Abstract
Previously, we observed that both major membrane protein II of Mycobacterium leprae (MMP-ML) and its fusion with M. bovis BCG (BCG)-derived heat shock protein 70 (HSP70) (Fusion-ML) are immunogenic and that recombinant BCG secreting either of these proteins effectively inhibits the multiplication of M. leprae in mice. Here, we purified M. tuberculosis-derived major membrane protein II (MMP-MTB) and its fusion with HSP70 (Fusion-MTB) in a lipopolysaccharide-free condition and evaluated their immunostimulatory abilities. Both MMP-MTB and Fusion-MTB activated monocyte-derived dendritic cells (DC) in terms of phenotype and interleukin-12 (IL-12) production, but Fusion-MTB more efficiently activated them than MMP-MTB did. The IL-12 production was a consequence of the ligation of those recombinant proteins with Toll-like receptor 2. The M. tuberculosis-derived and M. leprae-derived recombinant proteins activated naïve T cells of both CD4 and CD8 subsets, but M. tuberculosis-derived proteins were superior to M. leprae-derived proteins and fusion proteins were superior to MMP, regardless of the origin of the protein. Memory-type CD4+ T cells obtained from BCG-vaccinated healthy individuals seem to be primed with MMP-MTB by the vaccination, and both M. tuberculosis-derived recombinant proteins produced perforin-producing CD8+ T cells from memory-type CD8+ T cells. Further, infection of DC and macrophages with M. tuberculosis H37Ra and H37Rv induced the expression of MMP on their surface. These results indicate that M. tuberculosis-derived MMP, as a sole protein or as part of a fusion protein, may be useful for developing new vaccinating agents against tuberculosis.
Tuberculosis is a chronic infectious disease caused by intracellular infection with Mycobacterium tuberculosis (20). It is estimated that one-third of the global population is latently infected with this inhaled pathogen, which infects primarily macrophages and dendritic cells (DC), and tuberculosis is responsible for more than two million deaths yearly worldwide (11, 34, 36). The emergence of multidrug-resistant strains of M. tuberculosis mandates the development of more effective preventive and therapeutic strategies, including the development of improved vaccines (48). Protective immunity against M. tuberculosis is conducted chiefly by adaptive cellular immune responses, and gamma interferon (IFN-γ)-producing type 1 CD4+ T cells and CD8+ T cells are key components of this immunity (1, 12, 16). IFN-γ produced by activated T cells is believed to be an essential element of the host defense against M. tuberculosis (13). Further, the contribution of CD8+ T cells to protection by lysing infected cells is also important for bacterial killing (7, 19). CD8+ T cells can kill M. tuberculosis-infected host cells via a granule-dependent mechanism involving perforin and granulysin, which has a direct antimicrobial activity (42, 49).
The only approved vaccine currently available against tuberculosis is M. bovis bacillus Calmette-Guérin (BCG), an attenuated strain of M. bovis. More than four billion doses of BCG have been administered so far, and is established as a safe vaccine (29). BCG appears to be effective at preventing diseases such as tuberculous meningitis and miliary tuberculosis in newborns and toddlers; however, it has no apparent effect on pulmonary tuberculosis in adults (3, 9). The reason why BCG cannot prevent disease development is not fully known, but one of the reasons is based on the fact that BCG has a capacity to block phagosome maturation to inhibit antigen (Ag) processing and presentation to type 1 T cells (14, 32, 38). Indeed, although M. tuberculosis directly delivers Ag to the major histocompatibility complex (MHC) class I processing pathway, BCG was less able to activate CD8+ T cells (35, 41). Further, BCG growing in human macrophages was not recognized by immune CD4+ T cells, although BCG-infected macrophages continued to express MHC class II molecules (35). These observations indicate the need for the development of a new vaccine against tuberculosis.
Various new vaccine candidates which are based on Ags that are recognized in infected individuals are currently in clinical trials, including early secretory antigenic target 6 (ESAT-6), the Ag85 family, and a polyprotein Ag, designated Mtb72F, derived from M. tuberculosis proteins Mtb32 and Mtb59 (1, 2, 17, 18, 37, 39). However, a fully reliable new vaccine has not been established yet.
A situation similar to that of tuberculosis can be found in leprosy, which is caused by infection with M. leprae, and the development of a new vaccine capable of inhibiting the multiplication of M. leprae is highly desirable. In both tuberculosis and leprosy, the activation of T cells is induced by DC loaded with bacilli or their components, which display various antigenic molecules on their surface, including the immunodominant Ags (15, 30), although there are conflicting results indicating that M. leprae inhibits the activation and maturation of DC (33). We are of the opinion that future vaccines, to be successful, must (i) be highly antigenic, (ii) have the capacity to activate both naïve CD4+ T cells and CD8+ T cells, and (iii) have the ability to be expressed on the surface of mycobacterium-infected Ag-presenting cells (APCs) such as macrophages and DC. Previously, we identified major membrane protein (MMP; gene name, bfrA or ML2038) as one of the immunodominant Ags of M. leprae (21). M. leprae-derived MMP (MMP-ML) ligates Toll-like receptor 2 (TLR2) and consequently activates the NF-κB pathway of host cells (21). DC pulsed with MMP-ML activate memory-type CD4+ and CD8+ T cells to produce IFN-γ in an Ag-specific fashion (21, 26). Further, MMP-ML is supposed to be recognized in vivo by T cells of M. leprae-infected individuals, including paucibacillary leprosy patients (26).
