Abstract
A plasmid harboring eltB, the gene encoding heat-labile enterotoxin (LTB), was constructed by insertion of eltB into an Asd+ β-lactamase signal plasmid (pMMP65). This was introduced into the Δlon ΔcpxR Δasd Salmonella enterica serovar Typhimurium strain and designated the LTB adjuvant strain. LTB protein production and secretion from the strain were demonstrated with an immunoblot assay and enzyme-linked immunosorbent assay. The LTB strain was evaluated for enhancement of immunity and protection efficacy induced by a previously constructed live Salmonella vaccine candidate. In addition, immunization strategies using the LTB strain were optimized for effective salmonellosis protection. Seventy female BALB/c mice were divided into seven groups (A to G; n = 10 mice per group). Mice were primed at 6 weeks of age and boosted at 9 weeks of age. All mice were orally challenged with a virulent wild-type strain at week 3 postbooster. Serum IgG and IgA titers from mice immunized with the LTB strain alone or with a mixture of the LTB strain and the vaccine candidate were significantly increased. The secretory IgA titers from mice immunized with the LTB strain alone or with the mixture were at least 2.2 times greater than those of control mice. In addition, all group E mice (primed with the vaccine-LTB mixture and boosted with the vaccine candidate) were free of clinical signs of salmonellosis and survived a virulent challenge. In contrast, death due to the challenge was 100% in control mice, 80% in group A mice (single immunization with the vaccine candidate), 60% in group B mice (primed and boosted with the vaccine candidate), 40% in group C mice (single immunization with the LTB strain), 30% in group D mice (primed and boosted with the LTB strain), and 30% in group F mice (primed and boosted with the vaccine-LTB mixture). These results suggest that vaccination with the LTB strain, especially when added at the prime stage only, effectively enhances immune responses and protection against salmonellosis.
Nontyphoidal Salmonella serotypes are the leading cause of lethal food-borne infections worldwide (27, 50). Salmonella enterica serotype Typhimurium is the serotype most frequently associated with the diarrheal diseases and is commonly transmitted from animal to human through livestock- and domestic fowl-derived food products (34, 50). S. Typhimurium induces clinical enteric fever in mouse models with symptoms similar to human symptomology after S. enterica serovar Typhi infection (16, 25, 50). Infections may be asymptomatic or can result in enteric and fatal systemic disease. Asymptomatic animals may serve as potential carriers (4, 5, 39). Carriers are the primary sources of human and animal infection and also contribute to environmental contamination (3, 47). In addition, treatment of carriers with antibiotics fails to prevent S. Typhimurium shedding in the environment (39). Therefore, Salmonella prevention in domestic livestock and poultry industries is essential, and vaccination is an effective tool for salmonellosis prevention (1, 30, 39). Cell-mediated immune responses are crucial for effective protection postvaccination (23, 30, 39). Live vaccines for salmonellosis, particularly through the oral route, may confer effective protection against virulent challenges due to both cell-mediated and mucosal immune responses (24, 29, 48). However, oral immunization with live vaccines is frequently ineffective due to instability in the digestive tract, weak antigen uptake from mucosal surfaces, and difficult induction of immune responses against mucosally administered antigens (28, 32, 48). Powerful mucosal adjuvants, including the B subunit of the Escherichia coli heat-labile enterotoxin (LTB), may assist in resolving these problems (8, 28). Oral coimmunization with adjuvant LTB has resulted in the induction of protective robust mucosal and systemic immune responses (8, 28, 52).
We previously constructed a novel attenuated Salmonella vaccine candidate by deleting the cpxR and lon genes from a wild-type S. Typhimurium strain with an allelic exchange method (15). A balanced-lethal host-vector system based on the essential bacterial gene encoding aspartate β-semialdehyde dehydrogenase (asd) was used to deliver the adjuvant protein LTB from an Asd+ plasmid in the present study. The asd, lon, and cpxR genes were genetically deleted from the Salmonella delivery strain, and the Asd+ plasmid with the eltB gene encoding the LTB protein was transformed into the attenuated delivery strain and used as a mucosal adjuvant. This study evaluated whether the LTB strain enhanced immune responses and protective efficacy induced by oral administration of the live Salmonella vaccine candidate. Immunization strategies with the live vaccine candidate and the LTB strain were also optimized for effective protection against salmonellosis.
MATERIALS AND METHODS
Mice.
Five-week-old female BALB/c mice received water and food ad libitum. Experiments were conducted under the approval from the Chonbuk National University Animal Ethics Committee in accordance with the guidelines of the Korean Council on Animal Care.
