopy and analyzed by flow cytometry to measure platelet internalization of beads. As shown in Fig. 3 A, platelets bound and internalized IgG-coated beads. Internalized beads were discriminated from externally bound beads by using secondary PE-conjugated anti-human IgG F(ab′)2 fragments after fixing the platelets in paraformaldehyde. Using this technique, beads bound to the outside of platelets are stained with the PE antibody, while the internalized beads are not available for reaction with the secondary antibody and thus do not fluoresce (Fig. 3A, arrows).
FIG. 3.
Platelet internalization of IgG-coated beads. (A) Platelets were incubated with FITC-IgG-coated polystyrene beads (0.5 μm) at 4°C for 30 min and then warmed to 37°C for 30 min. Samples were then cooled to 4°C, fixed with 2% paraformaldehyde, stained with PE-conjugated anti-human IgG, and viewed by fluorescence microscopy for double-positive (external) or single-positive (internal) beads (arrows point to single-positive beads). (B) Flow cytometric analysis of internalization of IgG-coated 1.5-μm beads by platelets. Platelets were incubated with beads as described in panel A and then stained with APC-conjugated anti-CD42b MAb and PE-conjugated anti-human IgG. CD42b+ platelets were assessed for FITC and PE fluorescence by flow cytometry. Double-positive (external) and single-positive (internal) beads are shown in the upper right and lower right quadrants, respectively. (C) Internalization of IgG-coated beads by platelets. Samples treated as described in panel A were assessed for internalization by dividing the number of PE-negative beads (lower right quadrant) by the total number of beads (upper and lower right quadrants). Some samples were treated with 20 μM cytochalasin D (Cyto D) to inhibit actin rearrangement. Data are means ± SD representative of one experiment performed in triplicate. Experiments were repeated on 3 different days with platelets from three healthy volunteers with similar results. *, P < 0.01.
Using flow cytometry, platelets were further assessed for their ability to internalize IgG-coated beads. Platelets were exposed to FITC-IgG-coated polystyrene beads (or FITC-BSA-coated polystyrene beads as a control) on ice for 30 min; aliquots were warmed to 37°C in a water bath for 30 min to stimulate internalization. Samples were then placed on ice and fixed for 1 h with 2% paraformaldehyde. After being washed, samples were stained with APC-conjugated anti-CD42b and PE-conjugated anti-human IgG F(ab′)2 fragments, washed, and then analyzed by flow cytometry. CD42b+ events were gated on FITC to identify platelets that had interacted with beads (CD42b+ FITC+). As shown by a diminished PE signal shown in Fig. 3B and C, platelets can efficiently internalize IgG-coated polystyrene beads (Fig. 3B, lower right quadrant). Internalization takes place through actin-dependent mechanisms since incubation with 20 μM cytochalasin D blocked uptake by >75% (Fig. 3C).
IgG-coated beads induce cytokine release from platelets.
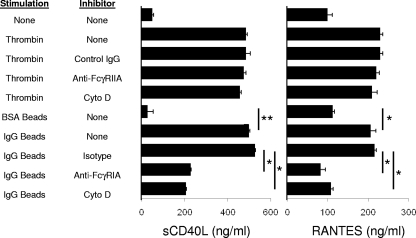
Platelets release multiple inflammatory molecules in response to various agonists in the clotting cascade (9). sCD40L (CD154) and RANTES (CCL5) are among the most abundant and commonly detected inflammatory mediators released by platelets. Therefore, ELISA was used to measure platelet-derived sCD40L and RANTES following activation by thrombin or IgG-coated polystyrene beads. Activation of platelets by thrombin resulted in high levels of CD154 sCD40L and RANTES released into the media (Fig. 4). Exposure of platelets to 0.5-μm or 1.5-μm IgG-coated beads results in platelet activation and triggered release of both sCD40L and RANTES into the media, while beads coated with BSA did not induce secretion of either sCD40L or RANTES. Consistent with the ability of cytochalasin D to inhibit platelet activation by IgG-coated beads, sCD40L and RANTES release was also inhibited by cytochalasin D, suggesting that cytokine release relies on internalization and not simply bead association. Moreover, cytochalasin D did not inhibit thrombin-induced cytokine release. We interpret these data to suggest that bead internalization is required for cytokine secretion.
FIG. 4.
