Abstract
The use of heat shock proteins (HSP) to enhance activation of the immune response to chaperoned antigen is being explored for immunotherapy. Hsp110 chaperones large protein substrates more effectively than Hsp70, offering the potential to use complex antigens containing multiple epitopes in HSP-based vaccines. In this study, we investigated the ability of recombinant bovine Hsp110 to chaperone E2 glycoprotein, the major envelope protein of bovine viral diarrhea virus (BVDV) and the dominant target of neutralizing antibodies. Hsp110 formed complexes with E2, as demonstrated by immunoprecipitation. When monocytes from BVDV-immunized cattle were stimulated with these complexes and incubated with autologous CD4+ T cells, enhanced levels of proliferation were observed. To determine the ability of these complexes to improve immunogenicity in vivo, cattle were vaccinated with either Hsp110-E2 complex or E2 only, combined with Quil-A adjuvant. In contrast to the in vitro data, cellular and humoral responses to E2 were greater in the E2-only vaccination group, indicating that complex formation had actually reduced the immunogenicity of E2. This study highlights the need for further understanding of the means by which HSP complexes are endocytosed and processed in vivo to enable the design of successful vaccine strategies.
Recognition of the importance of professional antigen-presenting cells (APC), particularly dendritic cells, in the induction of effector immune responses in natural infections has led to much research on how to manipulate these cells for the purposes of vaccination. Various methods to target antigen to APC and harness their inherent endocytic, processing, and presentation capabilities to initiate long-term immunity against defined diseases are currently being investigated (27).
Heat shock proteins (HSP) are ubiquitous constitutively or inducibly expressed proteins that act as molecular chaperones assisting in the assembly, folding, stabilization, and translocation of other cellular proteins. Under conditions of cell stress, HSP expression is elevated to enable them to bind unfolded, misfolded, or denatured proteins to prevent unwanted aggregation (25). Members of the HSP family have immunogenic properties, thought to be due to their ability to bind and stabilize antigen and protect it from degradation (28). The ability of HSP to nonspecifically stimulate the innate immune system remains controversial, with several studies pointing toward contaminating by-products from the HSP-producing bacterial expression systems as the source of the stimulation (8, 41). However, increasing evidence suggests that HSP can specifically enhance both CD4+ and CD8+ T cell responses due to improved delivery of antigen to APC.
We recently demonstrated enhanced in vitro proliferation of bovine CD4+ T cells in response to a foot-and-mouth disease virus (FMDV) peptide complexed to Hsp70 (21). Enhanced T cell proliferation was FMDV specific in the absence of bacterial contamination of HSP, illustrating the potential for such a vaccine strategy. However, the use of single-peptide vaccine strategies are limited due to their major histocompatibility complex (MHC) restriction and an inability to induce B cell help. To overcome these limitations, the ability of Hsp110 to chaperone whole viral proteins and improve antigen delivery was considered. Hsp110 effectively binds large protein substrates (up to approximately 100 kDa) and protects them from heat-induced aggregation (22). In support of its potential efficacy, several tumor antigens exhibit increased immunogenicity when complexed with Hsp110 before administration to mice (12, 17, 18, 36, 37).
Bovine viral diarrhea virus (BVDV) causes an economically important disease of cattle that occurs worldwide. Together with classical swine fever virus of pigs and border disease virus of sheep, BVDV belongs to the genus Pestivirus of the family Flaviviridae, which also contains human pathogens, including hepatitis C virus, dengue virus, and yellow fever virus. BVDV possesses a single-stranded positive-sense RNA genome with a single open reading frame. This is transcribed as a single polyprotein and cleaved co- and posttranslationally by host and viral proteases into the virus' structural and nonstructural proteins. The structural proteins comprise the C nucleocapsid protein and three envelope glycoproteins, Erns, E1, and E2 (10). Interestingly, the major envelope glycoproteins of other members of the family Flaviviridae are also being investigated as vaccine antigen candidates (20, 38). Here, we studied the ability of Hsp110 to enhance presentation of E2 to CD4+ T cells in vitro and to improve the immunogenicity of an E2 vaccine in cattle.
MATERIALS AND METHODS
Hsp110 and E2 protein preparation.
Bovine Hsp110 cDNA was obtained and amplified from total RNA extracted from calf testis (CTe) cells, using a OneStep RT-PCR Kit (Qiagen, Germantown, PA). Specific primers were designed from the HSP110 Bos taurus sequence (accession number BC122574): 5′-CCA TGG CGG TGG TGG GGC TG-3′ (sense) and 5′-GGT ACC GTC CAA GTC CAT ATT AAT G-3′ (antisense). An NcoI restriction site (indicated in the sense sequence in boldface) allowed the insertion of a Kozak sequence, and a KpnI restriction site (indicated in the antisense sequence in boldface) allowed the removal of a native stop codon and expression of the C-terminal His tag. Hsp110 cDNA was subcloned into an intermediate vector (pcDNA3.1/V5-His-TOPO), which was amplified in One Shot TOP10 Escherichia coli cells (Invitrogen, Karlsruhe, Germany). The insert was then cloned between the NcoI and KpnI restriction sites of the pTriex-1.1 vector (Novagen, Darmstadt, Germany), which contains flanking baculovirus sequences allowing the generation of recombinant baculoviruses. The derivative recombinant plasmid was designated pTriEx-1.1-Hsp110 and amplified in E. coli cells.
