Abstract
Vaccination of neonatal calves with Mycobacterium bovis bacillus Calmette-Guérin (BCG) induces a significant degree of protection against infection with virulent M. bovis, the causative agent of bovine tuberculosis (bTB). We compared two strains of BCG, Pasteur and Danish, in order to confirm that the current European human vaccine strain (BCG Danish) induced protective immunity in calves, and we assessed immune responses to determine correlates of protection that could assist future vaccine evaluation in cattle. Both vaccine strains induced antigen (purified protein derivate [PPD])-specific gamma interferon (IFN-γ) in whole-blood cultures. These responses were not significantly different for BCG Pasteur and BCG Danish and peaked at week 2 to 4 postvaccination. Vaccination with either BCG Danish or BCG Pasteur induced significant protection against bTB, with reductions in both lesion score and bacteriological burden evident in both groups of vaccinated calves compared with nonvaccinated control calves. Measurement of IFN-γ-expressing T lymphocytes postvaccination and postchallenge revealed both correlates and surrogates of protective efficacy. The frequency of central memory T lymphocytes present at 12 weeks postvaccination (at the time of M. bovis challenge) correlated significantly with protection. Conversely, the number of IFN-γ-expressing effector T cells present after M. bovis challenge was correlated with disease. These results demonstrate that vaccination of neonatal calves with either BCG Pasteur or BCG Danish induces protective immune responses against TB. In addition, we show that measurement of antigen-specific T lymphocyte populations may provide a reliable means for identifying protective vaccine candidates.
The persistence and/or increasing incidence of bovine tuberculosis (bTB) poses a human health risk and a major economic problem in developed countries, including the United Kingdom, the United States, and New Zealand. One potential control strategy is vaccination: numerous studies have been performed to generate, validate, and test the potential efficacy of TB vaccines (1, 21, 32). The live attenuated Mycobacterium bovis strain bacillus Calmette-Guérin (BCG) has been shown to have variable efficacy against TB in humans and cattle (6, 14). More recently, the optimization of the use of BCG for cattle and the application of alternative vaccination strategies such as neonatal vaccination (20, 24, 31) and heterologous prime-boost vaccination (13, 25, 26, 35, 36), have significantly improved the efficacy of BCG. Although a major constraint on the use of BCG as a vaccine in cattle is that it interferes with detection of bTB by tuberculin-based skin test and gamma interferon (IFN-γ) detection methods, diagnostic tests utilizing M. bovis-specific antigens are being developed (3, 5, 11, 34, 37).
Neonatal BCG vaccination of humans and cattle is associated with Th1-biased immune responses which may be critical for protective immunity. We and others have previously demonstrated that BCG vaccination of calves within the first weeks of life is associated with partial protection against challenge with virulent M. bovis and that protection is associated with antigen-specific IFN-γ secretion (2, 8, 20). The focus of this study was to provide additional information on the comparative protective efficacy of neonatal vaccination of cattle with the BCG strain licensed for humans in the United Kingdom, BCG Danish SSI, and BCG Pasteur. In previous studies (39), BCG Danish vaccination of calves was shown to induce poor IFN-γ responses but nevertheless induced a significant level of protective immunity against M. bovis challenge.
Here we have also assessed the potential for immunological measurements to provide correlates of BCG-induced protective immunity. Defining correlates of immunity can assist in the development of novel vaccines and vaccination strategies. Mechanisms to predict vaccine efficacy enable prioritization of vaccines for further study in experimental challenge studies. For cattle (and nonhuman primate) studies, where costs are relatively high and there are few suitable containment facilities, defining correlates which can be used to minimize the number of potential vaccine candidates is essential. We demonstrate that BCG Pasteur and BCG Danish induce similar levels of protective immunity in neonatal calves and that measurement of BCG vaccine-induced IFN-γ-expressing memory T lymphocytes could help predict vaccine efficacy.
MATERIALS AND METHODS
Bacteria and calf inoculations.
BCG Pasteur (P2; sourced from Veterinary Laboratories Agency, Weybridge) was diluted from previously titrated frozen (−70°C) stock grown in Middlebrook 7H9 broth containing 10% ADC supplement (19). Calves were inoculated with 2 ml BCG Pasteur subcutaneously (20). BCG Danish (batch number 104028A) was purchased from Statens Serum Institute, Denmark, and reconstituted from lyophilized vials in the supplied diluent according to the manufacturers' instructions (Sauton medium). Calves were inoculated subcutaneously with 0.5 ml of BCG Danish (5 times the recommended human dose). For each batch of BCG, the titer was assessed within 1 h of reconstitution/thawing by titration on 7H10 agar. The BCG dose was approximately 2 × 106 CFU per calf for both vaccines.