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Attenuated S. Typhimurium strain JOL911 was constructed by deletion of the cpxR and lon genes of the wild-type S. Typhimurium JOL401 isolate, as previously described (15). This strain was used as the vaccine. Strain JOL912 was constructed by deletion of the asd gene of strain JOL911 by allelic exchange, as previously described (14), and was used as the delivery strain for eltB, which encodes the LTB protein. Wild-type S. Typhimurium isolate JOL389 was used as the virulent challenge strain. An Asd+ plasmid, pMMP65, was used for eltB gene delivery. JOL911 and JOL389 were grown at 37°C in Luria-Bertani (LB) broth or agar or on brilliant green agar (BGA) (Becton Dickinson, Sparks, MD). Diaminopimelic acid (DAP; Sigma-Aldrich, St. Louis, MO) was added (50 μg/ml) to induce JOL912 growth.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| JOL500 | Wild-type F18+ LTB+ STa+ STb+ Stx2+ Stx2e+ ETEC isolate from pig | Present study |
| TOP10F′ | F′[lacIq Tn10 (Tetr)] mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74recA1araD139 Δ(ara-leu)7697galUgalKrpsL (Strr) endA1nupG | Invitrogen |
| BL21(DE3)pLysS | F−ompThsdSB (rB− mB−) dcmgal λ(DE3) pLysS Cmr | Promega |
| JOL470 | TOP10′ with pET28a-LTB | Present study |
| JOL471 | BL21(DE3)pLysS with pET28a-LTB | Present study |
| S. Typhimurium | ||
| JOL389 | Wild-type isolate from piglet with diarrhea; challenge strain | Present study |
| JOL401 | Wild-type isolate from chicken | Present study |
| JOL911 | ΔlonΔcpxR; a derivative of JOL401 | 14 |
| JOL912 | ΔlonΔcpxRΔasd; a derivative of JOL911 | Present study |
| JOL906 | JOL912 containing pMMP65-LTB | Present study |
| Plasmids | ||
| pMEG375 | Suicide vector | 14 |
| pET28a | IPTG-inducible expression vector; Kmr | Novagen |
| pET28a-LTB | pET28a derivative containing the eltB gene | Present study |
| pMMP65 | Asd+, pBR ori, β-lactamase signal sequence-based periplasmic secretion plasmid, 6× His tag | Present study |
| pMMP65-LTB | pMMP65 derivative containing eltB | Present study |
ETEC, enterotoxigenic E. coli; IPTG, isopropyl-β-d-thiogalactopyranoside.
Cloning of eltB.
The eltB gene was amplified by PCR using the eltB-F (5′-CCGCGAATTCGCTCCCCAGTCTATTACAG-3′) and eltB-R (5′-CCGCAAGCTTCTAGTTTTCCATACTGATTG-3′) primers. PCR products were inserted into pET28a (Table 1) and designated pET28a-LTB. This plasmid was transformed into E. coli BL21(DE3)pLysS, and the LTB protein from the strain was purified using Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Valencia, CA). pMMP65-LTB was constructed by inserting the eltB gene into pMMP65. This plasmid was introduced into JOL912 by electroporation and was designated JOL906. This strain was used as the LTB adjuvant strain.
Immunoblot analysis.
LTB antigen secretion from JOL906 was identified by immunoblot analysis. The strain was cultured in LB broth at 37°C for preparation of JOL906-secreted proteins. The culture was harvested by centrifugation at 3,400 × g for 20 min at an optical density at 600 nm (OD600) of 0.8. Culture supernatants were passed through a 0.22-μm-pore-size filter and precipitated overnight with 20% (vol/vol) trichloroacetic acid (TCA) to detect the secreted forms of proteins. TCA pellets were resuspended after centrifugation in cold phosphate-buffered saline (PBS) and acetone precipitation. Precipitates were washed with acetone and resuspended in PBS. JOL906 protein samples were boiled for 5 min and then separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The resolved proteins were transferred electrophoretically to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA) for immunoblotting. Membranes were blocked with 5% skim milk in PBS with 0.01% Tween 20 at 4°C overnight and were incubated with anti-His-tagged antibodies (IG Therapy, Chuncheon, South Korea) and horseradish peroxidase-conjugated goat anti-mouse IgG. Immunoreactive bands were developed with the addition of chemiluminescence dye and the West-one Western blot detection system (iNtRON, Seongnam, South Korea) and were detected using the multiwavelength illumination system Kodak Image Station 4000MM (Kodak, New Haven, CT).
Preparation of vaccine and LTB adjuvant strains.
The JOL911 and JOL906 strains were individually grown in LB broth and harvested as described for immunoblot analysis. The cells were resuspended in sterile PBS with 20% sucrose (PBS-sucrose) to 2 × 1011 CFU/ml. Mice were orally immunized with the strains on the day of preparation.
Immunization and sample collection.
Female 5-week-old BALB/c mice (n = 70) were divided into seven groups (n = 10 mice per group). Mice were orally primed at 6 weeks of age and orally boosted at 9 weeks of age. Immunization protocols are described in Table 2. The total number of inoculated bacterial cells per dose for all animals described below was 2 × 109 CFU in a volume of 20 μl. Group A mice were immunized with JOL911, group B mice were primed and boosted with JOL911, group C mice were immunized with JOL906, and group D mice were primed and boosted with JOL906. Group E mice were primed with a mixture consisting of 4 parts JOL911 (1.6 × 109 CFU) and 1 part JOL906 (0.4 × 109 CFU) and were boosted with JOL911 alone. Group F mice were primed and boosted with a mixture consisting of 4 parts JOL911 and 1 part JOL906. Group G mice were primed and boosted with PBS as a control. Food and water were withdrawn 4 h prior to immunization and resupplied 30 min after immunization. Blood samples were obtained by retro-orbital puncture with a Pasteur pipette at 0, 3, and 6 weeks postpriming immunization (PPI) to evaluate serum IgG and IgA titers. Sera were obtained from whole blood by centrifugation at 4,000 × g for 5 min. Vaginal secretions were collected by vaginal washes with 100 μl sterile PBS. All samples were stored at −80°C until use.
TABLE 2.
