Abstract
Human papillomavirus 16 (HPV 16) and HPV 18 antibody responses in a 2- versus 3-dose HPV vaccine (Gardasil) trial were measured by a pseudovirus neutralizing antibody (PsV NAb) assay and by the Merck competitive Luminex immunoassay (cLIA). Eight hundred twenty-four female subjects assigned to three dosing regimens (group 1, 9 to 13 years old; 2 doses, months 0 and 6 [n = 259]; group 2, 9 to 13 years old; 3 doses, months 0, 2, and 6 [n = 260]; group 3, 16 to 26 years old; 3 doses, months 0, 2, and 6 [n = 305]) had postvaccine responses assessed 1 month after the last dose. Of 791 subjects with baseline and 7-month sera, 15 (1.9%) and 9 (1.1%) were baseline seropositive for HPV 16 and HPV 18, respectively. All baseline-seronegative vaccinees seroconverted to both HPV 16 and HPV 18. Mean anti-HPV 16 levels were similar for groups 1 and 2 (for PsV NAb, P = 0.675; for cLIA, P = 0.874), and levels for both groups 1 and 2 were approximately 2-fold higher than that for group 3 (for PsV NAb and cLIA, P < 0.001). Mean anti-HPV 18 levels were approximately 1.4-fold lower in group 1 than in group 2 (for PsV, NAb P = 0.013; for cLIA, P = 0.001), and levels for both groups 1 and 2 were approximately 2.0- to 2.5-fold higher than that for group 3 (for PsV NAb and cLIA, P < 0.001). Pearson correlation coefficients for the assays were 0.672 for HPV 16 and 0.905 for HPV 18. Most of the discordant results were observed at lower cLIA signals. These results suggest that the PsV NAb assay could be a suitable alternative to cLIA for the measurement of postvaccine antibody responses.
Human papillomavirus (HPV) vaccines induce type-specific neutralizing antibodies (NAb) which correlate with immunity (5). The quadrivalent HPV (Q-HPV) vaccine (Gardasil; Merck Laboratories), which consists of HPV 6, HPV 11, HPV 16, and HPV 18 virus-like particles (VLPs), is currently licensed for a 3-dose regimen. Postvaccination antibody responses are typically measured by a proprietary competitive Luminex immunoassay (cLIA) (Merck Laboratories) (12), which is based on competitive binding of antibodies in human sera with labeled monoclonal antibodies directed against neutralizing epitopes of the respective VLP types. The cLIA simultaneously measures antibodies to the individual HPV vaccine types.
World Health Organization guidelines recommend that assays which assess antibody neutralizing potential should be the reference standard for measuring of vaccine responses (2, 14, 16). Conventional NAb assays for HPV are not feasible because of the inability of HPV to replicate in conventional cell cultures. This has been overcome by the use of HPV pseudoviruses (PsV) containing a reporter plasmid which is expressed when the PsV infect susceptible cells (1, 10). NAb prevent PsV from infecting cells and expressing the reporter gene product. PsV NAb assays are technically complex, are not commercially available, and have not yet been standardized, but they provide an independent bioassay-based alternative to vaccine manufacturers' assays to measure vaccine responses. In this study, we compare HPV 16 and HPV 18 antibody responses at 7 months in cohorts of females enrolled in an ongoing 2- versus 3-dose Q-HPV vaccine trial (4), using both the Merck cLIA and an in-house PsV NAb assay (10).
MATERIALS AND METHODS
Study population.