Further, when we introduced MMP-ML with the Ag85A secretion signal of M. tuberculosis into BCG, the modified BCG, termed BCG-SM, secreted MMP-ML, enhanced the ability of BCG to activate naïve CD4+, and further, successfully activated naïve CD8+ T cells (25). Furthermore, BCG-SM at least partially inhibited the growth of M. leprae in C57BL/6 mice subsequently challenged by injection in the footpads (22). These observations indicate that MMP-ML could be a target molecule to be further analyzed as a vaccine candidate, and the fact that BCG-SM can activate both subsets of naïve T cells to produce IFN-γ indicates that secretion of MMP-ML, presumably in the phagosome of APCs, is a useful strategy to activate T cells (25). We sought another strategy to further enhance the T cell-stimulating activity of BCG, especially of the ability to activate IFN-γ-producing CD8+ T cells quickly and strongly. To this end, we used heat shock protein 70 (HSP70) as a fusion partner (6, 10, 44, 45). The gene encoding HSP70 of BCG was directly linked with that of MMP and extrachromosomally transformed into BCG (BCG-70 M) (31). BCG-70 M secreted the HSP70-MMP fusion protein (Fusion-ML) and activated not only Ag-specific naïve CD8+ T cells polyclonally but also naïve CD4+ T cells strongly (31). Further, the secreted Fusion-ML protein activated DC in terms of phenotype and the production of cytokines such as interleukin-12 (IL-12) (31). Thus, the production and secretion of HSP70 in phagosomes along with MMP-ML, using BCG as a vector, seem to be effective in activating human naïve CD8+ T cells. These observations led us to speculate that the use of MMP, which is commonly present in pathogenic mycobacteria, or of the HSP70-MMP fusion protein may be useful in inhibiting the multiplication of M. tuberculosis. However, the MMP homology between M. leprae and M. tuberculosis (MMP-MTB; gene name, bfrA or Rv1876) is 90.6% at the amino acid level. Therefore, in this study, we purified M. leprae- or M. tuberculosis-derived MMP and a fusion protein composed of HSP70 and M. leprae- or M. tuberculosis-derived MMP by using M. smegmatis and evaluated their immunostimulatory activities.
MATERIALS AND METHODS
Preparation of cells and Ags.
Peripheral blood was obtained from healthy, purified protein derivative-positive individuals after informed consent was obtained. In Japan, BCG vaccination is compulsory for children (0 to 4 years old). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden) and cryopreserved in liquid nitrogen until use, as previously described (23). The viability of T cells obtained from cryopreserved PBMCs was more than 90%, and no functional selection was induced in either monocytes or T cells by the cryopreservation of PBMCs. For the preparation of peripheral monocytes, CD3+ T cells were removed from either freshly isolated heparinized blood or cryopreserved PBMCs using immunomagnetic beads coated with anti-CD3 monoclonal antibody (MAb; Dynabeads 450; Dynal Biotech, Oslo, Norway). The CD3− PBMC fraction was plated on collagen-coated plates, and the non-plastic-adherent cells were removed by extensive washing. The remaining adherent cells were used as monocytes (47). Monocyte-derived DC were differentiated as described previously (23, 28). Briefly, monocytes were cultured in the presence of 50 ng of recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF; PeproTech EC Ltd., London, England) and 10 ng of rIL-4 (PeproTech) per ml (28). On day 4 of culture, immature DC (purity, 70%) were pulsed with recombinant protein and on day 6 of culture, DC were used for further analyses of surface Ag and for mixed-lymphocyte assays. Macrophages were differentiated as described previously (24, 27). In brief, monocytes were cultured in the presence of 10 ng of rM-CSF (R&D Systems, Inc., Minneapolis, MN) per ml. On day 5 of culture, macrophages were pulsed with recombinant protein and on day 7 of culture, they were used for further analyses of surface Ag and for mixed-lymphocyte assays.
Preparation of M. tuberculosis.
M. tuberculosis strains H37Ra and H37Rv, which were originally purchased from the American Tissue Culture Collection, were kindly donated by T. Yamazaki, National Institute of Infectious Diseases. Both H37Ra and H37Rv were cultured in vitro using Middlebrook 7H9 broth (BD Biosciences, San Jose, CA) supplemented with 0.05% Tween 80 and 10% albumin-dextrose-catalase (BD Biosciences). The bacilli were grown to log phase and stored at 108 CFU/ml at −80°C. Before the infection of DC and macrophages, H37Ra and H37Rv bacteria were counted by the colony assay method.
Construction of vectors for production of M. leprae- or M. tuberculosis-derived recombinant MMP and HSP70-MMP fusion protein (Fusion-ML) or Fusion-MTB.
An Escherichia coli-Mycobacterium shuttle vector, pMV261 (43), was used as a parent vector. To replace the kanamycin resistance gene with a hygromycin resistance cassette, the XbaI-NheI fragment from pYUB854 (5) was cloned into SpeI-NheI-digested plasmids. The resultant vector was a hygromycin-resistant pMV261 vector, pMV261H. The acetamidase promoter was amplified from M. smegmatis mc2155 genomic DNA. The primers used were F Pace Xba (5′-TTA ATC TAG AGA AGT GAC GCG GTC TCA AGC GTC-3′ [underlining indicates an XbaI site]) and R Pace Bam (5′-TTT AGG ATC CGT GGA CTC CCT TTC TCT TA-3′ [underlining indicates a BamHI site]). The Hsp60 promoter region in pMV261H was replaced with the amplified PCR products, and the resulting vector was named p2H Pace.
We inserted an N-terminally His-tagged gene sequence encoding MMP-ML, MMP-MTB, HSP70-MMP-ML fusion (Fusion-ML), or HSP70-MMP-MTB (Fusion-MTB) into the p2H Pace vector using the In-Fusion Advantage PCR Cloning Kit (Clontech Laboratories, Inc.). Briefly, the linearized p2H Pace vector for the In-Fusion reaction was prepared by PCR with F ter (5′-TAG TTA ACT AGC GTA CGA T-3′) and R Pace H6 (5′-GTG ATG GTG GTG ATG GTG CAT GTG GAC TCC CTT TCT CTT AT-3′). PCR primers for inserts were designed that share 15 bases of homology with the sequences at the ends of linearized p2H Pace. These primers were used to amplify the insert DNAs for MMP-ML, MMP-MTB, Fusion-ML, and Fusion-MTB. The resulting PCR products were combined with the linearized vector in the In-Fusion cloning reaction and then transformed into E. coli. All clones was verified by sequencing.
Expression and purification of recombinant proteins in M. smegmatis.
M. smegmatis mc2155 was cultured in vitro using LB broth supplemented with 0.05% Tyloxapol (Sigma-Aldrich, St. Louis, MO). Expression vectors were introduced into M. smegmatis by electroporation (40). Transformants were selected on LB agar (BD Biosciences, San Jose, CA) plates containing 50 μg/ml hygromycin. The selected clone was grown in LB broth with 150 μg/ml hygromycin. During the logarithmic phase, acetamide was added to the culture medium at a final concentration of 0.2% (8). After an additional 16 h of culture, recombinant M. smegmatis was centrifuged and resuspended in lysis buffer (50 mM sodium phosphate, 300 mM NaCl, 3 M guanidine-HCl) containing proteinase inhibitor and disrupted by sonication. The suspension was centrifuged at 27,000 × g for 15 min. The supernatant was further filtered through a 0.45-μm filter and used as starting material. MMP-ML and MMP-MTB were purified by metal affinity chromatography (TALON Metal Affinity Resins; Clontech Laboratories). Fusion-ML and Fusion-MTB were purified by two purification steps. Passage through a metal affinity column (TALON) was also used in the first step. The eluted crude proteins were applied to a HiLoad Superdex 200 pg column (GE Healthcare, Buckinghamshire, England) for further purification by gel filtration. Three major fractions were detected after the second step; one of them contained the target protein. The purified proteins (MMP-ML, MMP-MTB, Fusion-ML, and Fusion-MTB) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie brilliant blue staining (see Fig. S1 in the supplemental material).
