Abstract
The persistence of anti-Leishmania donovani antibodies in past visceral leishmaniasis (VL) cases was retrospectively assessed by means of the direct agglutination test (DAT) and the rK39 enzyme-linked immunosorbent assay (ELISA). Antibody titers remained high for an extended period of time in past cases of VL. These results highlight the need to carefully elicit the history of patients with VL symptoms.
Visceral leishmaniasis (VL; kala-azar) is a systemic infection of the reticuloendothelial system. VL patients present with fever, weight loss, weakness, loss of appetite, and enlargement of the liver and spleen. Such progressive infection is associated with poor delayed-type hypersensitivity and high antibody production (11, 12). While the “gold standard” for diagnosis is still demonstration of parasites in splenic or bone marrow smears, serological tests such as the direct agglutination test (DAT) and rK39-based tests (i.e., immunochromatic strip tests or enzyme-linked immunosorbent assay [ELISA]) are increasingly used for diagnosis (3). It is well known that antileishmanial antibodies persist after clinical cure (4, 6, 13, 17); however, how long they persist in VL patients in the Indian subcontinent is unknown. In the present study, we used the DAT and the rK39 ELISA to assess the persistence of antibodies against Leishmania donovani, the VL agent in the Indian subcontinent, in past VL cases in Muzaffarpur District, India.
(The preliminary results of this study were presented at the 6th European Congress on Tropical Medicine and International Health in Verona, Italy, 6 to 10 September 2009.)
Three house-to-house surveys were conducted in 16 villages in which VL is endemic in Muzaffarpur District in November 2006, 2007, and 2008. Details on the selection of the study villages and their demographic characteristics are provided elsewhere (14). Age, gender, and history of VL were gathered from participants using semistructured questionnaires. Individuals who reported a past episode of VL were further interviewed (parents or guardians in the case of minors) to collect data on date of onset of symptoms, date and place of treatment, and type of drug used. Medical records, if available, were checked. The case ascertainment was made by a physician. A blood sample was collected from all consenting individuals over 2 years old by finger prick onto Whatman (Maidstone, United Kingdom) filter paper 3.
Self-reported VL cases treated with antileishmanial drugs before 1 November 2006 who provided a blood sample were included in this study. The time between VL treatment and blood sampling was calculated in months. The date of treatment was used instead of the onset of symptoms to minimize the recall bias. For those who reported several episodes of VL (relapse or recurrence), the most recent episode was taken into consideration.
DAT and rK39 ELISA were performed to detect L. donovani antibodies in blood samples, as detailed elsewhere (7-9). For DAT, sera were tested at titers of 1:400 up to 1:25,600. Sera that did not agglutinate were assigned a titer of 1:200, and those with an end titer above 1:25,600 were coded as 1:51,200. A titer of 1:3,200 was used as a cutoff (5). Results from the rK39 ELISA were expressed as percent positivity (PP) (8). A cutoff of 22 PP for ELISA was determined from 37 nonendemic control samples, as detailed elsewhere (8).
Spearman's correlation coefficient was used to evaluate the agreement between DAT and rK39 ELISA (8). The geometric means (GMs) (and 95% confidence interval) for DAT titers and rK39 PP were calculated and plotted for the following periods: 0 to 6, 7 to 12, 13 to 24, 25 to 36, and 37 to 48 months and 5 to 6, 7 to 8, 9 to 11, 12 to 17, 18 to 24, and over 24 years. The GMs were calculated on the basis of the number of past VL cases in each category, using Stata v10.
The study protocol was approved by Institutional Review Boards of the Banaras Hindu University and the University of Antwerp. Written informed consent was obtained from all participants or guardians before enrolling in the study.
Out of 13,343 subjects, 845 had been treated for VL before 1 November 2006. DAT and rK39 ELISA results were available for 780 (92.3%) past VL cases. Most of the participants were male (500/780) and reported a single VL episode (96.3%; 751/780). The median age when they suffered VL was 17 years old (range, <1 to 87 years old). Blood samples were taken at time points ranging from 1 month to 53 years postcompletion of therapy (median of 3 years).
The majority of VL cases were treated in private institutions or nongovernmental organizations, especially in the last 15 years (Table 1). The fact that incentives are now provided to patients attending public facilities (10) should increase the number of people treated in governmental facilities and reduce underreporting (15). A significant number of patients were still treated with sodium stibogluconate (SSG) (Table 1), even if SSG has progressively been replaced by amphotericin B since the mid-1990s (16). Miltefosine, the first-line treatment for VL in India since 2005 (1), was administered to only 30 (4%) patients, all of them after 2002.
