Abstract
In 2007, recommendations were proposed for the molecular typing of meningococci. Multilocus sequence typing (MLST) was recommended to guide national and international disease management and facilitate studies of population biology and evolution. Sequencing of porA variable regions (VRs) 1 and 2 and the fetA VR was recommended for monitoring antigenic distribution and investigating potential outbreaks. porB characterization was recommended if further resolution was required. Several investigational “group B” meningococcal vaccines, including two in the advanced stages of development, incorporate factor H-binding protein (fHBP). The requirement for routine surveillance of fhbp places additional pressure on reference laboratories, both financially and in terms of labor. This study investigated the optimal and most efficient molecular typing schemes for (i) routine meningococcal characterization and (ii) the investigation of potential outbreaks, in conjunction with routine surveillance of fhbp. All invasive disease isolates received by the Health Protection Agency Meningococcal Reference Unit between July 2007 and June 2008 (n = 613) were characterized in terms of capsular group, porA, fetA VR, fhbp, and sequence type (ST). Following capsular grouping and porA genosubtyping, several predominant capsular group-porA combinations were identified. The levels of additional resolution afforded by fetA and fhbp were comparable and partially complementary. fhbp constitutes an effective substitute for fetA as a routine marker of antigenic distribution, thereby reducing costs in conjunction with fhbp surveillance. MLST afforded markedly superior resolution overall and is the optimal scheme for investigating outbreaks in which (i) typing data are unavailable for the index case or (ii) the index case possesses a known, predominant capsular group-porA repertoire.
Neisseria meningitidis (the meningococcus) causes meningitis and septicemia, with an associated mortality approaching 10% and severe physical and neurological sequelae in approximately 20% of survivors (25). In previously healthy individuals, invasive disease is almost exclusively associated with encapsulated meningococci. It is this capsule (or lack thereof) that forms the basis of the primary classification scheme termed “grouping” (34). The vast majority of invasive meningococcal disease (IMD) is caused by just five (out of 13 known) meningococcal capsular groups, A, B, C, W135, and Y, each displaying distinct geographical tendencies (13). In addition to being the principal epidemiological marker, the existence of glycoconjugate vaccines against groups A, C, W135, and Y (23) bestows upon grouping an important role in directing the management of case contacts. With its relatively low resolution, however, grouping is of limited use when investigating potential outbreaks, especially in countries such as the United Kingdom, in which 90% of IMD is due to group B meningococci (MenB) (17, 18). Increased resolution has traditionally been obtained by immunological characterization of the major outer membrane proteins (OMPs) PorB (serotyping) and PorA (serosubtyping) (9). Genotypic characterization of these antigens increases resolution further and captures variants for which immunological reagents are unavailable (21). Further resolution may be achieved by genotyping additional antigens, e.g., FetA (previously FrpB), a diverse and immunogenic iron-regulated OMP with a variable region (VR) comparable to those of PorA (30). Another genotypic technique, multilocus sequence typing (MLST), involves genetic analysis of ∼500-bp regions of seven housekeeping genes distributed around the genome (6, 20). These genes, unlike those encoding PorA and other surface antigens, are under stabilizing selection, making MLST ideal for tracking long-term epidemiology on national and international scales (2).
In addition to providing enhanced resolution, genotypic methods are rapid, highly portable, and readily adapted for use with noncultured specimens, the proportion of which have increased in recent years (11) probably due to early antibiotic intervention (4). Therefore, where phenotypic data are not specifically required, molecular typing is regarded as the routine typing method of choice (15). To harmonize typing among reference laboratories, recommendations have been published regarding both the choice of targets and the nomenclature employed (15). MLST was recommended for application to (at least) a representative proportion of case and carriage isolates to guide national and international disease management and to facilitate studies of population biology and evolution. For investigating potential outbreaks and monitoring antigenic distribution, the porA VRs 1 and 2 and the fetA VR were recommended. porB genotyping was recommended where additional resolution is required, but this was dropped from the recommended nomenclature that takes the following form: sero-/genogroup:porA VR1,porA VR2:fetA VR:MLST sequence type (ST) (clonal complex [cc]), e.g., B:P1.19-1,15-11:F5-1:ST269 (cc269). The porA semivariable region VR3 was omitted from the proposed scheme, as it is less diverse and considered to add little information for the additional work its determination entails (15). The recommendation of porB genotyping is, perhaps, surprising. Unlike those of PorA, the epitopes of PorB are largely nonlinear and correspond to up to six variable regions (32, 36). The scheme is therefore relatively complex, entails an extended sequencing protocol (15, 31), and currently lacks a standardized nonculture procedure (8). Uptake of porB genotyping has been low among European reference laboratories, only two of which returned porB genotyping data for the 1st EU-IBIS external quality assessment (EQA) culture distribution. No data were subsequently reported for the 2nd distribution (5). As such, the genotyping of porB has not been adopted by the Health Protection Agency Meningococcal Reference Unit (HPA MRU).
Since the MenB capsule has largely been abandoned as a potential vaccine candidate, due to its poor immunogenicity in humans and the potential for eliciting autoimmune antibodies (35), a number of protein alternatives are currently being investigated. Two such investigational vaccines, in the advanced stages of development, are Novartis Vaccines' four-component MenB (4CMenB; previously rMenB-OMV) investigational vaccine (10) and the Pfizer (formerly Wyeth Vaccine Research) investigational MenB vaccine (24), currently undergoing phase III and phase II clinical trials, respectively. A common component to both of these vaccines is factor H-binding protein (fHBP; previously known as genome-derived neisserial antigen 1870 [GNA1870] or lipoprotein 2086 [LP2086]). fHBP is a virulence factor that recruits human factor H to the bacterial surface, thereby downregulating complement-mediated killing (19, 26). It exists in two immunologically distinct subfamilies, subfamily A (comprising phylogenetic variant groups 2 and 3) and subfamily B (comprising phylogenetic variant group 1) (7, 22). Nomenclature schemes for individual subvariants are available on the fHBP allele and peptide database hosted at http://pubmlst.org/neisseria. Another investigational vaccine with an fHBP component (currently undergoing phase I clinical trials) is that of the Walter Reed Army Institute of Research (37).