For challenge, M. bovis (strain AF 2122/97 [16]) was diluted immediately prior to inoculation from frozen stock in 7H9 medium. Cattle were inoculated intratracheally as previously described (36). The dose of M. bovis was confirmed by titration on modified 7H11 agar plates (15) as approximately 3 × 103 per calf.
Animals and experimental plan.
Cattle were British Holstein-Friesian calves (Bos taurus) bred at the Institute for Animal Health. The IAH herd has been confirmed free from bovine TB for more than 10 years. Animals were vaccinated with BCG at between 1 day and 27 days of age. Control animals were not vaccinated. Seven calves were assigned to each group according to age. Twelve weeks following vaccination, calves were transferred into a high-security ACDP category 3 animal housing unit and infected with M. bovis via the intratracheal route. Twelve weeks following infection, tuberculin skin tests were performed. The animals were killed 1 week later and postmortem examinations performed as described below. The experiments were approved by the local ethics committee according to United Kingdom national guidelines.
Postmortem examination and bacteriology.
Lymph nodes of the head (retropharyngeal, submandibular, and parotid) and thorax (mediastinal and four bronchus associated), tonsils, nasal, and tracheal mucosa and the seven pulmonary lobes were examined for gross lesions following the cutting of 0.5- to 1-cm slices. Macroscopic lesions were scored as previously described (33). Tissues were fixed in 10% formal saline and processed for histological examination following staining with hematoxylin and eosin. Tissues with typical lesions of TB evident by microscopy, but no gross lesions, were given a score of 1 in the overall pathology comparison. Tissue samples were frozen at −70°C for subsequent bacteriological examination by titration of tissue homogenates on modified 7H11 agar (15).
Antigens.
Purified protein derivatives from Mycobacterium avium (PPD-A) and M. bovis (PPD-B) were obtained from the tuberculin production unit at Veterinary Laboratories Agency (VLA), Weybridge. Recombinant ESAT-6 and CFP-10 were as described previously (11). These antigens are expressed by M. bovis but not by BCG and are used to discriminate between infected and vaccinated individuals.
Tuberculin skin tests.
The single comparative intradermal tuberculin test with avian and bovine PPD (PPD-A and PPD-B, respectively) was by intradermal inoculation of 0.1 ml of PPD-A and PPD-B, and reactions were read 72 h later. Results were recorded as increase in skin thickness at 72 h compared to thickness preinjection and interpreted according to the standard protocol (European Communities Commission regulation number 1226/2002) (27).
Immunological assays.
Blood was collected into heparin (10 U/ml). For cytokine assays, 4-ml aliquots of blood were incubated at 37°C for 24 h with PPD-A or PPD-B (final concentration of 20 μg per ml) diluted in RPMI with 50 μg/ml gentamicin. ESAT-6 and CFP-10 were used at a final concentration of 5 μg/ml. An equal volume of RPMI with gentamicin was used as a control. The supernatant was removed after centrifugation and stored at −20°C until assayed. IFN-γ was assayed by enzyme-linked immunosorbent assay (ELISA) as described previously, using recombinant bovine IFN-γ as a standard (23). Each sample assayed was measured in duplicate by ELISA; the variability between samples was less than 5%.
ELISPOT assay.
Secretion of IFN-γ by cultured peripheral blood mononuclear cells (PBMC) was also assessed by enzyme-linked immunospot (ELISPOT) assay (20, 41). For the cultured ELISPOT assay (40), PBMC were prepared and stimulated in 24-well plates (2 × 106 PBMC/ml; 1-ml aliquots) with PPD-B at 10 μg/ml. PBMC cultures were fed on days 5 and 8 with recombinant bovine interleukin-2 (IL-2) (final concentration, 10 U/ml). On days 10 and 12, half of the supernatant was removed and replaced with tissue culture medium without IL-2. Cells were washed four times on day 13 by centrifugation and counted, and 104 cells were added to wells of ELISPOT plates (20) and cultured together with antigen (PPD-A and PPD-B; 10 μg/ml). To each well, 1 × 103 autologous monocyte-derived dendritic cells (19) were added. Cells were cultured for 24 h, and spots were developed (20, 41). Results are expressed as spot-forming cells (SFC) per 106 PBMC.
Cytokine flow cytometry (CFC).
Whole-blood samples were incubated for 20 to 24 h at 37°C with 10 μg/ml PPD-B or an appropriate volume of phosphate-buffered saline (PBS) as a control. For the final 4 h of the incubation period, 10 μg/ml brefeldin A (BFA) was added. The expression of CD4 and intracellular IFN-γ was assessed as previously described (29). The cells were assayed using a flow cytometer (FACSCalibur; Becton Dickinson), and the data were analyzed using FCS Express (De Novo Software, Ontario, Canada). A minimum of 50,000 events were collected. The mononuclear cells were identified by light-scattering properties, and the percentages of CD4+ cells that expressed IFN-γ were calculated.