Immunizations, isolation of challenge strains from organs, and mortality after challenge
| Group | Immunizationa |
Challenge |
||||
|---|---|---|---|---|---|---|
| Mortality (%) | Isolation of challenge strainb |
|||||
| Prime | Booster | No. of infected livers/no. of livers tested | No. of infected spleens/no. of spleens tested | |||
| A | JOL911 | None | 10 | 80 | 2/2 | 2/2 |
| B | JOL911 | JOL911 | 10 | 60 | 1/4 | 1/4 |
| C | JOL906 | None | 10 | 40 | 4/6 | 4/6 |
| D | JOL906 | JOL906 | 10 | 30 | 3/7 | 3/7 |
| E | 4 parts JOL911 and 1 part JOL906 | JOL911 | 10 | 0 | 0/10 | 0/10 |
| F | 4 parts JOL911 and 1 part JOL906 | 4 parts JOL911 and 1 part JOL906 | 10 | 30 | 2/7 | 2/7 |
| G | PBS | PBS | 10 | 100 | ND | ND |
JOL911 is a vaccine strain, and JOL906 is an LTB protein-secreting strain. The total number of inoculated bacterial cells per dose for all animals was 2 × 109 CFU in a volume of 20 μl.
ND, not detected.
Immune response measurements.
Serum IgG and fecal secretory IgA (sIgA) antibodies against recombinant LTB (rLTB) were investigated by a standard enzyme-linked immunosorbent assay (ELISA) performed as previously described (53), with a slight modification. Briefly, ELISA plates (Greiner Bio-One GmbH, Frickenhausen, Germany) were coated with recombinant LTB proteins (0.5 μg per well) at 4°C overnight. Sera were diluted to 1:100 for examination of IgG titers. Fecal samples were diluted 1:3 for examination of sIgA titers. Plates were treated with horseradish peroxidase-conjugated goat anti-mouse antibodies (Southern Biotechnology, Birmingham, AL). Enzymatic reactions were developed with o-phenylenediamine (Sigma-Aldrich) and measured by an automated ELISA spectrophotometer (Tecan, Salzburg, Austria) at 492 nm. The standard curve describing the relation between the concentration of standards and their absorbance value was generated, and the concentration of antibody for each sample was expressed as the number of ng/ml.
In addition, Salmonella-specific IgG, IgG1, IgG2a, and IgA antibodies in serum and fecal sIgA were measured by ELISA. S. Typhimurium lipopolysaccharide (LPS) was prepared from the wild-type JOL389 strain with an LPS extraction kit (INtRON Biotechnology, Seongnam, Gyeonggi, South Korea), according to the manufacturer's instructions, and stored at −70°C until use. ELISA plates were coated with S. Typhimurium LPS (0.5 μg per well) at 4°C overnight. Sera were diluted 1:400 for examination of IgG, IgG1, and IgG2a titers. Serum and fecal samples were diluted 1:3 for examination of IgA and sIgA titers, respectively.
Fecal shedding of vaccine and LTB adjuvant strains.
Mice in each group (n = 5) were tested once daily for 3 weeks after inoculation to investigate the fecal shedding of the vaccine and adjuvant strains. Fecal samples were preenriched in buffered peptone water (BPW) for 18 h at 37°C. Preenriched broth (100 μl) was transferred to 10 ml of Rappaport-Vassiliadis R10 broth (Difco, Sparks, MD) and incubated under aerobic conditions for 24 h at 42°C. One hundred microliters of the enrichment medium was then streaked on BGA (Difco), and the resulting colonies were identified using an API 32E system (bioMérieux, Marcy l'Étoile, France).
Challenge experiment.
Virulent wild-type strain JOL389 was grown in LB broth overnight at 37°C, diluted 1:20 in LB broth, and grown to an OD600 of 0.8. Cells were harvested by centrifugation at 3,400 × g for 20 min and were diluted to approximately 2 × 108 CFU in 20 μl of sterile PBS-sucrose after being washed twice with sterile PBS. All mice were orally challenged with 20 μl of the diluted solution at week 6 PPI. All mice were subsequently monitored for mortality for 14 days postchallenge. The isolated strains were confirmed by PCR using the following Salmonella-specific primers: OMPC (OMPCF, 5′-ATCGCTGACTTATGCAATCG; OMPCR, CGGGTTGCGTTATAGGTCTG-3′) for Salmonella spp. and TYPH (TYPHF, 5′-TTGTTCACTTTTTACCCCTGAA-3′; TYPHR, 5′-CCCTGACAGCCGTTAGATATT-3′) for S. Typhimurium, cpxR (cpxR-F, 5′-GATAATTTACCGTTAACGAC-3′; cpxR-R, 5′-CATCATCTGCGGGTTGCAGC-3′) and lon (lon-F, 5′-CATCATCTGCGGGTTGCAGC-3′; lon-R, 5′-CCACACTCCGCTGTAGGTGA-3′) primers for the vaccine candidate (2), and LTB (LTB-F, 5′-GCTCCCCAGTCTATTACAG-3′; LTB-R, 5′-CTAGTTTTCCATACTGATTG-3′) primers for the adjuvant strain.
Bacteriological examinations of infected organs.
All mice who survived the initial challenge were sacrificed on day 14 postchallenge, and their liver and splenic tissues were harvested. Whole livers and spleens were homogenized and diluted 1:10 (wt/vol) (liver) and 1:3 (wt/vol) (spleen) in PBS. Homogenates were plated on BGA, and the challenge strain was isolated after incubation at 37°C for 24 h. The vaccine and challenge strains were confirmed by PCR using the previously described Salmonella-specific primers.
Statistical analyses.
Results are expressed as means ± standard deviations (SD). Significant differences in antibody titers between immunized and control groups and between IgG2a and IgG1 in immunized groups were determined by the Mann-Whitney U test using the SPSS 16.0 program (SPSS, Chicago, IL). Differences in postchallenge mortality were analyzed by a chi-square test using the SPSS 16.0 program. Statistical significance was established at P values of <0.05.
RESULTS
Secretion of LTB from JOL906.