The Joint Committee on Vaccination and Immunization has, therefore, recommended modification of the current English and Welsh surveillance program (27) to incorporate the routine genotypic characterization of fhbp. The expansion of the current system would put additional pressure on reference laboratories both financially and in terms of labor. If fhbp (for which an established and extensive database is hosted at http://Neisseria.org) should be found to provide equal or greater resolution compared with fetA, then it may be argued that routine characterization of fetA, for the purpose of general antigenic monitoring and outbreak investigation, is in excess of the requirement and may be discontinued. This is especially the case if both antigens are found to resolve the same isolates. Conversely, if they were found to complement one another in their resolving power or if a FetA vaccine is introduced, it may be deemed beneficial, or indeed necessary, to retain both in routine typing.
This study aimed to determine the optimal and most efficient molecular typing schemes for (i) routine meningococcal characterization and (ii) the investigation of potential outbreaks, in conjunction with the routine surveillance of fhbp. The resolving power of fetA, fhbp, MLST, and combinations thereof were determined across the full range of invasive disease isolates submitted to the HPA MRU during the epidemiological year from July 2007 to June 2008. The cumulative resolving power of (i) the capsular group, (ii) porA VRs 1 and 2, and (iii) fetA and/or fhbp and/or the ST were also investigated among the predominant lineages identified. The additional resolution afforded by porA VR3 was also investigated, and we report on the outcomes of case cluster (two or more spatially related cases of probable or confirmed invasive disease with dates of onset within 4 weeks) investigations carried out by the HPA MRU over the period studied and how these relate to the overall study findings.
MATERIALS AND METHODS
Specimens.
Isolates used in the study (n = 613) consisted of all English and Welsh invasive disease isolates received by the HPA MRU during the epidemiological year from July 2007 to June 2008, inclusively. Isolates were preserved at −80°C on Microbank cryovials (Prolab Diagnostics, Ontario, Canada). Overnight cultures were performed on Columbia agar plus 5% (vol/vol) horse blood (Oxoid, Basingstoke, United Kingdom) at 37°C in an atmosphere containing 5% CO2. Additionally, routinely obtained genotypic data (genogroup and genosubtype) pertaining to nonculture cases (n = 12) (specimens may include EDTA whole blood, coagulated whole blood, cerebrospinal fluid, serum, or plasma) have been reported for cases in which they formed part of a cluster investigation over the period specified.
Capsular grouping.
Sero-/genogroup characterization was performed by the HPA MRU at the point of receipt, as described by Gray et al. (11).
Genomic DNA extraction.
Genomic DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Crawley, United Kingdom). Briefly, 1 ml meningococcal suspensions in physiological saline (adjusted to an optical density equal to 0.1 at A650) were heat killed at 60°C for 70 min and then pelleted at 6,000 × g for 10 min. Following aspiration of the supernatant, DNA was extracted using the manufacturer's Gram-negative protocol (Qiagen).
Molecular typing.
Primers used for PCRs and sequence analyses are listed in Table S1 in the supplemental material. Thermocycle conditions can be viewed in Table S2 in the supplemental material. Thermocycling was performed on a Veriti 96-well thermal cycler (Applied Biosystems, CA).
PCR.
All PCRs were performed using the HotStarTaq DNA polymerase kit (Qiagen). PCR products were electrophoresed on 2% agarose gels. Target-specific methods were as follows: fhbp PCRs were performed in 25-μl reactions. Final concentrations were 10× PCR buffer (1×), MgCl2 (3.25 mM), forward primer (0.5 μM), reverse primer (0.5 μM), dNTPs (200 μM per dNTP), and Taq polymerase (0.625 units/reaction). Each reaction mixture contained 2 μl of extracted genomic DNA template. fhbp PCR products were cleaned using ExoSAP-IT (exonuclease I and shrimp alkaline phosphatase) (USB Corporation, Cleveland, OH), according to the manufacturer's instructions. Cleaned PCR products were diluted 1 in 3 in nuclease-free water prior to sequencing.
porA, fetA, and MLST PCRs were performed in 50-μl reaction mixtures. Final concentrations were 10× PCR buffer (1×), MgCl (1.5 mM), forward primer (0.5 μM), reverse primer (0.5 μM), dNTPs (200 μM per dNTP), and Taq polymerase (1 unit/reaction). Each reaction mixture contained 1 μl of extracted genomic DNA template. porA, fetA, and MLST PCR products were cleaned using the Millipore multiscreen plate (Millipore, MA), according to the manufacturer's protocol.
Sequence analyses.
fhbp and MLST sequence analyses were performed using the BigDye version 3.1 (v3.1) kit (Applied Biosystems) in 1-in-8-strength reactions, according to the manufacturer's instructions. Final primer concentrations were 0.33 μM, and each reaction mixture contained 1 μl of cleaned PCR product as a template.
fetA and porA sequence analyses were performed using the BigDye v1.1 kit (Applied Biosystems) in 1-in-16-strength reactions, according to the manufacturer's instructions. Final primer concentrations were 0.33 μM (fetA) and 0.66 μM (porA), and each reaction mixture contained 1 μl of cleaned PCR product as a template.
All sequencing products were cleaned using ethanol-sodium acetate cleanup, resuspended in 15 μl HiDi formamide (Applied Biosystems), and analyzed on a 3130xl sequence analyzer (Applied Biosystems). Contig assembly and manual adjustment of bases were performed using Sequencher v4.8 (Gene Codes Corporation, MI).
Assessing resolving power among typing schemes.
The resolving power of fetA, fhbp, MLST, and combinations thereof (fetA-fhbp and fetA-fhbp-ST), were each determined across the full range of isolates using Simpson's index of diversity (D) (14, 28), a measure of the probability that two unrelated strains sampled from a test population would be allocated to different types. The resolving power of fetA, fhbp, MLST, and combinations thereof were also determined within the predominant capsular group-porA VR1/VR2 combinations identified. Values for D and 95% confidence intervals were obtained according to the methods described by Hunter and Gaston (14) and Grundmann et al. (12), respectively.