Platelet cytokine secretion in response to IgG-coated targets. Platelets were stimulated with thrombin (20 U/ml) or 1.5-μm polystyrene beads (10 beads per platelet) coated with BSA or IgG and assessed for secretion of sCD40L and RANTES by ELISA. Other samples were pretreated with 5 μg/ml anti-FcγRIIA MAb or 5 μg/ml isotype-matched control IgG to assess the role of FcγRIIA. Other samples were pretreated with 20 μM cytochalasin D (Cyto D) to determine the role of actin rearrangement in cytokine secretion. Data are means ± SD representative of one experiment performed in triplicate. Experiments were repeated on 5 different days with platelets from five healthy volunteers with similar results. *, P < 0.01; **, P < 0.001.
DISCUSSION
The role of platelets in hemostasis is well established, but additional roles for platelets in various other physiologic processes, including host defense and inflammation, are not fully appreciated. Platelet activation has been described in response to many different stimuli, most extensively clotting factors such as thrombin and collagen.
Although observations of thrombosis in various inflammatory capacities were made nearly 50 years ago and platelets have been demonstrated to respond to immunological stimuli of various forms, the exact role that platelets play in these situations is only recently becoming more clear.
The capacity of platelets as phagocytic cells has been widely disputed. Many studies have shown that platelets appear to internalize particles but that these particles are located in the open canalicular system (OCS), as opposed to undergoing internalization into a phagosome (26, 28, 38, 43). These studies differ in their target choices, preparation techniques, and staining methods. Based on our previous observations in leukocytes obtained by identical fluorescence methods, we believe that the target is in a compartment that is not in communication with the extracellular environment and therefore not within the OCS. Our observations may be related to internalization through FcγRIIA, which is a very potent phagocytic receptor compared to some other innate surface receptors. Even so, the compartment may be part of the OCS that is now serving as a receptacle for internalized targets. Regardless of the compartment's composition/origin, the platelet is sequestering targets presumably in an attempt to prevent further dissemination.
During the process of phagocytosis, we observed a significant amount of sCD40L and RANTES being produced by platelets. Platelets have been reported to secrete inflammatory mediators after stimulation with immune complexes and thrombin (1). Platelets have also been shown to secrete an array of cytokines in response to thrombin, collagen, and ADP stimulation. Many of these cytokines may indeed be secreted by platelets, but some could be produced by small numbers of leukocytes remaining in the platelet preparation. We chose two molecules (sCD40L and RANTES) to analyze in these studies based on the number of reports that their release by platelets and the amount of each molecule released into the media suggest that they are indeed of platelet origin and not from contaminating leukocytes. After stimulation with thrombin, we observed a significant increase in both sCD40L (CD154) and RANTES (CCL5) by ELISA. Although IgG-coated beads stimulated platelets to secrete significant amounts of sCD40L and RANTES, the total amount is less than that of the thrombin-stimulated platelets. This observation could be due to the fact that presumably all platelets were stimulated by thrombin, while only a fraction are activated by IgG-coated targets. A second explanation is that thrombin binds to receptors covering the entire platelet surface, while only 1 to 2 IgG-coated beads bind to an individual platelet, suggesting that platelets may be release more sCD40L and RANTES during “global” stimulation versus “targeted” stimulation.
The fact that cytochalasin D inhibited platelet activation (measured by CD62P) and release of sCD40L and RANTES suggests that both responses are dependent upon conformational alteration of the actin cytoskeleton. However, cytochalasin D has been observed to affect platelet activation (measured by CD62P, CD62, and annexin V) in response to collagen as an agonist but not thrombin, suggesting that cytochalasin blocks actin-dependent signal transduction but not vesicle release (10). Therefore, we believe that our results are due to cytochalasin D blocking internalization of IgG-coated targets and not by blocking vesicle fusion. However, we cannot rule out that inhibition of α-granule fusion is not taking place. Further studies into the mechanisms of α-granule release need to be performed.
Acknowledgments
We thank the University of Toledo Clinical Cytometry Laboratory for assistance with designing flow cytometry experiments and William Gunning for critical reading of the manuscript.
This work is supported by an Arthritis Foundation Investigator Award (to R.G.W.) and a Translational Research Stimulation Award from the University of Toledo College of Medicine (to R.G.W.).
We have no competing financial interests.
Footnotes
Published ahead of print on 22 December 2010.