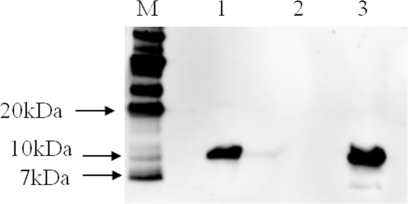
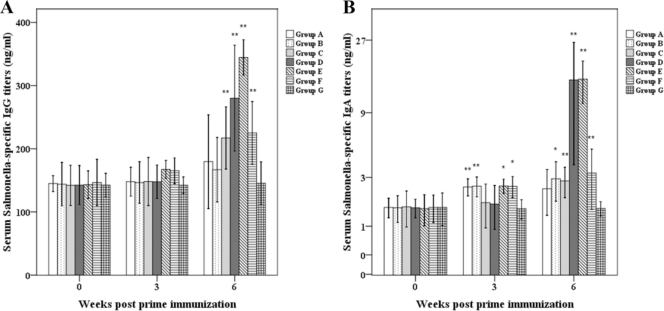
A 327-bp DNA fragment of the eltB gene from LTB+ E. coli was amplified using the specific primers and cloned into pMMP65. Subsequently, pMMP65 carrying eltB (pMMP65-LTB) was transformed into a ΔcpxR Δlon Δasd S. Typhimurium strain, which was designated JOL906. Immunoblots of trichloroacetic acid-precipitated culture supernatants were performed to examine the production and secretion of JOL906 LTB protein. S. Typhimurium-harboring pMMP65 only and purified LTB protein were used as negative and positive controls, respectively. A 11.6-kDa band (the monomer size of the LTB protein) was observed in precipitated culture supernatants and the positive control (Fig. 1). No band was evident in the negative control. In addition, to confirm whether rLTB was expressed and immunogenic in mice, serum IgG and fecal sIgA antibodies against LTB were investigated by ELISA. Serum IgG titers of group C (single administration of JOL906), group D (primed and boosted with JOL906), group E (primed with JOL911 plus JOL906 and boosted with JOL911 only), and group F (primed and boosted with JOL911 plus JOL906) were at least 1.3 times higher than those of the control group at 6 weeks PPI (P < 0.05), while serum IgG titers of group A (single administration with JOL911) and group B (primed and boosted with JOL911) were similar to those of the control group at 6 weeks PPI (Fig. 2A). Fecal sIgA titers of groups C and E were increased to 8.7 and 7.0 times higher than those of the control group at 6 weeks PPI (P < 0.05), while the fecal sIgA titer of group F was 3.7 times higher (Fig. 2B). The fecal sIgA titer of group D was higher than that of the control group, but the fecal sIgA titer of group D was lower than those of groups A and B at 6 weeks PPI (Fig. 2B).
FIG. 1.
Identification of the LTB protein by immunoblot analysis. The LTB protein, expressed and secreted by JOL906, was detected by immunoblotting with anti-His-tagged monoclonal antibody. JOL912 carrying pMMP65 only was used as a negative control, and recombinant LTB protein was used as a positive control. Lane M, size marker; lane 1, JOL906; lane 2, JOL912 (negative control); lane 3, LTB protein (positive control).
FIG. 2.
Immune responses to LTB in mice orally inoculated with or without the LTB adjuvant strain. Anti-LTB serum IgG (A) and fecal sIgA (B). Group A mice were immunized with JOL911, group B mice were primed and boosted with JOL911, group C mice were immunized with JOL906, group D mice were primed and boosted with JOL906, group E mice were primed with a JOL911-JOL906 mixture and boosted with JOL911, group F mice were primed and boosted with a JOL911-JOL906 mixture, and group G mice were inoculated with PBS as a control. The data shown are the mean values for all mice in each group, and error bars demonstrate standard deviations (SD). Asterisks indicate significant differences between group A to F values (*, P < 0.05; **, P < 0.01) and those of the control group.
Systemic and mucosal immune responses induced by vaccination.
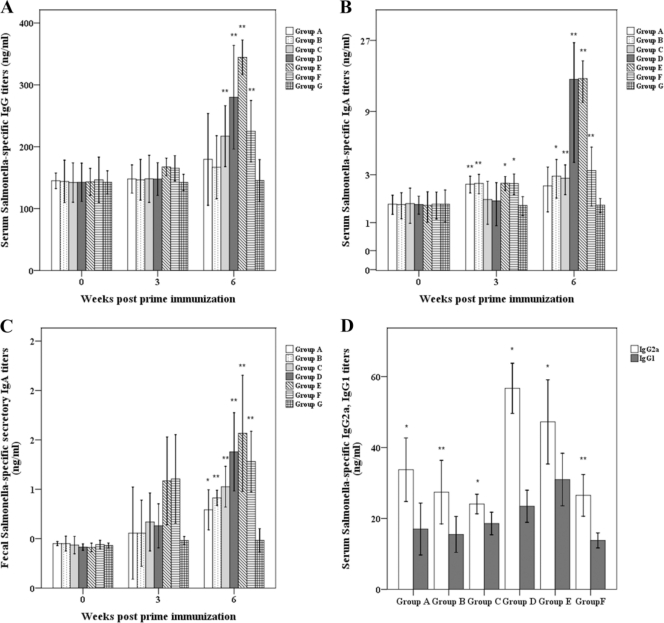
Antibody responses in the sera and the fecal samples of immunized mice to the Salmonella antigen are presented in Fig. 2. Serum IgG titers of groups A and B were increased approximately 1.1 times more than those of the control group at 6 weeks PPI. However, serum IgG titers of groups C to F were at least 1.5 times higher than those of the control group (P < 0.01) and were at least 1.3 times higher than those of groups A and B (Fig. 3A). Serum IgA titers of mice in groups C to F were also significantly higher than those of other mice at 6 weeks PPI (P < 0.05) (Fig. 3B). The sIgA titers of the fecal samples of these groups were also significantly increased (P < 0.05) (Fig. 3C).