RESULTS
Capsular grouping.
The capsular groups represented among the isolates were B (87.9%; n = 539/613), Y (4.6%; n = 28), W135 (3.9%; n = 24), C (2.6%; n = 16), 29E (0.3%; n = 2), A (0.2%; n = 1), and Z (0.2%; n = 1). Two isolates (0.3%) [P1.5,2,36-2:ST60 (cc60) and P1.18-23,25-1,38-1:ST198 (cc198), respectively] remained ungrouped, having tested negative in serological assays to detect capsular groups A, B, C, X, Y, Z, 29E, and W135 and in genotypic assays (siaD) to detect capsular groups B, C, Y, and W135.
porA subtyping.
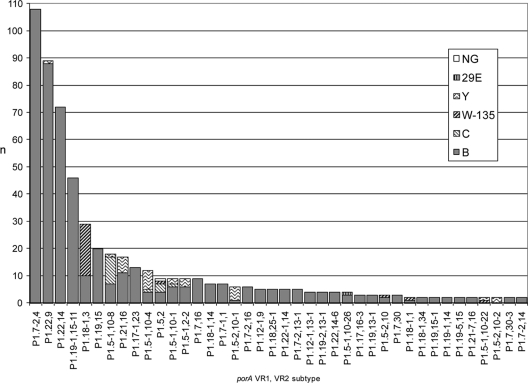
Two isolates gave no PCR product for porA. The 611 confirmed porA+ isolates represented 97 different VR1/VR2 combinations, 57 of which accounted for just a single isolate each (9.3% of isolates). Of the 40 remaining subtype combinations (Fig. 1), 30 accounted for between 2 and 10 isolates (21.3%; n = 130, collectively). The 10 major subtypes (n ≥ 12 isolates) (69.4%; n = 424, collectively) were P1.7-2,4 (17.7%; n = 108), P1.22,9 (14.6%; n = 89), P1.22,14 (11.8%; n = 72), P1.19-1,15-11 (7.5%; n = 46), P1.18-1,3 (4.7%; n = 29), P1.19,15 (3.3%; n = 20), P1.5-1,10-8 (2.9%; n = 18), P1.21,16 (2.8%; n = 17), P1.17-1,23 (2.1%; n = 13), and P1.5-1,10-4 (2.0%; n = 12).
FIG. 1.
porA subtypes (representing >1 isolate) among English and Welsh meningococcal invasive disease isolates from the epidemiological year 2007-2008, as resolved by capsular group. Collectively, the porA subtypes plotted (n = 40) represent 554/613 (90.3%) of the isolates under investigation. Each subtype is broken down into its constituent capsular groups. A further 57 porA subtypes (not plotted) represented a single isolate each (9.3% of total isolates). Two further isolates (not plotted; 0.3% of total isolates) gave no porA PCR product. NG, nongroupable; n, number of isolates.
Capsular group and porA VR1 and VR2 combined.
porA VR1/VR2 combinations representing >1 isolate were initially resolved in terms of capsular group (Fig. 1). Of the 10 major VR1/VR2 combinations identified, P1.7-2,4, P1.22,14, P1.19-1,15-11, P1.19,15, and P1.17-1,23 consisted solely of MenB isolates. P1.22,9 was predominantly MenB, with a single exception (Y:P1.22-9). The remaining major porA VR1/VR2 combinations included minor (non-B) capsular groups in greater proportions, with P1.18-1,3 comprising W135 (65.5%; n = 19) and B (34.5%; n = 10), P1.5-1,10-8 comprising C (55.6%; n = 10), B (38.9%; n = 7), and Y (5.6%; n = 1), P1.21,16 comprising B (64.7%; n = 11) and Y (35.3%; n = 6), and P1.5-1,10-4 comprising Y (58.3%; n = 7), B (33.3%; n = 4), and C (8.3%; n = 1) isolates.
Several major (n > 12 isolates) capsular group-porA VR1/VR2 combinations predominated (representing 366/613 isolates; 59.7%, collectively). These were B:P1.7-2,4 (n = 108; 17.6%), B:P1.22,9 (n = 88; 14.4%), B:P1.22,14 (n = 72; 11.7%), B:P1.19-1,15-11 (n = 46; 7.5%), B:P1.19,15 (n = 20; 3.3%), W135:P1.18-1,3 (n = 19; 3.1%), and B:P1.17-1,23 (n = 13; 2.1%). Among these, the four most populous, B:P1.7-2,4, B:P1.22,9, B:P1.22,14, and B:P1.19-1,15-11, accounted for 314/366 isolates (85.8%; 51.2% overall). These constitute the capsular group-porA VR1/VR2 combinations requiring the greatest degree of additional resolution from additional typing schemes, e.g., when investigating potential outbreaks.
fetA, fhbp, and ST.
All isolates were characterized in terms of fetA, fhbp, and ST. The resolving powers of each scheme and the combinations thereof were compared (i) across all 613 isolates collectively and (ii) within each of the predominant capsular group-porA VR1/VR2 combinations identified. Values for the diversity index (D), the number of types resolved (nt) and the frequency of the predominant type (fmax) for each individual scheme/combination are shown in Table 1. Individual frequencies for all identified types within the predominant capsular group-porA VR1/VR2 combinations are plotted schematically in Fig. 2. In all cases, where applicable, significance was inferred by the existence of nonoverlapping 95% confidence intervals.
TABLE 1.