Statistical analyses.
Analyses were performed using MINITAB. Differences in immunological responses, degree of pathology, and bacterial colonization between BCG-vaccinated and control calves were compared using the Mann-Whitney test. P values of <0.05 were considered significant.
RESULTS
Vaccination with BCG Pasteur or BCG Danish in neonatal calves induces comparable levels of protection against disease caused by M. bovis challenge.
One group of neonatal calves was vaccinated with BCG Pasteur and one group with BCG Danish. A third group of calves was inoculated with medium. Twelve weeks after BCG vaccination, calves were challenged intratracheally with M. bovis; the degree of protection afforded by each strain of BCG compared to nonvaccinated controls was assessed at 12 weeks postchallenge (Tables 1 and 2). None of the animals showed clinical signs of disease (anorexia, poor responsiveness, rapid or difficult breathing, or coughing). Lesions typical of TB were observed in all of the nonvaccinated control animals, in both lymph nodes and lung. Although the extent of lesions varied between animals, as reflected by the lesion scores (Table 1), in each case lesions were primarily restricted to the respiratory tract-associated lymph nodes and lungs. One control animal (no. 135) had a single lesion in the right submandibular lymph node. Both BCG Danish and BCG Pasteur conferred significant protection against disease, with reduced lesion scores noted in lymph nodes (P < 0.01) and lung (P < 0.05). This reflects both reduced number and size of TB lesions in vaccinated animals. There was no significant difference in the level of protection induced by BCG Danish versus BCG Pasteur.
TABLE 1.
Lesions at necropsy 12 weeks after M. bovis challenge in BCG-vaccinated and control animals
| Vaccination and animal no. | Total lesion scorea |
|
|---|---|---|
| Lymph nodesb | Lungc | |
| BCG Pasteur | ||
| 105 | 0 | 0 |
| 109 | 10 | 8 |
| 118 | 0 | 5 |
| 123 | 1 | 0 |
| 129 | 0 | 2 |
| 134 | 2 | 0 |
| 137 | 0 | 0 |
| Median | 0** | 0* |
| BCG Danish | ||
| 104 | 5 | 3 |
| 113 | 0 | 4 |
| 117 | 2 | 3 |
| 127 | 4 | 6 |
| 130 | 5 | 3 |
| 133 | 2 | 3 |
| 138 | 3 | 8 |
| Median | 3** | 3* |
| Control M. bovis alone | ||
| 101 | 11 | 7 |
| 103 | 16 | 10 |
| 106 | 8 | 8 |
| 108 | 6 | 10 |
| 128 | 4 | 6 |
| 131 | 13 | 6 |
| 135 | 11 | 5 |
| Median | 11 | 7 |
**, P < 0.01 compared to control M. bovis-alone group; *, P < 0.05 compared to control M. bovis-alone group.
Total lesion score for head lymph nodes (left and right parotid, submandibular, retropharyngeal, and tonsil) and respiratory tract-associated lymph nodes (mediastinal and four bronchial nodes).
Total lesion score for seven lung lobes (left and right apical, medial, diaphragmatic, and intermediate).
TABLE 2.
M. bovis viable counts in tissues taken postmortem from BCG-vaccinated and control animals
| Vaccination and animal no. | Total bacterial count (log10 CFU)a |
|
|---|---|---|
| Lymph nodesb | Lungc | |
| BCG Pasteur | ||
| 105 | 2.9 | ND |
| 109 | 4.6 | 3.5 |
| 118 | 4.2 | 3.5 |
| 123 | 4.3 | ND |
| 129 | 2.8 | ND |
| 134 | 4 | ND |
| 137 | 2.1 | ND |
| Median | 4.04** | 0* |
| BCG Danish | ||
| 104 | 4.6 | 4.4 |
| 113 | 4.3 | 2.3 |
| 117 | 4.3 | 3.4 |
| 127 | 4.1 | 3.7 |
| 130 | 4.6 | 1.7 |
| 133 | 4 | 3.2 |
| 138 | 4.2 | 3.5 |
| Median | 4.26* | 3.39 |
| Control M. bovis alone | ||
| 101 | 4.8 | 4.2 |
| 103 | 4.8 | 3.7 |
| 106 | 5.2 | 3.1 |
| 108 | 5 | 4 |
| 128 | 4.2 | 4.5 |
| 131 | 4.6 | 4.3 |
| 135 | 4.8 | 3.6 |
| Median | 4.78 | 3.96 |
Viable counts were recorded as log10 CFU per gram tissue. ND, not detected (below sensitivity limit of <5 CFU). **, P < 0.01 compared to control M. bovis-alone group; *, P < 0.05 compared to control M. bovis-alone group.
