Abstract
Vibrio cholerae O1 causes dehydrating diarrhea with a high mortality rate if untreated. The infection also elicits long-term protective immunity. Since V. cholerae is noninvasive, mucosal immunity is likely important for protection. In this study, we compared humoral immune responses in the duodenal mucosa and blood of cholera patients at different time points after the onset of disease and compared them with those of healthy controls (HCs). Immune responses to lipopolysaccharide (LPS) and the recombinant cholera toxin B subunit (rCTB) were assessed by enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunospot (ELISPOT) assay. Significant increases in V. cholerae LPS-specific IgA and IgG antibody levels were seen in duodenal extracts on day 30, but the levels decreased to baseline by day 180; plasma V. cholerae LPS-specific IgA levels remained elevated longer. Levels of mucosal CTB antibodies also peaked on day 30, but the increase reached statistical significance only for IgG. A significant correlation was found between the CTB antibody-secreting cell (ASC) response in the circulatory system on day 7 and subsequent CTB-specific IgA levels in duodenal extracts on day 30 and the numbers of CTB-specific IgA ASCs in duodenal tissues on day 180. The proportion (0.07%) of mucosal V. cholerae LPS IgA ASCs peaked on day 30 and remained elevated through day 180 compared to that of HCs (P = 0.03). These results suggest that protective immunity against V. cholerae is not likely mediated by the constitutive secretion of antibodies at the mucosal surface; our results are consistent with those of other studies that suggest instead that anamnestic immune responses of mucosal lymphocytes may play a major role in protection against cholera.
Vibrio cholerae O1 is a noninvasive mucosal pathogen that colonizes the surface of the small intestine and elaborates cholera toxin (CT), an ADP-ribosylating toxin that causes secretory diarrhea. Although infection with V. cholerae induces protection against subsequent disease for several years (7, 10), the mechanisms of protective immunity are not understood (13). However, because V. cholerae is noninvasive, it has been hypothesized that protection is effected by antibodies at the mucosal surface.
The best-characterized marker of protective immunity to cholera is the plasma vibriocidal antibody, a complement-dependent bactericidal antibody that increases with age in areas in which cholera is endemic and is associated with protection from infection with V. cholerae. However, it is unlikely that vibriocidal antibodies directly mediate protection because there is no threshold vibriocidal-antibody titer at which protection against cholera is achieved (19) and studies with volunteers from areas where cholera is not endemic demonstrate that protective immunity to cholera persists even after vibriocidal-antibody titers have declined to undetectable levels (11).
Secretory IgA antibody (sIgA) is the predominant isotype on mucosal surfaces. The local IgA response is believed to play a major role in protective immunity from diarrhea caused by V. cholerae, since cholera is a gut-restricted, noninvasive mucosal infection. Cholera also induces both plasma IgG and IgA responses to V. cholerae antigens, but only levels of circulating V. cholerae-specific IgA antibodies are associated with protection (9). However, like plasma vibriocidal-antibody titers, plasma IgA responses remain elevated for only 6 to 12 months after cholera infection (8), while protective immunity after clinical cholera infection lasts substantially longer (7).
Because protective immunity after V. cholerae infection persists longer than detectable increases in serum antibodies, it has been hypothesized that protective immunity is generated by rapid anamnestic responses of memory B cells in the gut-associated lymphoid tissue (GALT) or in the blood. In support of this hypothesis, we found that circulating V. cholerae-specific memory B cells remain detectable for at least 1 year after cholera infection and persist longer than traditional measures of immunity to cholera (8). Furthermore, volunteers rechallenged with cholera toxin B (CTB) 15 months after initial exposure were able to respond with a rapid rise in detectable levels of sIgA in intestinal lavage in as little as 3 days after exposure (21), which is suggestive of an anamnestic response in mucosal tissues.
An alternative hypothesis is that the mechanism of protective immunity against V. cholerae infection is the ability of antibodies produced by antibody-secreting cells (ASCs) located in the lamina propria to maintain protection against V. cholerae upon reexposure. This hypothesis is consistent with the finding that the majority of V. cholerae antigen-specific ASCs, which are detected only transiently in the blood after cholera infection, express intestinal rehoming markers (17) and likely remain localized in mucosal tissues long after infection. For this reason, mucosal antibody levels may not be adequately measured by monitoring peripheral markers of immunity, such as the vibriocidal antibody, V. cholerae-specific-IgA, or other short-lived ASCs in the blood.
