Abstract
Measles remains an important cause of morbidity and mortality among children in the developing world. The goal of this study was to examine measles virus-specific mucosal immune responses in healthy immune (n = 24; plaque reduction neutralization [PRN] titers of ≥200 mIU/ml) and nonimmune (n = 24) young adult volunteers who received the monovalent Moraten measles vaccine via intranasal (spray delivery) or subcutaneous immunization. Serum, oral fluid, and nasal wash samples were examined for measles virus-specific and total IgG and IgA on day 0 (prior to vaccination) and on days 14, 28, and 90 after vaccination. Nonimmune subjects vaccinated subcutaneously developed high levels of measles virus PRN, IgG, and IgA antibodies in serum, oral fluid, and nasal washes. Total IgG and secretory IgA (sIgA) titers were increased in nasal washes, and total IgG was increased in oral fluid specimens. There was a strong correlation between PRN and measles virus-specific IgG titers measured in serum, oral fluid, and nasal washes, whereas a weak correlation was found between PRN and measles virus-specific IgA titers. Notably, intranasal measles vaccination resulted in increased production of measles virus-specific sIgA in oral fluid and nasal washes in nonimmune individuals, without evidence of a systemic immune response. In contrast, no significant vaccine-induced responses were observed in immune subjects, regardless of the route of immunization. These results demonstrate that (i) intranasal measles immunization can elicit a mucosal response independent of the induction of serum antibodies and (ii) both mucosal and systemic antibody responses following nasal or subcutaneous immunization are blunted by preexisting measles immunity.
Measles remains a major cause of morbidity and mortality, particularly among infants in the developing world (11, 14). In recent years, mass immunization campaigns with the currently licensed measles vaccines have succeeded in reducing the measles mortality burden in sub-Saharan Africa (2, 19). Both the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF), through the Global Immunization Vision and Strategy (GIVS) for 2006 to 2015, are committed to reducing the measles disease burden by aggressively supporting routine and catch-up immunization and by improving existing vaccines (20). This strategy includes immunization using alternative routes of vaccine delivery, such as small-particle aerosol and large-droplet intranasal administration (6). The small-particle aerosol route is currently favored, as it has been shown to induce strong and long-lived systemic and mucosal immune responses (3, 7, 8).
In a recent clinical study, we examined whether healthy adult volunteers immunized intranasally (i.n.) with the live attenuated Moraten measles vaccine could elicit a protective immune response comparable to that induced by routine subcutaneous (s.c.) vaccination (16). We found that subjects who received the vaccine s.c. responded with a significant increase in serum plaque reduction virus-neutralizing (PRN) antibodies, the measles correlate of protection (5). In contrast, subjects who received the vaccine i.n. failed to develop systemic PRN responses (16). In this study, we examined the mucosal immune responses in these subjects in relation to their preexisting serum PRN antibody status and demonstrated that nonimmune individuals immunized i.n. with the measles vaccine elicited measles virus (MV)-specific secretory IgA (sIgA) that was detected in oral fluid and nasal washes, although they failed to mount a concomitant serum antibody response.
MATERIALS AND METHODS
Study design.
A clinical study was performed with healthy adult volunteers aged 18 to 45 years who were recruited and enrolled in Santiago, Chile, between September and October 2004 as described elsewhere (16). Serum PRN antibodies against MV were measured in all volunteers before vaccination, and those with titers of ≥200 mIU/ml were considered immune to measles (5). A total of 24 measles-immune and 24 measles-nonimmune volunteers were randomly assigned in a double-blinded fashion to receive the measles vaccine intranasally or subcutaneously. The study was approved by the Chilean Ethics Committee and the University of Maryland Institutional Review Board.
Vaccines and immunization.
Participants received the Moraten Berna live attenuated monovalent measles vaccine (Berna Biotech Ltd., Bern, Switzerland). The vaccine was prepared in MRC-5 human diploid cells from the attenuated MV Edmonston Zagreb strain. It was supplied in freeze-dried multidose vials and reconstituted with 0.9% NaCl to a volume of 0.5 ml/dose, as instructed by the manufacturer for s.c. injection, or of 0.2 ml/dose for i.n. administration (0.1 ml was administered into each nostril). Each dose contained 3.9 log10 50% tissue culture infective doses (TCID50) of MV. Placebo recipients were given 0.9% NaCl. Subcutaneous vaccination was performed following standard procedures. Intranasal administration was performed by inserting the tip of an Accuspray device (Becton Dickinson) inside the nose of an upright individual, holding the device at approximately 45° to the ground, and depressing the plunger to spray toward the occiput. The clinical assessment following vaccination is described in detail elsewhere (16).