Resolving power of fetA, fhbp, fetA-fhbp, ST, and fetA-fhbp-ST against English and Welsh meningococcal invasive disease isolates from the epidemiological year 2007-2008a
| Typing scheme/typing scheme combination | All isolates (n = 613) |
B:P1.7-2,4 (n = 108) |
B:P1.22,9 (n = 88) |
B:P1.22,14 (n = 72) |
B:P1.19-1,15-11 (n = 46) |
B:P1.19,15 (n = 20) |
W135:P1.18-1,3 (n = 19) |
B:P1.17-1,23 (n = 13) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nt | fmax | D (95% CI) | nt | fmax | D (95% CI) | nt | fmax | D (95% CI) | nt | fmax | D (95% CI) | nt | fmax | D (95% CI) | nt | fmax | D (95% CI) | nt | fmax | D (95% CI) | nt | fmax | D (95% CI) | |
| fetA | 49 | 22.8 | 88.1 (86.7-89.5) | 16 | 74.1 | 44.7 (33.0-56.4) | 13 | 63.6 | 57.3 (46.0-68.7) | 13 | 54.2 | 68.7 (57.6-79.8) | 4 | 89.1 | 20.6 (5.2-35.9) | 7 | 55.0 | 68.9 (48.9-89.0) | 5 | 78.9 | 38.6 (11.6-65.6) | 4 | 46.2 | 71.8 (57.3-86.3) |
| fhbp | 86 | 19.6 | 90.9 (89.7-92.0) | 11 | 73.1 | 45.0 (33.9-56.1) | 13 | 43.2 | 69.8 (63.6-76.0) | 21 | 37.5 | 83.8 (76.5-91.2) | 2 | 97.8 | 4.3 (0-12.4) | 6 | 65.0 | 57.9 (34.0-81.8) | 3 | 73.7 | 43.3 (21.0-65.6) | 7 | 30.8 | 84.6 (72.4-96.8) |
| fetA-fhbp | 195 | 16.0 | 94.9 (93.9-95.9) | 26 | 57.4 | 66.0 (56.0-76.1) | 25 | 37.5 | 81.3 (74.9-87.6) | 35 | 31.9 | 88.9 (82.6-95.2) | 5 | 89.1 | 20.7 (5.2-36.2) | 9 | 55.0 | 70.5 (49.2-91.9) | 6 | 57.9 | 64.3 (43.9-84.7) | 8 | 30.8 | 89.7 (79.3-100) |
| ST | 267 | 8.2 | 98.1 (97.7-98.5) | 46 | 38.0 | 84.3 (77.8-90.7) | 36 | 22.7 | 90.3 (86.6-94.0) | 29 | 44.4 | 79.4 (69.9-88.8) | 20 | 41.3 | 81.6 (71.2-92.1) | 14 | 35.0 | 88.9 (76.4-100) | 10 | 21.1 | 90.6 (84.5-96.8) | 7 | 30.8 | 84.6 (72.4-96.8) |
| fetA-fhbp-ST | 379 | 7.3 | 98.9 (98.6-99.2) | 56 | 33.3 | 88.3 (82.7-93.9) | 51 | 20.5 | 94.5 (91.3-97.6) | 49 | 22.2 | 95.0 (91.0-98.9) | 23 | 32.6 | 87.9 (80.2-95.7) | 14 | 35.0 | 88.9 (76.4-100) | 14 | 21.1 | 95.3 (89.7-100) | 10 | 23.1 | 94.9 (87.9-100) |
The resolving power of each typing scheme/combination of typing schemes is given for (i) all of the isolates collectively (all isolates; n = 613) and (ii) for all isolates harboring each of the seven predominant capsular groups: porA VR1/VR2 combinations identified in the corresponding year (B:P1.7-2,4, B:P1.22,9, B:P1.22,14, B:P1.19-1,15-11, B:P1.19,15, W135:P1.18-1,3, and B:P1.17-1,23). Resolving power is reported in terms of Simpson's index of diversity (D) (%) and is supplemented by the number of individual types resolved (nt) and the frequency of the predominant type (fmax). nt, number of individual types defined by typing scheme/typing scheme combination; fmax, frequency of predominant type (%).
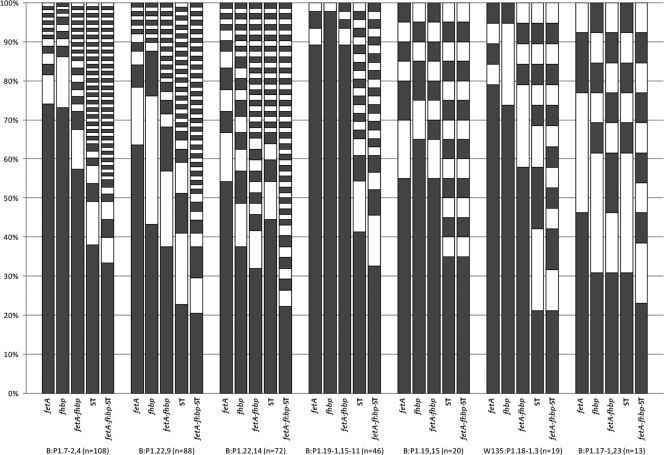
FIG. 2.
Resolution of predominant capsular group-porA VR1/VR2 combinations by fetA, fhbp, fetA-fhbp, ST, and fetA-fhbp-MLST among English and Welsh meningococcal invasive disease isolates from the epidemiological year 2007-2008. For each capsular group-porA VR1/VR2 combination, all individual types (each represented by an individual white or gray block) and their respective frequencies (size of block) are plotted for each of the typing schemes/typing scheme combinations under investigation, i.e., fetA, fhbp, fetA-fhbp, ST, and fetA-fhbp-MLST. The greater the number of types and the broader the distribution of types, the greater is the resolution afforded by the corresponding typing scheme. White/gray shading is arbitrary and intended to maximize contrast, thereby facilitating comparisons.
(i) fetA versus fhbp.
Among all 613 isolates collectively, fhbp afforded significantly better discrimination than that afforded by fetA. fhbp also afforded superior D values within four of the seven predominant capsular group-porA VR1/VR2 combinations, including the second and third most populous (B:P1.22,9 and B:P1.22,14). In the case of B:P1.22,9, both schemes resolved an equal number of types (nt = 13); however, the isolates were more evenly distributed by fhbp, the predominant type of which was therefore less frequent (fmax = 43.2% versus 63.6%). For B:P1.22,14, fhbp resolved 61.5% more types (nt = 21 versus 13) and yielded a less-frequent predominant type (fmax = 37.5% versus 54.2%). fetA afforded greater D values among two of the remaining three predominant capsular group-porA VR1/VR2 combinations, including the fourth most populous (B:P1.19-1,15-11), in which it was marginally superior in terms of D, fmax, and nt. Both schemes performed relatively poorly in this case, however (D = 20.6% and 4.3%, respectively). Against the most populous capsular group-porA VR1/VR2 combination (B:P1.7-2,4), fetA and fhbp afforded comparable levels of discrimination (D = 44.7% versus 45.0%, respectively). This was despite fetA resolving 45.5% more types overall (nt = 16 versus 11), since the predominant type in each case was similarly (and considerably) frequent (fmax = 74.1% and 73.1%, respectively). Within the predominant capsular group-porA VR1/VR2 combinations alone, 95% confidence intervals were unable to confer significance to any differences in D values between fetA and fhbp.