To evaluate these hypotheses regarding the nature of protective immunity to cholera, we directly measured mucosal immune responses in duodenal biopsy specimens from a cohort of 18 otherwise-healthy adults recovering from severe cholera infections. Specifically, we addressed the following questions: (i) whether the levels of V. cholerae antigen-specific ASCs and antibodies are increased for a longer period after cholera in the duodenal lamina propria than in the blood and (ii) whether there is a correlation between the levels of V. cholerae antigen-specific mucosal antibodies and ASCs in duodenal biopsy specimens and measurements of V. cholerae antigen-specific immune responses in the blood.
MATERIALS AND METHODS
Study subjects.
Eighteen individuals admitted to the hospital of the International Centre for Diarrhoeal Diseases Research, Bangladesh (ICDDR,B), with acute cholera were enrolled in the study. The study was approved by the institutional review boards of the ICDDR,B and Massachusetts General Hospital, Boston, Massachusetts. Five healthy adults with asymptomatic Helicobacter pylori infection, evaluated as part of a separate study, were used as the healthy control group for comparison. Duodenal biopsy specimens of around 1 mm3 in diameter were obtained by standard forceps (Radial Jaw megabyte; Boston Scientific) during acute infection (on the second day of hospitalization after stabilization, termed day 2 in the study) and again on days 30, 180, and 360. Venous blood samples were obtained on days 2, 7, 30, 90, 180, and 360 after the onset of illness. For each blood sample, we measured the vibriocidal-antibody titer, the levels of IgG and IgA antibodies, and the proportions of circulating IgG and IgA ASCs specific to CTB and the homologous serotype of V. cholerae lipopolysaccharide (LPS). From the gut biopsy specimens, we measured the levels of IgG and IgA antibodies to CTB and LPS in duodenal extracts and the proportions of antigen-specific IgG and IgA ASCs among extracted lamina propria lymphocytes (LPLs), using an enzyme-linked immunospot (ELISPOT) procedure.
Sample preparation.
Heparinized blood was diluted in phosphate-buffered saline. After the blood solution was centrifuged on a Ficoll-Isopaque instrument (Pharmacia, Piscataway, NJ), peripheral blood mononuclear cells (PBMCs) and plasma were separated. Plasma specimens were frozen at −80°C prior to use in immunologic assays. LPLs were isolated from the duodenal biopsy specimens by incubation in EDTA-dithiothreitol (DTT) and a subsequent treatment with collagenase-DNase, as described previously (12). The cell suspension was filtered through nylon mesh, and the number of lymphocytes was counted. Isolated LPLs were resuspended at a concentration of 1 × 106 cells/ml in RPMI complete medium (Gibco, Carlsbad, CA) with 10% heat-inactivated fetal bovine plasma (HyClone, Logan, UT). Resuspended LPLs and PBMCs were used immediately for detecting antigen-specific IgG and IgA ASCs by ELISPOT assay. Duodenal extracts were prepared as described previously (1). In brief, a single biopsy specimen was snap-frozen at −70°C at each time point. Specimens were then thawed overnight at 4°C in 150 μl of phosphate-buffered saline (10 mM, pH 7.3) containing 2% saponin (Sigma, Germany), 0.05 mg of phenyl-methyl-sulfonyl-fluoride (Sigma), 0.1 mg of soybean trypsin inhibitor (Sigma), and 0.1% bovine serum albumin (BSA). The samples were centrifuged at 13,800 × g for 5 min, and the supernatants were analyzed for specific IgA and IgG antibodies by enzyme-linked immunosorbent assay (ELISA) or were stored at −70°C.
Quantification of naïve and memory B cells in LPLs and PBMCs.