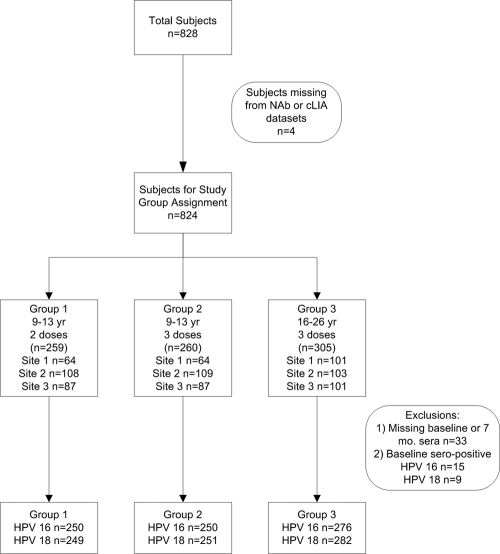
Eight hundred twenty-eight females of ages 9 to 26 years were enrolled in a 2- versus 3-dose Q-HPV vaccine trial at three sites in Canada: British Columbia, Québec, and Nova Scotia. Younger subjects (9 to 13 years old) were randomly assigned to receive 2 or 3 doses of Q-HPV vaccine (Gardasil; Merck Laboratories, Kirkland, Canada), whereas older subjects (ages 16 to 26 years) received only the standard 3-dose regimen. Sera were collected from the whole cohort at baseline, month 7, and month 24; in addition, half the cohort had serum collected at month 18, and the remaining half are to be collected at month 36. Of the 824 subjects for whom antibody levels at 7 months (i.e., 1 month after the final dose) were determined by both the PsV NAb and cLIA assays, distribution among the study arms was as follows: group 1 (9 to 13 years; mean age, 12.4 years; n = 259) received 2 doses at months 0 and 6; group 2 (9 to 13 years; mean age, 12.3 years; n = 260) received 3 doses at months 0, 2, and 6; and group 3 (16 to 26 years; mean age, 19.3 years; n = 305) received 3 doses at months 0, 2, and 6 (Fig. 1). Group 3 subjects also provided self-collected vaginal swabs (HC female swab specimen collection kit; Qiagen, Mississauga, Canada) at baseline to assess the prevalence of high-risk HPV DNA.
FIG. 1.
Distribution of subjects.
Antibody assays.
Merck cLIA testing was performed at Merck Research Laboratories as previously described (12), and antibody levels were expressed as milli-Merck units (mMU) per ml. The PsV NAb assay was performed as previously described (10). Briefly, HPV 16 and HPV 18 PsV containing a reporter plasmid encoding red fluorescent protein (RFP) were prepared and titrated in 293TT cells. Sera were serially diluted, mixed with 100 infectious units of the respective HPV PsV, and inoculated onto 293TT cells in microtiter plates. Cultures were read by fluorescence microscopy after 4 to 6 days. The endpoint was the highest dilution of serum which completely blocked expression of RFP (100% neutralization). Baseline and 7-month sera were tested in duplicate in the same assay run, and geometric mean titers (GMTs) were calculated. Subjects were considered to be NAb seropositive for the respective HPV type if the GMT was ≥40; the Merck cLIA was considered positive if the cLIA signal was ≥11 mMU for HPV 16 and ≥10 mMU for HPV 18. Testing laboratories were blinded to the dosing regimens.
HPV DNA testing.
Baseline self-collected vaginal swab specimens from 302 group 3 subjects were available for HPV DNA testing. Specimens were tested by the Roche Linear Array HPV genotyping test (LA) (Roche Diagnostics, Mississauga, Canada), which detects 37 high- and low-risk HPV types.
Statistical analysis.
Pearson correlation coefficients for the overall PsV NAb and cLIA antibody levels at 7 months were calculated. Mean log (ln) GMT and ln cLIA results at 7 months for the three study arms were compared using the Tukey-Kramer multiple-comparison method (3). Seven-month responses for baseline HPV 16- or HPV 18-seropositive versus baseline seronegative and for baseline HPV 16 or HPV 18 DNA-positive versus the respective baseline HPV 16- and HPV 18 DNA-negative subjects were compared by the Wilcoxon rank sum test (3). All statistical calculations were performed using the SAS v.9.1.3 software program (Statistical Analysis Software, Cary, NC).
Informed consent was obtained for all subjects. The study was approved by the University of British Columbia Clinical Research Ethics Board and by local research ethics boards at other sites. This Q-HPV 2-dose versus 3-dose noninferiority trial has been registered with ClinicalTrials.gov (NCT00501137).
