Abstract
Staphylococcus aureus superantigens (SAgs) are highly potent T cell mitogens. Antibodies against non-enterotoxin gene cluster (non-egc) SAgs are common in healthy adults, whereas neutralizing antibodies against egc SAgs are rare. We investigated the infecting S. aureus strains and the anti-SAg antibody response during S. aureus bacteremia (SAB). This prospective clinical study (www.clinicaltrials.gov, NCT00548002) included 43 injection drug users (IDUs) and 44 group-matched nonaddicts with SAB. spa genotypes and SAg gene patterns (multiplex PCR) of the S. aureus isolates were determined. The neutralizing capacities of sera obtained at the acute phase and the convalescent phase of SAB were tested against the SAg cocktail of the respective infecting strain and a panel of recombinant SAgs. The lineages CC59 and CC30 were more prevalent among bacteremia strains from IDUs than among strains from nonaddicts. SAg gene patterns in isolates from IDUs and nonaddicts were similar. At the acute phase of bacteremia, IDUs had more neutralizing antibodies against non-egc SAgs than did nonaddicts. Antibody titers frequently increased during infection. In contrast, there were no neutralizing antibodies against egc SAgs at disease onset and such antibodies were not induced by SAB. SAB triggers an antibody response only against non-egc SAgs. Preimmunization in IDU patients is probably due to previous exposure to the infecting strain.
Staphylococcus aureus is a major human pathogen that causes a wide spectrum of infections, such as toxin-mediated diseases and systemic infections, for instance, bacteremia and endocarditis. At the same time, S. aureus is a commensal that colonizes approximately 35% of the healthy population in the nose (46, 48).
Among the numerous toxins of S. aureus are the 21 known staphylococcal superantigens (SAgs): the toxic shock syndrome toxin (TSST-1), the staphylococcal enterotoxins (SEA to SEE and SEG to SEJ), and the staphylococcal enterotoxin-like toxins (SElK to SElU) (13, 24, 33, 35, 38). They are encoded on mobile genetic elements, like phages and pathogenicity islands (25). SAgs are the causative agents of food poisoning and toxic shock syndrome, but their role in bacteremia is not well defined (13, 28). They can activate a large fraction of T lymphocytes by directly cross-linking certain T cell receptor Vβ domains with conserved structures on major histocompatibility complex class II (MHC II) molecules. This results in a polyclonal T cell activation and massive cytokine release.
The more recently described enterotoxin gene cluster (egc) harbors five or six SAg genes (seg, sei, selm, seln, selo, and sometimes selu), which cluster on a staphylococcal pathogenicity island (νSaβ) (17, 23). In contrast to the non-egc SAgs, the egc is organized as an operon, and its genes are transcribed into a polycistronic mRNA (17). The egc genes are the most prevalent SAg genes in commensal and invasive S. aureus isolates, with frequencies ranging between 52 and 66% (4, 9, 14).
We previously reported that SAg genes are not randomly distributed but rather strongly associated with the clonal lineages (14). Thus, each lineage is characterized by a typical SAg gene profile. However, within each lineage, most SAg genes are mobile (except for egc SAgs). Therefore, several SAg genotypes can occur within one clonal complex (CC).
In addition to their superantigenicity, SAgs, like other proteins, also act as conventional antigens and induce a specific antibody response. Antibodies against non-egc SAgs (e.g., TSST-1, SEA, SEB, and SEC) are common in the healthy population (12, 21, 39, 41). In S. aureus carriers, these antibodies are highly specific for the SAgs of the colonizing strain and they effectively neutralize their mitogenic effects (15). Surprisingly, neutralizing antibodies against egc SAgs are very rare, even among carriers of egc-positive S. aureus strains (reference 12 and unpublished observations). This “egc gap” in the antibody response of healthy individuals was unexpected because of the high prevalence of egc SAg genes in clinical S. aureus isolates (4, 9, 14). A comparison of recombinant egc and non-egc SAgs revealed that they do not differ in any of the studied aspects of T cell activation, including gene regulation, cytokine secretion, or induction of T cell proliferation (10). Remarkably, egc SAgs are secreted by S. aureus during exponential growth in vitro, whereas non-egc SAgs—like most virulence factors—are expressed during stationary growth (10, 31).
The aim of our study was to determine SAg gene patterns of S. aureus bacteremia (SAB) strains and to test whether the differentially regulated egc SAgs and non-egc SAgs elicit an antibody response during systemic infection. In particular, we wanted to investigate the role of egc SAgs in SAB among nonaddicts previously less exposed to S. aureus and among injection drug users (IDUs) with more frequent contact with it.
In a prospective clinical study, we (i) determined the genotype and SAg gene patterns in bacteremia isolates from IDUs and matched nonaddicts and (ii) compared the SAg-neutralizing capacities of sera obtained at the acute phase of bacteremia and in the convalescent phase.