BAC10:KO1629 (kindly given by D. Chapman, Institute for Animal Health [IAH] Pirbright) is a genetically stable knockout form of bacmid unable to initiate virus infection unless rescued by recombination with an appropriate transfer vector (42). The bacmid and pTriEx-1.1-Hsp110 plasmid were first linearized (using Bsu36I) and cotransfected into Sf9 insect cells (33), which were maintained in sf-900 II SFM (Invitrogen) with 2% heat-inactivated fetal calf serum (FCS) at 28°C in a nonhumidified ambient-air-regulated incubator. Supernatants containing recombinant baculovirus were harvested after 5 days, and recombinant virus was amplified by three sequential passages in Sf9 cells. For protein expression, Sf9 cells were grown in suspension culture to mid-log phase and infected with recombinant viral stock at a multiplicity of infection (MOI) of 2 for 72 h. The centrifuged cell pellet was stored at −70°C until purification was done.
Recombinant Hsp110 protein was purified as previously described (35). Buffer was exchanged by dialysis against phosphate-buffered saline (PBS) in a Slide-A-Lyser 3.5-kDa molecular-mass-cutoff dialysis cassette (Thermo Scientific, Waltham, MA), and protein was concentrated using Centricon YM-50 spin columns (Millipore, Billerica, MA). Endotoxin was depleted using Detoxigel endotoxin-removing gel (Thermo Scientific), and the Hsp110 protein concentration was quantified using a bicinchoninic acid (BCA) reagent (Thermo Scientific) against a standard of bovine serum albumin (BSA). The level of remaining endotoxin content was assessed using the Limulus amebocyte lysate assay (Lonza, Basel, Switzerland) and was shown to be very low (0.45 endotoxin units [EU]/mg of purified Hsp110 protein).
For the expression of V5 epitope- and His-tagged recombinant E2 (a membrane glycoprotein from the NADL strain of BVDV), the cDNA of E2 was inserted into the pMT/Bip/V5-His plasmid. The resulting plasmid was transfected into Drosophila S2 cells grown in serum-free medium. Expression of E2 was induced by the addition of 0.7 mM copper sulfate. After 2 to 3 days, the culture supernatant was harvested and E2 was purified by metal affinity chromatography, followed by dialysis against PBS.
Luciferase aggregation assay.
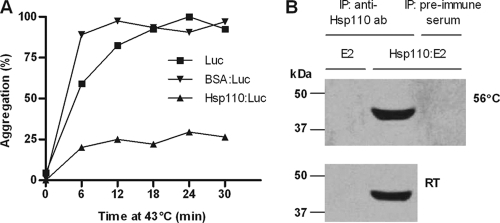
The heat-induced aggregation of luciferase protein results in an increase in light scattering and is measurable by optical density (OD), and Hsp110 has been shown to inhibit this effect (22). This was replicated to confirm the chaperoning activity of our preparation of recombinant bovine Hsp110. Recombinant luciferase protein (150 nM; Promega, Madison, WI), in the presence or absence of Hsp110 or BSA (1:1 molar ratio), was equilibrated to room temperature in 25 mM HEPES (pH 7.5). The mixture was then incubated at 43°C, and the optical density at 320 nm was determined every 6 min for 30 min, using a spectrophotometer. The optical density of the luciferase heated alone was set to 100%, and the relative percentage of aggregation was determined for each mixture/time point as follows: (OD of mixture at time T/OD of luciferase alone at 30 min) × 100.
Immunoprecipitation.
To demonstrate Hsp110-E2 complex formation, an immunoprecipitation experiment was performed as previously described with some modifications (19). Rabbit anti-mouse Hsp110 antiserum (Enzo Biochem, New York, NY) was preincubated with protein A beads in 1 ml PBS for 1 h at room temperature with rocking. The antibody (Ab)-bead complex was blocked with preimmune rabbit sera for a further 30 min at room temperature. V5-tagged E2 (10 μg) was incubated with Hsp110 (10 μg) for 30 min at room temperature or 56°C. The Hsp110-E2 complex was subsequently added to the blocked antibody-bead complex for 1 h with rocking. The beads were then washed 6 times with washing buffer (500 mM NaCl, 0.5 mM EDTA, 0.13% [vol/vol] Triton X-100) and subjected to SDS-PAGE on a 10% (vol/vol) acrylamide gel, followed by Western blot analysis using a horseradish peroxidase (HRP)-conjugated anti-V5 monoclonal antibody (MAb) (Invitrogen).
Cattle and immunization procedures.
The cattle used were conventionally reared British Holstein Friesians bred at the Institute for Animal Health, Compton. The three cattle used for assessing the proliferative response to Hsp110-E2 in vitro (see Fig. 2) had previously been challenged with noncytopathic live BVDV and were immune, with detectable recall responses, to whole BVDV. On the other hand, the BVDV-free status of the seven cattle, which were subsequently vaccinated (see Fig. 3 to 5), was determined by assaying the sera for BVDV-specific antibodies and viremia by virus neutralization assay and PCR, respectively.
Complexes of Hsp110 and E2 (30 μg each) were formed by incubation at 56°C for 1 h in glass vials. E2 alone was treated identically. The proteins were then diluted in 1 ml PBS, and Quil A was added to a final concentration of 1 mg/ml. The vaccine was incubated for a further 30 min at room temperature with rolling before use. Three cattle received Hsp110-E2 complex vaccine, three animals received E2 vaccine, and one animal remained unvaccinated (control). The vaccine was injected intramuscularly in the rump on days 0 and 21, and heparinized blood and serum were collected weekly from day 0 to day 35. All experiments were approved by the institute's ethical review process and were in accord with national guidelines on animal use.
Culture media.
The culture medium comprised RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (Autogen, Calne, United Kingdom), 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 10 μg/ml gentamicin, and 50 mM 2-mercaptoethanol, termed complete medium below.
Cells.