LPLs and PBMCs (5 × 104 cells per sample with a control) were stained for naïve and memory B cell markers, as well as a gut-homing marker, as described previously (3, 23), using anti-CD19-fluorescein isothiocyanate (FITC), anti-α4+β7+-phycoerythrin (PE), and anti-CD27-allophycocyanin (APC) (Becton Dickinson Immunocytometry Systems). Stained cells were fixed in formaldehyde before being analyzed by flow cytometry on a FACSCalibur instrument equipped with blue and red lasers (Becton Dickinson, San Jose, CA). Control samples were included in each run. Fluorescence-activated cell sorter (FACS) data were analyzed with FlowJo software (TreeStar, Inc.). Lymphocytes were gated by forward and side scatter, and B cells were gated based on surface expression of CD19+. CD27+ and α4+β7+ cell populations were gated from the CD19+ cell population. Control samples showed that contamination between groups was negligible.
Quantification of CTB and LPS IgA and IgG ASCs in blood and duodenal biopsy specimens.
CTB and LPS ELISPOT assays were performed as previously described (8, 17, 20). Briefly, nitrocellulose-bottomed plates (Millipore, Bedford, MA) were coated with affinity-purified goat anti-human Ig (5 μg/ml; Jackson Immunology Research, West Grove, PA), monosialotetrahexosylganglioside (GM1) ganglioside (3 nM/ml), LPS (25 μg/ml), or keyhole limpet hemocyanin (KLH) (2.5 μg/ml) and incubated overnight at 4°C (17). Prior to blocking, recombinant CTB (rCTB) (2.5 μg/ml) was applied to the GM1-coated plates, and the plates were incubated for 1 h at 37°C. All plates were blocked for 2 h at 37°C prior to use with RPMI complete medium. A total of 4 × 105 lymphocytes were added to the CTB-, and LPS-coated plates. Following 3 h of incubation at 37°C, plates were washed and IgA and IgG ASCs were detected using horseradish peroxidase-conjugated mouse anti-human IgA and alkaline phosphatase-conjugated IgG (1:500 dilution; SouthernBiotech, Birmingham, AL). After overnight incubation, IgG-conjugated plates were developed with 5-bromo-4-chloro-3-indolyl-phosphate-nitroblue tetrazolium and IgA-conjugated plates with 3-amino-9-ethylcarbazole. ASCs were quantified by two individuals using a stereomicroscope (Leica WILD M3Z) independently. The specific ASCs were expressed as the percentages of antigen-specific B cells of the total number of IgG- or IgA-expressing B cells. Wells were coated with KLH (Pierce Biotechnology, Rockford, IL) (2.5 μg/ml) for use as a negative control. For duodenal LPLs, LPS IgG ASC responses were not measured because of the limited number of cells obtained.
Vibriocidal-antibody assay and CTB and LPS ELISAs in plasma.
The vibriocidal assay was performed as previously described, using guinea pig complement and the homologous serotype of V. cholerae O1 Ogawa (X-25049) or Inaba (T-19479) as the target organism (17). The vibriocidal titer was defined as the reciprocal of the highest plasma dilution that resulted in more than a 50% reduction in the optical density compared to that of the control wells without plasma. The CTB- and LPS-specific IgA and IgG responses in extracts from biopsy specimens (1) and plasma of patients and healthy controls were quantified using standardized ELISA protocols (8, 14, 17). Briefly, to quantify antibodies to CTB, ELISA plates were coated with ganglioside GM1 (0.3 nM/ml) followed by recombinant CTB (2.5 μg/ml) (gifts from A. M. Svennerholm, Gothenburg University). To quantify antibodies to LPS, ELISA plates were coated with the homologous serotype of V. cholerae LPS (2.5 μg/ml) (14). For each antigen, 100 μl either of saponin-treated extracts of biopsy specimens (duodenal extracts, 1:10 dilution) or of plasma (1:100 dilution) diluted in 0.1% bovine plasma albumin in phosphate-buffered saline was added per well. Horseradish peroxidase-conjugated secondary antibodies to human IgG or IgA (Jackson Laboratories, Bar Harbor, ME) were applied in separate wells. After a 90-min incubation at 37°C, the plates were washed and then developed with ortho-phenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer and 0.1% hydrogen peroxide. Plates were read kinetically at 450 nm for 5 min. The maximal rate of change in optical density was expressed as milli-absorbance units per minute, and ELISA units were normalized by calculating the ratio of the optical density of the test sample to that of a standard of pooled convalescent-phase sera from patients recovered from cholera run as a positive control on each plate.
Statistical analyses.
Comparisons of immunologic responses were tested for significance using the Mann-Whitney U test. All reported P values are two tailed, with a cutoff P value of <0.05 considered the threshold for statistical significance.