MATERIALS AND METHODS
Patient population.
We prospectively collected 430 adult patients with blood cultures positive for methicillin-sensitive S. aureus (MSSA). Twelve university or central hospitals in Finland participated in this study between January 1999 and May 1999 and between January 2000 and August 2002 (36). In this study, 43 IDUs and 44 group-matched nonaddicts as controls were included (Table 1) (37). For each IDU with endocarditis (n = 19), we chose a nonaddict with preferably definite endocarditis (n = 20; 16 definite and 4 possible cases). For each IDU without endocarditis (n = 24), we chose an age (±15 years)- and sex-matched nonaddict, whose randomization time was the nearest possible.
TABLE 1.
Characteristics of IDUs and nonaddicts with methicillin-sensitive Staphylococcus aureus bacteremia (n = 87)
| Characteristic | No. (%) of patients |
|
|---|---|---|
| Injection drug users (n = 43) | Nonaddicts (n = 44) | |
| Age (yr, mean ± SD) | 29 ± 8 | 50 ± 19 |
| Male sex | 32 (74.4) | 28 (63.6) |
| Previous S. aureus infectiona | 7 (16) | 8 (18.2) |
| Underlying disease | ||
| Liver disease | 36 (83.7) | 5 (11.4) |
| HIV infection | 6 (13.9) | 0 (0.0) |
| Diabetes | 3 (7.0) | 12 (27.3) |
| Coronary artery diseases | 0 (0.0) | 8 (18.2) |
| Chronic renal failure | 0 (0.0) | 6 (13.6) |
| Malignancy | 0 (0.0) | 4 (9.1) |
| McCabe's classificationb | ||
| Healthy or nonfatal disease | 43 (100) | 33 (75.0) |
| Ultimately or rapidly fatal disease | 0 (0) | 11 (25.0) |
| Endocarditisc | 19 (44.2) | 20 (45.5) |
| Outcome: death within 3 mo | 2 (4.7) | 4 (9.1) |
Superficial S. aureus infections or S. aureus bacteremia (only IDUs).
Prognosis or severity of underlying diseases classified according to the criteria of McCabe and Jackson.
Classified as definite or possible by using the modified Duke criteria.
Written informed consent was obtained from all patients or their representatives. The study was approved by the ethics committees of all study sites and was conducted in accordance with the Declaration of Helsinki.
S. aureus identification and DNA isolation.
Routine bacteriological methods were used to detect S. aureus grown in blood (36). Total S. aureus DNA was isolated with the Promega Wizard DNA purification kit (Promega, Mannheim, Germany) according to the manufacturer's instructions.
Serum samples.
After the first positive culture for S. aureus, serum samples were collected at days 2 to 7 (acute phase) and at days 22 to 28 (convalescent phase) (37). Sera were stored at −20°C for further analysis. Both samples were available from 27 of 43 IDUs and from 37 of 44 nonaddicts. In the other 16 IDUs and 7 nonaddicts, one or both serum samples were missing.
spa genotyping.
PCR for amplification of the S. aureus protein A (spa) repeat region was performed according to the published protocols (1, 11). PCR products were purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany) and sequenced using both amplification primers by a commercial supplier (MWG Biotech, Ebersberg, Germany). The forward and reverse sequence chromatograms were analyzed with the Ridom StaphType software, version 1.99.11 (Ridom GmbH, Würzburg, Germany). With the BURP algorithm (Ridom GmbH), spa types were clustered into different groups (the parameter “calculated cost between members of a group” was set at ≤5). spa types shorter than five repeats were not grouped, because they do not allow the reliable deduction of ancestries. Since spa typing and multilocus sequence typing (MLST) are highly concordant (40), spa typing data could be easily mapped on MLST types by using the SpaServer database (www.spaserver.ridom.de).
Virulence gene detection by PCR.
PCR was used to screen for a total of 25 virulence genes. Single and multiplex PCRs were applied for the detection of genes for gyrase (gyr), methicillin resistance (mecA), Panton-Valentine leukocidin (PVL), staphylococcal enterotoxins (sea to selu), toxic shock syndrome toxin 1 (tst), and exfoliative toxins (eta and etd) and agr groups 1 to 4 as previously reported (14).
Neutralization assay.
Neutralization assays were performed as described before (12, 15, 47). Initially, the concentrations of bacterial supernatants and recombinant SEB, SEC, SElQ, TSST-1, and SEI which elicited submaximal proliferation were determined in T cell proliferation assays (between 1 and 100 pg/ml). The bacterial supernatants were collected at the stationary growth phase from cultures of the infecting S. aureus isolates grown in tryptone soy broth medium. The recombinant SAgs were produced in Escherichia coli and were efficiently lipopolysaccharide (LPS) depleted as described before (10). T cell proliferation assays were performed in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) using peripheral blood mononuclear cells (PBMCs) from three different, healthy blood donors. Differences in the proliferative responses between the PBMC donors concerned mainly the numbers of responding cells (cpm values) and not their response curves.