Total log10 CFU for head lymph nodes (left and right parotid, submandibular, retropharyngeal, and tonsil) and respiratory tract-associated lymph nodes (mediastinal and four bronchial nodes).
Total log10 CFU for seven lung lobes (left and right apical, medial, diaphragmatic, and intermediate).
BCG vaccination of neonatal calves reduces the level of bacterial colonization.
Bacteriological examination of tissues indicated that M. bovis was present in numbers of up to 7 × 104 CFU per gram from the majority of tissues with gross lesions (Table 2), with a significant correlation between the number of isolated bacteria and lesion score (P < 0.05; data not shown). Overall, significantly fewer bacteria were isolated from tissues from both the BCG Danish- and BCG Pasteur-vaccinated groups than from the control group (P < 0.05). However, when bacterial numbers in lymph nodes and lung were analyzed separately, differences between the vaccines were observed. A significant reduction in CFU was observed in the lymph nodes of calves vaccinated with BCG Danish and BCG Pasteur (P < 0.05), whereas BCG Pasteur induced significantly greater protection in lung tissue (P < 0.05).
Skin test reactivity in BCG-vaccinated and control calves following M. bovis challenge.
Reactions to PPD in the skin test were observed in all animals at 12 weeks after challenge with M. bovis (Table 3). Visible local clinical signs and increases in comparative skin thickness (skin test thickness response with PPD-B minus that with PPD-A) ranging from 8 to 30 mm were recorded. Low responses to PPD-A were noted, and all animals would be classified as reactors according to the standard interpretation of the skin test (European Communities Commission regulation number 1226/2002) (27). There were no significant differences between any of the groups.
TABLE 3.
Tuberculin skin test responses
| Vaccination and animal no. | Increase (mm)a |
||
|---|---|---|---|
| PPD-A | PPD-B | PPD-B − PPD-A | |
| BCG Pasteur | |||
| 105 | 3 | 16 | 13 |
| 109 | 8 | 34 | 26 |
| 118 | 5 | 17 | 12 |
| 123 | 5 | 28 | 23 |
| 129 | 6 | 23 | 17 |
| 134 | 6 | 27 | 21 |
| 137 | 4 | 16 | 12 |
| Mean | 17.1 | ||
| BCG Danish | |||
| 104 | 5 | 28 | 23 |
| 113 | 3 | 12 | 9 |
| 117 | 2 | 19 | 17 |
| 127 | 5 | 28 | 23 |
| 130 | 6 | 31 | 25 |
| 133 | NT | NT | NT |
| 138 | 4 | 29 | 25 |
| Mean | 21 | ||
| Control M. bovis alone | |||
| 101 | 4 | 33 | 29 |
| 103 | 4 | 28 | 24 |
| 106 | 1 | 9 | 8 |
| 108 | 4 | 26 | 22 |
| 128 | 5 | 28 | 23 |
| 131 | 6 | 26 | 20 |
| 135 | 6 | 36 | 30 |
| Mean | 22.3 | ||
Increase in skin thickness from day 0 to day 3. No significant differences between groups were noted, and all animals were classed as reactors according to the standard interpretation of the test. NT, not tested.
BCG Danish and BCG Pasteur induce similar kinetics and intensity of antigen-specific IFN-γ responses in whole-blood cultures.
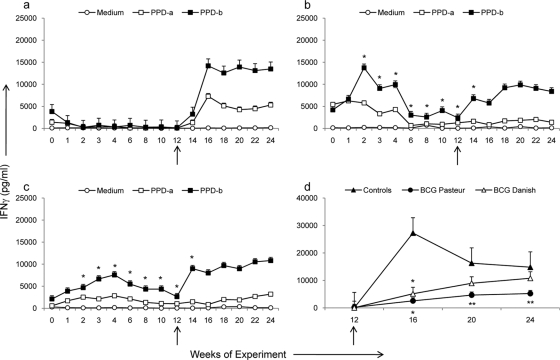
Immune responses were monitored for 12 weeks in nonvaccinated control (Fig. 1 a), BCG Pasteur-vaccinated (Fig. 1b), and BCG Danish-vaccinated (Fig. 1c) calves. At 12 weeks the calves were infected with M. bovis, and immune responses were monitored for an additional 12 weeks (the time of challenge is indicated by an arrow). Significant increases in PPD-B-specific IFN-γ secretion in response to vaccination with both BCG Pasteur (Fig. 1b) and BCG Danish (Fig. 1c) were evident as early as 2 weeks postvaccination (P < 0.05). The peak IFN-γ response to BCG was detected between 2 and 4 weeks postvaccination, and this had waned significantly by week 12 (P < 0.05). There were no significant differences detected between animals vaccinated with BCG Pasteur and those vaccinated with BCG Danish at any time point postvaccination. No significant PPD-B-specific IFN-γ was detected in the control group prior to M. bovis challenge at week 12 (Fig. 1a). There was no significant correlation between the overall IFN-γ produced during the 12-week vaccination period and the extent of protection against disease as assessed by lesion score (r2 = 0.115). Responses postvaccination to ESAT-6 and CFP-10 were absent or very low and intermittent (<200 pg/ml at any time [data not shown]).