FIG. 3.
Immune responses to Salmonella-specific antigens in mice orally inoculated with the Salmonella vaccine candidate with or without mucosal adjuvant strains. Serum Salmonella-specific IgG (A), IgA (B), and IgG1 and IgG2a (D) and fecal Salmonella-specific sIgA (C). Group A to G values refer to Fig. 2 and Table 2. The data shown are the mean values for all mice in each group, and error bars demonstrate standard deviations (SD). Asterisks indicate significant differences between group A to F values (*, P < 0.05; **, P < 0.01) and those of the control group.
IgG isotype analyses.
IgG isotype subclass IgG2a and IgG1 levels were measured at 6 weeks PPI. The IgG2a isotype was the dominant response in the sera of all immunized groups in response to the S. Typhimurium LPS antigen (Fig. 3D). The IgG2a/IgG1 ratios in groups A, B, C, D, E, and F were approximately 2.0, 1.8, 1.3, 2.4, 1.5, and 1.9, respectively (P < 0.05).
Fecal shedding of the vaccine and LTB strains.
Shedding of the vaccine and LTB strains in feces was investigated for 3 weeks following inoculation, and five mice from each group were tested daily. These strains were not detected in any fecal sample.
Protection after challenge.
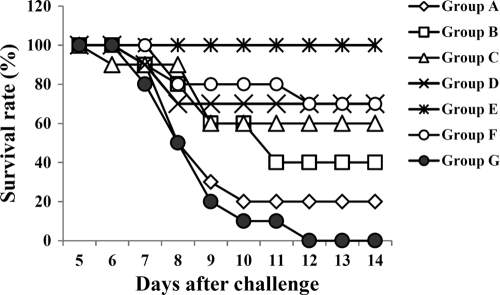
Mice from each group were orally challenged with the virulent S. Typhimurium strain (2 × 108 CFU) at 6 weeks PPI (Table 2). Group E mice (primed with the JOL911-JOL906 mixture and boosted with JOL911) did not demonstrate significant clinical signs and survived challenge through the end of the study (Fig. 4 and Table 2). However, all control group mice died between days 6 and 12 postchallenge. Deaths postchallenge in other groups of immunized mice were identified, except in group E mice.
FIG. 4.
Survival rates for mice challenged with a wild-type S. Typhimurium strain. Group A, B, C, D, E, F, and G values refer to Fig. 2 and Table 2. All mice in each group were orally challenged with 2 × 108 CFU of virulent wild-type S. Typhimurium at 6 weeks postprime immunization. Mice demonstrated mortality from day 6 postchallenge.
Isolation of the challenge strain from the organs.
Isolation of the challenge strain from the liver and splenic tissues of mice was performed on day 14 postchallenge. As shown in Table 2, the strain was not isolated from hepatic and splenic tissues from group E mice, who all survived the challenge. The strain was isolated from the organs of all surviving mice in group A. The strain was isolated from some mice in groups C (n = 4), D (n = 3), and F (n = 3).
DISCUSSION
A cpxR- and lon-deleted S. Typhimurium vaccine candidate was constructed in our previous study (15). The ability of an LTB adjuvant strain to enhance immune responses and protective efficacy induced by the vaccine candidate was evaluated in the present study. We constructed the LTB strain JOL906 to produce and secrete the B subunit of the E. coli heat-labile enterotoxin using an attenuated S. Typhimurium-harboring Asd+ plasmid. The present results indicate that the LTB adjuvant strain may enhance the efficacy of the vaccine candidate. The principle receptor for LTB is GM1 ganglioside, a glycosphingolipid ubiquitously identified on the surface of mammalian cells (9, 11, 12, 26). A principal effect of LTB interaction with mammalian cells is the stable cross-linking of GM1 at the cell surface, resulting in the uptake of coadministered proteins (9), and enhancement of both mucosal and cellular immune responses (8, 28, 52). Therefore, the nontoxic LTB adjuvant has been studied for various vaccines (19, 22, 38, 49, 51), and preparation of rLTB has been implemented (22). However, previous methods for rLTB preparation with a prokaryotic expression system were not efficient and were costly (22, 41, 42), since previously studied expression systems easily formed insoluble inclusion bodies and resulted in a lower yield of bioactive rLTB (22). Attenuated S. Typhimurium has been used as a host for delivery of recombinant proteins; there are many advantages to this, including high expression levels of many heterologous proteins and efficient secretion (6, 10, 18, 24, 40). In this study, the robust production and secretion of the LTB protein from the ΔcpxR Δlon Δasd S. Typhimurium strain harboring the eltB gene carried by plasmid pMMP65 were demonstrated by immunoblot assay. Plasmid pMMP65 contains pBR ori; plasmids containing pBR ori can be stably maintained over 50 generations in S. Typhimurium (14). In addition, β-lactamase encoded by the ampicillin resistance gene is secreted to the periplasmic space of Gram-negative bacteria, and its translocation depends on the presence of a 23-amino-acid residue signal sequence at the N terminus (13, 35) plus an additional 12 amino acids of the mature β-lactamase (14, 17, 44). It has been suggested that at least 50% of recombinant protein from the β-lactamase signal plasmid is secreted (14). It is, therefore, possible that the pMMP65 plasmid harboring the eltB gene encoding LTB is stably maintained in Salmonella strains, allowing copious amounts of the produced LTB protein to be secreted. To confirm whether the rLTB was expressed and immunogenic in mice, serum IgG and fecal sIgA antibodies against LTB were presently investigated by ELISA. The serum IgG and fecal sIgA titers of all groups immunized with JOL906 or with the JOL911-JOL906 mixture were significantly increased compared to those of the control group at 6 weeks PPI (P < 0.05), while those titers of group A (single administration with JOL911) and group B (primed and boosted with JOL911) were not. These results indicate that LTB can be secreted and is immunogenic in mice.