RESULTS
Study population.
Demographic, microbiologic, and clinical characteristics of the patients studied are presented in Table 1. Eighteen patients (median age, 30 years; male, 17; female, 1) were enrolled in the study. Patients and controls were excluded from the study if they had a history of illness consistent with cholera in the previous year.
TABLE 1.
Demographic, microbiologic, and clinical characteristics of the patients in this study
| Characteristic | Value |
|---|---|
| Median age (range) in yr | 30 (22-44) |
| Gender | |
| No. of males | 17 |
| No. of females | 1 |
| Duration (h) of diarrhea | |
| prior to admission | |
| (mean ± SD) | 19.83 (±13.97) |
| No. completing follow-up day: | |
| 2 | 18 |
| 30 | 17 |
| 180 | 9 |
| 360 | 7 |
| No. infected with Vibrio cholerae O1 serotype: | |
| Ogawa | 16 |
| Inaba | 2 |
| Blood group | |
| No. with O | 8 |
| No. with A | 3 |
| No. with B | 7 |
Vibriocidal responses.
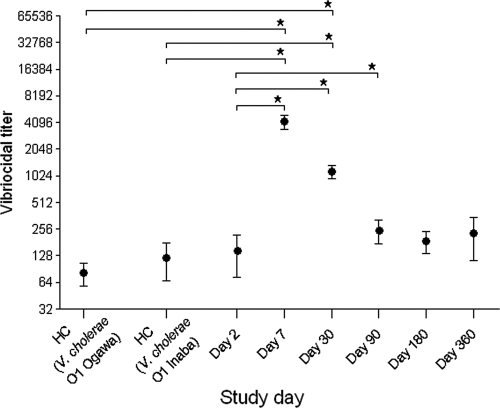
Consistent with those in previous studies, all patients in this cohort mounted strong vibriocidal responses and demonstrated seroconversion, represented by a 4-fold-or-greater rise in their vibriocidal titers (Fig. 1). The vibriocidal-antibody titer peaked on day 7 (geometric mean [GM] count, 2,281; 95% confidence interval [CI], 954.7 to 5,449; P < 0.001) and remained significantly elevated through day 90 before declining to baseline levels at day 180 (GM, 95; 95% CI, 24 to 363; P < 0.1).
FIG. 1.
Vibriocidal-antibody responses in plasma (means ± standard errors of the means [SEMs]; log 2). HC, healthy controls; *, statistically significant difference (P ≤ 0.05).
Antibody responses in plasma and in duodenal extracts.
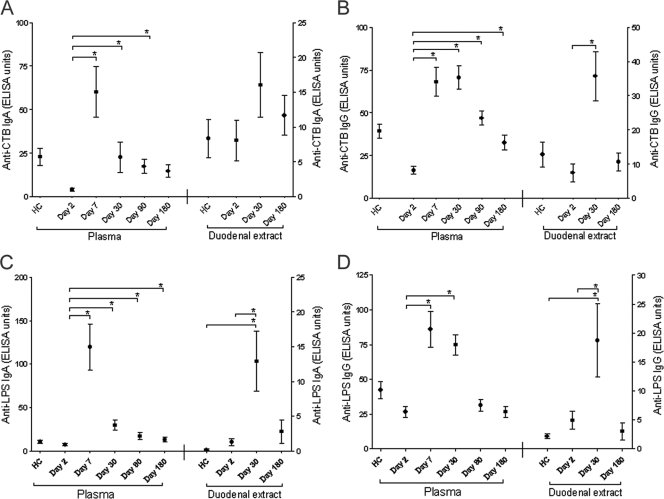
In duodenal extracts, CTB-specific IgG and IgA antibody responses peaked at day 30, although the difference between day 2 and day 30 was statistically significant only for IgG antibodies (Fig. 2). In contrast, increases in the levels of CTB-specific IgA antibodies in plasma were seen through day 90 and in the levels of CTB-specific IgG antibodies in plasma until day 180, suggesting that CTB-specific antibodies remain elevated for a longer time in the plasma than in the duodenal mucosa. Levels of V. cholerae O1 LPS-specific IgG and IgA antibodies in the duodenal extracts were significantly elevated on day 30 compared to those for day 2 samples and controls. These levels declined to baseline by day 180, even though V. cholerae O1 LPS-specific IgA antibodies in plasma remained elevated through day 180. These results suggest that after cholera infection, preformed antibodies do not persist longer at the intestinal mucosal surface than in the blood.
