Abstract
In 6 hematopoietic stem cell transplant (HSCT) recipients with candidemia, the (1,3)-β-d-glucan (BG) test was positive a median of 2.5 days after a positive blood culture. Only in 1 patient did BG positivity precede positive blood cultures. BG concentrations decreased in patients with clinical response, but positive BG results persisted long after blood cultures became sterile (median, 48 days).
The diagnosis of invasive candidiasis (IC) is challenging in hematological patients because of nonspecific clinical presentation and poor diagnostic yield of cultures. The (1,3)-β-d-glucan (BG) is a fungal cell wall component which circulates in the blood of patients with many fungal infections. High sensitivity of BG was reported in candidemia, ranging from 59 to 78% (2-6). A positive BG result was reported to precede the onset of invasive fungal infection (IFI)-related fever or diagnosis of IFI by other methods (1, 4, 6), and BG levels decreased in patients who responded to antifungal treatment (1, 6).
The aim of this study was to evaluate the relationship between positive blood cultures and a positive BG test in hematopoietic stem cell transplant (HSCT) recipients with documented candidemia.
HSCT recipients that developed candidemia were identified, and BG testing (Fungitell; Cape Cod, MA) was performed on samples collected for galactomannan screening. All samples were stored at −20°C. A BG serum concentration of ≥80 pg/ml was considered positive, and assays were performed in duplicate according to the manufacturer's recommendations. The day of the first blood culture positive for yeasts was considered day 0. All clinical data were retrieved from our prospectively collected computerized database. To be included in the study, patients had to have at least one blood culture positive for Candida, blood cultures performed after candidemia to document its clearance, and serum samples from before and after candidemia available for BG testing. Six patients fulfilling the above criteria were identified. Four of them (patients 1, 2, 4, and 6) had repeatedly negative blood cultures during the days preceding candidemia.
The characteristics of the patients and the results of BG testing are shown in Table 1. Five patients were severely neutropenic (less than 100 neutrophils/ml) and on long-term antifungal prophylaxis, while patient 1 had a white blood cell count of 2,700 cells/ml and started fluconazole 4 days before candidemia. Except for patient 6, none of the others was colonized by Candida on routine surveillance cultures of throat swab and urine samples. Only patient 6 had been colonized with Candida krusei 19 days before candidemia. All of the patients were fitted with a tunneled central venous catheter (CVC), and only patient 5 had her CVC removed (because of persistent fungemia due to Candida parapsilosis).
TABLE 1.
Patient characteristics and results of (1,3)-β-d-glucana
| Patient | Age (yr)/sex | Underlying condition(s) | Time between candidemia and HSCT | Antifungal prophylaxis | Candida species | No. of positive blood cultures | Treatment | No. of days to: |
BG value (pg/ml) on day shown vs first positive blood culture for Candida (day 0) |
Outcome (day of last follow-up) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Last positive blood culture | First negative blood culture | −7 | −4 | 0 | +2b | +7 | +14 | +21 | +28 | +35 | |||||||||
| 1 | 57/male | ALL, relapsed | 669 days | FLU | C. albicansc | 1 | CAS | 1 | 37 | <7 | 76 | 174 | 137 | 107 | 123 | 153 | 127 | Deceased due to relapse (144) | |
| 2 | 39/male | ALL, relapsed | 17 days | FLU | C. krusei | 4 | CAS | 3 | 4 | <7 | <7 | 47 | 28 | 40 | 104 | <7 | <7 | <7 | Deceased due to BSI and relapse (63) |
| 3 | 33/female | AML | 9 days | FLU | C. krusei | 3 | CAS | 7 | 8 | <7 | <7 | <7 | 179 | 117 | 300 | 492 | 383 | >523 | Alive |
| 4 | 54/male | ALL | 5 days | FLU | C. krusei | 3 | CAS | 4 | 5 | <7 | <7 | >523 | >523 | >523 | 248 | 410 | Deceased due to MOF (21) | ||
| 5 | 63/female | MF | 17 days | CAS | C. parapsilosis | 13 | L-AmB | 24 | 27 | <7 | <7 | <7 | <7 | 263 | 293 | >523 | >523 | >523 | Deceased due to relapse (119) |
| 6 | 63/male | MF, relapsed | >10 yr | FLU | C. krusei | 3 | CAS | 5 | 7 | 96 | 110 | 115 | 84 | 259 | 330 | 220 | 175 | 105 | Deceased due to relapse (45) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BSI, bloodstream infection; CAS, caspofungin; FLU, fluconazole; L-AmB, liposomal amphotericin B; MF, myelofibrosis; MOF, multiorgan failure.
Sample from day +3 for patients 1 and 6 and from day +1 for patient 2.
Susceptible to fluconazole.