Bovine peripheral blood mononuclear cells (PBMC) were obtained from heparinized blood by density gradient centrifugation (1,200 × g for 30 min over 1.083 g/ml Histopaque; Sigma-Aldrich, Poole, United Kingdom). For some experiments, monocytes were isolated from PBMC using anti-human CD14 paramagnetic microbeads (Miltenyi-Biotec, Woking, United Kingdom). The labeled cells were isolated from Midimacs LS columns (Miltenyi-Biotec) according to the manufacturer's instructions. CD4+ cells were isolated from PBMC using mouse anti-bovine CD4 antibody (clone CC30; IAH, Compton), followed by rat anti-mouse IgG1 microbeads (Miltenyi-Biotec) as described above. Typically, the purity of the resulting CD4+ T cell and CD14+ monocyte populations was over 95%, as determined by flow cytometry.
Proliferation assays.
Proliferation assays were set up on the day of blood collection. PBMC (2 × 105 cells/well) or monocytes (5 × 103/well) plus CD4+ cells (2 × 105/well) were incubated in a total volume of 200 μl of complete medium in U-bottom 96-well microtiter plates in the presence of various antigens: E2 (used at 5, 25, 50, or 100 ng/ml), Hsp110 (used at 5, 50, or 500 ng/ml), Erns (100 ng/ml), Hsp110-E2 complexes (formed by incubating E2 and Hsp110, at various concentrations, for 30 min at 56°C or room temperature), or a combination of these. Medium alone and pokeweed mitogen (PWM) (2 μg/ml) were used as negative and positive controls, respectively. After 5 days, the cells were pulsed with 37 Bq per well of [3H]thymidine and incubated for a further 16 h before being harvested onto filter mats. The radioactive thymidine incorporated into the DNA of proliferating cells was determined by liquid scintillation counting using a Trilux Microbeta counter (Wallac, Beaconsfield, United Kingdom) and expressed as counts per minute (cpm).
ELISA and enzyme-linked immunospot (ELISPOT) assays.
Supernatants from PBMC cultured for 72 h in the presence of various antigens (similar to those used in proliferation assays) were assayed for gamma interferon and interleukin 10 (IL-10) by capture enzyme-linked immunosorbent assay (ELISA), as previously described (15, 21). For the cattle study, serum was stored at −20°C before being assayed for E2-specific IgG antibody by indirect ELISA, as previously described, except that the plates were coated with E2 protein at 1 μg/ml (16). Detection of E2-specific IgG-secreting cells (plasma cells) in freshly isolated PBMC was performed as previously described, except that the plates were coated with 100 μl of a preparation of E2 protein diluted at 1/200 (16).
Data analysis.
Statistical analyses were performed with the statistical software GraphPad Prism 5, using a one-way analysis of variance (ANOVA) followed by a Bonferroni posthoc test. P values of <0.05 were considered statistically significant.
RESULTS
Interaction of bovine Hsp110 with large protein substrates.
As a functional test of the chaperoning capacity of recombinant bovine Hsp110, a luciferase aggregation assay was performed as described previously (22). Heat shock leads to the denaturation and aggregation of luciferase protein, which can be detected by an increase in optical density measured at 320 nm. In the presence of recombinant bovine Hsp110, the heat-induced aggregation of luciferase was reduced by approximately 75%. BSA, used as a negative control, had no effect on luciferase aggregation (Fig. 1 A).
FIG. 1.
Bovine Hsp110 chaperones large proteins. (A) Aggregation assay. Luciferase protein (Luc) was incubated either alone or in the presence of Hsp110 or BSA at 43°C for 30 min. Aggregation was monitored by measuring the increase in optical density at 320 nm. The data show the means of two independent experiments. (B) Western blot showing immunoprecipitation (IP) of E2 alone and Hsp110-E2 complexes formed at 56°C or at room temperature (RT), using a rabbit anti-Hsp110 or preimmune Ab conjugated to protein A-Sepharose beads. The proteins were resolved on a 10% SDS-PAGE gel, followed by immunoblotting with anti-V5-HRP antibody to detect V5-tagged E2 protein. The experiment was performed twice with similar results.
The formation of Hsp110-E2 complexes was confirmed by immunoprecipitation and subsequent analysis of Western blots for V5-tagged E2. As Fig. 1B shows, V5-tagged E2 was indeed coimmunoprecipitated with Hsp110 using rabbit antiserum to Hsp110, indicating a physical interaction between the two proteins. No such precipitation was observed using preimmune rabbit serum or in the absence of Hsp110, confirming the specificity of the assay. Interestingly, Hsp110-E2 complexes formed at both room temperature and 56°C, unlike ovalbumin, which bound Hsp110 only at 80°C (data not shown).
Enhanced proliferation of antigen-specific CD4+ T cells in response to Hsp110-chaperoned E2 in vitro.
Using three BVDV-immune cattle, recall responses to E2 protein alone or as an Hsp110-E2 complex were evaluated by proliferation assay as previously described (21). First, monocytes were incubated with increasing amounts of E2 alone or chaperoned with 500 ng/ml Hsp110 and allowed to stimulate autologous CD4+ cells from BVDV-immune cattle. Our results showed that 5 ng/ml E2 induced significantly higher levels of proliferation when chaperoned by Hsp110. However, strong proliferative responses to E2 alone were detected at higher concentrations of E2 (25 and 50 ng/ml), abrogating the enhancement effect of Hsp110 chaperoning on T cell proliferation (Fig. 2 A). In subsequent experiments, E2 was used at 5 ng/ml in order to study the effects of Hsp110 under limiting conditions. A titration of Hsp110 was also performed in the presence of a constant amount of E2 (5 ng/ml) to determine the appropriate molar ratio for the optimal enhancement of T cell proliferation. An Hsp110-to-E2 molar ratio of approximately 40:1 (500 ng/ml Hsp110 and 5 ng/ml E2) induced the highest proliferative responses and was used thereafter (Fig. 2B).