TABLE 1.
Number of past VL cases per period, where the cases were treated, and antileishmania drug received
| Time between VL treatment and November 2006 blood sampling (no. of subjects) | % (no.) of cases treated by facilitya: |
% (no.) of cases treated withd: |
% (no.) of cases positive bye: |
|||
|---|---|---|---|---|---|---|
| Governmentb | Private/NGOc | SSG | Amphotericin B | DAT | rK39 ELISA | |
| <1 yr (124) | 19.4 (24) | 80.6 (100) | 31.5 (39) | 61.3 (76) | 93.5 (116) | 86.3 (107) |
| 1-<5 yr (447) | 16.1 (72) | 83.7 (374) | 38.0 (170) | 55.9 (250) | 89.3 (399) | 68.2 (305) |
| 5-<10 yr (123) | 13.0 (16) | 87.0 (107) | 53.7 (66) | 45.5 (56) | 80.5 (99) | 57.7 (71) |
| 10-<15 yr (35) | 25.7 (9) | 74.3 (26) | 68.6 (24) | 31.4 (11) | 68.6 (24) | 42.9 (15) |
| ≥15 yr (51) | 29.4 (15) | 62.7 (32) | 84.3 (43) | 7.8 (4) | 52.9 (27) | 39.2 (20) |
Other sites and “unknown” not shown.
District Hospital, Medical College, and Community Health Centres.
Private hospitals or health centers and nongovernmental organizations (NGO; i.e., Kala Azar Medical Research Centre in Muzaffapur).
Other drugs (i.e., miltefosine) and “unknown” not shown. SSG, sodium stibogluconate.
Cutoff values: DAT, titer of 1:3,200; rK39 ELISA, 22% positivity.
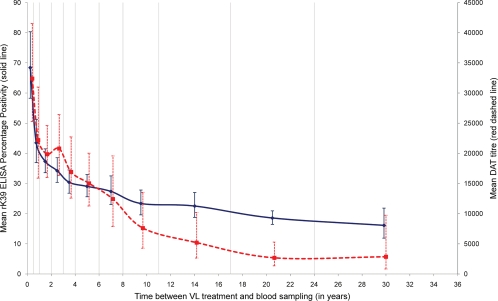
We found a modest correlation between the DAT and rK39 ELISA results (Spearman's rho = 0.57; P < 0.001). The agreement between tests was better than the one observed in asymptomatic individuals in Nepal (8). The kinetics of L. donovani antibodies over time detected by both tests were similar. Titers (DAT) and PP values (rK39 ELISA) were high for recent cases, decreased rapidly in the first 12 months, and then declined more slowly (Fig. 1). There were, however, some differences between rK39 ELISA and DAT: e.g., the early decline was steeper for ELISA than for agglutination antibodies (Fig. 1). This difference could be related to differences in ELISA and DAT antibody half-life times (6). Nevertheless, antibody titers remained high for an extended period of time: e.g., a significant number of individuals who suffered from VL 15 or more years before the blood sample was taken tested positive by DAT (53%) and rK39 ELISA (39%) (Table 1). In areas of endemicity, antileishmanial antibodies remain detectable many years after the episode of VL. This may be due to repeated exposure to L. donovani or incomplete elimination of the parasite, as suggested in a study in Nepal where 26.1% (6/23) of past VL cases were PCR positive (2). The study samples could not be tested by PCR as this method has not been validated for filter paper samples (2).
FIG. 1.
Kinetics of Leishmania donovani antibodies on past visceral leishmaniasis (VL) cases. Shown are the geometric mean and 95% confidence interval for direct agglutination test (DAT) titers (red dashed line) and rK39 ELISA percentage positivity (PP) (blue solid line) per period between the reported date of VL treatment and the date of blood sampling. Vertical lines indicate the periods considered to calculate the geometric means: 0 to 6, 7 to 12, 13 to 24, 25 to 36, and 37 to 48 months and 5 to 6, 7 to 8, 9 to 11, 12 to 17, 18 to 24, and over 24 years.