Mucosal sIgA antibodies block secondary Salmonella infection in the intestinal lumen by inhibition of bacterial adhesion and invasion of epithelial and M cells (1, 5, 26). In addition, systemic antibodies eliminate Salmonella from the blood and promote Salmonella phagocytosis by opsonization (5, 23). LTB not only is highly immunogenic itself but also can act as a potent adjuvant or carrier to increase immune responses to other antigens. In a previous trial, major structural proteins such as VP2 and VP4 of porcine parvovirus were expressed in Lactobacillus casei fused with LTB as a mucosal adjuvant (20, 36). The levels of IgG and sIgA from mice orally immunized with the fusion proteins were significantly higher than those from mice receiving VP2 or VP4 only without LTB. Presently, serum IgG and IgA titers to the Salmonella-specific antigen from mice immunized together with the LTB strain were significantly higher than those of the control (P < 0.01) and other nonadjuvant vaccinated groups. The sIgA titers to Salmonella from all mice immunized with vaccine and/or LTB strains were also significantly increased (P < 0.05). This suggests that the LTB adjuvant strain can effectively induce systemic and mucosal immune responses. Conversely, Th1 cells direct cell-mediated immunity and promote class switching to IgG2a in T-cell-dependent, antigen-specific immune responses. Th2 cells direct humoral immunity and promote class switching to IgG1 and IgA (31, 43). Presently, levels of IgG2a and IgG1 subunits in the groups inoculated with the adjuvant strain tended to increase (Fig. 3D), suggesting that immunization with the strain may enhance the induction of both humoral and cellular immune responses.
We also optimized the immunization strategy with the vaccine candidate and adjuvant strains to maximize the protective efficacy against salmonellosis. Our results demonstrate that all mice from group E were fully protected against salmonellosis induced by the virulent wild-type challenge, while all control group mice, 80.0% of group A mice (single immunization with the vaccine candidate), 60% of group B mice (primed and boosted with vaccine candidate), 40% of group C mice, 30% of group D mice (primed and boosted with LTB strain), and 30% of group F mice (primed and boosted with a mixture of the vaccine and LTB strain) died after challenge. Furthermore, the challenge strain was not isolated from the hepatic and splenic tissues of all group E mice postchallenge, whereas the strains were isolated in tissues obtained from the other groups. Oral administration of antigens along with toxin B subunits such as LTB may induce profound tolerance to the same antigens when the adjuvant is subsequently inoculated (11, 46). These observations suggest that boosting with the vaccine strain is necessary, while the adjuvant is required only during priming to establish the most efficient vaccine strategy for protection against salmonellosis. The mucosal immune system comprises approximately 80% of all immunocytes and has developed effective means for protecting animals as well as human beings against mucosal infections and harmful immune responses to ingested or inhaled antigens (45). LTB can promote powerful immune responses to prevent mucosal pathogens as an effective mucosal adjuvant but can also induce oral tolerance against mucosal antigens presented with LTB by modulating interleukin-10 and transforming growth factor β1 and promoting the development of Forkhead box P3 (Foxp3)-positive T cells while at the same time inhibiting the immune response (7, 21, 33, 37). Presently, the immune responses of group E were higher than those of group F, and all mice of group E survived after challenge with virulent wild-type S. Typhimurium, whereas 70% of group F mice were protected against the challenge. This suggests that priming along with the LTB strain and boosting without the LTB strain can improve immune responses and can provide the best protection efficacy against salmonellosis. On the contrary, the booster administration of the adjuvant strain may instead inhibit the induction of immune responses and decrease the protection efficacy against Salmonella infections by virtue of the potency of LTB to induce mucosal tolerance.
To examine whether the LTB strain was excreted in feces, we attempted to isolate the strain from daily fecal samples obtained from randomly selected mice from each immunized group (n = 5) for 3 weeks postinoculation. No adjuvant strains were identified in fecal samples from any mice, suggesting that the strain may not be excreted into feces and may not contaminate the environment. Adverse reactions (including diarrhea and weight loss) were not identified in this group during the experimental period. This suggests that the adjuvant strain is safe for use in hosts and in the environment.