Analysis of cell surface Ag.
The expression of cell surface Ag on DC and lymphocytes was analyzed using a FACScalibur. Dead cells were eliminated from the analysis by staining with propidium iodide (Sigma-Aldrich), and 1 × 104 live cells were analyzed. For cell surface Ag analysis, fluorescein isothiocyanate (FITC)-conjugated MAbs against HLA-ABC (G46-2.6; BD Biosciences), HLA-DR (L243; BD Biosciences), CD86 (FUN-1; BD Biosciences), and CD83 (HB15a; Immunotech, Marseille, France) were used.
The expression of MMP on Ag-pulsed DC or DC infected with M. tuberculosis at an indicated multiplicity of infection (MOI) was determined using the MAb against MMP-ML (M270-13, IgM, kappa), which probably detects MMP complexed with MHC molecules on the surface of DC (26), followed by FITC-conjugated anti-mouse Ig Ab (Tago Immunologicals, Camarillo, CA). The intracellular production of perforin was assessed as follows. Memory-type CD8+ T cells were stimulated with Ag-pulsed DC for 5 days in the presence of memory-type CD4+ T cells, and CD8+ T cells were surface stained with phycoerythrin-labeled MAb to CD8 and fixed in 2% formaldehyde. Subsequently, the cells were permeabilized using permeabilizing solution (BD Biosciences) and stained with an FITC-conjugated MAb to perforin (δG9; BD Biosciences) or an FITC-labeled isotype control.
APC functions of DC.
The ability of Ag-pulsed DC and macrophages to stimulate T cells was assessed using an autologous APC-T cell coculture as previously described (15, 28). Purification of CD4+ and CD8+ T cells was conducted by using negative-isolation kits (Dynabeads 450; Dynal Biotech) (28). The purity of the CD4+ and CD8+ T cells was more than 95% as assessed by FACScalibur. Naïve CD4+ and CD8+ T cells were produced by further treatment of these T cells with MAb to CD45RO, which was followed by beads coated with MAb to goat anti-mouse IgG (Dynal Biotech). The purity of both subsets of naïve T cells was more than 97%. However, there was no contamination of memory-type T cells in the naïve T cell preparations. More than 98% of the CD45RA+ T cells were positive for expression of the CCR7 molecule. Memory-type T cells were similarly produced by the treatment of cells with a MAb to CD45RA Ag. The purified responder cells (1 × 105 per well) were plated in 96-well round-bottom tissue culture plates, and DC or macrophages pulsed with Ag were added to give the indicated APC/T cell ratio. Supernatants of APC-T cell cocultures were collected on day 4, and the cytokine levels were determined. In some cases, Ag-pulsed DC were treated with MAbs to HLA-ABC (W6/32; mouse IgG2a, kappa), HLA-DR (L243; mouse IgG2a, kappa), CD86 (IT2.2; mouse IgG2b, kappa, BD Biosciences), or MMP (M270-13) or normal mouse IgG or IgM. The treatment of DC with these MAbs did not affect the viability of the DC (not shown). Also, in some cases, Ag-pulsed DC were costimulated with CD40 ligand (CD40L; 1 μg/ml; PeproTech). The optimal concentration was determined in advance.
Measurement of cytokine production.
Levels of the following cytokines were measured: IFN-γ produced by CD4+ and CD8+ T cells and IL-12p40 produced by DC stimulated for 24 h with Ag. The concentrations of these cytokines were quantified with enzyme-linked immunosorbent assay (ELISA) kits (Opt EIA Human ELISA Set; BD Biosciences). The detection limit of the IFN-γ ELISA kit is 3.0 pg/ml.
Statistical analysis.
Student's t test was used to determine statistically significant differences.
RESULTS
Activation of DC by M. tuberculosis-derived recombinant proteins.
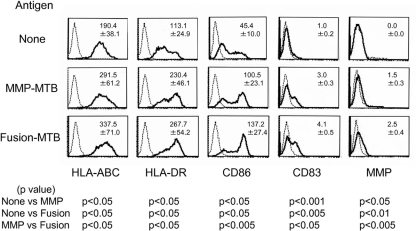
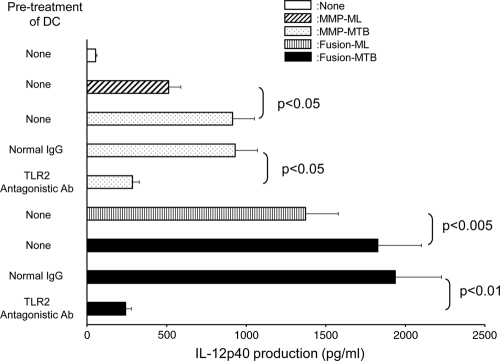
For a recombinant protein to activate T cells, it must have the ability to activate APCs. We assessed the phenotypic change induced in DC by stimulation with MMP-MTB (gene name, Rv1876 or bfrA) and a fusion protein composed of BCG-derived HSP70 and MMP-MTB (Fusion-MTB) (Fig. 1). Both recombinant proteins upregulated the surface expression of HLA-ABC, HLA-DR, CD86, and CD83. However, Fusion-MTB more efficiently enhanced the expression of all of these molecules. Further, MMP-MTB- or Fusion-MTB-pulsed DC expressed molecules which react with anti-MMP-ML MAbs. Again, Fusion-MTB was more efficient than MMP-MTB in the induction of expression of the molecules. These results indicated that both recombinant proteins may have the ability to activate DC. To confirm this point, we measured the IL-12p40 production of DC by stimulation with the recombinant proteins (Fig. 2). We comparatively analyzed MMP-ML, MMP-MTB, Fusion-ML, and Fusion-MTB. All of the recombinant proteins induced the production of IL-12p40, but the levels of IL-12p40 produced by stimulation were as follows: MMP-MTB > MMP-ML, Fusion-MTB > Fusion-ML, and Fusion-MTB > MMP-MTB. In order to reveal the mechanisms leading to the activation of DC by MMP-MTB or Fusion-MTB, we pretreated immature DC with TLR2-antagonistic Ab and subsequently stimulated the treated DC with recombinant proteins, since both MMP-ML and Fusion-ML are reported to activate the NF-κB pathway through ligation with TLR2 (21, 31). While pretreatment of DC with normal murine IgG did not affect the production of IL-12 by stimulation with recombinant proteins, the pretreatment with TLR2-antagonistic Ab significantly inhibited the cytokine production caused by MMP-MTB and Fusion-MTB (Fig. 2). Also, we tested the effect of TLR4-antagonistic Ab on IL-12 production; however, the pretreatment of DC with TLR4-antagonistic Ab did not inhibit cytokine production (not shown).