Previous cohort studies of past VL showed that rK39 ELISA detected anti-Leishmania antibodies for up to 4 years in India (13) and cases remained positive for up to 12 years in Brazil (4) and 24 months in Sudan (17). Similarly, 89% of past VL cases were DAT positive 56 to 90 months posttreatment in Ethiopia (6). In contrast to our retrospective study, cohort designs have fewer problems with recall and misclassification bias but are limited by the number of patients that can be followed for a long time. The results of this study highlight the need to carefully elicit the history of patients with VL symptoms, as serological tests used for diagnosis may continue to produce positive results for a long time in past VL patients.
Acknowledgments
We thank S. G. Reed, Infectious Disease Research Institute, Seattle, WA, for kindly providing the rk39 antigen.
This work was financially supported by the EU-funded INCO-DEV KALANET project (EU contract no. 015374). Kamlesh Gidwani was supported by a fellowship from the Council of Scientific and Industrial Research, New Delhi, India.
The authors report they have no conflicts of interest.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1.Bhattacharya, S. K., D. Sur, P. K. Sinha, and J. Karbwang. 2006. Elimination of leishmaniasis (kala-azar) from the Indian subcontinent is technically feasible & operationally achievable. Indian J. Med. Res. 123:195-196. [PubMed] [Google Scholar]
- 2.Bhattarai, N. R., et al. 2009. PCR and direct agglutination as Leishmania infection markers among healthy Nepalese subjects living in areas endemic for Kala-Azar. Trop. Med. Int. Health 14:404-411. [DOI] [PubMed] [Google Scholar]
- 3.Boelaert, M., et al. 2008. Diagnostic tests for kala-azar: a multi-centre study of the freeze-dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Trans. R. Soc. Trop. Med. Hyg. 102:32-40. [DOI] [PubMed] [Google Scholar]
- 4.De Almeida Silva, L., et al. 2006. Immunologic tests in patients after clinical cure of visceral leishmaniasis. Am. J. Trop. Med. Hyg. 75:739-743. [PubMed] [Google Scholar]
- 5.el Harith, A., et al. 1988. Improvement of a direct agglutination test for field studies of visceral leishmaniasis. J. Clin. Microbiol. 26:1321-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hailu, A. 1990. Pre- and post-treatment antibody levels in visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 84:673-675. [DOI] [PubMed] [Google Scholar]
- 7.Jacquet, D., et al. 2006. Comparative evaluation of freeze-dried and liquid antigens in the direct agglutination test for serodiagnosis of visceral leishmaniasis (ITMA-DAT/VL). Trop. Med. Int. Health 11:1777-1784. [DOI] [PubMed] [Google Scholar]
- 8.Khanal, B., et al. Serological markers for Leishmania donovani infection in Nepal: agreement between direct agglutination test and rK39 ELISA. Trop. Med. Int. Health, in press. [DOI] [PubMed]
- 9.Kurkjian, K. M., et al. 2005. Application of an improved method for the recombinant K39 enzyme-linked immunosorbent assay to detect visceral leishmaniasis disease and infection in Bangladesh. Clin. Diagn. Lab. Immunol. 12:1410-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NVBDCP. Template for developing district action plan (kala-azar), in press. National Vector Borne Disease Control Programme, Delhi, India.
- 11.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 12.Sharma, U., and S. Singh. 2009. Immunobiology of leishmaniasis. Indian J. Exp. Biol. 47:412-423. [PubMed] [Google Scholar]
- 13.Singh, S., V. Kumari, and N. Singh. 2002. Predicting kala-azar disease manifestations in asymptomatic patients with latent Leishmania donovani infection by detection of antibody against recombinant K39 antigen. Clin. Diagn. Lab. Immunol. 9:568-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh, S. P., et al. 2010. The epidemiology of Leishmania donovani infection in high transmission foci in India. Trop. Med. Int. Health 15(Suppl. 2):12-20. [DOI] [PubMed] [Google Scholar]
- 15.Singh, S. P., D. C. Reddy, M. Rai, and S. Sundar. 2006. Serious underreporting of visceral leishmaniasis through passive case reporting in Bihar, India. Trop. Med. Int. Health 11:899-905. [DOI] [PubMed] [Google Scholar]
- 16.Thakur, C. P., et al. 2008. Impact of amphotericin-B in the treatment of kala-azar on the incidence of PKDL in Bihar, India. Indian J. Med. Res. 128:38-44. [PubMed] [Google Scholar]
- 17.Zijlstra, E. E., et al. 1998. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin. Diagn. Lab. Immunol. 5:717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]