Biological specimens.
Blood, oral fluid, and nasal wash specimens were obtained for antibody measurements prior to immunization and on days 14, 28, and 90 after immunization. Oral fluid was collected using a sponge swab (Oracol; Malvern Medical Developments Limited, Worcester, United Kingdom) as previously described (17). Fluid was extracted, and an equal volume of phosphate-buffered saline (PBS) containing 0.05% Tween 20 and 0.01% chlorhexidine digluconate was added as a preservative. Nasal washes were obtained by instilling 2.5 ml of saline into each nostril for 15 s with the head reclined, followed by collection of the specimen into a sterile cup. Samples were centrifuged for 20 min at 2,500 rpm and immediately frozen. All specimens were cryopreserved at −80°C until assayed.
Measles virus PRN antibodies.
Measles virus-neutralizing antibodies were measured by PRN assay as previously described (18), using an adaptation of the method described by Albrecht et al. (1). Samples were tested in parallel with the 2nd WHO international measles reference serum (66/202).
Measles virus-specific IgG and IgA.
Measles virus-specific IgG and IgA in serum were measured by enzyme-linked immunosorbent assay (ELISA) (18). Briefly, plates were coated with MV lysate (Advanced Biotechnologies, Inc., Columbia, MD) at 5 μg/ml in carbonate buffer and blocked with 10% milk in PBS. Samples were tested in serial 2-fold dilutions in PBS containing 0.05% Tween 20 (PBST) in duplicate wells. Antibodies were detected with horseradish peroxidase (HRP)-labeled goat anti-human Fc-specific IgG and IgA (Jackson ImmunoResearch, West Grove, PA), diluted 1:5,000 and 1:2,000, respectively, in PBST. Tetramethylbenzidine (TMB; Kirkegaard & Perry Laboratories, Gaithersburg, MD) was used as a substrate. Measles virus-specific IgG and IgA in oral fluid and nasal washes were measured as described above, using biotin-labeled goat anti-IgA and anti-IgG (MP Biomedical, Solon, OH) diluted 1:2,000 in PBST followed by avidin peroxidase (Sigma, St. Louis, MO) diluted 1:400 in 1% bovine serum albumin (BSA) in PBS. Titers were calculated as the inverse of the dilution that produced an absorbance value of 0.2 above the blank value and are reported in ELISA units (EU)/ml.
Total IgG and IgA.
Total IgG and IgA antibodies were measured in oral fluid and nasal washes by using a sandwich ELISA as previously described (17). Plates were coated with purified anti-IgG (γ-chain specific; Jackson ImmunoResearch) or anti-IgA (α-chain specific) at 1 μg/ml in PBS. HRP-labeled anti-IgA and anti-IgG (Jackson ImmunoResearch) were used as conjugates, at dilutions of 1:20,000 and 1:15,000, respectively. Standard curves for purified human IgG and IgA (Calbiochem, Madison, WI) were used to calculate the amounts of IgG and IgA in each sample. Results are expressed in μg/ml. Positive and negative controls were included in all assays.
Statistical analysis.
A vaccine response was defined as achieving a PRN titer of ≥200 mIU/ml or a 4-fold increase in ELISA antibody measurements. The statistical analytical strategy included analyses of distribution, point estimates, and 95% confidence intervals (CI) for geometric mean titers (GMTs) for immune and nonimmune subjects immunized i.n. or s.c. Maximum GMT responses on day 14, 28, or 90 were used to calculate the maximum fold increase, % response point estimates, and 95% CI. The Shapiro-Wilk test was used to assess the normal distribution. Two-tailed nonparametric tests were applied to log-transformed continuous end points, including the Wilcoxon signed rank test for pre- and postvaccine responses and the Wilcoxon rank sum test for comparing i.n. and s.c. administration. Fisher's exact test was used to compare the proportions of responders. Spearman's rank correlation coefficient was used to assess correlations of log-transformed continuous variables by group, and the kappa statistic was used to assess agreement between dichotomous outcome variables. No adjustments were made for multiple comparisons.