RESULTS
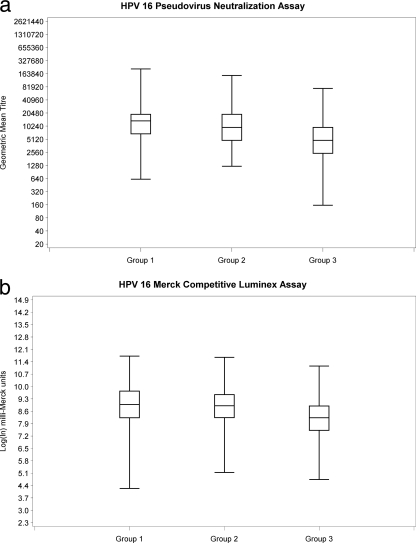
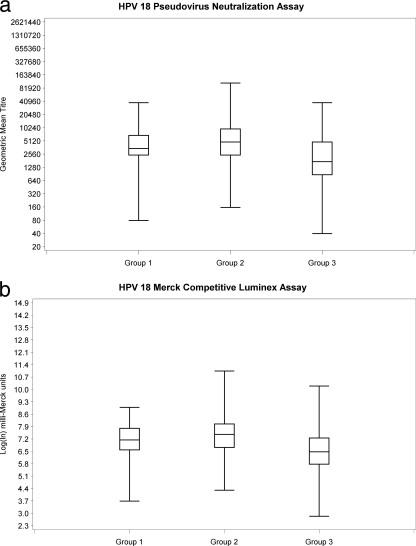
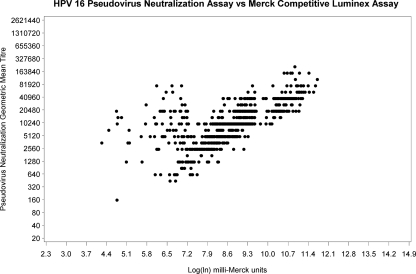
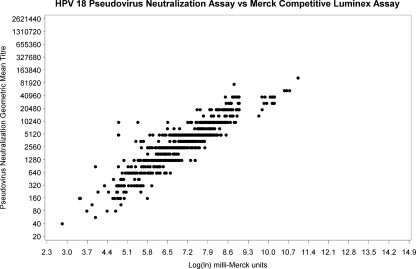
Subjects missing baseline or 7-month sera (n = 33) and subjects who were NAb or cLIA seropositive at baseline (15/791 [1.9%] for HPV 16 and 9/791 [1.1%] for HPV 18) were excluded from the 7-month response analysis (Fig. 1). At 7 months, all subjects who were seronegative at baseline seroconverted for both HPV 16 and HPV 18. NAb GMT and cLIA levels are shown in Fig. 2 a and b and 3 a and b. For 9- to 13- year-old subjects (groups 1 and 2), there was no significant difference in mean HPV 16 NAb or cLIA antibody levels between those receiving 2 doses and those receiving 3 doses (Table 1); however, for HPV 18, mean NAb and cLIA levels were significantly higher for those receiving 3 doses (P = 0.013 and P = 0.001, respectively). Older subjects (group 3; 16 to 26 years) had significantly lower mean HPV 16 and HPV 18 NAb and cLIA antibody levels at 7 months than younger subjects who received either 2 or 3 doses of vaccine (P < 0.001). Pearson correlation coefficients for the NAb and cLIA assays were 0.672 for HPV 16 and 0.905 for HPV 18 (Fig. 4 and 5). For a subset of subjects who displayed lower cLIA signals, HPV 16 NAb GMTs were higher than the respective cLIA signals; this was less frequently observed for HPV 18.
FIG. 2.
HPV 16 antibody responses at 7 months.
FIG. 3.
HPV 18 antibody responses at 7 months.
TABLE 1.
HPV 16 and HPV 18 antibody levels at 7 months, by study group
| Virus and assay (units for antibody level)a | Antibody levelb |
P valuec |
||||
|---|---|---|---|---|---|---|
| Group 1 (9-13 yr, 2 doses) | Group 2 (9-13 yr, 3 doses) | Group 3 (16-26 yr, 3 doses) | Group 1 vs group 2 | Group 1 vs group 3 | Group 2 vs group 3 | |
| HPV 16 | ||||||
| PsV NAb (ln GMT) | 9.47 (0.98) | 9.39 (1.05) | 8.67 (1.05) | 0.675 | <0.001 | <0.001 |
| cLIA (ln mMU) | 8.90 (1.39) | 8.96 (1.24) | 8.16 (1.06) | 0.874 | <0.001 | <0.001 |
| HPV 18 | ||||||
| PsV NAb (ln GMT) | 8.20 (1.06) | 8.49 (1.15) | 7.50 (1.22) | 0.013 | <0.001 | <0.001 |
| cLIA (ln mMU) | 7.09 (1.01) | 7.45 (1.15) | 6.48 (1.13) | <0.001 | <0.001 | <0.001 |
PsV NAb (ln GMT), pseudovirus neutralizing antibody, natural log; geometric mean titer; cLIA (ln mMU), competitive Luminex assay, natural log milli-Merck units.