FIG. 1.
Expression of APC-associated molecules and MMP on DC by stimulation with recombinant proteins. Immature DC obtained from monocytes in the presence of rGM-CSF and rIL-4 were pulsed with either MMP-MTB or Fusion-MTB at 10 μg/ml on day 4 of culture. The DC were gated and analyzed on day 6 after the start of culture. Dotted lines, isotype-matched control IgG or IgM (for MMP); solid lines, the indicated test MAb. Representative results of three separate experiments are shown. The value in the top right corner of each graph is the mean fluorescence intensity of three independent experiments with a control Ig or the test MAb ± the standard deviation. Titers were statistically compared using Student's t test.
FIG. 2.
IL-12p40 production by DC stimulated with recombinant proteins. Monocyte-derived DC from 5 days of culture in the presence of rGM-CSF and rIL-4 were stimulated with the indicated recombinant protein at 10 μg/ml for 24 h. In some cases, immature DC were pretreated with normal murine IgG or TLR2-antagonistic Ab (10 μg/ml) and subsequently stimulated with recombinant protein for 24 h. The concentration of IL-12p40 was determined by the ELISA method. A representative of three separate experiments is shown. Assays were performed in triplicate, and the results are expressed as the mean ± the standard deviation. Titers were statistically compared by Student's t test.
Activation of T cells by recombinant protein.
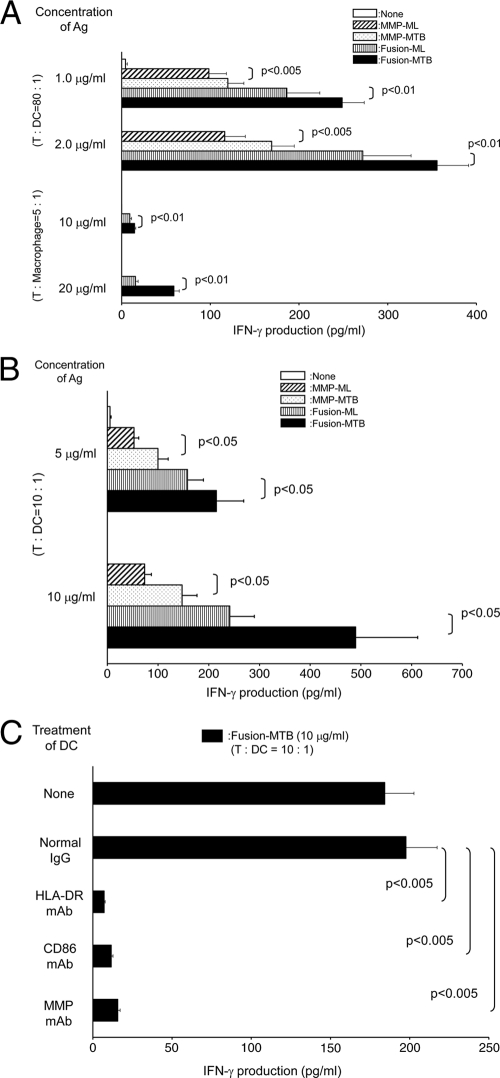
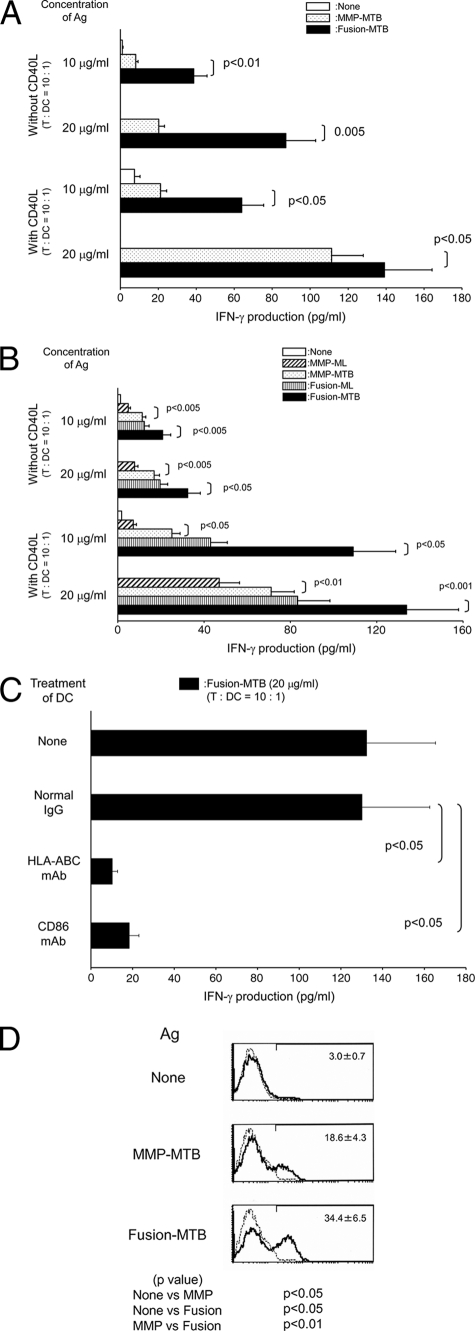
The enhanced activation of DC by M. tuberculosis-derived MMP and fusion proteins may enable autologous T cell activation. The T cell-activating ability of the recombinant proteins was assessed chiefly by using DC as APCs. Memory-type CD4+ T cells were purified from healthy, BCG-vaccinated individuals. All of the recombinant proteins activated the CD4+ T cells with a small dose (∼2.0 μg/ml) of Ags and a small dose of recombinant protein-pulsed DC (T cell/DC ratio, 80:1) (Fig. 3 A). However, MMP-MTB and Fusion-MTB more efficiently activated the T cells than M. leprae-derived proteins did, and Fusion-MTB induced a significantly larger amount of IFN-γ than MMP-MTB did. When we used a higher concentration of recombinant proteins, such as 5 or 10 μg/ml, and used a higher number of DC, such as a T cell/DC ratio of 10:1, as a stimulator, similar statistically significant differences were observed (not shown). Further, only Fusion-MTB successfully activated memory-type CD4+ T cells through macrophages, although a large dose of Ag and a high number of Ag-pulsed macrophages were required (T cell/macrophage ratio, 5:1) (Fig. 3A). The CD4+ T cell-stimulating activity of MMP-MTB and Fusion-MTB was confirmed by using CD45RO-negative naïve CD4+ T cells. All of the recombinant proteins, including MMP-ML, MMP-MTB, Fusion-ML, and Fusion-MTB, activated naïve CD4+ T cells, and Fusion-MTB was the most effective (Fig. 3B). Compared to memory CD4+ T cells, naïve CD4+ T cells required a larger dose of Ag (∼10 μg/ml) and a higher number of Ag-pulsed DC (T cell/DC ratio, 10:1) to be activated. To address the mechanisms leading to the activation of naïve CD4+ T cells by Fusion-MTB, Fusion-MTB-pulsed DC were treated with MAbs against HLA-DR, CD86, and MMP-ML molecules and subsequently used to stimulate naïve CD4+ T cells (Fig. 3C). IFN-γ production by these naïve CD4+ T cells was significantly inhibited by the surface treatment of the DC with the MAbs, and similarly, IL-2 production by naïve CD4+ T cells was inhibited (not shown). The ability of MMP-MTB and Fusion-MTB to activate memory-type CD8+ T cells was then assessed (Fig. 4 A). Although, in contrast to memory-type CD4+ T cells, a large dose of recombinant proteins was required, both M. tuberculosis-derived recombinant proteins induced significant production of IFN-γ from memory-type CD8+ T cells. Further, the additional treatment of Ag-pulsed DC with CD40L upregulated the production of IFN-γ by CD8+ T cells. In both cases, i.e., without and with CD40L treatment, Fusion-MTB induced significantly greater IFN-γ production than MMP-MTB did. In order to confirm the CD8+ T cell-stimulating abilities of both MMP-MTB and Fusion-MTB, naïve CD8+ T cells were also examined as responders. In this case, purified proteins from M. leprae were used as a control (Fig. 4B). Both MMP-MTB and Fusion-MTB activated naïve CD8+ T cells to produce IFN-γ; however, the concentration of IFN-γ released from naïve CD8+ T cells was low and a cytokine concentration of less than 35 pg/ml was produced, and the concentration of IFN-γ produced from naïve CD8+ T cells by stimulation with Fusion-MTB was significantly lower than that from memory CD8+ T cells (P < 0.005). The naïve CD8+ T cell-stimulating activities of the recombinant proteins were as follows: MMP-MTB > MMP-ML, Fusion-MTB > Fusion-ML, and Fusion-MTB > MMP-MTB. The IFN-γ production by naïve CD8+ T cells was enhanced by the additional treatment of Ag-pulsed DC with CD40L, and the highest production of IFN-γ was achieved by Fusion-MTB; in this case, Fusion-MTB could induce an IFN-γ concentration of more than 100 pg/ml. To elucidate the mechanisms of the activation of naïve CD8+ T cells by Fusion-MTB, Fusion-MTB pulsed DC were treated with MAbs to HLA-ABC and CD86 and subsequently used as a stimulator (Fig. 4C). IFN-γ production by naïve CD8+ T cells was significantly inhibited by the treatment of the DC. One of the aims of CD8+ T cell activation in terms of the host defense against M. tuberculosis is to produce cytotoxic CD8+ T cells. To measure the production of cytotoxic CD8+ T cells, we assessed the intracellular production of perforin in CD8+ T cells which were stimulated with MMP-MTB or Fusion-MTB in the presence of CD4+ T cells (Fig. 4D). Both recombinant proteins produced perforin-producing CD8+ T cells, and Fusion-MTB seemed to produce them more efficiently.
FIG. 3.
(A) IFN-γ production by memory-type CD4+ T cells stimulated with recombinant proteins. Monocyte-derived DC or macrophages were pulsed with the indicated recombinant protein at the indicated concentration and used to stimulate memory-type CD4+ T cells in a 4-day culture. Responder CD4+ T cells (1 × 105) were stimulated with the indicated dose of Ag-pulsed DC or macrophages. (B) IFN-γ production by naïve CD4+ T cells by stimulation with recombinant protein. Monocyte-derived DC were pulsed with the indicated recombinant protein at 5 or 10 μg/ml and used to stimulate naïve CD4+ T cells in a 4-day culture. Responder CD4+ T cells (1 × 105) were stimulated with the Ag-pulsed DC at a T cell/DC ratio of 10:1. (C) Inhibition of naïve CD4+ T cell activation by treatment of Ag-pulsed DC with MAb. Monocyte-derived DC were pulsed with Fusion-MTB at 10 μg/ml and subsequently treated at 10 μg/ml with MAb to HLA-DR, CD86, MMP, or normal murine IgG or IgM. These DC were used to stimulate naïve CD4+ T cells (1 × 105) at a T cell/DC ratio of 10:1. IFN-γ produced from T cells was measured by the ELISA method. A representative of three separate experiments is shown. Assays were performed in triplicate, and the results are expressed as means ± standard deviations. Titers were statistically compared by Student's t test.
FIG. 4.