RESULTS
Mucosal and serum antibodies induced by live attenuated measles vaccine administered s.c. or i.n. in the presence or absence of measles immunity.
A clinical study was conducted in healthy adults in Santiago, Chile, to investigate the safety and immunogenicity of the licensed live attenuated monovalent measles vaccine administered by s.c. injection (the recommended route for use in humans) versus large-droplet intranasal spray delivery. To evaluate the influence of baseline measles immunity on the responses to systemic versus mucosal immunization, subjects were prescreened for serum PRN antibodies. Twenty-four nonimmune (<200 mIU/ml) and 24 immune (≥200 mIU/ml) individuals were randomly allocated into 2 groups of 12 subjects each, to receive the vaccine s.c. or i.n. An extensive clinical assessment showed that the vaccine was well tolerated regardless of the route of immunization. No differences were found in safety and reactogenicity among groups, and no serious adverse events occurred (16).
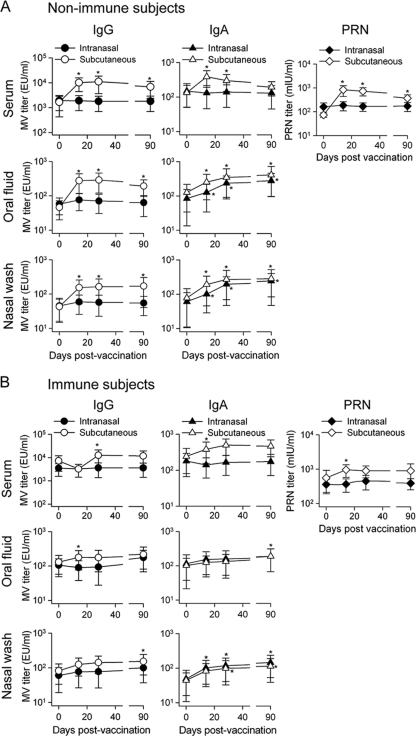
Antibody responses were measured in serum, oral fluid, and nasal washes at different time points after vaccination. All 12 nonimmune subjects who received the vaccine s.c. responded with significant increases in measles virus-specific serum IgG, IgA, and PRN titers (Fig. 1 A). The highest PRN titers were observed on days 14 and 28 and decreased thereafter. Similarly, measles virus-specific serum IgA and IgG antibodies peaked on days 14 and 28 postvaccination (Fig. 1A). Increased titers of measles virus-specific IgG and IgA were also found in oral fluid and nasal washes; IgG titers rose to peak levels by day 28 and remained steady thereafter, whereas measles virus-specific IgA levels increased up to day 90 after immunization (Fig. 1A). In contrast, nonimmune subjects immunized i.n. failed to develop serum antibody responses. Nevertheless, these individuals exhibited increased levels of measles virus-specific sIgA in both oral fluid and nasal washes, which increased gradually up to day 90 following vaccination. No IgG responses were detected in these oral fluid and nasal wash samples. These results indicate that despite being unable to stimulate a systemic (serum antibody) response, intranasal measles vaccination succeeded in eliciting a sustained mucosal sIgA response (Fig. 1A). Overall, the peak responses elicited by s.c. immunization surpassed those elicited by i.n. delivery, except for the sIgA responses in oral fluid and nasal washes.
FIG. 1.
Measles virus-specific systemic and mucosal antibody responses. Healthy nonimmune (A) and immune (B) volunteers (n = 24 for each cohort) were vaccinated s.c. or i.n. with one dose of the monovalent attenuated Moraten measles virus vaccine. MV-specific IgG and IgA were measured in serum, oral fluid, and nasal washes and reported as end-point dilution titers (EU/ml). PRN antibodies were measured in serum and reported in mIU/ml. Results are shown as GMTs for all subjects in each cohort, with 95% CI. *, P < 0.04 compared to prevaccination titers.