FIG. 2.
Proliferation of E2-specific CD4+ T cells is enhanced following treatment of monocytes with Hsp110-E2 complexes. (A to F) Monocytes were incubated with E2 or Hsp110-E2 complexes and autologous CD4+ T cells from a BVDV-immune animal for 5 days before being pulsed with [3H]thymidine, incubated for a further 16 h, and harvested. Incorporated radioactivity was determined by liquid scintillation counting and expressed as cpm. The culture conditions were as follows: increasing concentrations of E2 only or Hsp110-E2 complexes formed at 56°C with a constant concentration of 500 ng/ml Hsp110 (results are expressed as the means of triplicate determinations plus standard deviations [SD]) (A); increasing concentrations of Hsp110 only or Hsp110-E2 complex formed at 56°C with a constant concentration of 5 ng/ml E2 (results are expressed as the means of triplicate determinations plus SD) (B); 5 ng/ml E2 and 500 ng/ml Hsp110 or Hsp110-E2 complexes formed at 56°C using the same final protein concentrations (results are expressed as the means of 10 independent experiments plus standard errors of the mean [SEM]) (C); experiment performed as in panel C, but complexes were formed at room temperature (results are expressed as the means of six independent experiments plus SEM) (D); experiment performed as in panel C, but as a control, monocytes were also incubated with a combination of noncomplexed E2 and Hsp110 (results are expressed as the means of three independent experiments plus SEM) (E); experiment was performed as in panel C, but after 72 h, supernatants were harvested and assayed for gamma interferon production (results are expressed as the means of three independent experiments plus SEM) (F).*, P < 0.05; **, P < 0.01; ***, P < 0.001; one-way ANOVA followed by a Bonferroni posthoc test.
In cells from all three animals tested, CD4+ T cell proliferation induced by Hsp110-chaperoned E2 was significantly higher than that induced by E2 alone. This occurred when complexes were formed at either 56°C (Fig. 2C) or room temperature (Fig. 2D). Hsp110 alone did not stimulate proliferation above background levels (10,000 cpm) in any of the experiments. Complex formation was essential for Hsp110-mediated enhancement of T cell proliferation, since when E2 was preincubated at 56°C and then mixed with Hsp110 immediately before being added to the cells, i.e., before complex formation had time to occur, no enhancement of proliferation was observed (Fig. 2E).
Gamma interferon production by CD4+ T cells induced by Hsp110-E2 complexes (Fig. 2F) was assessed using the same culture conditions as for cell proliferation. After 72 h, the concentration of gamma interferon in culture supernatants was measured by capture ELISA and was found to correlate closely with the level of CD4+ T cell proliferation. Indeed, gamma interferon production was significantly increased when cells were stimulated with Hsp110-E2 complexes compared to E2 alone.
Comparison of Hsp110-E2 and E2-only vaccines in cattle.
To determine whether the in vitro proliferative enhancement mediated by Hsp110 chaperoning translated into improved in vivo responses, a pilot study was conducted in cattle. BVDV-free animals were vaccinated with either E2 protein or Hsp110-E2 protein complexes and boosted 21 days later. Quil A was included as an adjuvant.
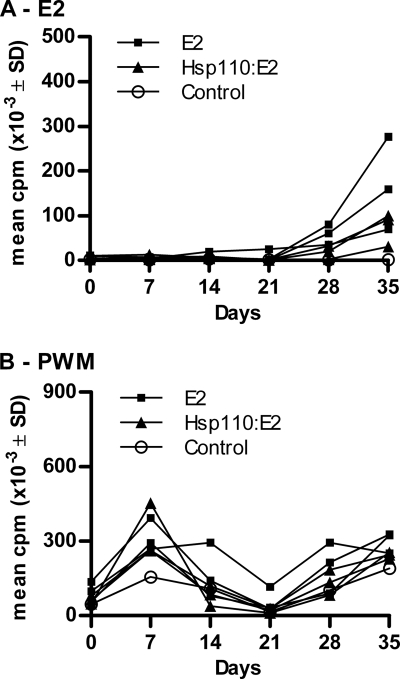
Responses to E2 in PBMC proliferation assays were at background levels (below 4,000 cpm) at day 0 in all animals and throughout the experiment in the unvaccinated control animal. At day 35, recall responses to E2 in E2-vaccinated cattle were significantly higher than in the Hsp110-E2 group and the control animal (Fig. 3 A). Although responses were evident in the Hsp110-E2 vaccination group (mean, 75,000 cpm), they did not reach a level of statistical significance compared to the control. Responses to Erns, the control protein, remained completely negative throughout the study in all groups (data not shown). No significant differences in responses to the positive-control pokeweed mitogen were detected (Fig. 3B).
FIG. 3.
Vaccination with E2 alone results in higher cellular responses than vaccination with Hsp110-E2 complexes. Cattle (three per group) were vaccinated with E2 or Hsp110-E2 complexes and boost vaccinated 21 days later. One animal remained unvaccinated (control). PBMC (2 × 105 per well) were cultured weekly in the presence of 100 ng/ml E2 (A) or 2 μg/ml PWM as a positive control (B). After 5 days, the cells were pulsed with [3H]thymidine and incubated for a further 16 h before being harvested. Incorporated radioactivity was determined by liquid scintillation counting and expressed as cpm.