(ii) fetA or fhbp versus fetA-fhbp.
Among all 613 isolates collectively, the combination fetA-fhbp afforded significantly better discrimination than either of the antigens individually. fetA-fhbp was also largely superior within the predominant capsular group-porA VR1/VR2 combinations. Exceptions were B:P1.19-1,15-11 and B:P1.19,15, in which fetA-fhbp performed comparably with fetA alone (D = 20.7% versus 20.6%, and 70.5% versus 68.9%, respectively). In all cases, the predominant fetA-fhbp type comprised the predominant individual fetA and fhbp types for the respective capsular group-porA VR1/VR2 combination. Nonoverlapping 95% confidence intervals indicated that fetA-fhbp afforded significantly better discrimination than fetA alone versus the second and third most populous capsular group-porA VR1/VR2 combinations, B:P1.22,9 and B:P1.22,14. Significant differences in D values could not be inferred among the remaining predominant capsular group-porA VR1/VR2 combinations, with respect to fetA-fhbp versus fetA or fhbp alone.
(iii) fetA or fhbp versus ST.
Among all 613 isolates collectively, the ST afforded significantly better resolution than that of either fetA or fhbp. Against the predominant capsular group-porA combinations, the D values afforded by MLST exceeded those of fetA and fhbp for seven and five of the predominant capsular group-porA VR1/VR2 combinations, respectively. The difference was shown to be significant (against both individual antigenic typing schemes) for three of the four most populous capsular group-porA VR1/VR2 combinations (B:P1.7-2,4, B:P1.22,9, and B:P1.19-1,15-11) and the sixth most populous (W135:P1.18-1,3). Against the third most populous capsular group-porA VR1/VR2 combination (B:P1.22,14), fhbp afforded superior D and fmax values versus those of MLST, despite MLST defining 38.0% more types (nt = 29 [MLST] versus 21 [fhbp]). In this case, however, the difference was not shown to be significant. MLST and fhbp performed equally in terms of D, fmax, and nt against the least populous capsular group-porA VR1/VR2 combination, B:P1.17-1,23.
(iv) fetA-fhbp versus ST.
Among all 613 isolates collectively, ST afforded significantly better resolution than that of combined fetA-fhbp. ST also afforded greater D values within five of the seven predominant capsular group-porA VR1/VR2 combinations. Nonoverlapping 95% confidence intervals conferred significance in the case of the most populous combination (B:P1.7-2,4) and the fourth most populous (B:P1.19-1,15-11), for which the difference was considerable (D = 81.6% versus 20.7%). fetA-fhbp afforded greater D values for the remaining two combinations, i.e., those for which fhbp alone was superior. In neither case, however, was the difference shown to be significant.
(v) fetA-fhbp-ST versus all.
Among all 613 isolates collectively, a combination of all three schemes (fetA-fhbp-ST) afforded significantly better resolution than any of the respective schemes individually or the combination fetA-fhbp. fetA-fhbp-ST also afforded superior D, fmax, and nt values within all of the predominant capsular group-porA VR1/VR2 combinations except B:P1.19,15, for which ST alone performed equally in all respects, and W135:P1.18-1,3, for which ST afforded an equal value for fmax. With respect to the predominant capsular group-porA VR1/VR2 combinations, nonoverlapping 95% confidence intervals were able to confer significance to the superiority of fetA-fhbp-MLST over ST alone only in the case of B:P1.22,14.
porA VR3.
Among the seven predominant capsular group-porA VR1/VR2 combinations described, B:P1.19,15 and B:P1.17-1,23 were each homogeneous with respect to porA VR3, which therefore afforded no further resolution. The remaining five combinations were also relatively conserved in terms of VR3; however, a number of VR3 “outliers” existed for each, comprising 6/108 (5.6%) B:P1.7-2,4 isolates (across four additional VR3s), 5/88 (5.7%) B:P1.22,9 isolates (across five additional VR3s), 2/72 (2.8%) B:P1.22,14 isolates (across two additional VR3s), 1/46 (2.2%) B:P1.19-1,15-11 isolates, and 1/19 (5.3%) W135:P1.18-1,3 isolates. With the exception of fetA in W135:P1.18-1,3 and of fetA-fhbp-ST in B:P1.22,14 and W135:P1.18-1,3, the resolution afforded by VR3 was, at least partially, complementary to that of the additional typing schemes compared herein (Table 2).
TABLE 2.
Resolution of porA VR3 outliers within predominant capsular group-porA (VR1 andVR2) populations among English and Welsh meningococcal invasive disease isolates from the epidemiological year 2007-2008a
| Population (n) | Total no. of porA VR3 outliers | No. of porA VR3 outliers further discriminated by VR3 after initial typing with: |
||||
|---|---|---|---|---|---|---|
| fetA | fhbp | fetA-fhbp | MLST | fetA-fhbp-MLST | ||
| B:P1.7-2,4 (108) | 6 | 6 | 6 | 4 | 2 | 2 |
| B:P1.22,9 (88) | 5 | 3 | 5 | 3 | 4 | 2 |
| B:P1.22,14 (72) | 2 | 2 | 2 | 1 | 1 | 0 |
| B:P1.19-1,15-11 (46) | 1 | 1 | 1 | 1 | 1 | 1 |
| W135:P1.18-1,3 (19) | 1 | 0 | 1 | 1 | 1 | 0 |
Where applicable, the total number of porA VR3 outliers, i.e., those not harboring the respective predominant porA VR3 subtype, is reported for each of the predominant capsular group-porA populations. Also reported are the number of these porA VR3 outliers which, following initial resolution of their respective capsular group-porA population by a given typing scheme, can still be more finely discriminated by virtue of their outlying porA VR3. For example, in the case of B:P1.7-2,4 there are a total of six porA VR3 outliers among 108 isolates. Following initial resolution of B:P1.7-2,4 by MLST, however, only two of the original porA VR3 outliers can be more finely discriminated by virtue of their porA VR3.