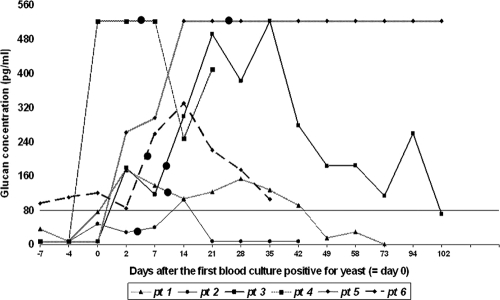
All of the patients had at least one sample positive for BG, but only one patient had positive BG test before the day of candidemia (patient 6). Four and 7 days before candidemia, the BG test was positive, while blood cultures remained negative. The median time between the positive BG test and positive blood culture was 2.5 days (range, from 7 days before candidemia to 14 days after). BG positivity persisted long after blood cultures became sterile. (In 3 patients in whom the BG test became negative, the median time between the first negative blood culture and the first negative BG result was 48 days, and the range was 17 to 102 days). The kinetics of BG are outlined in Fig. 1. Patients who responded to antifungal therapy had BG levels diminishing slowly over time, while patient 5, who had persistently high BG, was diagnosed with splenic candidiasis.
FIG. 1.
The kinetics of (1,3)-β-d-glucan in each of six patients (pt 1 to 6), compared to those on day 0, the day of the first positive blood culture. Large black dots denote the day of the first blood culture negative for Candida. The vertical line at 80 pg/ml represents the cutoff positivity of (1,3)-β-d-glucan, as recommended by the manufacturer.
In our study, BG results were positive in all patients with candidemia; however, in most cases, the BG result did not precede the positive blood culture. BG persisted long after cultures became negative, even in patients clinically responding to treatment.
In particular, samples collected before positive blood cultures were negative in all cases but one: in 4 patients from which the samples were drawn on the day of candidemia but before the onset of fever, the BG test was negative. The patient with a positive BG result on day 0 developed the symptoms during the night before. It is worth noting that in the most of the patients, BG positivity came after the blood culture positivity. On the contrary, Persat and colleagues found that BG positivity preceded blood cultures in 4 of 7 patients with candidemia (4). The reason for the BG result becoming positive at the same time or after the blood cultures might be that candidemia in HSCT recipients is a result of abrupt entrance of yeast through a catheter from skin or the gastrointestinal (GI) tract. These patients, who routinely receive fluconazole prophylaxis, do not usually experience an overgrowth of Candida in the GI tract or skin, unless fluconazole-resistant species are present, as happens in intensive care unit (ICU) patients in whom multifocal colonization precedes candidemia.
The BG result persisted as positive long after clinical response (negative blood cultures and no fever), but similarly to the results reported by Senn and colleagues, a trend toward BG reduction was evident (6). The only patient with repeatedly high BG levels had splenic IC. In the case of candidemia, the removal of CVC is generally advised, but its risks and benefits remain controversial in neutropenic patients. One of the reasons for long-term BG positivity might be the fact that most of the patients retained their CVC, because blood cultures became negative soon after starting the antifungal treatment. The presence of CVC that was involved in candidemia might have caused a slow clearance of BG. As all of the patients had normal clearance of creatinine, reduced renal elimination of BG could not be the cause of BG persistence.
Despite the low number of cases reported, our data suggest that BG might be useful for diagnosing candidemia in HSCT recipients, particularly because the results can be obtained in 2 h, compared to at least 48 h for traditional microbiological cultures. However, only in 1 of 6 patients was the BG test positive before blood cultures. The time to BG negativity in patients responding to treatment and with already negative blood cultures was long, and further studies are warranted to identify reasons for the persistence of positive BG results in this population. The repeatedly high levels of BG might indicate that the infection has become chronic, even if blood cultures are negative.
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1.Ellis, M., et al. 2008. Assessment of the clinical utility of serial beta-d-glucan concentrations in patients with persistent neutropenic fever. J. Med. Microbiol. 57:287-295. [DOI] [PubMed] [Google Scholar]
- 2.Koo, S., J. M. Bryar, J. H. Page, L. R. Baden, and F. M. Marty. 2009. Diagnostic performance of the (1→3)-beta-d-glucan assay for invasive fungal disease. Clin. Infect. Dis. 49:1650-1659. [DOI] [PubMed] [Google Scholar]
- 3.Ostrosky-Zeichner, L., et al. 2005. Multicenter clinical evaluation of the (1→3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654-659. [DOI] [PubMed] [Google Scholar]
- 4.Persat, F., et al. 2008. Contribution of the (1→3)-beta-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 46:1009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickering, J. W., H. W. Sant, C. A. Bowles, W. L. Roberts, and G. L. Woods. 2005. Evaluation of a (1→3)-beta-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 43:5957-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senn, L., et al. 2008. 1,3-Beta-d-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin. Infect. Dis. 46:878-885. [DOI] [PubMed] [Google Scholar]