When we analyzed the vaccine-induced responses among immune subjects, we found that only those immunized s.c. developed significant, albeit modest, increases in measles virus-specific serum IgG, IgA, and PRN antibodies after immunization (Fig. 1B). Measles virus-specific IgG and IgA were also slightly elevated in oral fluid and nasal washes (Fig. 1B), and the kinetics of these responses resembled those described above for nonimmune subjects. Subjects immunized i.n. failed to mount measles virus-specific IgG and IgA responses in serum or oral fluid. However, significant measles virus-specific sIgA responses were detected in oral fluid and nasal washes (Fig. 1B), with sIgA titers increasing steadily up to day 90. The serological responses exhibited by immune individuals immunized s.c. surpassed those of subjects immunized i.n., except for sIgA responses.
Fold increases in antibody titers and proportions of responders after s.c. versus i.n. measles vaccination.
We also examined the antibody responses to s.c. or i.n. measles immunization in terms of fold increases in mean antibody titers, comparing baseline and peak postvaccination time points (Table 1). Subjects immunized s.c. had larger fold increase responses in MV-specific serum PRN, IgG, and IgA than those immunized i.n.; the difference was particularly evident for subjects lacking preexisting immunity. Nonimmune subjects immunized s.c. also had the highest fold rises in measles virus-specific IgG in oral fluid and nasal washes. Regardless of the route of immunization, nonimmune individuals also exhibited multifold increases in measles virus-specific sIgA in oral fluid and nasal washes.
TABLE 1.
Fold increases and % responses for antibodies elicited by s.c. and i.n. measles vaccination among measles-immune and -nonimmune adults
| Antibody and sample type (day of peak response) | Nonimmune adults |
Immune adults |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fold increasea |
P valuec | % Responseb |
P valued | Fold increasea |
P valuec | % Responseb |
P valued | |||||
| s.c. (n = 12) | i.n. (n = 12) | s.c. (n = 12) | i.n. (n = 12) | s.c. (n = 12) | i.n. (n = 12) | s.c. (n = 12) | i.n. (n = 12) | |||||
| Serum | ||||||||||||
| PRN (14) | 9.8 (6.1-15.8) | 1.1 (0.9-1.4) | <0.001 | 100 (74-100) | 42 (15-72) | 0.002 | 1.9 (1.3-2.8) | 1.0 (0.8-1.2) | 0.003 | 58.0 (28-85) | 92 (62-100) | 0.080 |
| MV-specific IgG (28) | 6.7 (4.7-9.5) | 0.9 (0.8-1.1) | <0.001e | 83 (52-98) | 0 (0-27) | <0.001 | 1.7 (1.3-2.3) | 1.0 (0.7-1.6) | 0.002e | 8.3 (0-39) | 0 (0-27) | 0.500 |
| MV-specific IgA (28) | 2.9 (day 14) (1.9-4.4) | 0.9 (0.8-1.1) | <0.001 | 17 (2-48) | 0 (0-27) | 0.200 | 2.1 (1.0-4.1) | 0.9 (0.7-1.2) | 0.020e | 0.0 (0-27) | 17 (2-48) | 0.200 |
| Oral fluid | ||||||||||||
| MV-specific IgG (28) | 6.3 (4.2-9.4) | 1.2 (0.9-1.7) | <0.001 | 83 (52-98) | 8 (0-39) | <0.001 | 1.3 (1.1-1.7) | 0.9 (0.6-1.4) | 0.070 | 0.0 (0-27) | 0 (0-27) | 1.000 |
| MV-specific sIgA (90) | 3.4 (2.1-5.7) | 3.3 (2.1-5.2) | 0.900 | 33 (10-65) | 50 (21-79) | 0.300 | 1.2 (0.9-1.5) | 1.6 (1.2-2.3) | 0.070 | 8.0 (0-39) | 0 (0-27) | 0.500 |
| Nasal wash | ||||||||||||
| MV-specific IgG (90) | 4.0 (2.9-5.4) | 1.2 (0.7-2.0) | 0.002 | 50 (21-79) | 8 (0-39) | 0.030 | 1.9 (1.3-2.6) | 1.6 (1.1-2.4) | 0.600 | 8.0 (0-39) | 0 (0-27) | 0.500 |
| MV-specific sIgA (90) | 3.6 (2.7-4.9) | 4.0 (2.5-6.4) | 0.200e | 58 (28-85) | 67 (35-90) | 0.500 | 2.6 (1.7-3.9) | 3.0 (1.9-4.5) | 0.600 | 33.0 (10-65) | 17 (2-48) | 0.300 |
Calculated between baseline and peak postvaccination levels. Data are GMTs ± 95% CI.