Cluster investigation.
Over the period studied, the HPA MRU investigated 20 clusters in terms of capsular group and porA VR1, VR2, and VR3 (11). Seven clusters were incompletely characterized due to negative or indeterminate PCR results. Details of the 13 characterized clusters can be viewed in Table 3. The constituent cases of four of the clusters could be differentiated on the basis of capsular group-porA VR1/VR2 and so were deemed to be unrelated. With the exception of several more-diffuse regional cases, the constituent cases of the nine remaining clusters were not differentiated on this basis. Two of these provided cultures in all constituent cases and so (in the course of this study) were further characterized in terms of fetA, fhbp, and ST. In neither case did the additional characterization serve to differentiate the constituent isolates. The remaining seven undifferentiated clusters provided only noncultured or a mixture of cultured and noncultured specimens and so were not characterized in terms of fetA, fhbp, and ST.
TABLE 3.
Meningococcal case clusters characterized by the HPA MRU over the epidemiological year 2007-2008
| Cluster no. | No. of cases | Nature of specimens | Typing data | Spatiotemporal data (interval [days]) |
|---|---|---|---|---|
| 1 | 2 | Culture | B:P1.22,14,36 | Patients attended same school (12) |
| Culture | B:P1.22,9,35-1 | |||
| 2 | 2 | Culture | 29E:P1.18-7,9,35-1 | Patient/career contact (16) |
| Culture | W135:P1.18-1,3,38 | |||
| 3 | 2 | Nonculture | B:P1.5-1,10,36-2 | Patients lived locally, close-knit community (3) |
| Culture | B:P1.7-2,4,37 | |||
| 4 | 2 | Culture | Y:P1.5,10 | Patients attended same college (2) |
| Culture | B:P1.4 | |||
| 5 | 2 | Nonculture | B:P1.7-2,4,37 | Extended-family members (<1) |
| Nonculture | B:P1.7-2,4,37 | |||
| 6 | 2 | Nonculture | B:P1.19,13-1,35-1 | Patients attended same school (2) |
| Nonculture | B:P1.19,13-1,35-1 | |||
| 7 | 2 | Nonculture | B:P1.22,14,36 | Extended family with mutual friends (29) Previous, indistinguishable case occurred on same road (156) |
| Nonculture | B:P1.22,14,36 | |||
| 8 | 2 | Nonculture | B:P1.17-1,23,37 | Patient/health professional contact (5) |
| Culture | B:P1.17-1,23,37 | |||
| 9 | 2 | Culture | B:P1.22,9,35-1 | Patients attended same nursery (9) |
| Nonculture | B:P1.22,9,35-1 | |||
| 10 | 2 | Nonculture | B:P1.7-2,4,37 | Patients attended same work party (7) |
| Nonculture | B:P1.7-2,4,37 | |||
| 11 | 2 | Nonculture | B:P1.7-2,4,37 | Work colleagues, same department (1) |
| Culture | B:P1.7-2,4,37 | |||
| 12 | 2 | Culture | B:P1.7-2,4,37:F1-5:ST41 (cc41/44); fhbp, 1.4 | Extended-family members, attended same school (1) |
| Culture | B:P1.7-2,4,37:F1-5:ST41 (cc41/44); fhbp, 1.4 | |||
| 13 | 2 | Culture | B:P1.19-5,15,36:F5-1:ST479 (cc269); fhbp, 1.2 | Patients attended same school, different classes (8) |
| Culture | B:P1.19-5,15,36:F5-1:ST479 (cc269); fhbp, 1.2 |
DISCUSSION
Several investigational MenB vaccines, including two in the advanced stages of development, incorporate fHBP. The antigen has therefore been recommended for routine genotypic surveillance in England and Wales. This study aimed to evaluate the discriminatory value of fhbp and several existing (sero-/genogroup, porA subtype, and MLST) and recommended (fetA) typing schemes to investigate the optimal and most efficient typing repertoire for (i) routine antigenic monitoring and (ii) the investigation of potential outbreaks. All invasive disease isolates received by the HPA MRU in 2007-2008 were characterized in terms of capsular group, porA VRs 1, 2, and 3, fetA VR, fhbp, and ST.
When attempting to differentiate between unrelated isolates, the ideal typing scheme would afford a diversity index of 100% (complete diversity); however, a value of >90% is widely regarded as desirable (14, 33). In this respect, fhbp performed significantly better than fetA against all invasive disease isolates received by the HPA MRU in the epidemiological year 2007-2008. Both loci are comparable in terms of their proximity to the porA typing locus (∼506 kb versus ∼626 kb) (29), and both are covered by extensive databases at http://pubmlst.org. They each also encode candidate MenB vaccine antigens; however, FetA-based vaccines are in the relatively early stages of development, whereas fHBP-containing vaccines are likely to be licensed in the near future. The ensuing requirement for the routine surveillance of fhbp and its comparability with fetA in terms of diversity and genomic proximity to the core antigenic typing locus (porA) suggest that fhbp would be an effective substitute for fetA as a means of routine antigenic monitoring, thereby saving on costs and labor. Therefore, the HPA MRU shall not be adopting fetA as a routine target for the purpose of monitoring antigenic distribution. A nonculture protocol is currently being devised for fhbp to account for the high proportion of nonculture, laboratory case confirmations in England and Wales (approximately 60%).