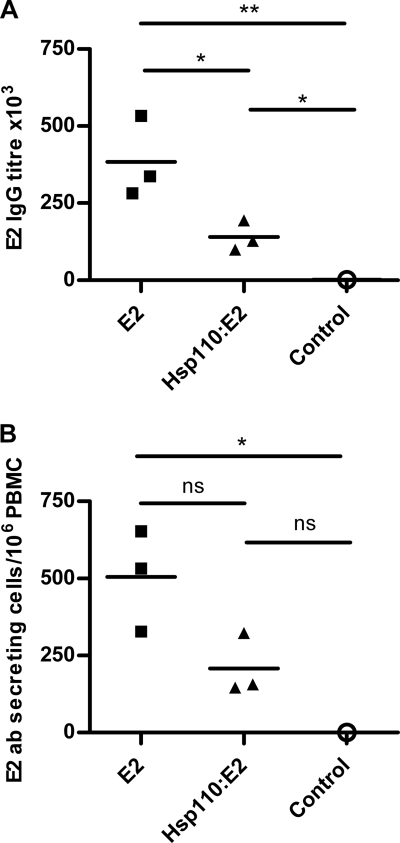
At day 0, anti-E2 IgG serum antibody titers were <2,500 in all animals (data not shown). At day 28, E2-specific serum antibody titers had significantly increased to over 280,000 (mean titer, 384,000 ± 76,000; n = 3) for each animal in the E2-vaccinated group and to over 98,000 (mean titer, 140,000 ± 28,000; n = 3) for those in the Hsp110-E2 group compared to the control (titer, 1,906), which remained negative (Fig. 4 A). However, anti-E2 IgG antibody titers were also significantly lower in cattle from the Hsp110-E2 group than in those from the E2-only group (Fig. 4A). Similarly, we also detected a significantly higher number of E2-specific plasma cells at day 25 (day 4 postboost) in the blood of E2-vaccinated cattle (mean, 504 ± 164 Ab-secreting cells per 106 PBMC; n = 3) compared to both Hsp110-E2-vaccinated (mean, 208 ± 99 Ab-secreting cells per 106 PBMC; n = 3) and control (no E2-specific Ab-secreting cells detected) cattle (Fig. 4B). The clear correlation observed between the Ab titer data and plasma cell number data obtained for each of these groups of cattle is in full agreement with a previously published study (16).
FIG. 4.
Vaccination with E2 alone results in higher humoral responses than Hsp110-E2 complexes. (A) At day 28, serum samples were analyzed by indirect ELISA for E2-specific IgG antibody titers. Results are expressed as the mean of duplicate determinations for each animal. (B) At day 25, the number of E2-specific IgG plasma cells in the blood was determined using a B cell ELISPOT assay. Results are expressed as the mean of duplicate determinations for each animal. *, P < 0.05; **, P < 0.01; one-way ANOVA followed by Bonferroni posthoc test.
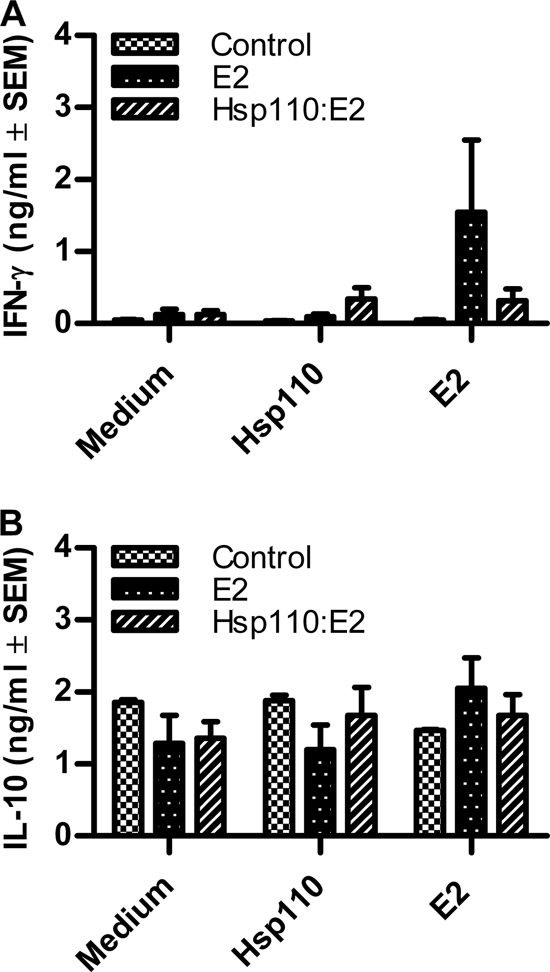
We next investigated whether the differences in cellular and humoral responses between the different groups of vaccinated cattle were the result of a qualitatively different immune response. For this purpose, the supernatants from PBMC cultures at day 28 post-primary vaccination were assayed for gamma interferon and IL-10 (Fig. 5 A and B, respectively). Consistent with the pattern of cellular and humoral responses, gamma interferon production in response to E2 stimulation was higher in E2-vaccinated animals (mean, 1.5 ± 1.0 ng/ml; n = 3) than in Hsp110-E2-vaccinated (mean, 0.3 ± 0.17 ng/ml; n = 3) or control (mean, 0.05 ng/ml) animals, but this was not statistically significant (Fig. 5A). No differences in IL-10 production were observed between the groups of cattle (Fig. 5B).
FIG. 5.
Cytokine profiles in culture supernatants from PBMC of vaccinated animals. Supernatants from PBMC collected at day 28 and stimulated with Hsp110 (500 ng/ml) or E2 (100 ng/ml) for 72 h were assayed by capture ELISA for gamma interferon (IFN-γ) (A) or IL-10 (B) production. Results are expressed as the means of duplicate determinations for each vaccination group plus SEM.