Acknowledgments
This study was supported by the Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1.Abd El Ghany, M., et al. 2007. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect. Immun. 75:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, J., et al. 2004. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. J. Clin. Microbiol. 42:1734-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessa, M. C., et al. 2007. Phenotypic and genetic characterization of Salmonella enterica subsp. enterica serovar Typhimurium isolated from pigs in Rio Grande do Sul, Brazil. Res. Vet. Sci. 83:302-310. [DOI] [PubMed] [Google Scholar]
- 4.Boyen, F., et al. 2008. Non-typhoidal Salmonella infections in pigs: a closer look at epidemiology, pathogenesis and control. Vet. Microbiol. 130:1-19. [DOI] [PubMed] [Google Scholar]
- 5.Brumme, S., et al. 2007. Impact of Salmonella Typhimurium DT104 virulence factors invC and sseD on the onset, clinical course, colonization patterns and immune response of porcine salmonellosis. Vet. Microbiol. 124:274-285. [DOI] [PubMed] [Google Scholar]
- 6.Chen, H., and D. M. Schifferli. 2003. Construction, characterization, and immunogenicity of an attenuated Salmonella enterica serovar Typhimurium pgtE vaccine expressing fimbriae with integrated viral epitopes from the spiC promoter. Infect. Immun. 71:4664-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaldson, D. S., K. K. Tong, and N. A. Williams. 2010. Mucosal administration of the B subunit of E. coli heat-labile enterotoxin promotes the development of Foxp3-expressing regulatory T cells. Mucosal Immunol. doi: 10.1038/mi.2010.65. [DOI] [PubMed]
- 8.Fingerut, E., B. Gutter, M. Goldway, D. Eliahoo, and J. Pitcovski. 2006. B subunit of E. coli enterotoxin as adjuvant and carrier in oral and skin vaccination. Vet. Immunol. Immunopathol. 112:253-263. [DOI] [PubMed] [Google Scholar]
- 9.Freytag, L. C., and J. D. Clements. 2005. Mucosal adjuvants. Vaccine 23:1804-1813. [DOI] [PubMed] [Google Scholar]
- 10.Garmory, H. S., et al. 2003. The use of live attenuated bacteria as a delivery system for heterologous antigens. J. Drug Target. 11:471-479. [DOI] [PubMed] [Google Scholar]
- 11.Hajishengallis, G., S. Arce, C. M. Gockel, T. D. Connell, and M. W. Russell. 2005. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: application for oral infections. J. Dent. Res. 84:1104-1116. [DOI] [PubMed] [Google Scholar]
- 12.Holmgren, J., M. Lindblad, P. Fredman, L. Svennerholm, and H. Myrvold. 1985. Comparison of receptors for cholera and Escherichia coli enterotoxins in human intestine. Gastroenterology 89:27-35. [DOI] [PubMed] [Google Scholar]
- 13.Kadonaga, J. T., A. Plückthun, and J. R. Knowles. 1985. Signal sequence mutants of β-lactamase. J. Biol. Chem. 260:16192-16199. [PubMed] [Google Scholar]
- 14.Kang, H. Y., J. Srinivasan, and R. Curtiss III. 2002. Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect. Immun. 70:1739-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, S. W., et al. 2009. Changes of physiological and biochemical properties of Salmonella enterica serovar Typhimurium by deletion of cpxR and lon genes using allelic exchange method. J. Microbiol. Methods 79:314-320. [DOI] [PubMed] [Google Scholar]
- 16.Kodama, C., et al. 2005. Evaluation of the Lon-deficient Salmonella strain as an oral vaccine candidate. Microbiol. Immunol. 49:1035-1045. [DOI] [PubMed] [Google Scholar]
- 17.Koshland, D., and D. Botstein. 1980. Secretion of beta-lactamase requires the carboxy end of the protein. Cell 20:749-760. [DOI] [PubMed] [Google Scholar]
- 18.Kwon, Y. M., M. M. Cox, and L. N. Calhoun. 2007. Salmonella-based vaccines for infectious diseases. Expert Rev. Vaccines 6:147-152. [DOI] [PubMed] [Google Scholar]
- 19.Lim, J. U., et al. 2009. Expression of functional pentameric heat-labile enterotoxin B subunit of Escherichia coli in Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 19:502-510. [DOI] [PubMed] [Google Scholar]
- 20.Liu, D., X. Wang, J. Ge, S. Liu, and Y. Li. 2010. Comparison of the immune responses induced by oral immunization of mice with Lactobacillus casei-expressing porcine parvovirus VP2 and VP2 fused to Escherichia coli heat-labile enterotoxin B subunit protein. Comp. Immunol. Microbiol. Infect. Dis. doi: 10.1016/j.cimid. 2010.02.004. [DOI] [PMC free article] [PubMed]
- 21.Luross, J. A., T. Heaton, T. R. Hirst, M. J. Day, and N. A. Williams. 2002. Escherichia coli heat-labile enterotoxin B subunit prevents autoimmune arthritis through induction of regulatory CD4+ T cells. Arthritis Rheum. 46:1671-1682. [DOI] [PubMed] [Google Scholar]
- 22.Ma, X., B. Yao, W. Zheng, and L. Li. 2010. Comparative study on characterization of recombinant B subunit of E. coli heat-labile enterotoxin (rLTB) prepared from E. coli and P. patoris. J. Microbiol. Biotechnol. 20:550-557. [PubMed] [Google Scholar]
- 23.Mastroeni, P., J. A. Chabalgoity, S. J. Dunstan, D. J. Maskell, and G. Dougan. 2001. Salmonella: immune responses and vaccines. Vet. J. 161:132-164. [DOI] [PubMed] [Google Scholar]
- 24.Matic, J. N., et al. 2009. Development of non-antibiotic-resistant, chromosomally based, constitutive and inducible expression systems for aroA-attenuated Salmonella enterica serovar Typhimurium. Infect. Immun. 77:1817-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui, M., A. Takaya, and T. Yamammoto. 2008. σ32-Medited negative regulation of Salmonella pathogenicity island 1 expression. J. Bacteriol. 190:6636-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McSorley, S. J., and M. K. Jenkins. 2000. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect. Immun. 68:3344-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mead, P. S., et al. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyle, P. M., R. P. McGeary, J. T. Blanchfield, and I. Toth. 2004. Mucosal immunization: adjuvants and delivery system. Curr. Drug Deliv. 1:385-396. [DOI] [PubMed] [Google Scholar]