The ability to distinguish between unrelated isolates is crucial when investigating potential outbreaks, so as to avoid unnecessary intervention beyond close contacts. Against all invasive disease isolates received by the HPA MRU in the epidemiological year 2007-2008, ST afforded significantly better discrimination than either fetA or fhbp. The combination fetA-fhbp performed significantly better than either fetA or fhbp individually; however, the individual schemes often differentiated the same groups of isolates, such that their respective predominant types tended to coincide. As such, ST also performed significantly better than the combination fetA-fhbp. The superiority of ST was magnified among the predominant capsular group-porA combinations in which fetA and fhbp afforded inconsistent and often poor levels of discrimination. The range of D values afforded by ST against these populations was 79.4% to 90.6% (versus 20.7% to 89.7% for fetA-fhbp). Even against the predominant capsular group-porA combination B:P1.7-2,4 (representing approximately 1 in 6 isolates across the entire year), fetA-fhbp afforded a D value of only 66.0% compared with 84.3% for ST. Thus, when investigating a potential outbreak (or cluster) in which (i) typing data are not yet available for the index case or (ii) the index case possesses a known predominant capsular group-porA VR1/VR2 repertoire, it seems prudent to immediately apply MLST (alongside routine characterization, including fhbp) to all potentially related cases. Conversely, where the index case is non-MenB or possesses a minor capsular group-porA subtype (approximately 40% of isolates in 2007-2008), MLST may not be required since routine antigenic typing would likely differentiate any unrelated cases. The finding that MLST affords greater resolution than either fetA or fhbp is not, perhaps, surprising, given that there are seven loci characterized by MLST (versus just one or two loci for fetA or fhbp or fetA-fhbp). While this bias undoubtedly contributed to the superiority of MLST in resolving the isolates in this study, it is possible that combinations of housekeeping alleles are inherently better suited to this purpose. A study by Buckee et al. (3) showed strong temporal associations between the fetA VR and those of porA, with minimal overlap between combinations. Conversely, the MLST alleles (which displayed a strong lineage structure and temporally fluctuating antigenic associations) demonstrated considerable overlap among the various combinations, thus allowing more combinations among isolates with a common porA subtype.
From a practical point of view, MLST is already adapted for nonculture work (1) and so is available as a tool for outbreak investigation both in the presence and absence of laboratory culture. The relative resource and labor intensity of MLST are unlikely to preclude its use in outbreak investigations since the indication for its immediate use is likely to be a relatively rare occurrence (over the epidemiological year studied, the HPA MRU investigated 20 clusters as potential outbreaks), thus minimizing additional costs. The extended turnaround time associated with MLST applies to genotypic schemes in general. The HPA MRU, for example, quotes a minimum 3-working-day turnaround for porA genotyping (currently recommended for outbreak investigations). Therefore, any genotypic data pertaining to outbreak investigations may often be examined retrospectively after the initiation of management action based on, e.g., spatiotemporal evidence and more rapidly attainable serogrouping and serosubtyping data (routinely performed on cultures within the HPA MRU). A potential drawback pertaining to nonculture MLST in particular is its relatively high consumption of DNA template. Therefore, when DNA content is low, or in cases involving infants (from whom relatively low blood/cerebrospinal fluid [CSF] volumes are obtained), there may be insufficient sample remaining following preliminary molecular testing. Therefore, while MLST constitutes the optimal technique for investigating potential outbreaks, the scope of genotypic investigations may ultimately be limited by the volume/quality of the specimen received. A possible solution may be to employ a targeted approach in which the most discriminatory MLST alleles are prioritized, based on previous data (local epidemiological knowledge), as has been alluded to previously (20).
Inclusion of porA VR3 further resolved up to 5.7% of the isolates among five of the predominant capsular group-porA VR1/VR2 combinations. Up to 100% of this additional resolution complemented that of fetA, fhbp, and ST, either alone or in combination. Therefore, while the additional resolution afforded by VR3 is limited, it seems wasteful not to process the data when it already exists (having been generated alongside that of VR2), especially when an outbreak is suspected. Consistent with the recommendations on molecular targets for meningococci (15), however, the limited potential for resolving clusters does not warrant the extra work of specifically pursuing VR3.
The finer aspects of typing applied in this study (fetA, fhbp, and MLST) were undertaken largely retrospectively. During the period under investigation, clusters were typically investigated by the HPA MRU using only sero-/genogrouping and porA genosubtyping (VR1, VR2, and VR3). The number of clusters investigated (n = 20) was too small to enable any statistical analyses; however, they serve to illustrate several important points. First, approximately a third of the clusters were incompletely characterized due to negative/indeterminate PCR results, reflecting, in some cases, poor specimen quality. This represents an area for future development in (i) increasing the sensitivity of nonculture laboratory methods and (ii) educating clinicians to ensure that optimal specimens are submitted. Second, the constituent cases of four of the clusters were differentiated on the basis of capsular group and porA alone, illustrating that even apparently strong spatiotemporal links can often be coincidental. Third, nine of the clusters were unresolved, which would serve to bolster decisions (initially based on strong spatiotemporal associations) regarding intervention among case contacts and possibly the wider community. Seven of these comprised cases with predominant capsular group-porA repertoires, thus reducing any certainty the cases were indeed linked and highlighting the need to apply MLST in order to maximize discriminatory potential. Even when MLST fails to discriminate, however, it should be remembered that, while an outbreak may be discounted with 100% certainty, the same is not true of outbreak confirmation, which therefore relies heavily on spatiotemporal and circumstantial evidence.
In summary, fhbp is to be included within the routine meningococcal typing scheme in England and Wales to monitor the potential coverage of fHBP-containing vaccines. The present study has shown fhbp and fetA to be comparable in terms of diversity. fhbp shall therefore provide a practical and effective substitute for fetA as a routine marker of antigenic variation in England and Wales. MLST outperformed fetA, fhbp, and fetA-fhbp when resolving the predominant capsular group-porA VR1/VR2 combinations. Therefore, in cases in which (i) typing data are not yet available for the index case or (ii) the index case possesses a predominant capsular group-porA VR1/VR2 repertoire, MLST should be the method of choice for investigating possible outbreaks. Genetic diversity tends to be higher among invasive capsular group B isolates (13); therefore, the situation described may be different in countries experiencing a greater balance among, e.g., capsular groups, or indeed if additional targets are required for routine surveillance.