FIG. 2.
Mean normalized antigen-specific IgA and IgG antibody responses in plasma and duodenal extracts (with standard-error bars). Trends for plasma and duodenal extract anti-CTB IgA (A) and IgG (B) and plasma anti-LPS IgA (C) and IgG (D) levels are shown in ELISA units on different study days for patients and healthy controls. In panels A and B, plasma and duodenal extract data are shown on the left and right axes, respectively. *, statistically significant difference (P ≤ 0.05).
Peripheral and mucosal antibody-secreting cell responses.
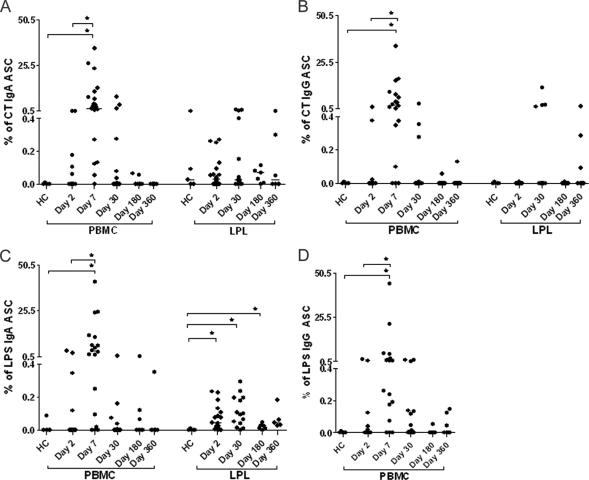
Circulating V. cholerae antigen-specific ASC responses are short-lived after cholera infection (16). IgG and IgA ASC responses against V. cholerae LPS and CTB were all significantly increased on day 7 but declined to baseline levels by day 30 (Fig. 3). The proportions of mucosal CTB-specific IgA and IgG ASCs were slightly elevated on day 30 but did not differ significantly between study days or between patients and healthy controls. In contrast, the numbers of V. cholerae O1 LPS-specific IgA ASCs in LPLs peaked on day 30 and were found to be significantly higher in cholera patients than in healthy controls on days 2, 30, and 180, suggesting a relatively early response to LPS and the long-term persistence of LPS-specific IgA ASCs in the duodenal mucosa.
FIG. 3.
Peripheral blood and duodenal antigen-specific IgA and IgG ASC responses. Trends for anti-CTB IgA (A)-, anti-CTB IgG (B)-, anti-LPS IgA (C)-, and anti-LPS IgG (D)-specific ASC levels in patients and healthy controls on different study days are shown. Data are normalized to total IgA or IgG and expressed as percentages. *, statistically significant differences (P ≤ 0.05).
Mucosal and systemic B cell responses.
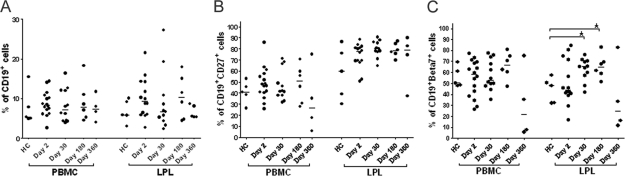
The percentages of B cell, memory B cell, and gut-homing B cell populations in both duodenal LPLs and PBMCs from patients and healthy controls were quantified on different study days. We observed significantly higher gut-homing CD19+ cells (CD19+ β7+) at day 30 (P < 0.007) and day 180 (P < 0.008) in biopsy specimens from patients than in those from healthy controls (Fig. 4 C), although there were no differences in the overall levels of CD19+ (Fig. 4A) and CD19+ CD27+ (Fig. 4B) B cells in the blood or duodenal mucosa of patients and those of healthy controls.
FIG. 4.
Peripheral blood and duodenal B cell responses in cholera patients on different days after the onset of infection compared to those in healthy controls. (A) Percentages of CD19+ cells in total lymphocytes. (B and C) Percentages of CD19+ CD27+ (B) or CD19+ β7+ (C) cells among total CD19+ cells. *, statistically significant difference (P ≤ 0.05).