A response is defined as a titer of >200 mIU/ml for PRN antibody and a >4-fold increase for all other immune markers. Measles-immune subjects had PRN titers of ≥200 mIU/ml before vaccination. Percent responses for PRN antibody indicate subjects with immune status (does not imply that they are vaccine responders). Data are GMTs ± 95% CI.
For i.n. versus s.c. response by t test on log-transformed data. Values shown in bold indicate significant differences.
For i.n. versus s.c. response by Fisher's exact test. Values shown in bold indicate significant differences.
The Wilcoxon rank sum test was used when the log-transformed data were not normally distributed.
We defined “responders” as those individuals who exhibited PRN titers of ≥200 mIU/ml and showed >4-fold increases in antibody levels (Table 1). None of the immune subjects qualified as “responders.” Among the nonimmune subjects, the group immunized s.c. had the largest number of responders for serum PRN and MV-specific IgG and for oral fluid and nasal wash IgG. Interestingly, there was a greater proportion of oral fluid and nasal wash sIgA “responders” among those immunized by the nasal route.
Measles vaccination increases total mucosal IgG and IgA.
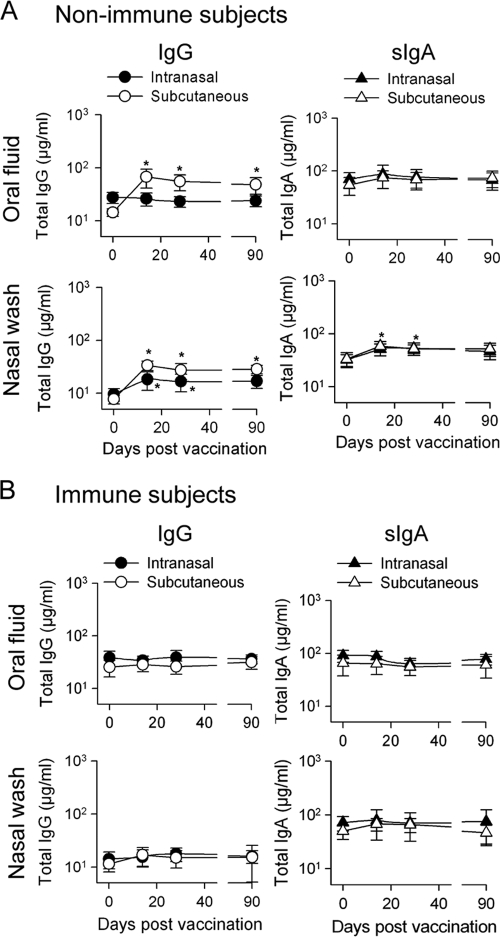
Antibodies are secreted locally in response to antigenic stimuli, and the proportion of antigen-specific antibodies in the total immunoglobulin population must be taken into consideration in assessing responses in mucosal tissues. We examined the levels of total IgG and IgA in oral and nasal fluids of vaccinated adults. Nonimmune subjects vaccinated s.c. showed increased levels of total IgG in oral fluid and nasal washes as well as increased levels of sIgA in nasal washes, which peaked on day 14 (Fig. 2 A). Subjects immunized i.n. also had increased levels of total IgG and sIgA in nasal washes (Fig. 2A). No differences were seen in immune individuals after vaccination (Fig. 2B).
FIG. 2.
Total IgG and IgA measured in oral fluid and nasal washes. Nonimmune (A) and immune (B) volunteers were vaccinated as described in the legend to Fig. 1. Total IgG and sIgA were measured in oral fluid and nasal washes and reported in μg/ml. Results are expressed as GMTs with 95% CI. *, P < 0.03 compared to prevaccination titers.
Additionally, we determined the ratios of specific MV antibodies to total IgG and IgA levels in oral fluid and nasal wash samples and found no significant differences between nonimmune and immune individuals by any route of vaccination (data not shown). Due to the rise in total IgG and IgA levels, the responses to the vaccine were muted when reported as ratios of measles virus-specific to total antibodies.