Afterwards, four sets of neutralization assays were performed with PBMCs from different donors. The appropriate concentrations of supernatant or recombinant SAgs were incubated in the presence of heat-inactivated patient sera serially diluted in RPMI 1640-10% FBS. For control, supernatants or recombinant SAgs were incubated without human serum and titrated over a broad range. After 20 min, 105 PBMCs were added to measure mitogenic potency and neutralizing serum capacity. T cell proliferation was determined by the incorporation of [3H]thymidine after 72 h, quantified by calculating the area under the proliferation curve (AUC), and expressed as a percentage of the control without human serum. All measurements were performed in triplicate. Two IDU patients (T-29359 and T-37698) were excluded because their invasive S. aureus isolate was SAg negative.
Statistical analysis.
Differences in the spa genotype and virulence gene patterns between IDUs and nonaddicts were assessed using the chi-square test. The Mann-Whitney test was used to compare the neutralizing capacities of sera from IDUs and nonaddicts. The neutralizing capacities of the acute- and convalescent-phase serum samples from the bacteremic patients were compared with the paired t test. P values of ≤0.05 were considered statistically significant.
RESULTS
Genotypes of SAB isolates from IDUs and nonaddicts.
Among the SAB isolates from 43 IDUs and 44 nonaddicts we found 46 different spa types, which were assigned to 12 CCs (CC1, -5, -8, -9, -12, -15, -20, -22, -25, -30, -45, and -59). Moreover, we observed six singletons. Eight isolates could not be clustered by BURP analysis, because clustering parameters excluded spa types shorter than five repeats, and two strains were nontypeable by spa PCR.
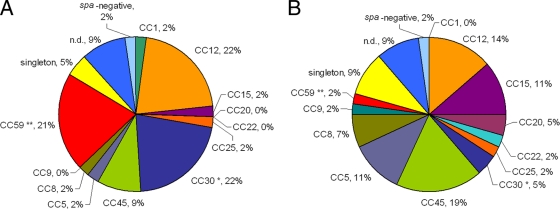
The genetic diversities of SAB strain collections from IDUs and nonaddicts were similar, but CC59 and CC30 were significantly overrepresented among isolates from IDUs in comparison to those from nonaddicts (CC59, 20.9% versus 2.3%, P ≤ 0.01, and CC30, 20.9% versus 4.5%, P ≤ 0.05, respectively) (Fig. 1). No CC or spa type was associated with endocarditis.
FIG. 1.
Clonal distribution of SAB isolates from IDUs (A) and nonaddicts (B). spa types were clustered into 12 CCs by BURP analysis. MLST-CC nomenclature was deduced from spa-CCs using the Ridom SpaServer database. CC59 and CC30 were overrepresented among SAB isolates from IDUs. Significance was determined by the chi-square test (*, P ≤ 0.05; **, P ≤ 0.01). n.d., excluded.
Virulence gene repertoire of SAB isolates from IDUs and nonaddicts.
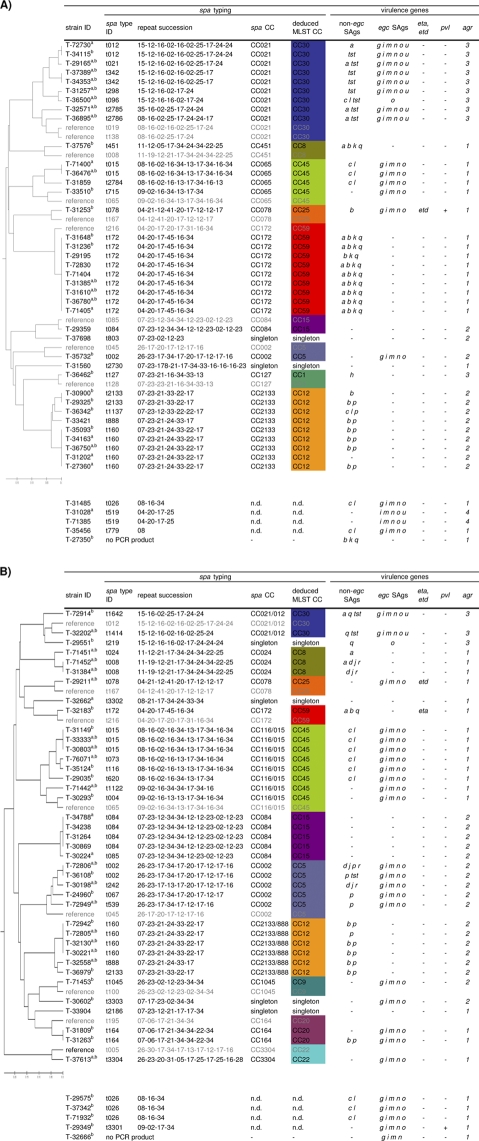
To test whether SAB isolates from IDUs and nonaddicts differ in their virulence gene patterns, we next determined the accessory gene regulator (agr) type and SAg, Panton-Valentine leukocidin (PVL), and exfoliative toxin genes. agr is a global regulator of virulence gene expression, and four different agr subgroups, agr 1 to 4, are known. In agreement with previous studies (14, 16, 30, 34, 49), we observed a strict linkage of agr subgroups with the spa-derived lineages (Fig. 2). PVL and exfoliative toxin genes (eta and etd) occurred only rarely.