(A) IFN-γ production by memory-type CD8+ T cells by stimulation with recombinant protein. Monocyte-derived DC were pulsed with MMP-MTB or Fusion-MTB at 10 or 20 μg/ml, costimulated with or without CD40L (1.0 μg/ml), and used to stimulate memory-type CD8+ T cells in a 4-day culture. Responder CD8+ T cells (1 × 105) were stimulated with the Ag-pulsed DC at a T cell/DC ratio of 10:1. (B) IFN-γ production by naïve CD8+ T cells stimulated with recombinant proteins. Monocyte-derived DC were pulsed with the indicated recombinant protein at 10 or 20 μg/ml, further costimulated with or without CD40L (1.0 μg/ml), and used to stimulate naïve CD8+ T cells in a 4-day culture. Responder CD8+ T cells (1 × 105) were stimulated with the Ag-pulsed DC at a T cell/DC ratio of 10:1. (C) Inhibition of naïve CD8+ T cell activation by treatment of Fusion-MTB-pulsed DC with MAb. Monocyte-derived DC were pulsed with MMP-MTB at 20 μg/ml, costimulated with CD40L (1.0 μg/ml), and subsequently treated at 10 μg/ml with MAb to HLA-ABC, CD86, or normal murine IgG. These DC were used to stimulate naïve CD8+ T cells (1 × 105) at a T cell/DC ratio of 10:1. IFN-γ produced by T cells was measured by the ELISA method. A representative of three separate experiments is shown. Assays were performed in triplicate, and the results are expressed as means ± standard deviations. Titers were statistically compared by Student's t test. (D) Intracellular production of perforin by CD8+ T cells. Monocyte-derived DC were pulsed at 10 μg/ml with either MMP-MTB or Fusion-MTB and cultured with unseparated memory-type T cells (T cell/DC ratio, 40:1) for 5 days. The stimulated CD8+ T cells were gated and analyzed for perforin production. Values are the mean percentages of the CD8+ T cell population that were perforin positive in three independent experiments and the standard deviations. Titers were statistically compared using Student's t test. A representative of three separate experiments is shown.
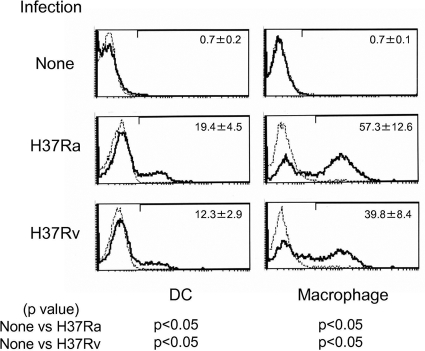
Expression of MMP on APCs infected with M. tuberculosis.
The molecule used as a vaccinating agent should be expressed on APCs infected with the pathogen. To reveal the expression of MMP on the surface of APCs, DC and macrophages were infected with M. tuberculosis H37Ra and H37Rv and analyzed by flow cytometry (Fig. 5). Both DC and macrophages expressed MMP molecules on their surface after infection with H37Ra and H37Rv. Expression levels seemed to be dependent on the dose of M. tuberculosis used for infection (not shown).
FIG. 5.
Expression of MMP on DC and macrophage infected with M. tuberculosis. Monocyte-derived DC or macrophages were infected with either H37Ra or H37Rv at an MOI of 1.0 and cultured for another 2 days in the presence of rGM-CSF plus rIL-4 or rM-CSF, respectively. The DC and macrophages were gated and analyzed on day 5 after the start of culture. Dotted lines, control IgM; solid lines, MMP MAb. Results representative of three separate experiments are shown. The values are the mean percentages of major membrane protein II-positive DC or macrophages in three independent experiments and the standard deviations. Titers were statistically compared using Student's t test.
DISCUSSION
In vivo studies using various knockout mice indicate that adaptive immunities play an important role in inhibiting the multiplication of M. tuberculosis and that the activation of both CD4+ T cells and CD8+ T cells is an essential element of the control of M. tuberculosis infection (1, 12, 16). While CD4+ T cells chiefly act in the initial phase of infection, CD8+ T cells either producing IFN-γ or having cytotoxic killing activity contribute to the chronic or stationary phase of infection (7, 19, 46). Thus, the antigenic molecules which are used as an essential component of a vaccine should have the ability to activate not only naïve CD4+ T cells and CD8+ T cells but also APCs, including DC. So far, we have found MMP to be one of the immunodominant Ags of M. leprae (21) and found evidence that MMP-ML activated DC through ligation with TLR2, which resulted in the activation of the NF-κB pathway of host cells, and that DC pulsed with MMP-ML stimulated both CD4+ and CD8+ T cells to produce IFN-γ in an Ag-specific manner (21, 26). Further, MMP-ML is supposed to be recognized in vivo by both T cell subsets of M. leprae-infected individuals, including paucibacillary leprosy patients (26).
In addition, HSP70, one of the heat shock proteins, plays various roles in the upregulation of the ability of APCs to stimulate T cells (6, 10, 44, 45). Further, HSPs of both mammalian host cell and bacterial origins are reported to have chaperon activity (6, 44) and can effectively prime a cytolytic response (10, 45). In fact, we previously reported that HSP70 effectively induced the cross-priming of CD8+ T cells through the cytosolic pathway when secreted from recombinant BCG in the phagosome of DC as part of a fusion protein (31). Also, others have reported that HSP65 activated naïve CD8+ T cells and a DNA vaccine containing the hsp65 gene inhibited the development of tuberculosis that is induced by the multiplication of subsequently challenged M. tuberculosis (50). Furthermore, vaccination of mice with recombinant BCG that secreted either MMP-ML or Fusion-ML, in which BCG was used as a vehicle, efficiently inhibited the multiplication of subsequently challenged M. leprae, although the fusion protein was more efficient in both activating naïve T cells and inhibiting M. leprae multiplication (22, 25, 31).
MMP is commonly expressed in both pathological mycobacteria and BCG, so that it may be that MMP-MTB plays a substantial role in inhibiting the replication of M. tuberculosis; however, the homology of MMP between M. leprae (ML2038) and M. tuberculosis (Rv1876) is only 90.6% at the amino acid level. Therefore, we assessed the immunostimulatory activity of M. tuberculosis-derived MMP and its fusion with BCG-derived HSP70 by using MMP-ML and Fusion-ML as controls.
As expected, MMP derived from M. tuberculosis activated DC in terms of phenotypic change and cytokine production, and the cytokine production was associated with the ability of MMP-MTB to ligate with TLR2. MMP-MTB-pulsed DC activated both CD4+ and CD8+ T cells. In this respect, only a very small amount of MMP was required to induce vigorous activation of CD4+ T cells, but not CD8+ T cells, obtained from BCG-vaccinated healthy donors. These results may indicate that some subsets of CD4+ T cells are primed with MMP by vaccination with BCG, whose MMP is 100% homologous to that of M. tuberculosis, as in the case of leprosy patients whose T cells were primed by M. leprae infection. However, in contrast to leprosy patients, only CD4+ T cells are primed with MMP by BCG vaccination, which may be linked with the fact that the parent BCG less efficiently activates naïve CD8+ T cells. Activation of T cells usually depends on APCs expressing Ags, so that successful production of MMP-reactive memory-type T cells could be achieved by administration of MMP since MMP could be expressed on the surface of DC after infection with M. tuberculosis H37Ra and H37Rv. This speculation might be supported by our preliminary experiments in which administration of MMP-MTB to C57BL/6 mice produced memory-type splenic T cells reactive to MMP-MTB in vitro, which produced IFN-γ because of this stimulation.