Mean (SD).
Boldface indicates statistical significance.
FIG. 4.
HPV 16 PsV NAb assay (GMT) versus Merck cLIA (ln) correlation at 7 months (r2 = 0.672).
FIG. 5.
HPV 18 PsV NAb assay (GMT) versus Merck cLIA (ln) correlation at 7 months (r2 = 0.905).
Subjects who were seropositive at baseline demonstrated increases in median anti-HPV 16 and anti-HPV 18 levels at 7 months compared to baseline levels. There was no significant difference in the antibody response (NAb and cLIA) for baseline seropositive versus baseline seronegative subjects (Table 2).
TABLE 2.
HPV 16 and HPV 18 antibody levels at 7 months, by baseline HPV serologic result
| Baseline serologic result | Antibody levela |
|
|---|---|---|
| PsV NAb (ln GMT) | cLIA (ln mMU) | |
| Anti-HPV 16b | ||
| Negative | 9.23 (8.54; 9.93) | 8.64 (7.92; 9.35) |
| Positive | 8.54 (8.19; 9.23) | 8.53 (8.25; 8.73) |
| P value | 0.066 | 0.376 |
| Anti-HPV 18c | ||
| Negative | 8.19 (7.15; 8.89) | 7.03 (6.24; 7.76) |
| Positive | 8.19 (7.85; 8.54) | 6.86 (5.45; 7.24) |
| P value | 0.821 | 0.177 |
Antibody level is expressed as median value (quartile 1; quartile 3). PsV NAb (ln GMT), pseudovirus neutralizing antibody, natural log geometric mean titer; cLIA (ln mMU), competitive Luminex assay, natural log milli-Merck units.
For negative serologic result at baseline, n = 776 subjects; for positive result, n = 15 subjects.
For negative serologic result at baseline, n = 782 subjects; for positive result, n = 9 subjects.
Baseline HPV DNA results from self-collected vaginal swab samples were available for 302/305 group 3 subjects. Of these, 218 (72.2%) were HPV negative; 18 (6.0%) were HPV 16 positive, 3 (1.0%) were HPV 18 positive, 3 (1.0%) were both HPV 16 and HPV 18 positive, 38 (12.6%) were positive for other high-risk HPV (non-HPV 16 or -HPV 18), and 22 (7.3%) were positive for low-risk HPV only. Among the HPV 16 and HPV 18 baseline seropositive subjects, 4/15 and 1/9, respectively, also had detectable corresponding HPV 16 and HPV 18 DNA on vaginal self-collection. Median anti-HPV 16 levels at 7 months for HPV 16 DNA-positive versus DNA-negative subjects were not significantly different (Table 3). For HPV 18 DNA-positive vaccinees, median anti-HPV 18 levels were higher at 7 months than those for HPV 18 DNA-negative vaccinees, but this was not statistically significant.
TABLE 3.
Group 3 antibody levels at 7 months by baseline HPV DNA result
| Baseline DNA result | Antibody levela |
|
|---|---|---|
| PsV NAb (ln GMT) | cLIA (ln mMU) | |
| HPV 16b | ||
| Negative | 7.85 (5.08; 8.54) | 8.24 (7.53; 8.83) |
| Positive | 8.19 (7.15; 8.54) | 8.50 (7.96; 9.03) |
| P value | 0.733 | 0.125 |
| HPV 18c | ||
| Negative | 7.50 (6.81; 8.54) | 6.47 (5.72; 7.24) |
| Positive | 8.89 (8.19; 8.89) | 7.59 (7.26; 7.65) |
| P value | 0.051 | 0.061 |
Antibody level is expressed as median value (quartile 1; quartile 3). PsV NAb (ln GMT), pseudovirus neutralizing antibody, natural log geometric mean titer; cLIA (ln mMU), competitive Luminex assay, natural log milli-Merck units.
For negative result, n = 270 subjects; for positive result, n = 19 subjects.
For negative result, n = 286 subjects; for positive result, n = 3 subjects.