FIG. 2.
Distribution of virulence genes within spa-defined CCs among SAB isolates from IDUs (A) and nonaddicts (B). For construction of the phylogenetic tree, several reference strains were included in the BURP clustering (shaded in gray). Virulence genes (SAg genes, agr, eta, etd, mecA, and pvl genes) were determined by multiplex PCR. SAB strains from IDUs and nonaddicts did not differ in their virulence gene patterns. Staphylococcal enterotoxins (SEs) are indicated by single letters (a = sea, etc.). tst, toxic shock syndrome toxin; egc, enterotoxin gene cluster; eta and etd, exfoliative toxins a and d; agr, accessory gene regulator; pvl, Panton-Valentine leukocidin (lukPV). a, patients with infective endocarditis; b, serum analyzed in neutralization assays. n.d., excluded.
Multiplex PCR was applied to detect 19 SAg genes. SAg genes were highly prevalent among SAB isolates from both collections (IDUs, 90.7%; nonaddicts, 84.1%), and SAg gene patterns differed remarkably. As previously reported, SAg genes were linked to staphylococcal lineages (14).
The egc SAg genes were by far the most prevalent (IDUs, 44.2%; nonaddicts, 61.4%). seb and sea were overrepresented among IDU isolates (seb, 44.2% versus 15.9%, P ≤ 0.01; sea, 30.2% versus 9.1%, P ≤ 0.05), but this was due to the high prevalence of sea/seb-positive CC59 and sea-positive CC30 isolates among IDU strains. This emphasizes the importance of a simultaneous analysis of virulence genes and genetic background. The comparison of SAg patterns within certain CCs revealed no major differences between isolates from IDUs and those from nonaddicts. Furthermore, we found no association of SAg genes with endocarditis.
Neutralizing serum antibodies in IDUs and nonaddicts.
To test whether egc SAgs elicit an antibody response during infection, we analyzed the neutralizing antibody responses of SAB patients (i) against the supernatant of their infecting strain and (ii) against representative recombinant SAgs. Supernatants were obtained from S. aureus cultures in stationary phase and contain both egc SAgs, which are expressed at exponential growth phase and remain in the culture, and non-egc SAgs, which are typically secreted in stationary phase.
At the onset of bacteremia, many patients already possessed neutralizing serum antibodies against the SAg cocktail produced by their infecting strain (Fig. 3 and 4 A). While this neutralizing capacity was mostly low in nonaddicts, IDUs already showed high antibody titers at the acute phase. This suggests that they were preimmunized with the SAgs of their infecting strain.
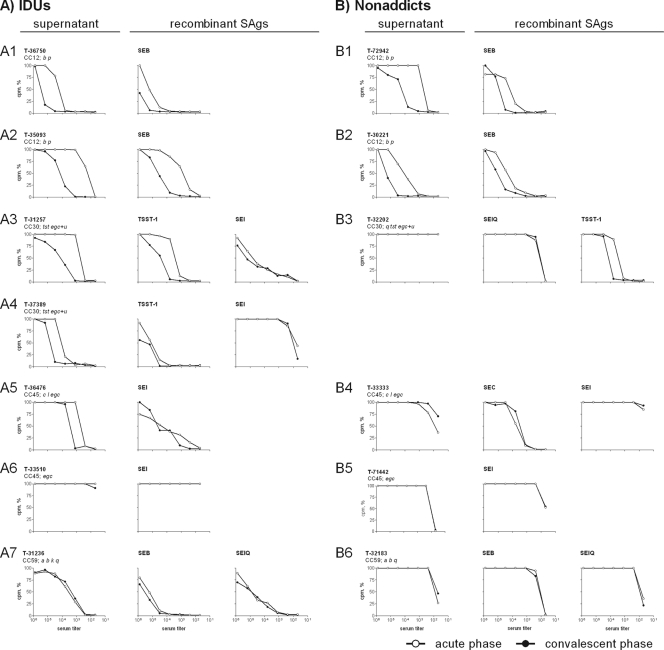
FIG. 3.