FIG. 1.
Secretion of antigen-specific IFN-γ in whole-blood cultures. (a to c) Levels of IFN-γ released from bovine whole-blood cultures stimulated with PPD-A, PPD-B, or medium from nonvaccinated controls challenged with M. bovis at week 12 (arrow) (a), animals vaccinated with BCG Pasteur at week 0 and challenged with M. bovis at week 12 (b), or animals vaccinated with BCG Danish at week 0 and challenged with M. bovis at week 12 (c). (d) Whole-blood IFN-γ responses to stimulation with ESAT-6/CFP-10 cocktail were measured after M. bovis challenge in control calves, BCG Pasteur-vaccinated calves, and BCG Danish-vaccinated calves. IFN-γ levels are expressed as group mean values (± standard deviation [SD]) for plasma concentrations (pg/ml). *, P < 0.05; **, P < 0.01 (compared to nonvaccinated control group). No significant differences were observed between vaccines.
After challenge with M. bovis, a rapid induction of PPD-B-specific IFN-γ was observed in both vaccinated groups. Two weeks following challenge (week 14), PPD-B-specific responses were elevated in vaccinated calves (Fig. 1b and c) and were significantly higher than those in control calves (Fig. 1a) (P < 0.05). By week 4 postchallenge (Fig. 1, week 16), all calves displayed strong IFN-γ responses, and no significant differences were detected between groups from this point on. No significant differences in PPD-B-specific IFN-γ secretion between BCG Danish-vaccinated and BCG Pasteur-vaccinated groups were noted at any time point throughout the time period. Reactivity to ESAT-6 and CFP-10 was detected in all animals by week 4 postchallenge (Fig. 1d, week 16) but was significantly lower in all BCG-vaccinated animals than in nonvaccinated animals (P < 0.05). Animals vaccinated with BCG Pasteur also had significantly lower ESAT-6/CFP-10 responses than nonvaccinated animals at weeks 20 and 24 (Fig. 1d) (P < 0.01). The total ESAT-6/CFP10-specific IFN-γ measured postchallenge correlated with lesion score (r2 = 0.78; P < 0.001). No significant differences between BCG Pasteur or BCG Danish groups were noted at any time point after M. bovis challenge.
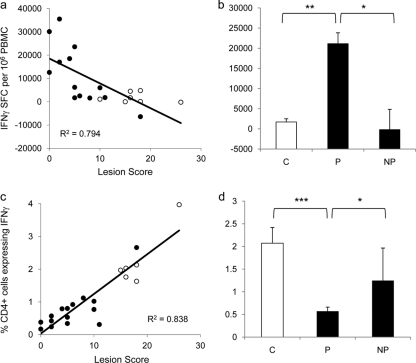
The frequency of antigen-specific IFN-γ-secreting memory cells induced postvaccination correlates with protective immunity.
At 12 weeks postvaccination, immediately prior to M. bovis challenge, PBMC were isolated from all calves and cultured in the presence of antigen and IL-2 to establish short-term T cell lines. Following 2 weeks of culture, the presence of memory T cells secreting IFN-γ was assessed by ELISPOT assay. The frequency of PPD-B-specific SFC (PPD-B minus PPD-A) was calculated (Fig. 2 a). Vaccination of cattle with either BCG Danish or BCG Pasteur (Fig. 2a, closed symbols) induced IFN-γ-expressing central memory T lymphocytes, and these were significantly higher than those observed in nonvaccinated animals (Fig. 2a, open symbols) at the same time point (P < 0.05). The number of central memory lymphocytes present at the time of M. bovis challenge (week 12 post-BCG) was negatively correlated with the severity of disease, as assessed by lesion score (Pearson correlation, −0.794; P < 0.0001).
FIG. 2.
