Abstract
Use of a heterologous prime-boost strategy based on a combination of nonreplicative immunogens and candidate attenuated virus vaccines against dengue virus in the same schedule is an attractive approach. These combinations may result in a condensed immunization regime for humans, thus reducing the number of doses with attenuated virus and the time spacing. The present work deals with the evaluation of the heterologous prime-boost strategy combining a novel chimeric protein (domain III-capsid) of dengue virus serotype 2 (DEN-2) and the infective homologous virus in the same immunization schedule in monkeys. Primed monkeys received one dose of infective DEN-2 and were then vaccinated with the recombinant protein. We found that animals developed a neutralizing antibody response after the infective dose and were notably boosted with a second dose of the chimeric protein 3 months later. The neutralizing antibodies induced were long lasting, and animals also showed the ability to induce a specific cellular response 6 months after the booster dose. As a conclusion, we can state that the domain III region, when it is properly presented as a fusion protein to the immune system, is able to recall the neutralizing antibody response elicited following homologous virus infection in monkeys. Further prime-boost approaches can be performed in a condensed regime combining the chimeric domain III-capsid protein and candidate live attenuated vaccines against DEN-2.
Dengue is a mosquito-transmitted viral infection of high incidence worldwide. It is caused by four antigenically related but distinct dengue virus (DEN) serotypes which belong to the family Flaviviridae, genus Flavivirus (2), and which have been estimated to cause up to 100 million infections annually (11).
Despite the high incidence of this disease, currently there is no vaccine against dengue. At present, the live attenuated viruses (LAVs) are the most advanced candidate vaccines against the infection. These candidate vaccines achieve a broad-spectrum immune response due to their replicative capacity (12, 13, 24). However, for the same characteristic, they have been associated with nonpredictable interactions among the four virus serotypes when they are administered in tetravalent formulations (13, 23, 24). This is why it becomes difficult to reach a satisfactory balance between immunogenicity and attenuation (18). To solve this problem, the administration of several spaced doses is required for current candidates based on this technology, including immunization programs that can take up to a year to be completed (13, 22-24).
One of the attractive alternatives to solve the previous disadvantages is the use of a heterologous prime-boost strategy based on a combination of nonreplicative immunogens and candidate attenuated virus vaccines in the same schedule (25). These combinations may result in condensed immunization schedules for humans, thus reducing the number of doses with attenuated virus and the time spacing. On the other hand, the use of a suitable combination using nonreplicative immunogens, without the viral interference phenomenon, can help to induce a balanced response against the fours serotypes. In this sense, we previously described two studies in monkeys combining recombinant protein PD5 (domain III of the envelope [E] protein from DEN serotype 2 [DEN-2] fused to the protein carrier P64k) and infective DEN in the same immunization regime. In the first study, monkeys received four doses of the protein PD5 and were subsequently infected with one dose of DEN. The antibody response measured after virus inoculation in the primed monkeys was significantly higher than that in nonprimed monkeys and comparable to that elicited following two doses of infective virus (27). In the second study, monkeys were infected with one dose of the virus and subsequently boosted with one dose of the recombinant protein, reaching high levels of neutralizing antibodies which were still detectable 14 months after the last immunization (27).
In addition, in 2009 Simmons and colleagues proposed a prime-boost regime employing nonreplicating variants (a tetravalent DNA formulation or a tetravalent preparation based on the inactivated virus) with the replicative candidate of GSK (live attenuated tetravalent vaccine) (25). In this study, animals which received the combinations of the inactivated formulations and the LAV were completely protected after the viral challenge (25).
The present work deals with the evaluation of a prime-boost approach including a novel chimeric protein (domain III-capsid) and infective DEN-2 in the same immunization schedule in monkeys. The recombinant domain III-capsid protein comprises the domain III region of the envelope protein and the capsid protein, both from DEN-2. Previously, this protein in aggregated form was able to induce neutralizing antibodies, cell-mediated immunity (CMI), and protection against DEN-2 challenge in mice (26). In the present study, primed animals received one dose of the infective DEN-2 and were then vaccinated with the recombinant domain III-capsid protein. We found that primed monkeys developed an immune response and were notably boosted after inoculation of the chimeric protein 3 months later. The neutralizing antibodies induced were long lasting, and animals also showed the ability to induce a specific cellular immune response after the booster dose.
MATERIALS AND METHODS
Animals.