REFERENCES
- 1.Antczak, A. J., N. Singh, S. R. Gay, and R. G. Worth. 2010. IgG-complex stimulated platelets: a source of sCD40L and RANTES in initiation of inflammatory cascade. Cell. Immunol. 263:129-133. [DOI] [PubMed] [Google Scholar]
- 2.Boehlen, F., and K. J. Clemetson. 2001. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus. Med. 11:403-417. [DOI] [PubMed] [Google Scholar]
- 3.Booth, J. W., M. K. Kim, A. Jankowski, A. D. Schreiber, and S. Grinstein. 2002. Contrasting requirements for ubiquitylation during Fc receptor-mediated endocytosis and phagocytosis. EMBO J. 21:251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt, E., A. Ludwig, F. Petersen, and H. D. Flad. 2000. Platelet-derived CXC chemokines: old players in new games. Immunol. Rev. 177:204-216. [DOI] [PubMed] [Google Scholar]
- 5.Bubel, S., D. Wilhelm, M. Entelmann, H. Kirchner, and H. Kluter. 1996. Chemokines in stored platelet concentrates. Transfusion 36:445-449. [DOI] [PubMed] [Google Scholar]
- 6.Byrne, M. F., et al. 2003. Helicobacter pylori binds von Willebrand factor and interacts with GPIb to induce platelet aggregation. Gastroenterology 124:1846-1854. [DOI] [PubMed] [Google Scholar]
- 7.Cognasse, F., et al. 2008. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br. J. Haematol. 141:84-91. [DOI] [PubMed] [Google Scholar]
- 8.Coppinger, J. A., et al. 2004. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 103:2096-2104. [DOI] [PubMed] [Google Scholar]
- 9.Coppinger, J. A., et al. 2007. Moderation of the platelet releasate response by aspirin. Blood 109:4786-4792. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Ricart, M., et al. 2002. Inhibition of cytoskeletal assembly by cytochalasin B prevents signaling through tyrosine phosphorylation and secretion triggered by collagen but not by thrombin. Am. J. Pathol. 160:329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elzey, B. D., et al. 2003. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 19:9-19. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald, J. R., T. J. Foster, and D. Cox. 2006. The interaction of bacterial pathogens with platelets. Nat. Rev. Microbiol. 4:445-457. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald, J. R., et al. 2006. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol. Microbiol. 59:212-230. [DOI] [PubMed] [Google Scholar]
- 14.Franchini, M., and D. Veneri. 2004. Helicobacter pylori infection and immune thrombocytopenic purpura: an update. Helicobacter 9:342-346. [DOI] [PubMed] [Google Scholar]
- 15.Henn, V., et al. 1998. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391:591-594. [DOI] [PubMed] [Google Scholar]
- 16.Holme, P. A., et al. 1998. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 12:79-89. [DOI] [PubMed] [Google Scholar]
- 17.Huang, Z. Y., et al. 2006. Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis. J. Leukoc. Biol. 80:1553-1562. [DOI] [PubMed] [Google Scholar]
- 18.Israels, E. D., G. Nisli, F. Paraskevas, and L. G. Israels. 1973. Platelet Fc receptor as a mechanism for Ag-Ab complex-induced platelet injury. Thromb. Diath. Haemorrh. 29:434-444. [PubMed] [Google Scholar]
- 19.Jerushalmy, Z., A. Kohn, and A. De Vries. 1961. Interaction of myxoviruses with human blood platelets in vitro. Proc. Soc Exp. Biol. Med. 106:462-466. [DOI] [PubMed] [Google Scholar]
- 20.Kameyoshi, Y., A. Dorschner, A. I. Mallet, E. Christophers, and J. M. Schroder. 1992. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J. Exp. Med. 176:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelton, J. G., J. W. Smith, A. V. Santos, W. G. Murphy, and P. Horsewood. 1987. Platelet IgG Fc receptor. Am. J. Hematol. 25:299-310. [DOI] [PubMed] [Google Scholar]
- 22.King, M., P. McDermott, and A. D. Schreiber. 1990. Characterization of the Fc gamma receptor on human platelets. Cell. Immunol. 128:462-479. [DOI] [PubMed] [Google Scholar]