SAg-neutralizing capacities of selected SAB sera from IDUs (A) and nonaddicts (B). The neutralizing capacities of SAB sera (acute phase and convalescent phase) against (i) the supernatant from the infecting strain and (ii) representative recombinant SAgs were determined. Therefore, S. aureus culture supernatants or recombinant SAgs were incubated with serial dilutions of SAB sera. After 20 min, 105 PBMCs were added and T cell proliferation was measured after 72 h by [3H]thymidine incorporation. The graph depicts the SAg-induced proliferation in the presence of serum, expressed as percentages of the control without human serum. In contrast to nonaddicts, most IDUs had neutralizing antibodies already at the acute phase of SAB and antibody titers increased more frequently. In general, neutralizing antibodies against egc SAgs were absent and were not induced. Representative data sets are depicted (IDUs, 7/25; nonaddicts, 6/37).
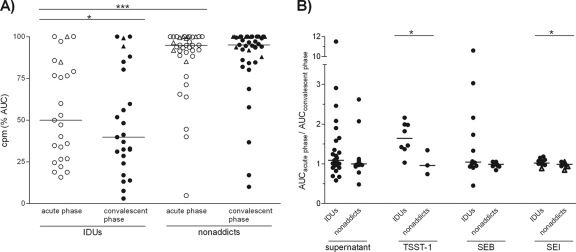
FIG. 4.
SAg-neutralizing capacities of SAB sera from IDUs and nonaddicts. S. aureus culture supernatants or recombinant SAgs were incubated with serial dilutions of SAB sera (acute phase and convalescent phase). After 20 min, 105 PBMCs were added and T cell proliferation was measured after 72 h by [3H]thymidine incorporation. (A) The neutralizing capacities of SAB sera against the supernatant of the infecting strain. SAg-induced proliferation in the presence of serum, quantified by calculating the area under the proliferation curve (AUC) and expressed as percentages of the control without human serum, for all data sets (IDUs, 25; nonaddicts, 37). In contrast to nonaddicts, most IDUs showed an effective antibody response already at the onset of bacteremia. (B) Fold changes of the neutralizing capacities of SAB sera (acute phase and convalescent phase) against the S. aureus culture supernatants and representative recombinant SAgs. The ratio of the AUCs from both serum samples of one patient was calculated. IDUs frequently showed an increase in neutralizing antibody titers against the SAg cocktail and recombinant non-egc SAgs. In general, neutralizing capacity against egc SAgs (triangles indicate SAB isolates which were only egc positive) was very low or absent and not triggered during bloodstream invasion. P values were calculated using the paired t test (acute phase versus convalescent phase) or the Mann-Whitney test (IDUs versus nonaddicts); median values are indicated (*, P ≤ 0.05; ***, P ≤ 0.001).
In several cases, we observed a rise in antibody concentrations during SAB, again especially among IDUs (Fig. 3 and 4). In some individuals, titers increased more than 100-fold (T-30900 and T-35093 [Fig. 3, panel A2]). However, this was different in patients infected with CC59 strains. These S. aureus isolates harbored a number of non-egc SAgs (sea, seb, sek, and selq), and their supernatants were strongly mitogenic. IDUs infected with these strains had neutralizing antibodies at diagnosis of SAB, but serum concentrations did not further increase thereafter.
The SAg cocktails in the S. aureus culture supernatants may be close to the clinical situation, but their SAg composition is not known. For molecular definition, we complemented analysis by neutralization assays with recombinant SAgs (TSST-1, SEB, SEC, SElQ, and SEI), which in most patients confirmed the results obtained with bacterial supernatants. SEI served as a representative for the coexpressed egc SAgs. Sera from six patients neutralized recombinant SAgs but not the bacterial supernatants (e.g., Fig. 3, panel B3). The neutralizing effect against individual SAgs was probably obscured by the mitogenic effects of others that were also present in the supernatant.
Notably, neutralizing antibodies against egc SAgs were rare exceptions at the acute phase of SAB, and such antibodies were not induced during bacteremia. The findings were similar for supernatants from egc-positive strains (Fig. 3, panels A6 and B5; Fig. 4) and for recombinant SEI (Fig. 3, panels A4, A6, B4, and B5; Fig. 4B). High titers of anti-SEI antibodies were present in only 2 of the 62 tested patient sera (T-31257 and T-36476, Fig. 3, panels A3 and A5). Both patients were IDUs, and their anti-SEI antibody serum concentrations did not increase during SAB.
DISCUSSION
Neutralizing antibodies against non-egc SAgs are common in healthy adults (21, 39, 41), whereas neutralizing antibodies against egc SAgs are very rare (12). Remarkably, the regulation of SAg release differs fundamentally between the two groups of SAgs (10, 31). These findings raised the question of how this differential regulation of egc and non-egc SAgs affects the anti-SAg antibody response during SAB. Comparison of SAB isolates from IDUs and nonaddicts revealed a high prevalence of CC59 and CC30 strains among IDUs but no differences in SAg gene patterns. We observed a boost of neutralizing antibody titers against non-egc SAgs during bloodstream infection. In contrast, egc SAgs (supernatants and recombinant SEI) did not elicit a boost or de novo generation of antibodies.