The frequency of antigen-specific IFN-γ-secreting T lymphocytes correlates with protective immunity. (a) At 12 weeks after BCG vaccination, memory cell responses were assessed by cultured ELISPOT assay. The number of PPD-B-specific IFN-γ SFC induced by vaccination (black circles) or in control calves (white circles) correlates with protection against TB as determined by lesion score. (b) Cultured ELISPOT responses in relation to vaccine success (vaccinated/protected, P) or failure (vaccinated/unprotected, NP). C, nonvaccinated controls. (c) At 12 weeks after M. bovis challenge, the capacity for CD4+ T cells to express PPD-B-specific IFN-γ was assessed by flow cytometric analysis. The number of CD4+ T cells expressing PPD-B-specific IFN-γ was assessed for vaccinated (black circles) and control calves (white circles). (d) Number of CD4+ T cells expressing IFN-γ in flow cytometric analysis in relation to vaccine success (vaccinated/protected, P) or failure (vaccinated/unprotected, NP). C, nonvaccinated controls. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
By subdividing the groups of animals into nonvaccinated control animals (C), vaccinated/protected animals (P), and vaccinated/unprotected animals (NP) as previously described (36), 11 animals were defined as protected (lesion score of less than 10) and 3 as not protected (lesion score of greater than 10). Assessment of the cultured ELISPOT responses showed that the vaccinated/protected animals had significantly (P < 0.05) higher responses postvaccination than the vaccinated/unprotected animals, which had cultured ELISPOT responses similar to those of the control group (Fig. 2b). Thus, higher numbers of central memory cells were associated with protective immunity induced by BCG.
The frequency of antigen-specific IFN-γ-secreting CD4+ T cells induced postchallenge is a surrogate for protective immunity.
At 12 weeks after M. bovis challenge, the percentage of CD4+ T lymphocytes expressing IFN-γ in response to PPD-B stimulation of whole blood was measured (Fig. 2c). The frequency of CD4+ T lymphocytes expressing PPD-B-specific IFN-γ was significantly higher in animals that were not vaccinated with BCG prior to M. bovis infection (Fig. 2c, open symbols) than in those which were previously BCG vaccinated (Fig. 2c, closed symbols) (P < 0.05). The number of IFN-γ-expressing antigen-specific CD4+ T lymphocytes correlated with the severity of disease; those animals with high lesion scores had elevated numbers of antigen-specific IFN-γ-expressing lymphocytes (Pearson correlation, 0.838; P < 0.0001). The vaccinated/protected animals had significantly lower numbers of IFN-γ-expressing CD4+ T cells (Fig. 2d) than both the control animals (P < 0.001) and the vaccinated/unprotected animals (P < 0.05).
DISCUSSION
Vaccination of neonatal calves with BCG Pasteur has been demonstrated to induce significant protection against experimental challenge with virulent M. bovis (2, 8, 20). However, of the available daughter strains of BCG, only commercially produced BCG Danish is licensed for use in humans in the United Kingdom. The focus of this study was therefore to provide additional information on the comparative protective efficacies of neonatal vaccination of cattle with the human BCG Danish vaccine strain (SSI) and BCG Pasteur.
Vaccination with either BCG Pasteur or BCG Danish significantly reduced the lesion score after M. bovis challenge, and no significant differences between vaccines were noted. Similar protective efficacies of BCG Danish and BCG Pasteur were reported in other studies of M. bovis infection in cattle (39) and in white-tailed deer (28) and for protection against Mycobacterium tuberculosis infection in mice (10). Some differences were observed between vaccines in terms of reduction of bacterial colonization: commercially produced BCG Danish appeared to be less effective in controlling M. bovis growth in the lungs than laboratory-grown BCG Pasteur. This contrasts with previously published data which showed that BCG Danish was more effective than BCG Pasteur in reducing M. bovis counts in the lung (39). These modest differences between studies may simply reflect relatively small group sizes and variability in sampling.
The identification of immunological correlates of protection is essential to refine vaccine trials, to reduce the costs of challenge experiments, and to prioritize vaccine candidates entering efficacy trials. IFN-γ is an essential component of protective immune responses to M. bovis; vaccines which fail to prime for the production of antigen-specific IFN-γ recall memory responses invariably fail to protect against virulent challenge, and in some studies, postvaccination levels of IFN-γ do correlate well with protection (reviewed in references 7, 18, and 22). Here, both BCG Pasteur and BCG Danish induced strong PPD-B-specific IFN-γ responses, but the overall level of IFN-γ detected during the vaccination period did not correlate with protective efficacy (r2 = 0.115). Interestingly, a previous study (39) found that BCG Danish vaccination of calves induced poor IFN-γ responses compared with BCG Pasteur vaccination, but this did not affect the protective immunity induced by BCG Danish.