Correlation between PRN titers and MV-specific IgG and IgA in serum and oral and nasal fluids.
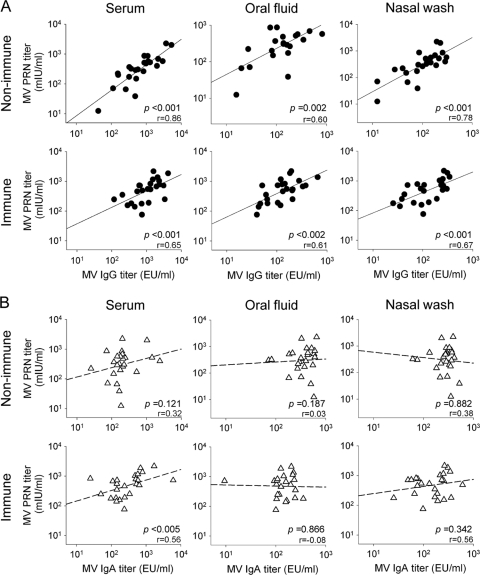
In an attempt to relate the serum PRN titers with the antibody responses obtained in systemic and mucosal tissues, we performed regression analyses comparing PRN and peak GMT titers for serum, oral fluid, and nasal wash MV-specific IgG and IgA. A significant correlation was found between serum PRN and measles virus-specific IgG antibody titers. To a lesser degree, PRN titers also correlated with oral fluid and nasal wash IgG titers for both immune and nonimmune vaccine recipients (Fig. 3 A). There was some correlation between PRN and IgA titers in sera and nasal washes, particularly for the immune vaccine recipients, whereas no correlation was established between serum PRN and oral fluid antibody responses (Fig. 3B). No correlation was found between serum measles virus-specific IgA and sIgA antibodies in any group (data not shown).
FIG. 3.
Correlations between serum PRN and MV-specific IgG and IgA titers in serum, oral fluid, and nasal washes for nonimmune and immune subjects vaccinated i.n. and s.c. The graphics show correlations between peak serum PRN (day 14) and MV-specific peak IgG (A) and IgA (B) titers measured in serum (day 28), oral fluid (days 28 and 90, respectively), and nasal washes (day 90). Data represent Spearman's correlations of log-transformed individual titers. The correlation coefficient is indicated (r).
DISCUSSION
The most significant observation made in this study is that large-droplet intranasal spray delivery of a monovalent live attenuated measles vaccine failed to elicit a serum antibody response but stimulated an sIgA response in oral fluid and nasal washes. Antibodies at the mucosal surfaces, particularly sIgA antibodies, play important roles in virus exclusion, intracellular neutralization, and reduction of viral shedding (4, 12, 21). Measles virus-specific IgA has been detected in sera, saliva, and tears of children infected with wild-type MV (9, 10). We previously reported the presence of MV-specific sIgA with virus-neutralizing capacity in the milk of adult mothers (13). It is hypothesized that mucosal sIgA antibodies contribute to protection against measles disease and exert an epidemiologic impact by diminishing virus excretion and transmission to susceptible individuals.
Bellanti et al. reported significantly higher levels of measles virus-specific IgA and IgG in serum and nasal secretions in response to a live attenuated measles vaccine administered by aerosol than in response to s.c. immunization (3). Although the correlate of protection against measles is serum PRN antibody (5), with the understanding that virus-neutralizing antibodies can block cell invasion, additional mechanisms may also be involved in protection. Measles virus is transmitted by both large respiratory droplets and droplet nucleus aerosols (19), and anti-measles virus sIgA antibodies may act as the first layer of defense, preventing infection by blocking virus entry into cells of the respiratory tract mucosa. It has been problematic to differentiate the relative contribution of mucosal sIgA antibodies from that of serum IgG (or IgA) antibodies in mediating protection (4). Mechanistically, it may be reasonable to assume that they both play critical roles but work in a stepwise manner, with mucosal sIgA representing an earlier defense.
The mucosal sIgA response observed in this study following i.n. administration of the live attenuated measles vaccine, without a concomitant rise in serum antibodies, may be due to the inability of the vaccine to reach systemic lymphoid tissue, limiting the responses to local mucosal antibodies. It is also conceivable that the host's mucosal defenses might prevent replication and further dissemination of the virus. The immune responses triggered locally could be sufficient to clear the live attenuated virus in such a way that systemic immune responses (in the form of serum antibodies) are not induced.