Fourteen amino acids of M. leprae MMP differ from those of M. tuberculosis MMP, and substitutions of amino acids between these mycobacteria are known to occur randomly. However, a MAb which recognizes the epitope expressed on DC pulsed with M. leprae-derived MMP could also detect a peptide expressed on the surface of DC pulsed with M. tuberculosis-derived MMP or infected with M. tuberculosis. The MAb against MMP-ML inhibited the activation of naïve CD4+ T cells by stimulation with MMP-MTB-pulsed DC. These observations indicated that the regions common to the MMPs of M. leprae and M. tuberculosis were chiefly used as antigenic epitopes of CD4+ T cells. However, the T cell activation by M. tuberculosis-derived MMP and Fusion-MTB is significantly stronger than that by M. leprae-derived proteins. The exact mechanism leading to the difference between the T cell-stimulating activities of the MMPs derived from these two pathological mycobacterial strains remains to be elucidated, but one possibility is that some parts of M. tuberculosis-derived MMP other than common regions have APC-immunomodulating activities that are associated with T cell activation. In fact, M. tuberculosis-derived MMP more efficiently activated DC than MMP-ML did, in terms of IL-12 production. However, both MMP-ML and MMP-MTB ligate TLR2; thus, MMP-MTB may have other unknown mechanisms that can induce the activation of DC more strongly. In this respect, we assessed the IL-1β-producing ability of MMP, but there was no apparent difference between the MMPs obtained from M. tuberculosis and M. leprae (not shown). It has been reported that the replacement of one amino acid of the T cell epitope of the antigenic determinant of Ag85B of M. tuberculosis strongly affects its T cell-stimulating activity, i.e., the ability to induce IFN-γ production (4). Therefore, a similar change may have occurred in the MMP system, although it has not been clearly defined.
When we compared the immunostimulating activities of MMP-MTB and Fusion-MTB in terms of the activation of APC and T cells, the latter showed higher activity in the activation of both DC and CD4+ and CD8+ T cells. The exact mechanism of the high immunostimulating activity of the fusion protein is not fully known, but it may be associated with previous reports indicating that HSPs play a varied role in enhancing the ability of APCs to stimulate T cells (6, 10, 44, 45). In fact, the fusion protein induced the expression of higher levels of APC-associated molecules on DC than MMP did. Further, Fusion-MTB may be useful to produce cytotoxic CD8+ T cells because the fusion protein efficiently produced perforin-producing CD8+ T cells, although both MMP-MTB and Fusion-MTB produced cytotoxic CD8+ T cells. Moreover, the fusion protein upregulated the expression of CD40 on DC (not shown) and treatment of Fusion-MTB-pulsed DC with CD40L induced the production of a larger dose of IFN-γ from both naïve CD4+ T cells (not shown) and naïve CD8+ T cells (Fig. 4B). These results indicate that the use of HSP70 as part of a fusion protein may make APCs susceptible to various conditioning molecules, including CD40L. This observation is in the line with the fact that only Fusion-MTB-pulsed monocyte-derived macrophages successfully activated CD4+ T cells, probably MMP primed, when conditioned with CD40L.
Taken together, the data present here suggest that MMP, alone or as part of fusion protein, is highly immunogenic and may be useful for developing new vaccine against tuberculosis, at least in combination with BCG, ESAT-6, or other molecules.
Supplementary Material
Acknowledgments
We thank M. Kujiraoka for her technical support and the Japanese Red Cross Society for kindly providing PBMCs from healthy donors.
This work was supported in part by a Grant-in-Aid for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labour, and Welfare of Japan.
Footnotes
Published ahead of print on 15 December 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Aagaard, C. S., T. T. K. T. Hoang, C. Vingsbo-Lundberg, J. Dietrich, and P. Andersen. 2009. Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J. Immunol. 183:2659-2668. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, P. 2007. Tuberculosis vaccines: an update. Nat. Rev. Microbiol. 5:484-487. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P., and T. M. Doherty. 2005. The success and failure of BCG: implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3:656-662. [DOI] [PubMed] [Google Scholar]
- 4.Ariga, H., et al. 2007. Instruction of naïve CD4+ T-cell fate to T-bet expression and T helper 1 development: roles of T-cell receptor-mediated signals. Immunology 122:210-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardarov, S., et al. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148(Pt. 10):3007-3017. [DOI] [PubMed] [Google Scholar]
- 6.Binder, R. J., and P. K. Srivastava. 2005. Peptides chaperoned by heat-shock proteins are necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat. Immunol. 6:593-599. [DOI] [PubMed] [Google Scholar]
- 7.Caccamo, N., et al. 2006. Phenotypical and functional analysis of memory and effector human CD8 T cells specific for mycobacterial antigens. J. Immunol. 177:1780-1785. [DOI] [PubMed] [Google Scholar]
- 8.Daugelat, S., et al. 2003. The RD1 proteins of Mycobacterium tuberculosis: expression in Mycobacterium smegmatis and biochemical characterization. Microbes Infect. 5:1082-1095. [DOI] [PubMed] [Google Scholar]
- 9.Doherty, T. M., and P. Andersen. 2005. Vaccines for tuberculosis: novel concepts and recent progress. Clin. Microbiol. Rev. 18:687-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flechtner, J. B., et al. 2006. High-affinity interactions between peptides and heat shock protein 70 augment CD8+ T lymphocyte immune responses. J. Immunol. 177:1017-1027. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 12.Flynn, J. L., M. M. Goldstein, K. J. Triebold, B. Koller, and B. R. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U. S. A. 89:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes, E. K., et al. 2008. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 181:4955-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grode, L., et al. 2005. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Invest. 115:2472-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto, K., et al. 2002. Mycobacterium leprae infection in monocyte-derived dendritic cells and its influence on antigen-presenting function. Infect. Immun. 70:5167-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoebe, K., E. Janssen, and B. Beutler. 2004. The interface between innate and adaptive immunity. Nat. Immunol. 5:971-974. [DOI] [PubMed] [Google Scholar]