- 29.Nagarajan, A. G., et al. 2009. sopB of Salmonella enterica serovar Typhimurium is a potential DNA vaccine candidate in conjugation with live attenuated bacteria. Vaccine 27:2804-2811. [DOI] [PubMed] [Google Scholar]
- 30.Norimatsu, M., V. Chance, G. Dougan, C. J. Howard, and B. Villarreal-Ramos. 2004. Live Salmonella enterica serovar Typhimurium (S. Typhimurium) elicit dendritic cell responses that differ from those induced by killed S. Typhimurium. Vet. Immunol. Immunopathol. 98:193-201. [DOI] [PubMed] [Google Scholar]
- 31.O'Garra, A., and N. Arai. 2000. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell. Biol. 10:542-550. [DOI] [PubMed] [Google Scholar]
- 32.Ogra, P. L., H. Faden, and R. C. Welliver. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14:430-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ola, T. O., and N. A. Williams. 2006. Protection of non-obese diabetic mice from autoimmune diabetes by Escherichia coli heat-labile enterotoxin B subunit. Immunology 117:262-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen, S. J., L. C. MacKinnon, J. S. Goulding, N. H. Bean, and L. Slutsker. 2000. Surveillance for foodborne-disease outbreaks—United States, 1993-1997. MMWR CDC Surveill. Summ. 49:1-62. [PubMed] [Google Scholar]
- 35.Plückthun, A., and J. R. Knowles. 1987. The consequences of stepwise deletion from the signal-processing site of β-lactamase. J. Biol. Chem. 262:3951-3957. [PubMed] [Google Scholar]
- 36.Qiao, X., et al. 2009. Recombinant porcine rotavirus VP4 and VP4-LTB expressed in Lactobacillus casei induced mucosal and systemic antibody responses in mice. BMC Microbiol. doi: 10.1186/1471-2180-9-249. [DOI] [PMC free article] [PubMed]
- 37.Raveney, B. J., et al. 2008. The B subunit of Escherichia coli heat-labile enterotoxin inhibits Th1 but not Th17 cell responses in established experimental autoimmune uveoretinitis. Invest. Ophthalmol. Vis. Sci. 49:4008-4017. [DOI] [PubMed] [Google Scholar]
- 38.Richards, C. M., A. T. Aman, T. R. Hirst, T. J. Hill, and N. A. Williams. 2001. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J. Virol. 75:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roesler, U., P. Heller, K. H. Waldmann, U. Truyen, and A. Hensel. 2006. Immunization of sows in an integrated pig-breeding herd using a homologous inactivated Salmonella vaccine decreases the prevalence of Salmonella Typhimurium infection in the offspring. J. Vet. Med. B Infect. Dis. Public Health 53:224-228. [DOI] [PubMed] [Google Scholar]
- 40.Roland, K. L., S. A. Tinge, K. P. Killeen, and S. K. Kochi. 2005. Recent advances in the development of live, attenuated bacterial vectors. Curr. Opin. Mol. Ther. 7:62-72. [PubMed] [Google Scholar]
- 41.Ruitenberg, K. M., J. R. Gilkerson, J. E. Wellington, D. N. Love, and J. M. Whalley. 2001. Equine herpesvirus 1 glycoprotein D expressed in Pichia pastoris is hyperglycosylated and elicits a protective immune response in the mouse model of EHV-1 disease. Virus Res. 79:125-135. [DOI] [PubMed] [Google Scholar]
- 42.Sagt, C. M., et al. 2000. Introduction of an N-glycosylation site increases secretion of heterologous proteins in yeasts. Appl. Environ. Microbiol. 66:4940-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spellberg, B., and J. E. Edwards, Jr. 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76-102. [DOI] [PubMed] [Google Scholar]
- 44.Summers, R. G., and J. R. Knowles. 1989. Illicit secretion of a cytoplasmic protein into the periplasm of Escherichia coli requires a signal peptide plus a portion of the cognate secreted protein. J. Biol. Chem. 264:20074-20081. [PubMed] [Google Scholar]
- 45.Sun, J. B., C. Czerkinsky, and J. Holmgren. 2010. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand. J. Immunol. 71:1-11. [DOI] [PubMed] [Google Scholar]
- 46.Sun, J. B., J. Holmegren, and C. Czerkinsky. 1994. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc. Natl. Acad. Sci. U. S. A. 91:10795-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swanenburg, M., H. A. P. Urlings, and J. M. A. Snijders. 2001. Salmonella in slaughter pigs: prevalence, serotypes and critical control points during slaughter in two slaughterhouses. Int. J. Food Microbiol. 70:243-254. [DOI] [PubMed] [Google Scholar]
- 48.Vasserman, Y., and J. Pitcovski. 2006. Genetic detoxification and adjuvant-activity retention of Escherichia coli enterotoxin LT. Avian Pathol. 35:134-140. [DOI] [PubMed] [Google Scholar]
- 49.Wagner, B., et al. 2004. Expression of the B subunit of the heat-labile enterotoxin of Escherichia coli in tobacco mosaic virus-infected Nicotiana benthamiana plants and its characterization as mucosal immunogen and adjuvant. J. Immunol. Methods 287:203-215. [DOI] [PubMed] [Google Scholar]
- 50.Weening, E. H., et al. 2005. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in Mice. Infect. Immun. 73:3358-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weltzin, R., B. Guy, W. D. Thomas, Jr., P. J. Giannasca, and T. P. Monath. 2000. Parenteral adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection. Infect. Immun. 68:2775-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams, N. A. 2000. Immune modulation by the cholera-like enterotoxins B-subunits: from adjuvant to immunotherapeutic. Int. J. Med. Microbiol. 290:447-453. [DOI] [PubMed] [Google Scholar]
- 53.Yoon, H. A., et al. 2007. Correlation between the nature of immunity induced by different immunogens and the establishment of latent infection by wild-type pseudorabies virus. Res. Vet. Sci. 83:73-81. [DOI] [PubMed] [Google Scholar]