Nonimmune individuals had the best responses to vaccination regardless of the route used, providing a compelling argument for taking into consideration the baseline immunity during evaluation of measles vaccine candidates or improved immunization regimens. In assessing pre- and postvaccine responses by measuring fold increases, the power to detect differences is diminished in an immune population with high baseline levels. Because the primary goal for measles reduction campaigns is to immunize nonimmune individuals (who include previously uninfected and previously unimmunized subjects and those with primary vaccine failure), we conclude that it is particularly important to test new measles vaccines or novel routes of administration of existing measles vaccines in a measles-nonimmune population. The recruitment of such individuals for phase 1 and 2 studies, however, is difficult. For safety reasons, we included immune (serum PRN antibody titers of >200 mIU/ml) adults in our study.
Assessment of the ratio of vaccine-specific antibody to total antibody is usually performed to account for interspecimen variability of total antibody in nonhomogeneous fluids, such as oral fluid and nasal wash samples. In this study, due to the concurrent increases in total IgG, increases in ratios of specific to total antibody were lower than increases in the absolute levels of measles virus-specific antibodies. When absolute levels of measles virus-specific antibodies were measured, significant increases in oral fluid IgG were found for nonimmune individuals (Table 1). Similarly, because total IgA increased in the nasal washes of nonimmune individuals, assessing only ratios of specific to total IgA in this tissue decreases the power to detect vaccine-induced responses.
A response was defined as reaching a seroprotective PRN level of ≥200 mIU/ml. However, the protective thresholds for other immunological markers, such as measles virus-specific serum IgG or mucosal sIgA, are not known. Correlation with the PRN titer was strongest for serum IgG, followed by oral fluid and nasal wash IgG (as expected, due to transudation of IgG from serum to the mucosal surfaces). This correlation was higher for the nonimmune population due to the more homogeneous and lower baseline levels. A poor correlation was obtained with sIgA, which represents a different effector mechanism at the mucosal surfaces and would not necessarily be expected to correlate with a serum PRN response. The relatively strong association between serum PRN and IgG levels, as opposed to serum IgA levels, suggests that IgG plays a greater role in virus neutralization in the circulation. Serum IgG also transudes to the mucosal surfaces, where it may help to prevent infection through neutralization of viral particles.
Clinical trials are currently exploring the possibility of enhancing measles immunization through small-particle aerosol delivery of the attenuated EZ measles vaccine (15). While for regulatory reasons the primary end point of such studies is the attainment of protective levels of serum antibodies, it would be useful to include an analysis of mucosal responses, which may shed light on the mechanisms of protection in humans. Mucosal immunological readouts might also provide a noninvasive readout that could serve as a surrogate of protection.
The results of our clinical trial indicate that intranasal administration of a live measles vaccine can stimulate mucosal sIgA locally, even in the absence of evidence of a systemic immune response. The extent to which sIgA contributes to protection in the absence of serum antibodies, however, remains to be determined. The induction of both mucosal and systemic immunity is desirable to enhance protection against measles virus and other respiratory viruses. Our findings also highlight some important considerations for the evaluation of immune responses to measles virus, including (i) the need to assess novel vaccines or routes of administration in nonimmune individuals, (ii) the importance of absolute levels of measles virus-specific antibodies, and (iii) the role of additional immunological readouts (e.g., sIgA) to establish vaccine take.
Acknowledgments
We thank the volunteers and personnel from CVD Chile for their assistance with the clinical study. We also thank Yu Lim and Mardi Reymann (Applied Immunology Section, CVD Baltimore) for performing immunological assays and William Blackwelder for assistance with statistical analysis.
This work was funded by the Bill and Melinda Gates Foundation.
Jean Francois Viret died before the manuscript was prepared. His posthumous inclusion as an author recognizes his strong support and collaboration in the design and performance of this study.
J.F.V. was an employee of Berna Biotech, a Crucell Company.
Footnotes
Published ahead of print on 12 January 2011.