Correlation between peripheral and duodenal markers of mucosal immunity to cholera.
Because the peak in circulating ASCs includes a large population of cells that are rehoming to the gut mucosa (16), we evaluated the correlation between the magnitude of the peak V. cholerae antigen-specific ASC response on day 7 and the subsequent levels of antigen-specific ASCs and IgA antibodies in gut mucosa (Table 2). We observed a significant correlation between the peak CTB ASC response on day 7 in the blood and the subsequent amount of CTB-specific IgA antibodies in duodenal tissue on day 30 and the number of CTB-specific IgA ASCs in duodenal tissue on day 180. However, there were no correlations observed between the peak in circulating LPS ASC responses on day 7 and subsequent mucosal LPS responses. Also of note, there was no significant correlation observed between simultaneous measurements of antigen-specific IgA in plasma and in duodenal tissue (data not shown). Finally, as expected for sequential events, there was no correlation observed between simultaneously measured levels of circulating and duodenal antigen-specific ASCs.
TABLE 2.
Correlations between day 7 circulatory system IgA ASC responses and subsequent duodenal responses to specific antigens
| Antigen | First measurement (day) | Second measurement (day) | Ra | P valueb |
|---|---|---|---|---|
| CTB | Circulating IgA ASCs (7) | Duodenal ASCs (30) | 0.42 | 0.11 |
| Antibodies in duodenal extract (30) | 0.78 | 0.008 | ||
| Duodenal ASCs (180) | 0.77 | 0.04 | ||
| LPS | Circulating IgA ASCs (7) | Duodenal ASCs (30) | 0.13 | 0.67 |
| Antibodies in duodenal extract (30) | 0.16 | 0.77 | ||
| Duodenal ASCa (180) | 0.24 | 0.61 |
Spearman rank correlation coefficient showing the correlation between the first and second measurements.
P values showing the significance between the first and second measurements.
DISCUSSION
Peripheral markers of humoral immunity to cholera decrease to baseline levels before protective immunity against cholera ends. Possible explanations are that constitutive antibody secretion at the mucosal surface maintains protection upon reexposure and that increased levels of mucosal antibody secretion persist longer than detectable increases in circulating antibodies. However, in direct opposition, we found that the increases in circulating CTB- and LPS-specific antibodies actually persist for a longer time than the increases in the levels of these antibodies in duodenal tissue following cholera infection. This finding suggests that it is unlikely that protective immunity to cholera is mediated by constitutive antibody secretion at the mucosal surface.
In contrast, we did find that cholera patients had persistent increases in V. cholerae LPS-specific ASCs in duodenal tissue after cholera infection. V. cholerae LPS-specific IgA ASCs remained at significantly increased levels compared to those for healthy controls at least until day 180, long after circulating LPS-specific IgA ASCs returned to baseline levels. Although V. cholerae LPS-specific ASCs on day 180 represented only 0.07% of all lamina propria IgA ASCs, the lamina propria harbors 80% of all ASCs in humans (6), and it is possible that even a relatively small proportion of V. cholerae LPS-specific ASCs in the lamina propria may allow protective immunity against cholera.
Interestingly, the persistent increase in V. cholerae LPS-specific ASCs remained detectable despite the absence of detectable increases in IgA antibodies in mucosal tissues. This finding is of potential interest, since it has been recently demonstrated that IgA plasma cells may not secrete antibodies until induced to do so by the cessation of ongoing stimulation with the cytokine B cell-activating factor (5) or upon an encounter with an antigen (22). The lack of correlation between the proportion of LPS-specific ASCs in the lamina propria and tissue antibody levels seen in our study raises the possibility that some of the persistent V. cholerae LPS-specific ASCs detected by ELISPOT assay in the lamina propria may produce substantial quantities of antibodies only in response to certain stimuli on reexposure.
While such long-lasting V. cholerae LPS-specific mucosal ASCs may contribute to protective immunity, an alternative possibility is that memory B cells that are capable of both proliferation and differentiation into plasma cells may mediate anamnestic responses that result in the amplification of humoral immunity on reexposure to an antigen. We have already found evidence that V. cholerae antigen-specific memory B cells persist in the blood much longer than antibodies and thus may contribute to long-term protection upon reexposure. However, we were not able to measure memory B cells in the duodenal biopsy specimens in this study because of the limited numbers of cells obtained.