SAB strains from IDUs and nonaddicts had a highly diverse population structure. In total, 12 different staphylococcal lineages and some singletons were observed. The variability of genotypes appeared to be lower among addicts, where the two lineages CC30 and CC59 accounted for over 40% of all strains. While the prevalence of CC30 among IDU isolates was in the same range as that reported for S. aureus colonization (16 to 27%) (6, 8, 14, 29), CC59 strains are rare in healthy European carriers. Prevalences range from 0% to 4.5% in nasal isolates from the United Kingdom, Poland, Netherlands, and Germany (6, 8, 14, 27, 29). However, in IDUs from the United Kingdom, methicillin-sensitive S. aureus strains isolated from abscesses and soft-tissue infections frequently belonged to CC59 (30), indicating that this lineage spreads in the internationally connected community of IDUs. We found no correlation of certain clonal lineages or SAg gene patterns with endocarditis.
We previously reported that every S. aureus clonal lineage is characterized by a consensus repertoire of agr subgroup and virulence genes (14). The SAB isolates in the present study fit well into this picture. In some CCs, the frequencies of the typical SAg genotypes differed between Finland and Germany (14).
Neutralizing antibodies against non-egc SAgs are frequent in the healthy population (12, 21, 39, 41), and in the present study, this was also found to be the case in SAB patients at the acute phase of bacteremia. However, neutralizing antibody titers were low in most nonaddicts. A possible explanation would be that the majority of them suffered from exogenous infections (15). Unfortunately, the carrier status of our patient cohort is not known. The SAg-neutralizing capacity was much higher in IDUs, suggestive of intensive exposure to S. aureus, probably even to the invasive strain. Repeated bacterial inoculation could be the reason for this, because frequent injections, contaminated drugs, sharing of drug use equipment, and poor hygiene usually characterize the living conditions of IDUs (2, 26). In agreement with this, studies from the 1970s showed higher colonization rates and more frequent endogenous infections in IDUs than in the general population (43, 44).
In IDUs but not in nonaddicts, bacteremia increased the neutralizing serum capacity, extending earlier reports about staphylococcal infections (e.g., bacteremia and wound infection) (18, 20). While other groups measured antibody binding, we employed a functional assay, because it has been shown that SAg-neutralizing antibodies can be protective in patients as well as in animal models (3, 22, 32, 45). The high titers and rapid increases of SAg-neutralizing antibodies are indicative of a vigorous antibody response to many S. aureus antigens in IDUs (37). In addition to other factors, such as younger age, lack of preexisting heart disease, and the predominance of right-sided heart involvement (5, 7, 19), an efficient and protective antibody response might contribute to the more favorable outcome of S. aureus endocarditis in IDUs than in the general population. Only a few nonaddicts responded to bacteremia with an increase of SAg-neutralizing antibodies. It appears that bacteremia rarely primes high-affinity antibody responses, which would be required for SAg neutralization, but it seems that this immune stimulus is strong enough to boost preexisting B cell memory.
Overall, the boost of neutralizing antibody titers clearly shows that the immune system is exposed to non-egc SAgs during S. aureus bloodstream invasion. This remains open for egc-encoded SAgs, which did not elicit a boost or de novo generation of specific antibodies, neither against supernatants of egc-positive strains nor against recombinant SEI. As we demonstrated previously, the immune cell-activating properties of egc and non-egc SAgs are very similar (induction of T cell proliferation, cytokine secretion, and gene regulation) and cannot explain the striking differences in the immune response to egc and non-egc SAgs (10). Because the amino acid sequences of the egc SAgs are more closely related to those of individual non-egc SAgs than to each other (17, 42), it also appears unlikely that the two groups of SAgs differ systematically in their immunogenicities. Remarkably, egc SAgs are secreted during exponential growth in vitro, whereas non-egc SAgs—like most virulence factors—are expressed in stationary growth (10, 31). It remains to be shown whether SAgs are also differentially regulated in vivo and whether this might explain the egc gap in S. aureus carriers and noncarriers (12), which is not closed following bloodstream invasion.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (GRK840, “Host-Pathogen Interactions in Generalized Bacterial Infections,” SFB-TR34) and the Alfried Krupp Wissenschaftskolleg Greifswald (“A Functional Genomics Approach to Infection Biology”).
We thank the FINTROVA and FINLEVO study group investigators for their invaluable participation in patient collection.