DISCUSSION
We demonstrated that recombinant purified Hsp110 can robustly bind complete viral antigens and enhance monocyte-stimulated recall CD4+ T cell proliferation in vitro but is not able to stimulate significantly improved primary immune responses in vivo.
Hsp110 is part of the HSP multigene family alongside the better-characterized Hsp70, which facilitates antigen presentation to both CD4+ T cells (7, 21, 26, 32, 34) and CD8+ T cells (4-6, 14, 23, 24, 26, 30, 31) and is currently being tested in clinical trials (3, 13, 29, 40). While less is known about Hsp110, it has been shown to bind and stabilize large protein substrates significantly more efficiently than Hsp70 (22). This is potentially a major advantage for vaccine design, as it allows larger proteins with multiple T cell epitopes to be chaperoned, extending the use of a more diverse MHC-peptide complex repertoire.
Previous studies of the immunogenic potential of Hsp110 have been restricted to mouse models using several tumor antigens. In both tumor treatment and tumor prevention studies, improved immunogenicity has been achieved using Hsp110-chaperoned antigen. Increased gamma interferon production and cytotoxic T lymphocyte (CTL) activity by antigen-specific CD4+ and CD8+ T cells has been suggested as a mechanism underlying this enhancement (18). In this study, we used E2, an envelope glycoprotein of BVDV, to further our understanding of whether Hsp110 could improve the immunogenic potential of a viral antigen and how it might be used in future vaccines.
The capacity of HSP to bind other proteins is central to their function as molecular chaperones in vivo and is likely to be key to their ability to enhance the immunogenicity of chaperoned antigens (2). We therefore confirmed that our recombinant bovine Hsp110 was able to bind E2 glycoprotein (by immunoprecipitation with Western blotting) and to inhibit the heat-induced denaturation/aggregation of luciferase protein. The latter methodology has previously been validated by others using recombinant mouse Hsp110 (22). Some protein substrates have been shown to bind Hsp110 only under conditions of heat denaturation, presumably when hydrophobic regions become exposed (e.g., ovalbumin). However, E2 bound Hsp110 at both room temperature and heat shock temperature (56°C). A similar observation has been reported for the renal cell carcinoma protein carbonic anhydrase IX (CA9) (12), suggesting that both the CA9 and E2 proteins contain domains accessible to Hsp110, even at low temperatures.
We have previously demonstrated that bovine Hsp70 can improve antigen-specific proliferation of CD4+ T cells in vitro (21). A similar experimental design was used here to determine if Hsp110 also modulated MHC II-restricted T cell proliferation. Monocytes and CD4+ T cells from three BVDV-immune cattle were incubated in vitro with either E2 chaperoned by Hsp110 or E2 alone, and proliferative responses were determined by thymidine incorporation. Increased proliferation in response to Hsp110-E2 complexes compared to E2 alone was consistently observed for all three animals. The Hsp110 component itself did not independently stimulate T cell proliferation above background levels. As previously reported for HSP-mediated enhancement of proliferation, this effect was apparent only at limiting antigen concentrations (9). Complex formation is vital for this process, as Hsp110 and E2 added to the cells without preincubation (i.e., in the absence of complex formation) did not enhance cell proliferation. Hsp110-E2 complexes not only stimulated proliferation, but also induced gamma interferon production by purified CD4+ T cells, indicating the effector status of these cells.
A major concern of research into HSP has been the possibility of contaminants from prokaryotic expression systems, mainly lipopolysaccharide (LPS), acting as adjuvants (8). To eliminate this possibility, we cloned and expressed Hsp110 in a eukaryotic insect cell system. To remove any environmental contamination that might have occurred during the purification process, the purified preparation was treated with polymixin B, a peptide antibiotic with high affinity for the lipid A moiety of most endotoxins. The LPS concentration of the final Hsp110 preparation was extremely low, i.e., 0.45 EU/mg of protein. The absence of proliferation or gamma interferon production by cells stimulated with Hsp110 only or Hsp110 in the presence of uncomplexed E2 shows that contaminants, LPS or others, potentially present in the Hsp110 preparation were not responsible for the effects observed.
Our interest in HSP is focused on their potential use in vaccines. Therefore, a study was performed in cattle to evaluate whether the enhanced CD4+ proliferative responses seen in vitro also resulted in greater immunogenicity of E2 in vivo. Three animals were vaccinated with E2 alone, and a further three animals were vaccinated with Hsp110-E2 complexes. Both vaccine preparations included the adjuvant Quil A, and all six animals were subsequently boosted with the same vaccines 21 days after the primary vaccination. One animal remained unvaccinated and was used as a control. We assessed the levels of E2-specific PBMC proliferation and antibody titers at selected time points postvaccination. The unvaccinated control animal did not seroconvert or respond to recall antigen in proliferation assays throughout the duration of the study. Significant recall responses were only detectable from 7 and 14 days after the boost vaccination. At these time points, the E2-vaccinated animals showed higher E2-specific proliferative responses and antibody titers than the Hsp110-E2 complex-vaccinated and control animals.
The reduced cellular and antibody responses to the Hsp110-E2 vaccine versus the E2-only vaccine were unexpected, since our in vitro studies showed enhanced CD4+ T cell-proliferative responses to Hsp110-E2 complexes under limiting conditions when monocytes were used as APC. It is possible that, in vivo, these complexes are internalized and processed by a variety of APC, including dendritic cells, macrophages, and B cells. Moreover, the immunizing antigen may be recognized and initiate a response from a variety of effector cells, including CD4+, but also CD8+ and gamma/delta T cells. The expansion of HSP-specific IL-10-producing T regulatory cells has been reported to occur following vaccination with Hsp70 and Hsp60 (1, 39). Therefore, we also assessed whether higher levels of IL-10, and also of gamma interferon, were produced in response to Hsp110. Raised gamma interferon production was observed in response to E2 in the E2-only vaccination group, in line with the proliferation data, but no differences in IL-10 production were detected in response to either Hsp110 or E2, suggesting that Hsp110-specific regulatory cells were not responsible for dampening the immune response.