In our previous study of memory B cell responses in blood after cholera infection (8), there were significant differences in the development of memory B cells into the T-cell-independent antigen LPS and the T-cell-dependent antigen CTB. We previously observed that peripheral IgG memory B cell responses to the protein antigen CTB persisted for a full year after cholera infection, but IgG memory B cell responses to LPS in blood waned more rapidly. Here, in mucosal tissues, we found the converse: V. cholerae LPS-specific IgA ASC responses in lamina propria persisted longer than IgA ASC responses to CTB in lamina propria. The lamina propria is a site of T-cell-independent antigen-induced IgA class switch recombination in the intestine, while T-cell-dependent IgA class switching is restricted to follicular lymphoid tissues (4). Our results suggest that LPS-specific IgA ASCs, derived from T-cell-independent IgA class switch recombination, may contribute to repopulating the lamina propria for a longer time than ASCs derived from follicular T-cell-dependent class switch recombination.
We found that the levels of circulating CT IgG and LPS IgG ASCs increased significantly at day 7 compared to those at the acute stage of infection and in healthy controls and then declined by day 30. However, we were not able to see any major differences in IgG ASC responses against CT in the gut. It has been shown previously that CTB IgG responses increased in the circulatory system after oral immunization with killed whole-cell enterotoxigenic Escherichia coli (ETEC) vaccine containing toxoid antigen (15, 24), as well as after natural cholera infection (16, 17) or ETEC diarrhea (16). Moreover, Quiding et al. (18) observed striking differences between duodenal and peripheral CTB ASC responses after oral immunization with B-subunit whole-cell (B-WC) cholera vaccine (18), with responses higher in the duodenum than in the circulatory system after vaccination. However, here we saw a different response following severe cholera infection. Further studies using intestinal immunocytes of the IgG antibody isotype (20) are needed.
The lack of correlation between concomitant measures of plasma V. cholerae antigen-specific IgA and ASCs and mucosal-tissue IgA and ASCs underscores the difficulty in identifying circulating markers of a protective immune response that is primarily dependent on anamnestic mucosal immune responses. Based on this study, the initial peak in CTB-specific ASCs is the best marker for a subsequent mucosal response to CTB. However, the T-cell-independent response to LPS appears to have different kinetics, and the initial ASC response in blood is not predictive of the ultimate magnitude of the mucosal anti-LPS response. The development of novel noninvasive assays for measuring anamnestic mucosal immune responses to antigenic challenge (somewhat analogous to the use of a tuberculin skin test) may ultimately provide better measurements of protective immunity against cholera and other mucosal infections than the measurement of circulating antibodies or ASC responses.
By flow cytometry, we have previously shown that the level of gut-homing B cells in the blood increases on day 7 compared to those in healthy controls and on day 2 of the illness (2). In this study, we found that the numbers of gut-homing CD19+ cells also increased significantly in the lamina propria late after infection, on days 30 and 180, compared to those on day 2 and in healthy controls. This finding suggests that gut-homing CD19+ cells may take some time to home back to the gut from the circulatory system. The baseline levels of CD19+ and gut-homing CD19+ cells in biopsy specimens were also relatively high in our healthy Bangladeshi controls, perhaps triggered by infection with other pathogens and the resulting recruitment of lymphocytes to this site (3). Further studies are needed to better understand the kinetics of antigen-specific B cell responses in the duodenal mucosa of V. cholerae-infected patients.
Taken together, our data are consistent with those of previous studies and provide additional evidence suggesting that protective immunity to cholera is not likely to be mediated by constitutive antibody secretion at the mucosal surface at the time of reinfection with V. cholerae. However, our data do not differentiate between the contributions to protective immunity of persistent V. cholerae LPS-specific IgA ASCs in the lamina propria or memory B cells capable of anamnestic immune responses upon reexposure. Additional studies are needed to address whether canonical memory B cells are present in mucosal tissue and whether these and/or circulating memory B cells contribute to protective immunity after cholera infection.