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1.Aires-de-Sousa, M., et al. 2006. High interlaboratory reproducibility of DNA sequence-based typing of bacteria in a multicenter study. J. Clin. Microbiol. 44:619-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassetti, S., and M. Battegay. 2004. Staphylococcus aureus infections in injection drug users: risk factors and prevention strategies. Infection 32:163-169. [DOI] [PubMed] [Google Scholar]
- 3.Bavari, S., B. Dyas, and R. G. Ulrich. 1996. Superantigen vaccines: a comparative study of genetically attenuated receptor-binding mutants of staphylococcal enterotoxin A. J. Infect. Dis. 174:338-345. [DOI] [PubMed] [Google Scholar]
- 4.Becker, K., A. W. Friedrich, G. Peters, and C. von Eiff. 2004. Systematic survey on the prevalence of genes coding for staphylococcal enterotoxins SElM, SElO, and SElN. Mol. Nutr. Food Res. 48:488-495. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, H. F., O. M. Korzeniowski, and M. A. Sande. 1983. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine 62:170-177. [PubMed] [Google Scholar]
- 6.Donker, G. A., et al. 2009. The population structure of Staphylococcus aureus among general practice patients from The Netherlands. Clin. Microbiol. Infect. 15:137-143. [DOI] [PubMed] [Google Scholar]
- 7.Fowler, V. G., Jr., et al. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012-3021. [DOI] [PubMed] [Google Scholar]
- 8.Fowler, V. G., Jr., et al. 2007. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J. Infect. Dis. 196:738-747. [DOI] [PubMed] [Google Scholar]
- 9.Fueyo, J. M., M. C. Mendoza, M. A. Alvarez, and M. C. Martin. 2005. Relationships between toxin gene content and genetic background in nasal carried isolates of Staphylococcus aureus from Asturias, Spain. FEMS Microbiol. Lett. 243:447-454. [DOI] [PubMed] [Google Scholar]
- 10.Grumann, D., et al. 2008. Immune cell activation by enterotoxin gene cluster (egc)-encoded and non-egc superantigens from Staphylococcus aureus. J. Immunol. 181:5054-5061. [DOI] [PubMed] [Google Scholar]
- 11.Harmsen, D., et al. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtfreter, S., et al. 2004. egc-encoded superantigens from Staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect. Immun. 72:4061-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtfreter, S., and B. M. Broker. 2005. Staphylococcal superantigens: do they play a role in sepsis? Arch. Immunol. Ther. Exp. (Warsz) 53:13-27. [PubMed] [Google Scholar]
- 14.Holtfreter, S., et al. 2007. Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J. Clin. Microbiol. 45:2669-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtfreter, S., et al. 2006. Staphylococcus aureus carriers neutralize superantigens by antibodies specific for their colonizing strain: a potential explanation for their improved prognosis in severe sepsis. J. Infect. Dis. 193:1275-1278. [DOI] [PubMed] [Google Scholar]
- 16.Jarraud, S., et al. 2002. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarraud, S., et al. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166:669-677. [DOI] [PubMed] [Google Scholar]
- 18.Jozefczyk, Z. 1974. Specific human antibodies to enterotoxins A, B, and C1 of Staphylococcus: their increased synthesis in staphylococcal infection. J. Infect. Dis. 130:1-7. [DOI] [PubMed] [Google Scholar]
- 19.Julander, I. 1985. Unfavourable prognostic factors in Staphylococcus aureus septicemia and endocarditis. Scand. J. Infect. Dis. 17:179-187. [DOI] [PubMed] [Google Scholar]
- 20.Kanclerski, K., B. Soderquist, M. Kjellgren, H. Homberg, and R. Mollby. 1996. Serum antibody response to Staphylococcus aureus enterotoxins and TSST-1 in patients with septicaemia. J. Med. Microbiol. 44:171-177. [DOI] [PubMed] [Google Scholar]
- 21.Kunstmann, G., E. Schroder, H. Hasbach, and G. Pulverer. 1989. Immune response to toxic-shock-syndrome toxin-1 (TSST-1) and to staphylococcal enterotoxins A, B and C in Staphylococcus aureus infections. Zentralbl. Bakteriol. 271:486-492. [DOI] [PubMed] [Google Scholar]
- 22.LeClaire, R. D., R. E. Hunt, and S. Bavari. 2002. Protection against bacterial superantigen staphylococcal enterotoxin B by passive vaccination. Infect. Immun. 70:2278-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letertre, C., S. Perelle, F. Dilasser, and P. Fach. 2003. Identification of a new putative enterotoxin SEU encoded by the egc cluster of Staphylococcus aureus. J. Appl. Microbiol. 95:38-43. [DOI] [PubMed] [Google Scholar]
- 24.Lina, G., et al. 2004. Standard nomenclature for the superantigens expressed by Staphylococcus. J. Infect. Dis. 189:2334-2336. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay, J. A., and M. T. Holden. 2006. Understanding the rise of the superbug: investigation of the evolution and genomic variation of Staphylococcus aureus. Funct. Integr. Genomics 6:186-201. [DOI] [PubMed] [Google Scholar]