A number of studies have shown significant correlations between protective immunity and cultured ELISPOT responses (9, 17, 30, 36). Although we have not shown definitively for cattle that the cultured ELISPOT assay measures only central memory, and not effector, T cell responses (due to a lack of specific reagents for central memory T cells in cattle, such as CCR7), a recent study revealed that PPD-specific cultured ELISPOT responses in humans were principally those of central memory T cells, as depletion of these cells ablated the cultured ELISPOT response (30). As shown in other cattle vaccine trials (36, 38), we demonstrated here that cultured ELISPOT responses predict vaccine success and that the number of IFN-γ-secreting memory T cells induced by vaccination correlates with protective efficacy. Endsley et al. showed that BCG vaccination of cattle induced memory CD4+ T cell populations with the capacity to kill infected macrophages (12) that may play important roles in protective immunity against virulent challenge, providing additional evidence that the quality of the CD4+ T cell response induced by vaccination is a key factor in vaccine success. Postchallenge, the number of antigen-specific IFN-γ-secreting effector CD4+ T cells correlated with the extent of disease, suggesting that this parameter can be used as a surrogate or predictor of disease as well as vaccine failure (29). Postchallenge IFN-γ measurements in whole blood stimulated by either PPD-B or the M. bovis-specific antigens ESAT-6 and CFP-10 also showed a correlation between extent of disease and IFN-γ levels. As demonstrated previously, low levels of ESAT-6/CFP-10-specific IFN-γ were detected in vaccinated/protected animals compared to the nonvaccinated controls, and these responses are inverse correlates of protection (20, 33). The responses to M. bovis-specific antigens such as ESAT-6 and CFP10 are central to immunodiagnostic tests differentiating between infected and vaccinated animals (DIVA) (4, 37).
In summary, we have shown that vaccination of neonatal calves with either BCG Pasteur or BCG Danish significantly reduces disease and bacterial load following challenge with M. bovis. Furthermore, we provide additional evidence that measurement of central memory T cells induced by vaccination can predict vaccine success or failure. This adds to the accumulating body of evidence suggesting that BCG vaccination, either alone or in prime-boost strategies, may be a useful tool for the control of bovine tuberculosis.
Acknowledgments
This work was supported by grants from the Department for the Environment, Food and Rural Affairs (DEFRA), United Kingdom, and the Biotechnology and Biological Science Research Council (BBSRC), United Kingdom. J. C. Hope, H. M. Vordermeier, and R. G. Hewinson are Jenner Institute Investigators.
We gratefully acknowledge the staff of the IAH animal facilities for care of the cattle and members of the bovine immune mechanisms group for help with necropsy, histological examination, and bacteriology.
Footnotes
Published ahead of print on 12 January 2011.
REFERENCES
- 1.Buddle, B. M. 2010. Tuberculosis vaccines for cattle: the way forward. Expert Rev. Vaccines 9:1121-1124. [DOI] [PubMed] [Google Scholar]
- 2.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 3.Buddle, B. M., et al. 2003. Use of mycobacterial peptides and recombinant proteins for the diagnosis of bovine tuberculosis in skin test-positive cattle. Vet. Rec. 153:615-620. [DOI] [PubMed] [Google Scholar]
- 4.Buddle, B. M., et al. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buddle, B. M., T. J. Ryan, J. M. Pollock, P. Andersen, and G. W. de Lisle. 2001. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 6.Buddle, B. M., B. J. Wards, F. E. Aldwell, D. M. Collins, and G. W. de Lisle. 2002. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 20:1126-1133. [DOI] [PubMed] [Google Scholar]
- 7.Buddle, B. M., D. N. Wedlock, M. Denis, and M. A. Skinner. 2005. Identification of immune response correlates for protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 108:45-51. [DOI] [PubMed] [Google Scholar]
- 8.Buddle, B. M., et al. 2003. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect. Immun. 71:6411-6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calarota, S. A., et al. 2008. HIV-1-specific T cell precursors with high proliferative capacity correlate with low viremia and high CD4 counts in untreated individuals. J. Immunol. 180:5907-5915. [DOI] [PubMed] [Google Scholar]
- 10.Castillo-Rodal, A. I., et al. 2006. Mycobacterium bovis BCG substrains confer different levels of protection against Mycobacterium tuberculosis infection in a BALB/c model of progressive pulmonary tuberculosis. Infect. Immun. 74:1718-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cockle, P. J., et al. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endsley, J. J., et al. 2007. Mycobacterium bovis BCG vaccination induces memory CD4+ T cells characterized by effector biomarker expression and anti-mycobacterial activity. Vaccine 25:8384-8394. [DOI] [PubMed] [Google Scholar]