An alternative explanation for the reduced responses to the Hsp110-E2 vaccine could come from the antigenic competition between the two components, which might occur via several mechanisms. Competition may occur at the level of antigen processing and binding to MHC molecules, and immunodominant Hsp110 peptides may have inhibited the loading of E2 peptides with a lower affinity to a given MHC molecule (11). Alternatively, binding of Hsp110 to E2 may have masked some B cell epitopes of E2 and therefore reduced E2-specific responses.
The reasons for the discrepancies between our results and those previously reported in mouse tumor studies are unclear. Indeed, the efficacy of treatment with Hsp110-tumor antigen complexes in most of those studies was defined by a reduction in tumor size and an increase in antigen-specific gamma interferon-producing cells (12, 17, 18, 36, 37). However, it is worth mentioning that in these mouse studies adjuvants were not added to some of the vaccine preparations. The presence of QuilA adjuvant in our study may therefore have masked any additional immunogenicity induced by Hsp110-E2 complexes over E2 protein alone. Kim et al. (12) showed that despite higher levels of antigen-specific gamma interferon-producing cells and better protection from tumor growth in the CA9-Hsp110-vaccinated animals, CA9-specific antibody titers were higher in animals vaccinated with CA9 in the presence of Freund's adjuvant than in those vaccinated with Hsp110-CA9 alone, which is in accordance with the antibody data presented in our study. Therefore, our results suggest that under these conditions, Hsp110 is less effective than traditional adjuvants and raise the question of whether HSP constitute weak adjuvants or only act as vehicles to protect and stabilize antigens.
Our findings echo those of a phase I trial using a recombinant human Hsp70 preparation complexed to a herpes simplex virus (HSV) peptide (AG-702), which gave poor results, as no peptide-specific responses were detected in patients or healthy controls (13). A new formulation, which uses a combination of HSV peptides and adjuvants, is currently in phase I trials, and the results are anticipated soon. The site of vaccination is also likely to be important for maximizing the responses to HSP-based vaccines. Intradermal vaccination was shown to be most effective at generating improved responses to HSP complexes (36), probably due to the presence of appropriate APC, including dendritic cells, at the site of vaccination.
Conclusion.
The current consensus is that an HSP-chaperoned protein is more immunogenic than the protein alone. Our study shows that this might not always be the case. Therefore, in order to develop effective vaccines, a more integrated consideration of how HSP complexes are handled in vivo by the immune system is required. Specifically, it will be essential to understand how HSP-protein complexes interact with APC at the vaccination site and how that impacts effector cells in order to determine if HSP-based vaccines can be used in the future to elicit a stronger immune response.
Acknowledgments
We thank D. Chapman for providing the bacmid DNA and also those involved in the care of the experimental animals.
Financial support for the research was provided by the Biotechnology and Biological Sciences Research Council. B.C. is a Jenner Institute fellow.
Footnotes
Published ahead of print on 8 December 2010.
REFERENCES
- 1.Anderton, S., R. van der Zee, B. Prakken, A. Noordzij, and W. van Eden. 1995. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J. Exp. Med. 181:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendz, H., et al. 2007. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J. Biol. Chem. 282:31688-31702. [DOI] [PubMed] [Google Scholar]
- 3.Binder, R. J. 2006. Heat shock protein vaccines: from bench to bedside. Int. Rev. Immunol. 25:353-375. [DOI] [PubMed] [Google Scholar]
- 4.Blachere, N. E., et al. 1997. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J. Exp. Med. 186:1315-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogers, W. M. J. M., et al. 2004. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. AIDS 18:25-36. [DOI] [PubMed] [Google Scholar]
- 6.Ciupitu, A.-M. T., et al. 1998. Immunization with a Lymphocytic Choriomeningitis Virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J. Exp. Med. 187:685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doody, A. D. H., et al. 2004. Glycoprotein 96 can chaperone both MHC Class I- and Class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J. Immunol. 172:6087-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, B., and M.-F. Tsan. 2003. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of Tumor Necrosis Factor alpha release by murine macrophages. J. Biol. Chem. 278:174-179. [DOI] [PubMed] [Google Scholar]
- 9.Haug, M., et al. 2005. The heat shock protein Hsp70 enhances antigen-specific proliferation of human CD4+ memory T cells. Eur. J. Immunol. 35:3163-3172. [DOI] [PubMed] [Google Scholar]
- 10.Hietala, S. K., and B. M. Crossley. 2005. Virus replication, p. 81-89. In S. M. Goyal and J. M. Ridpath (ed.), Bovine diarrhea virus: diagnosis, management and control. Wiley-Blackwell, Oxford, United Kingdom.