DISCUSSION
The 7-month anti-HPV 16 and -HPV 18 responses with the PsV NAb and cLIA assays were compared as part of this ongoing Q-HPV vaccine dosing study. Good correlation was observed between NAb and cLIA, but there was closer correlation for anti-HPV 18 than for anti-HPV 16. Most discordant results occurred for sera with lower cLIA signals, and a subset of vaccinees with low cLIA signals displayed high NAb GMTs. This suggests that the PsV NAb assay may identify potential neutralizing epitopes not detected by the cLIA (8, 14). Neutralization can result from antibody directly binding viral neutralizing epitopes or from antibody binding other epitopes which might sterically prevent binding of virus to the target cell. These findings are consistent with those of Dessy et al. (2), who showed that an HPV direct enzyme-linked immunosorbent assay (ELISA) based on multiple epitopes had a higher sensitivity and correlated better with PsV NAb than a single-epitope-based inhibition ELISA. Furthermore, a new immunoassay developed by Merck Research Laboratories (13) demonstrated an improvement in analytical sensitivity over the cLIA, reportedly due to its ability to measure antibodies that bind other sites on the VLP rather than a single neutralizing epitope. The differences we observed might also be explained by the different HPV capsids used in the cLIA and PsV NAb assays. Our NAb assay utilized PsV generated in cells transfected by plasmids obtained from the National Institutes of Health which consist of both the L1 and L2 proteins and which may more closely resemble natural HPV virions (6). The HPV VLPs used in the cLIA are concordant with those in the Gardasil vaccine and contain only the L1 protein. In addition, there are small sequence variations between the Merck VLPs and NIH PsV (Alfred Saah, Merck Research Laboratories, personal communication), which might also be an explanation for differences in the detected serological responses.
Our observation that older (group 3) subjects had approximately 2.0- to 2.5-fold-lower antibody responses for both HPV 16 and HPV 18 at 7 months than younger individuals (groups 1 and 2) is consistent with what has been reported (2, 7). For younger individuals, anti-HPV 16 responses at 7 months were similar for 2 versus 3 doses; however, for anti-HPV 18, 3 doses resulted in approximately 1.4-fold-higher antibody levels. Other vaccine trials have demonstrated higher anti-HPV 16 than anti-HPV 18 cLIA responses following use of the Gardasil vaccine (7, 15), whereas Kemp et al. (9) demonstrated similar postvaccination titers for both HPV 16 and HPV 18 using the Cervarix vaccine. The only head-to-head comparison of these two vaccines published to date (6) demonstrated that anti-HPV 16 levels were higher than anti-HPV 18 levels for both vaccines. This may be a function of the type-specific VLP immunogenicity or the assay methodology used to measure antibody responses. Given the lack of assay standardization and reports that the cLIA is known to underestimate the total antibody response to VLPs (13), caution should be exercised in interpreting quantitative differences between assays (15).
Individuals who were seropositive at baseline for either HPV 16 or HPV 18 demonstrated boosting of antibodies to levels similar to levels for those who were seronegative at baseline. This is consistent with the findings of Ngan et al. using the Cervarix vaccine (11). In contrast, Giuliano et al. (7) and Villa et al. (15) reported that individuals who were seropositive at baseline to a Gardasil vaccine type demonstrated significantly higher anti-HPV responses than those who were seronegative at baseline. Since the number of baseline seropositive subjects in our study was small, our data lack the statistical power to demonstrate a difference.
Group 3 individuals with HPV 16 infection at baseline (i.e., HPV 16 DNA positive) also demonstrated antibody levels at 7 months after vaccination which were similar to those for vaccinees without detectable HPV 16 DNA at baseline; however, anti-HPV 18 responses at 7 months were higher for baseline HPV 18 DNA-positive subjects, but the difference was not statistically significant. Giuliano et al. (7) showed that antibody responses were similar for baseline HPV DNA-negative and -positive subjects except when baseline DNA-positive subjects were also seropositive at baseline. Villa et al. (15) also demonstrated that baseline HPV DNA-positive, seronegative subjects had postvaccine antibody responses similar to those of subjects who were naive to the relevant HPV type at enrollment. Opalka et al. (13) reported that baseline HPV DNA-positive subjects generally had higher titers at 48 months than subjects who were HPV DNA negative at day zero or month seven. Further studies using larger data sets and longer follow-up periods would be required to better understand how past infection and/or repeated natural exposures to HPV might affect the type-specific antibody response in vaccinees.