Supplementary Material
Acknowledgments
We thank Maurizio Comanducci, Mariagrazia Pizza, Alessandro Muzzi, and Stefania Bambini for their ongoing assistance relating to fhbp characterization.
fhbp characterization and a proportion of the MLST and porA genosubtyping were funded by Novartis Vaccines.
Footnotes
Published ahead of print on 1 December 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Birtles, A., et al. 2005. Multilocus sequence typing of Neisseria meningitidis directly from clinical samples and application of the method to the investigation of meningococcal disease case clusters. J. Clin. Microbiol. 43:6007-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brehony, C., K. A. Jolley, and M. C. Maiden. 2007. Multilocus sequence typing for global surveillance of meningococcal disease. FEMS Microbiol. Rev. 31:15-26. [DOI] [PubMed] [Google Scholar]
- 3.Buckee, C. O., S. Gupta, P. Kriz, M. C. Maiden, and K. A. Jolley. 2010. Long-term evolution of antigen repertoires among carried meningococci. Proc. Biol. Sci. 277:1635-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartwright, K., S. Reilly, D. White, and J. Stuart. 1992. Early treatment with parenteral penicillin in meningococcal disease. BMJ 305:143-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EU-IBIS. May 2007. EUIBIS N. meningitidis EQA. http://www.euibis.org/documents/emgm_07_pdfs/eqa_af.pdf. Health Protection Agency, London, United Kingdom.
- 6.Feil, E. J., M. C. Maiden, M. Achtman, and B. G. Spratt. 1999. The relative contributions of recombination and mutation to the divergence of clones of Neisseria meningitidis. Mol. Biol. Evol. 16:1496-1502. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher, L. D., et al. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 72:2088-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox, A. J., M. K. Taha, and U. Vogel. 2007. Standardized nonculture techniques recommended for European reference laboratories. FEMS Microbiol. Rev. 31:84-88. [DOI] [PubMed] [Google Scholar]
- 9.Frasch, C. E., W. D. Zollinger, and J. T. Poolman. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev. Infect. Dis. 7:504-510. [DOI] [PubMed] [Google Scholar]
- 10.Giuliani, M. M., et al. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834-10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray, S. J., et al. 2006. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J. Med. Microbiol. 55:887-896. [DOI] [PubMed] [Google Scholar]
- 12.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison, L. H., C. L. Trotter, and M. E. Ramsay. 2009. Global epidemiology of meningococcal disease. Vaccine 27(Suppl. 2):B51-B63. [DOI] [PubMed] [Google Scholar]
- 14.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jolley, K. A., C. Brehony, and M. C. Maiden. 2007. Molecular typing of meningococci: recommendations for target choice and nomenclature. FEMS Microbiol. Rev. 31:89-96. [DOI] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.Lucidarme, J., et al. 2009. Characterization of fHbp, nhba (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B meningococcal ST269 clonal complex isolates from England and Wales. J. Clin. Microbiol. 47:3577-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucidarme, J., et al. 2010. Characterization of fHbp, nhba (gna2132), nadA, porA, and sequence type in group B meningococcal case isolates collected in England and Wales during January 2008 and potential coverage of an investigational group B meningococcal vaccine. Clin. Vaccine Immunol. 17:919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madico, G., et al. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiden, M. C., et al. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiden, M. C., and I. M. Feavers. 1994. Meningococcal typing. J. Med. Microbiol. 40:157-158. [DOI] [PubMed] [Google Scholar]
- 22.Masignani, V., et al. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pace, D., A. J. Pollard, and N. E. Messonier. 2009. Quadrivalent meningococcal conjugate vaccines. Vaccine 27(Suppl. 2):B30-B41. [DOI] [PubMed] [Google Scholar]
- 24.Pillai, S., et al. 2005. Outer membrane protein (OMP) based vaccine for Neisseria meningitidis serogroup B. Vaccine 23:2206-2209. [DOI] [PubMed] [Google Scholar]
- 25.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 26.Schneider, M. C., et al. 2006. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 176:7566-7575. [DOI] [PubMed] [Google Scholar]
- 27.Shigematsu, M., K. L. Davison, A. Charlett, and N. S. Crowcroft. 2002. National enhanced surveillance of meningococcal disease in England, Wales and Northern Ireland, January 1999-June 2001. Epidemiol. Infect. 129:459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson, E. H. 1949. Measurement of diversity. Nature 163:688. [Google Scholar]
- 29.Tettelin, H., et al. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 30.Thompson, E. A., I. M. Feavers, and M. C. Maiden. 2003. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 149:1849-1858. [DOI] [PubMed] [Google Scholar]
- 31.Urwin, R. 2001. Nucleotide sequencing of antigen genes of Neisseria meningitidis, p. 157-172. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal disease: methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 32.Urwin, R., et al. 2004. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect. Immun. 72:5955-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Belkum, A., et al. 2007. Guidelines for the validation and application of typing methods for use in bacterial epidemiology. Clin. Microbiol. Infect. 13(Suppl. 3):1-46. [DOI] [PubMed] [Google Scholar]
- 34.Vedros, N. A. 1987. Development of meningococcal serogroups, p. 33-37. In N. A. Vedros (ed.), Evolution of meningococcal disease. CRC Press Inc., Boca Raton, FL.
- 35.Wyle, F. A., et al. 1972. Immunologic response of man to group B meningococcal polysaccharide vaccines. J. Infect. Dis. 126:514-521. [DOI] [PubMed] [Google Scholar]
- 36.Zapata, G. A., W. F. Vann, Y. Rubinstein, and C. E. Frasch. 1992. Identification of variable region differences in Neisseria meningitidis class 3 protein sequences among five group B serotypes. Mol. Microbiol. 6:3493-3499. [DOI] [PubMed] [Google Scholar]
- 37.Zollinger, W. D., et al. 2008. Programme Abstr. 16th Int. Pathog. Neisseria Conf., abstr. O41. Rotterdam, Netherlands, 7 to 12 September.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.