- 13.Ferraz, J. C., et al. 2004. A heterologous DNA priming-Mycobacterium bovis BCG boosting immunization strategy using mycobacterial Hsp70, Hsp65, and Apa antigens improves protection against tuberculosis in mice. Infect. Immun. 72:6945-6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher, J., and D. M. Horwill. 1977. A selective oleic acid albumin agar medium for the cultivation of Mycobacterium bovis. J. Hyg. (Lond.) 79:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garnier, T., et al. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. U. S. A. 100:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goletti, D., et al. 2006. Region of difference 1 antigen-specific CD4+ memory T cells correlate with a favorable outcome of tuberculosis. J. Infect. Dis. 194:984-992. [DOI] [PubMed] [Google Scholar]
- 18.Hogarth, P. J., R. G. Hewinson, and H. M. Vordermeier. 2006. Development of vaccines against bovine tuberculosis. J. Pharm. Pharmacol. 58:749-757. [DOI] [PubMed] [Google Scholar]
- 19.Hope, J. C., L. S. Kwong, P. Sopp, R. A. Collins, and C. J. Howard. 2000. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in Bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand. J. Immunol. 52:285-291. [DOI] [PubMed] [Google Scholar]
- 20.Hope, J. C., et al. 2005. Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. bovis. Clin. Exp. Immunol. 139:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hope, J. C., and B. Villarreal-Ramos. 2008. Bovine TB and the development of new vaccines. Comp. Immunol. Microbiol. Infect. Dis. 31:77-100. [DOI] [PubMed] [Google Scholar]
- 22.Hope, J. C., and H. M. Vordermeier. 2005. Vaccines for bovine tuberculosis: current views and future prospects. Expert Rev. Vaccines 4:891-902. [DOI] [PubMed] [Google Scholar]
- 23.Kwong, L. S., et al. 2002. Development of an ELISA for bovine IL-10. Vet. Immunol. Immunopathol. 85:213-223. [DOI] [PubMed] [Google Scholar]
- 24.Marchant, A., et al. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 25.McShane, H., and A. Hill. 2005. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 7:962-967. [DOI] [PubMed] [Google Scholar]
- 26.McShane, H., et al. 2005. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis (Edinb.) 85:47-52. [DOI] [PubMed] [Google Scholar]
- 27.Morrison, W. I., et al. 2000. Pathogenesis and diagnosis of infections with Mycobacterium bovis in cattle. Independent Scientific Group on Cattle TB. Vet. Rec. 146:236-242. [PubMed] [Google Scholar]
- 28.Palmer, M. V., T. C. Thacker, and W. R. Waters. 2009. Vaccination with Mycobacterium bovis BCG strains Danish and Pasteur in white-tailed deer (Odocoileus virginianus) experimentally challenged with Mycobacterium bovis. Zoonoses Public Health 56:243-251. [DOI] [PubMed] [Google Scholar]
- 29.Sopp, P., C. J. Howard, and J. C. Hope. 2006. Flow cytometric detection of gamma interferon can effectively discriminate Mycobacterium bovis BCG-vaccinated cattle from M. bovis-infected cattle. Clin. Vaccine Immunol. 13:1343-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todryk, S. M., et al. 2009. The relationship between human effector and memory T cells measured by ex vivo and cultured ELISPOT following recent and distal priming. Immunology 128:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vekemans, J., et al. 2001. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 32.Vordermeier, H. M., M. A. Chambers, B. M. Buddle, J. M. Pollock, and R. G. Hewinson. 2006. Progress in the development of vaccines and diagnostic reagents to control tuberculosis in cattle. Vet. J. 171:229-244. [DOI] [PubMed] [Google Scholar]
- 33.Vordermeier, H. M., et al. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vordermeier, H. M., P. J. Cockle, A. O. Whelan, S. Rhodes, and R. G. Hewinson. 2000. Toward the development of diagnostic assays to discriminate between Mycobacterium bovis infection and bacille Calmette-Guerin vaccination in cattle. Clin. Infect. Dis. 30(Suppl. 3):S291-S298. [DOI] [PubMed] [Google Scholar]
- 35.Vordermeier, H. M., et al. 2004. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guerin. Immunology 112:461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vordermeier, H. M., et al. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77:3364-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vordermeier, H. M., et al. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waters, W. R., et al. 2009. Efficacy and immunogenicity of Mycobacterium bovis DeltaRD1 against aerosol M. bovis infection in neonatal calves. Vaccine 27:1201-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wedlock, D. N., M. Denis, H. M. Vordermeier, R. G. Hewinson, and B. M. Buddle. 2007. Vaccination of cattle with Danish and Pasteur strains of Mycobacterium bovis BCG induce different levels of IFNgamma post-vaccination, but induce similar levels of protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 118:50-58. [DOI] [PubMed] [Google Scholar]
- 40.Whelan, A. O., et al. 2008. Evidence for enhanced central memory priming by live Mycobacterium bovis BCG vaccine in comparison with killed BCG formulations. Vaccine 26:166-173. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, Y., et al. 2003. CpG ODN 2006 and IL-12 are comparable for priming Th1 lymphocyte and IgG responses in cattle immunized with a rickettsial outer membrane protein in alum. Vaccine 21:3307-3318. [DOI] [PubMed] [Google Scholar]