Our study has some limitations. A single batch of HPV 16 and HPV 18 PsV was used for the NAb assays, so we are unable to evaluate potential variability in GMTs between different PsV lots. NAb GMT determination using PsV containing the RFP reporter plasmid is subjective, while readings from the cLIA luminometer are objective. However, validation studies with our PsV NAb assay demonstrated excellent inter- and intra-assay reproducibility (data not shown).
In conclusion, this study demonstrated a high concordance between HPV antibody levels measured by the PsV NAb assay and those measured by the Merck cLIA. Among all of the study groups, type-specific PsV GMTs were slightly higher than levels measured by the cLIA. This suggests that PsV-based NAb assays are more sensitive and are able to detect a broader array of HPV type-specific NAb. Further studies will be required to elucidate the observed discordance between PsV NAb and cLIA antibody levels.
Although the results of this study demonstrate that the 2-dose Q-HPV vaccine regimen elicits a good immune response in young subjects, this analysis was focused on interassay comparison and was not intended to assess the noninferiority of the 2-dose regimen. Only month seven serological results were available for analysis, and a key challenge relating to HPV vaccination is to understand the long-term durability of HPV type-specific antibody responses. Such data will require longer-term follow-up of our and other study cohorts.
Acknowledgments
This work was supported by a grant from the Michael Smith Foundation for Health Research (PJ-HPV-002078).
We thank S. Pang and C. Buck (National Institutes of Health, Bethesda, MD) for providing HPV and reporter protein plasmids, 293TT cells, rabbit antisera, and technical advice. We acknowledge the support of Merck Research Laboratories for performing the cLIA assessments. This work was performed at the British Columbia Centre for Disease Control and Merck Research Laboratories.
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 119:445-462. [DOI] [PubMed] [Google Scholar]
- 2.Dessy, F. J., et al. 2008. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the ASO4-adjuvanted HPV-16/18 cervical cancer vaccine. Hum. Vaccin. 4:425-434. [DOI] [PubMed] [Google Scholar]
- 3.Devore, J. L. 1995. Probability and statistics for engineering and the sciences, 4th Ed. Duxbury Press, Belmont, CA.
- 4.Dobson, S., et al. 25 October 2010. A two-dose HPV vaccine schedule in girls: immunogenicity at 24 months, abstr. P-690. Twenty-sixth International Papillomavirus Conference, Montreal, Canada. https://iseventsolutions.com/∼hpv2010/main/index.php?option=com_conference&view=presentation&id=1883&conference=1&Itemid=100.
- 5.Einstein, M. 2008. Acquired immune response to oncogenic human papillomavirus associated with prophylactic cervical cancer vaccines. Cancer Immunol. Immunother. 57:443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Einstein, M., et al. 2009. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum. Vaccin. 5:705-719. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano, A. R., et al. 2007. Impact of baseline covariates on the immunogenicity of a quadrivalent (types 6, 11, 16, and 18) human papillomavirus virus-like-particle vaccine. J. Infect. Dis. 196:1153-1162. [DOI] [PubMed] [Google Scholar]
- 8.Joura, E. A., et al. 2008. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine 26:6844-6851. [DOI] [PubMed] [Google Scholar]
- 9.Kemp, T. J., et al. 2008. Evaluation of systemic and mucosal anti-HPV 16 and anti-HPV 18 antibody responses from vaccinated women. Vaccine 26:3608-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krajden, M., et al. 2009. Prevalence of human papillomavirus 16 and 18 neutralizing antibodies in prenatal women in British Columbia. Clin. Vaccine Immunol. 16:1840-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngan, H. Y., et al. 2010. Human papillomavirus-16/18 ASO4-adjuvanted cervical cancer vaccine: immunogenicity and safety in healthy Chinese women from Hong Kong. Hong Kong Med. J. 16:171-179. [PubMed] [Google Scholar]
- 12.Opalka, D., et al. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed Luminex assay. Clin. Diagn. Lab. Immunol. 10:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opalka, D., et al. 2010. Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin. Vaccine Immunol. 17:818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiller, J. T., and D. R. Lowy. 2009. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J. Infect. Dis. 200:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villa, L. L., et al. 2006. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 24:5571-5583. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. 2006. Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus-like particle vaccines. World Health Organization, Geneva, Switzerland. http://www.who.int/biologicals/publications/trs/areas/vaccines/human_papillomavirus/HPVg%20Final%20BS%202050%20.pdf.