- 23.Klinger, M. H., et al. 1995. Immunocytochemical localization of the chemokines RANTES and MIP-1 alpha within human platelets and their release during storage. Int. Arch. Allergy Immunol. 107:541-546. [DOI] [PubMed] [Google Scholar]
- 24.Levi, M., T. T. Keller, E. van Gorp, and H. ten Cate. 2003. Infection and inflammation and the coagulation system. Cardiovasc. Res. 60:26-39. [DOI] [PubMed] [Google Scholar]
- 25.Loughman, A., et al. 2005. Roles for fibrinogen, immunoglobulin and complement in platelet activation promoted by Staphylococcus aureus clumping factor A. Mol. Microbiol. 57:804-818. [DOI] [PubMed] [Google Scholar]
- 26.Male, R., W. E. Vannier, and J. D. Baldeschwieler. 1992. Phagocytosis of liposomes by human platelets. Proc. Natl. Acad. Sci. U. S. A. 89:9191-9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mero, P., et al. 2006. Phosphorylation-independent ubiquitylation and endocytosis of Fc gammaRIIA. J. Biol. Chem. 281:33242-33249. [DOI] [PubMed] [Google Scholar]
- 28.Movat, H. Z., W. J. Weiser, M. F. Glynn, and J. F. Mustard. 1965. Platelet phagocytosis and aggregation. J. Cell Biol. 27:531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller-Eckhardt, C., and E. F. Luscher. 1968. Immune reactions of human blood platelets. II. The effect of latex particles coated with gammaglobulin in relation of complement activation. Thromb. Diath. Haemorrh. 20:168-179. [PubMed] [Google Scholar]
- 30.Power, C. A., J. M. Clemetson, K. J. Clemetson, and T. N. Wells. 1995. Chemokine and chemokine receptor mRNA expression in human platelets. Cytokine 7:479-482. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld, S. I., et al. 1985. Human platelet Fc receptor for immunoglobulin G. Identification as a 40,000-molecular-weight membrane protein shared by monocytes. J. Clin. Invest. 76:2317-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schattner, M., et al. 1993. Activation of human platelets by immune complexes prepared with cationized human IgG. Blood 82:3045-3051. [PubMed] [Google Scholar]
- 33.Shibazaki, M., M. Nakamura, and Y. Endo. 1996. Biphasic, organ-specific, and strain-specific accumulation of platelets induced in mice by a lipopolysaccharide from Escherichia coli and its possible involvement in shock. Infect. Immun. 64:5290-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth, S. S., et al. 2009. Platelet functions beyond hemostasis. J. Thromb. Haemost. 7:1759-1766. [DOI] [PubMed] [Google Scholar]
- 35.Terada, H., M. Baldini, S. Ebbe, and M. A. Madoff. 1966. Interaction of influenza virus with blood platelets. Blood 28:213-228. [PubMed] [Google Scholar]
- 36.Ts'ao, C. H., D. Green, and K. Schultz. 1976. Function and ultrastructure of platelets of neonates: enhanced ristocetin aggregation of neonatal platelets. Br. J. Haematol. 32:225-233. [DOI] [PubMed] [Google Scholar]
- 37.Warkentin, T. E., et al. 1994. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood 84:3691-3699. [PubMed] [Google Scholar]
- 38.White, J. G. 2005. Platelets are covercytes, not phagocytes: uptake of bacteria involves channels of the open canalicular system. Platelets 16:121-131. [DOI] [PubMed] [Google Scholar]
- 39.White, J. G. 2006. Why human platelets fail to kill bacteria. Platelets 17:191-200. [DOI] [PubMed] [Google Scholar]
- 40.Worth, R. G., et al. 2006. Platelet FcgammaRIIA binds and internalizes IgG-containing complexes. Exp. Hematol. 34:1490-1495. [DOI] [PubMed] [Google Scholar]
- 41.Worth, R. G., et al. 2001. The cytoplasmic domain of FcgammaRIIA (CD32) participates in phagolysosome formation. Blood 98:3429-3434. [DOI] [PubMed] [Google Scholar]
- 42.Worth, R. G., L. Mayo-Bond, J. G. van de Winkel, R. F. Todd III, and H. R. Petty. 1996. CR3 (alphaM beta2; CD11b/CD18) restores IgG-dependent phagocytosis in transfectants expressing a phagocytosis-defective Fc gammaRIIA (CD32) tail-minus mutant. J. Immunol. 157:5660-5665. [PubMed] [Google Scholar]
- 43.Youssefian, T., A. Drouin, J. M. Masse, J. Guichard, and E. M. Cramer. 2002. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood 99:4021-4029. [DOI] [PubMed] [Google Scholar]