- 26.Lowy, F. D., and M. Miller. 2002. New methods to investigate infectious disease transmission and pathogenesis—Staphylococcus aureus disease in drug users. Lancet Infect. Dis. 2:605-612. [DOI] [PubMed] [Google Scholar]
- 27.Masiuk, H., et al. 2010. Association of recurrent furunculosis with Panton-Valentine leukocidin and the genetic background of Staphylococcus aureus. J. Clin. Microbiol. 48:1527-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 29.Monecke, S., C. Luedicke, P. Slickers, and R. Ehricht. 2009. Molecular epidemiology of Staphylococcus aureus in asymptomatic carriers. Eur. J. Clin. Microbiol. Infect. Dis. 28:1159-1165. [DOI] [PubMed] [Google Scholar]
- 30.Monk, A. B., S. Curtis, J. Paul, and M. C. Enright. 2004. Genetic analysis of Staphylococcus aureus from intravenous drug user lesions. J. Med. Microbiol. 53:223-227. [DOI] [PubMed] [Google Scholar]
- 31.Munson, S. H., M. T. Tremaine, M. J. Betley, and R. A. Welch. 1998. Identification and characterization of staphylococcal enterotoxin types G and I from Staphylococcus aureus. Infect. Immun. 66:3337-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson, I. M., M. Verdrengh, R. G. Ulrich, S. Bavari, and A. Tarkowski. 1999. Protection against Staphylococcus aureus sepsis by vaccination with recombinant staphylococcal enterotoxin A devoid of superantigenicity. J. Infect. Dis. 180:1370-1373. [DOI] [PubMed] [Google Scholar]
- 33.Ono, H. K., et al. 2008. Identification and characterization of two novel staphylococcal enterotoxins, types S and T. Infect. Immun. 76:4999-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peacock, S. J., et al. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proft, T., and J. D. Fraser. 2003. Bacterial superantigens. Clin. Exp. Immunol. 133:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruotsalainen, E., et al. 2006. Levofloxacin does not decrease mortality in Staphylococcus aureus bacteraemia when added to the standard treatment: a prospective and randomized clinical trial of 381 patients. J. Intern. Med. 259:179-190. [DOI] [PubMed] [Google Scholar]
- 37.Ruotsalainen, E., et al. 2008. Methicillin-sensitive Staphylococcus aureus bacteraemia and endocarditis among injection drug users and nonaddicts: host factors, microbiological and serological characteristics. J. Infect. 56:249-256. [DOI] [PubMed] [Google Scholar]
- 38.Schlievert, P. M., and G. A. Bohach. 2007. Staphylococcal and streptococcal superantigens: an update, p. 21-36. In M. Kotb and J. Fraser (ed.), Superantigens: molecular basis for their role in human diseases. ASM Press, Washington, DC.
- 39.Schroder, E., G. Kunstmann, H. Hasbach, and G. Pulverer. 1988. Prevalence of serum antibodies to toxic-shock-syndrom-toxin-1 and to staphylococcal enterotoxins A, B and C in West-Germany. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 270:110-114. [DOI] [PubMed] [Google Scholar]
- 40.Strommenger, B., et al. 2006. Assignment of Staphylococcus isolates to groups by spa typing, SmaI macrorestriction analysis, and multilocus sequence typing. J. Clin. Microbiol. 44:2533-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takei, S., Y. K. Arora, and S. M. Walker. 1993. Intravenous immunoglobulin contains specific antibodies inhibitory to activation of T cells by staphylococcal toxin superantigens. J. Clin. Invest. 91:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas, D., S. Chou, O. Dauwalder, and G. Lina. 2007. Diversity in Staphylococcus aureus enterotoxins. Chem. Immunol. Allergy 93:24-41. [DOI] [PubMed] [Google Scholar]
- 43.Tuazon, C. U., and J. N. Sheagren. 1974. Increased rate of carriage of Staphylococcus aureus among narcotic addicts. J. Infect. Dis. 129:725-727. [DOI] [PubMed] [Google Scholar]
- 44.Tuazon, C. U., and J. N. Sheagren. 1975. Staphylococcal endocarditis in parenteral drug abusers: source of the organism. Ann. Intern. Med. 82:788-790. [DOI] [PubMed] [Google Scholar]
- 45.Ulrich, R. G., M. A. Olson, and S. Bavari. 1998. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine 16:1857-1864. [DOI] [PubMed] [Google Scholar]
- 46.van Belkum, A., et al. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 199:1820-1826. [DOI] [PubMed] [Google Scholar]
- 47.Verkaik, N. J., et al. 2009. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J. Infect. Dis. 199:625-632. [DOI] [PubMed] [Google Scholar]
- 48.Wertheim, H. F., et al. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5:751-762. [DOI] [PubMed] [Google Scholar]
- 49.Wright, J. S., III, et al. 2005. The agr radiation: an early event in the evolution of staphylococci. J. Bacteriol. 187:5585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]