Acknowledgments
This research was supported by the ICDDR,B Centre for Health and Population Research and by the following grants: U01 AI058935 (S.B.C.), RO3 AI063079 (F.Q.), U01 AI077883 (E.T.R.), and International Research Scientist Development Award KO1 TW07409 (J.B.H.). F.C., A.I.K., and T.R.B. are recipients of Fogarty International Clinical Research Scholars awards from the Fogarty International Center of the National Institutes of Health (D43 TW005572 and R24TW007988).
We are grateful for the participation of patients and the work of the field staff at the ICDDR,B.
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1.Bergquist, C., A. Mattsson-Rydberg, H. Lonroth, and A. Svennerholm. 2000. Development of a new method for the determination of immune responses in the human stomach. J. Immunol. Methods 234:51-59. [DOI] [PubMed] [Google Scholar]
- 2.Bhuiyan, T. R., et al. 2009. Cholera caused by Vibrio cholerae O1 induces T-cell responses in the circulation. Infect. Immun. 77:1888-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhuiyan, T. R., et al. 2008. Comparison of mucosal B- and T-cell responses in Helicobacter pylori-infected subjects in a developing and a developed country. FEMS Immunol. Med. Microbiol. 54:70-79. [DOI] [PubMed] [Google Scholar]
- 4.Cerutti, A. 2008. The regulation of IgA class switching. Nat. Rev. Immunol. 8:421-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darce, J. R., B. K. Arendt, S. K. Chang, and D. F. Jelinek. 2007. Divergent effects of BAFF on human memory B cell differentiation into Ig-secreting cells. J. Immunol. 178:5612-5622. [DOI] [PubMed] [Google Scholar]
- 6.Fagarasan, S., S. Kawamoto, O. Kanagawa, and K. Suzuki. 2010. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 28:243-273. [DOI] [PubMed] [Google Scholar]
- 7.Glass, R. I., et al. 1982. Endemic cholera in rural Bangladesh, 1966-1980. Am. J. Epidemiol. 116:959-970. [DOI] [PubMed] [Google Scholar]
- 8.Harris, A. M., et al. 2009. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 77:3850-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris, J. B., et al. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koelle, K., M. Pascual, and M. Yunus. 2005. Pathogen adaptation to seasonal forcing and climate change. Proc. Biol. Sci. 272:971-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine, M. M., et al. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818-820. [DOI] [PubMed] [Google Scholar]
- 12.Lundgren, A., et al. 2005. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 73:523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson, E. J., J. B. Harris, J. G. Morris, Jr., S. B. Calderwood, and A. Camilli. 2009. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 7:693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qadri, F., et al. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qadri, F., et al. 2003. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi children 18-36 months of age. Vaccine 21:2394-2403. [DOI] [PubMed] [Google Scholar]
- 16.Qadri, F., et al. 1998. Enteric infections in an endemic area induce a circulating antibody-secreting cell response with homing potentials to both mucosal and systemic tissues. J. Infect. Dis. 177:1594-1599. [DOI] [PubMed] [Google Scholar]
- 17.Qadri, F., et al. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quiding, M., et al. 1991. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J. Clin. Invest. 88:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha, D., et al. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318-2322. [DOI] [PubMed] [Google Scholar]
- 20.Shamsuzzaman, S., et al. 2009. Robust gut associated vaccine-specific antibody-secreting cell responses are detected at the mucosal surface of Bangladeshi subjects after immunization with an oral killed bivalent V. cholerae O1/O139 whole cell cholera vaccine: comparison with other mucosal and systemic responses. Vaccine 27:1386-1392. [DOI] [PubMed] [Google Scholar]
- 21.Svennerholm, A. M., et al. 1984. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J. Infect. Dis. 149:884-893. [DOI] [PubMed] [Google Scholar]
- 22.Tengvall, S., A. Lundgren, M. Quiding-Jarbrink, and A. M. Svennerholm. 2010. BAFF, stimulatory DNA and IL-15 stimulates [sic] IgA(+) memory B cells and provides [sic] a novel approach for analysis of memory responses to mucosal vaccines. Vaccine 28:5445-5450. [DOI] [PubMed] [Google Scholar]
- 23.Weil, A. A., et al. 2009. Memory T-cell responses to Vibrio cholerae O1 infection. Infect. Immun. 77:5090-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wennerås, C., A. M. Svennerholm, C. Ahren, and C. Czerkinsky. 1992. Antibody-secreting cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect. Immun. 60:2605-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]