- 11.Insel, R. A. 1995. Potential alterations in immunogenicity by combining or simultaneously administering vaccine components. Ann. N. Y. Acad. Sci. 754:35-48. [DOI] [PubMed] [Google Scholar]
- 12.Kim, H. L., X. Sun, J. R. Subjeck, and X. Y. Wang. 2007. Evaluation of renal cell carcinoma vaccines targeting carbonic anhydrase IX using heat shock protein 110. Cancer Immunol. Immunother. 56:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koelle, D. M., et al. 2008. Phase I dose-escalation study of a monovalent heat shock protein 70-herpes simplex virus type 2 (HSV-2) peptide-based vaccine designed to prime or boost CD8 T-cell responses in HSV-naive and HSV-2-infected subjects. Clin. Vaccine Immunol. 15:773-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumaraguru, U., M. Gierynska, S. Norman, B. D. Bruce, and B. T. Rouse. 2002. Immunization with chaperone-peptide complex induces low-avidity cytotoxic T lymphocytes providing transient protection against herpes simplex virus infection. J. Virol. 76:136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwong, L. S., et al. 2002. Development of an ELISA for bovine IL-10. Vet. Immunol. Immunopathol. 85:213-223. [DOI] [PubMed] [Google Scholar]
- 16.Lefevre, E. A., B. V. Carr, H. Prentice, and B. Charleston. 2009. A quantitative assessment of primary and secondary immune responses in cattle using a B cell ELISPOT assay. Vet. Res. 40:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manjili, M. H., et al. 2002. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 62:1737-1742. [PubMed] [Google Scholar]
- 18.Manjili, M. H., et al. 2003. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J. Immunol. 171:4054-4061. [DOI] [PubMed] [Google Scholar]
- 19.Manjili, M. H., et al. 2002. Immunotherapy of cancer using heat shock proteins. Front. Biosci. 7:d43-d52. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Donato, G., et al. 2010. Ratio of HCV structural antigens in protein based vaccine formulations is critical for functional immune response induction. Biotechnol. Appl. Biochem. 56:111-118. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin, K., J. Seago, L. Robinson, C. Kelly, and B. Charleston. 2010. Hsp70 enhances presentation of FMDV antigen to bovine CD4+ T cells in vitro. Vet. Res. 41:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh, H. J., X. Chen, and J. R. Subjeck. 1997. Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J. Biol. Chem. 272:31636-31640. [DOI] [PubMed] [Google Scholar]
- 23.Pack, C. D., U. Kumaraguru, S. Suvas, and B. T. Rouse. 2005. Heat-shock protein 70 acts as an effective adjuvant in neonatal mice and confers protection against challenge with Herpes Simplex Virus. Vaccine 23:3526-3534. [DOI] [PubMed] [Google Scholar]
- 24.Peng, M., et al. 2006. Novel vaccines for the treatment of chronic HBV infection based on mycobacterial heat shock protein 70. Vaccine 24:887-896. [DOI] [PubMed] [Google Scholar]
- 25.Pockley, A. G. 2003. Heat shock proteins as regulators of the immune response. Lancet 362:469-476. [DOI] [PubMed] [Google Scholar]
- 26.SenGupta, D., et al. 2004. Heat shock protein-mediated cross-presentation of exogenous HIV antigen on HLA class I and class II. J. Immunol. 173:1987-1993. [DOI] [PubMed] [Google Scholar]
- 27.Shortman, K., M. H. Lahoud, and I. Caminschi. 2009. Improving vaccines by targeting antigens to dendritic cells. Exp. Mol. Med. 41:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srivastava, P. 2002. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 20:395-425. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava, P. K. 2006. Therapeutic cancer vaccines. Curr. Opin. Immunol. 18:201-205. [DOI] [PubMed] [Google Scholar]
- 30.Su, C., et al. 2007. Heterologous expression of FMDV immunodominant epitopes and HSP70 in P. pastoris and the subsequent immune response in mice. Vet. Microbiol. 124:256-263. [DOI] [PubMed] [Google Scholar]
- 31.Tobian, A. A., D. H. Canaday, W. H. Boom, and C. V. Harding. 2004. Bacterial heat shock proteins promote CD91-dependent class I MHC cross-presentation of chaperoned peptide to CD8+ T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages. J. Immunol. 172:5277-5286. [DOI] [PubMed] [Google Scholar]
- 32.Tobian, A. A., D. H. Canaday, and C. V. Harding. 2004. Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells. J. Immunol. 173:5130-5137. [DOI] [PubMed] [Google Scholar]
- 33.Vaughn, J. L., R. H. Goodwin, G. J. Tompkins, and P. McCawley. 1977. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 13:213-217. [DOI] [PubMed] [Google Scholar]
- 34.Wang, R., J. T. Kovalchin, P. Muhlenkamp, and R. Y. Chandawarkar. 2006. Exogenous heat shock protein 70 binds macrophage lipid raft microdomain and stimulates phagocytosis, processing, and MHC-II presentation of antigens. Blood 107:1636-1642. [DOI] [PubMed] [Google Scholar]
- 35.Wang, X.-Y., et al. 2004. Development of cancer vaccines using autologous and recombinant high molecular weight stress proteins. Methods 32:13-20. [DOI] [PubMed] [Google Scholar]
- 36.Wang, X.-Y., et al. 2010. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J. Immunol. 184:6309-6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, X. Y., et al. 2003. Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100. Cancer Res. 63:2553-2560. [PubMed] [Google Scholar]
- 38.Webster, D. P., J. Farrar, and S. Rowland-Jones. 2009. Progress towards a dengue vaccine. Lancet Infect. Dis. 9:678-687. [DOI] [PubMed] [Google Scholar]
- 39.Wendling, U., et al. 2000. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J. Immunol. 164:2711-2717. [DOI] [PubMed] [Google Scholar]
- 40.Wood, C., et al. 2008. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet 372:145-154. [DOI] [PubMed] [Google Scholar]
- 41.Ye, Z., and Y.-H. Gan. 2007. Flagellin contamination of recombinant heat shock protein 70 is responsible for its activity on T cells. J. Biol. Chem. 282:4479-4484. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, Y., D. A. G. Chapman, and I. M. Jones. 2003. Improving baculovirus recombination. Nucleic Acids Res. 31:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]