REFERENCES
- 1.Albrecht, P., K. Herrmann, and G. R. Burns. 1981. Role of virus strain in conventional and enhanced measles plaque neutralization test. J. Virol. Methods 3:251-260. [DOI] [PubMed] [Google Scholar]
- 2.Arevshatian, L., et al. 2007. An evaluation of infant immunization in Africa: is a transformation in progress? Bull. World Health Organ. 85:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellanti, J. A., et al. 2004. Immunologic studies of specific mucosal and systemic immune responses in Mexican school children after booster aerosol or subcutaneous immunization with measles vaccine. Vaccine 22:1214-1220. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P. 2007. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25:5467-5484. [DOI] [PubMed] [Google Scholar]
- 5.Chen, R. T., et al. 1990. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162:1036-1042. [DOI] [PubMed] [Google Scholar]
- 6.Cutts, F. T., C. J. Clements, and J. V. Bennett. 1997. Alternative routes of measles immunization: a review. Biologicals 25:323-338. [DOI] [PubMed] [Google Scholar]
- 7.Dilraj, A., et al. 2000. Response to different measles vaccine strains given by aerosol and subcutaneous routes to schoolchildren: a randomised trial. Lancet 355:798-803. [DOI] [PubMed] [Google Scholar]
- 8.Dilraj, A., R. Sukhoo, F. T. Cutts, and J. V. Bennett. 2007. Aerosol and subcutaneous measles vaccine: measles antibody responses 6 years after re-vaccination. Vaccine 25:4170-4174. [DOI] [PubMed] [Google Scholar]
- 9.El Mubarak, H. S., et al. 2004. Measles virus protein-specific IgM, IgA, and IgG subclass responses during the acute and convalescent phase of infection. J. Med. Virol. 72:290-298. [DOI] [PubMed] [Google Scholar]
- 10.Friedman, M. G., M. Phillip, and R. Dagan. 1989. Virus-specific IgA in serum, saliva, and tears of children with measles. Clin. Exp. Immunol. 75:58-63. [PMC free article] [PubMed] [Google Scholar]
- 11.Grais, R. F., et al. 2007. Unacceptably high mortality related to measles epidemics in Niger, Nigeria, and Chad. PLoS Med. 4:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmgren, J., and C. Czerkinsky. 2005. Mucosal immunity and vaccines. Nat. Med. 11:S45-S53. [DOI] [PubMed] [Google Scholar]
- 13.Mandomando, I. M., et al. 2008. Measles-specific neutralizing antibodies in rural Mozambique: seroprevalence and presence in breast milk. Am. J. Trop. Med. Hyg. 79:787-792. [PubMed] [Google Scholar]
- 14.Moss, W. J. 2007. Measles still has a devastating impact in unvaccinated populations. PLoS Med. 4:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omer, S. B., G. S. Hiremath, and N. A. Halsey. 2010. Respiratory administration of measles vaccine. Lancet 375:706-708. [DOI] [PubMed] [Google Scholar]
- 16.Simon, J. K., et al. 2007. A clinical study to assess the safety and immunogenicity of attenuated measles vaccine administered intranasally to healthy adults. Hum. Vaccin. 3:54-58. [DOI] [PubMed] [Google Scholar]
- 17.Tapia, M. D., et al. 2006. Measurement of tetanus antitoxin in oral fluid: a tool to conduct serosurveys. Pediatr. Infect. Dis. J. 25:819-825. [DOI] [PubMed] [Google Scholar]
- 18.Tapia, M. D., et al. 2005. A serosurvey to identify the window of vulnerability to wild-type measles among infants in rural Mali. Am. J. Trop. Med. Hyg. 73:26-31. [PubMed] [Google Scholar]
- 19.World Health Organization. 2009. Measles. Fact sheet 286. World Health Organization, Geneva, Switzerland.
- 20.World Health Organization and UNICEF. 2010. Global immunization vision and strategy. World Health Organization, Geneva, Switzerland. http://www.who.int/immunization/givs/en/index.html. [DOI] [PMC free article] [PubMed]
- 21.Yan, H., M. E. Lamm, E. Bjorling, and Y. T. Huang. 2002. Multiple functions of immunoglobulin A in mucosal defense against viruses: an in vitro measles virus model. J. Virol. 76:10972-10979. [DOI] [PMC free article] [PubMed] [Google Scholar]