- 17.Hoft, D. F. 2008. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet 372:164-175. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz, M. A., B. W. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann, S. H. 1988. CD8+ T lymphocytes in intracellular microbial infections. Immunol. Today 9:168-174. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann, S. H., and A. J. McMichael. 2005. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat. Med. 11:S33-S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda, Y., T. Mukai, J. Spencer, and M. Makino. 2005. Identification of immunomodulating agent from Mycobacterium leprae. Infect. Immun. 73:2744-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda, Y., T. Tamura, M. Matsuoka, and M. Makino. 2009. Inhibition of the multiplication of Mycobacterium leprae by vaccination with a recombinant M. bovis BCG strain that secretes major membrane protein-II in mice. Clin. Vaccine Immunol. 16:1399-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino, M., and M. Baba. 1997. A cryopreservation method of human peripheral blood mononuclear cells for efficient production of dendritic cells. Scand. J. Immunol. 45:618-622. [DOI] [PubMed] [Google Scholar]
- 24.Makino, M., Y. Maeda, Y. Fukutomi, and T. Mukai. 2007. Contribution of GM-CSF on the enhancement of the T cell-stimulating activity of macrophages. Microbes Infect. 9:70-77. [DOI] [PubMed] [Google Scholar]
- 25.Makino, M., Y. Maeda, and K. Inagaki. 2006. Immunostimulatory activity of recombinant Mycobacterium bovis BCG that secretes major membrane protein II of Mycobacterium leprae. Infect. Immun. 74:6264-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makino, M., Y. Maeda, and N. Ishii. 2005. Immunostimulatory activity of major membrane protein-II from Mycobacterium leprae. Cell. Immunol. 233:53-60. [DOI] [PubMed] [Google Scholar]
- 27.Makino, M., Y. Maeda, M. Kai, T. Tamura, and T. Mukai. 2009. GM-CSF-mediated T-cell activation by macrophages infected with recombinant BCG that secretes major membrane protein-II of Mycobacterium leprae. FEMS Immunol. Med. Microbiol. 55:39-46. [DOI] [PubMed] [Google Scholar]
- 28.Makino, M., S. Shimokubo, S. Wakamatsu, S. Izumo, and M. Baba. 1999. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J. Virol. 73:4575-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittrücker, H.-W., et al. 2007. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 104:12434-12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modlin, R. L., et al. 1988. Learning from lesions: patterns of tissue inflammations in leprosy. Proc. Natl. Acad. Sci. U. S. A. 85:1213-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukai, T., et al. 2009. Induction of cross-priming of naïve CD8+ T lymphocytes by recombinant bacillus Calmette-Guérin that secretes heat shock protein 70-major membrane protein-II fusion protein. J. Immunol. 183:6561-6568. [DOI] [PubMed] [Google Scholar]
- 32.Mukai, T., Y. Maeda, T. Tamura, Y. Miyamoto, and M. Makino. 2008. CD4+ T-cell activation by antigen-presenting cells infected with urease-deficient recombinant Mycobacterium bovis bacillus Calmette-Guérin. FEMS Immunol. Med. Microbiol. 53:96-106. [DOI] [PubMed] [Google Scholar]
- 33.Murray, R. A., M. R. Siddiqui, M. Mendillo, J. Krahenbuhl, and G. Kaplan. 2007. Mycobacterium leprae inhibits dendritic cell activation and maturation. J. Immunol. 178:338-344. [DOI] [PubMed] [Google Scholar]
- 34.North, R. J., and Y. J. Jung. 2004. Immunity to tuberculosis. Annu. Rev. Immunol. 22:599-623. [DOI] [PubMed] [Google Scholar]
- 35.Pancholi, P., A. Mirza, N. Bhardwaj, and R. M. Steinman. 1993. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science 260:984-986. [DOI] [PubMed] [Google Scholar]
- 36.Raviglione, M. C., D. E. Snider, Jr., and A. Kochi. 1995. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273:220-226. [PubMed] [Google Scholar]
- 37.Reed, S. G., et al. 2009. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc. Natl. Acad. Sci. U. S. A. 106:2301-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyrat, J. M., F. X. Berthet, and B. Gicquel. 1995. The urease locus of Mycobacterium tuberculosis and its utilization for the demonstration of allelic exchange in Mycobacterium bovis bacillus Calmette-Guérin. Proc. Natl. Acad. Sci. U. S. A. 92:8768-8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skeiky, Y. A., et al. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 172:7618-7628. [DOI] [PubMed] [Google Scholar]
- 40.Snapper, S. B., et al. 1988. Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc. Natl. Acad. Sci. U. S. A. 85:6987-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soualhine, H., et al. 2007. Mycobacterium bovis bacillus Calmette-Guérin secreting active cathepsin S stimulates expression of mature MHC class II molecules and antigen presentation in human macrophages. J. Immunol. 179:5137-5145. [DOI] [PubMed] [Google Scholar]
- 42.Stenger, S., et al. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 43.Stover, C. K., et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 44.Tobian, A. A. R., D. H. Canaday, W. H. Boom, and C. V. Harding. 2004. Bacterial heat shock proteins promote CD91-dependent class I MHC cross-presentation of chaperoned peptide to CD8+ T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages. J. Immunol. 172:5277-5286. [DOI] [PubMed] [Google Scholar]
- 45.Tobian, A. A. R., C. V. Harding, and D. H. Canaday. 2005. Mycobacterium tuberculosis heat shock fusion protein enhances class I MHC cross-processing and -presentation by B lymphocytes. J. Immunol. 174:5209-5214. [DOI] [PubMed] [Google Scholar]
- 46.van Pinxteren, L. A., J. P. Cassidy, B. H. Smedegaard, E. M. Agger, and P. Andersen. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 30:3689-3698. [DOI] [PubMed] [Google Scholar]
- 47.Wakamatsu, S., M. Makino, C. Tei, and M. Baba. 1999. Monocyte-driven activation-induced apoptotic cell death of human T-lymphotropic virus type I-infected T cells. J. Immunol. 163:3914-3919. [PubMed] [Google Scholar]
- 48.WHO. 2007. Global MDR-TB and XDR-TB response plan 2007-2008, p. 1-48. In WHO report 2007. World Health Organization, Geneva, Switzerland.
- 49.Woodworth, J. S., Y. Wu, and S. M. Behar. 2008. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J. Immunol. 181:8595-8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida, S., et al. 2006. DNA vaccine using hemagglutinating virus of Japan-liposome encapsulating combination encoding mycobacterial heat shock protein 65 and interleukin-12 confers protection against Mycobacterium tuberculosis by T cell activation. Vaccine 24:1191-1204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.