Healthy adult green monkeys (Cercopithecus aethiops sabaeus) were obtained from CENPALAB (Havana, Cuba). All animals were screened for previous exposure to dengue virus by enzyme-linked immunosorbent assay (ELISA) and plaque reduction neutralization test (PRNT). Animals were considered naive when antigen-specific antibodies were undetectable by ELISA (titer < 1:100) and PRNT (titer < 1:10). Monkeys were maintained in accordance with the Cuban guidelines for the care and use of laboratory animals.
Viruses.
A preparation from a suckling mouse brain infected with DEN-2 (strain New Guinea C) was used as a sucrose-acetone antigen (5) for immunoassays. A similar preparation obtained from the brain of a noninoculated mouse was used as a negative control. For the PRNT, cell culture supernatant harvested from Vero cells infected with DEN-2 strain SB8553 (kindly provided by M. J. Cardosa, University Sarawak, Malaysia) was used. A viral stock for the challenge study was prepared with DEN-2 strain SB8553 in Vero cells using fresh supplemented RPMI 1640 medium (5% heat-inactivated fetal bovine serum [FBS], 2 mM l-glutamine, 100 U/ml of penicillin [Gibco], 100 μg/ml streptomycin [Gibco]). The supernatant was harvested 144 h later and then aliquoted, stored at −70°C, and titrated by plaque formation on BHK-21 cells.
Recombinant protein.
The chimeric domain III-capsid protein is formed by the fusion of the domain III moiety from DEN-2 in the N-terminus region of the capsid protein of the same virus (12). To obtain the particulate form of the protein, the recombinant domain III-capsid molecule was purified as described previously (26). The purified preparation was incubated with a mixture of 50-base-long DNA oligonucleotides (ODNs) at a 3:1 protein/oligonucleotide molecular ratio basically according to the procedure described by Valdés et al. in 2009 (26).
Animal immunizations and viral isolation.
Three monkeys were subcutaneously inoculated in the upper arms with 4 log10 PFU infective virus, DEN-2 strain SB8553. Blood was collected daily for 10 days to detect viremia. Sera from clotted blood were stored at −70°C until viremia was analyzed.
The presence of virus in the sera was determined by inoculating 0.15 ml of serum diluted 1:10 onto Vero cells grown in 25-cm2 flasks at a final volume of 2.5 ml. After 1 h of incubation at 37°C, an additional 2.5 ml of fresh supplemented RPMI 1640 medium was added to the flasks and the cultures were incubated for 6 days. The culture supernatants were collected and assayed for the presence of virus by ELISA.
Three months after the viral inoculation, monkeys were subcutaneously injected with 100 μg of the aggregated domain III-capsid protein using aluminum hydroxide (alum) at a final concentration of 1.44 mg/ml. For serological studies, additional blood samples were taken at days 0, 30, 60, 90, 120, 150, 180, 210, and 270 after inoculations.
Analysis of antibody response.
The anti-DEN IgG antibodies stimulated by immunization were monitored by an amplified sandwich ELISA system as previously described (28). The absorbance at 492 nm was read in a microplate reader (SensIdent Scan; Merck, Germany). Titers were defined as the dilution of serum giving twice the absorbance value of the negative-control serum.
The functionality of the antibodies was measured by neutralization of DEN-2 infectivity by a PRNT on BHK-21 cell culture as previously described (21).
Virus detection by enzyme-linked immunosorbent assay.
Flat-bottom 96-well plates (Costar) were coated with anti-DEN human IgG (5 μg/ml) for 2 h at 37°C. The plates were then blocked with 2% bovine serum albumin and incubated for 1 h at 37°C. After two washes with phosphate-buffered saline-Tween 20 (PBS-T), culture supernatant was added in triplicate to each well, and the plates were incubated for 2 h at 37°C. Three additional washes were performed, and then polyclonal hyperimmune mouse ascitic fluid diluted 1:2,000 in PBS-T with 1% normal mouse brain antigen was added to the plates. After 1 h of incubation at 37°C, the plates were washed again three times and then anti-mouse IgG-peroxidase conjugate (Sigma) was added. The plates were incubated for 1 h at 37°C and washed three times, and 0.04% substrate solution (o-phenylenediamine in buffer consisting of 2% Na2HPO4, 1% citric acid, and 30% H2O2, pH 5.0) was added. The reaction was stopped 30 min later by addition of 12.5% H2SO4, and the absorbance at 492 nm was read in a microplate reader (SensIdent Scan; Merck). A value of absorbance of 2-fold the absorbance of the supernatant from an uninfected culture was considered a positive result.
Measurement of cell-mediated immunity.
Blood was obtained by venipuncture at day 180 after the dose with the domain III-capsid formulation. The peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque Plus (Amersham Biosciences AB, Uppsala, Sweden) density gradient centrifugation. Cells were washed twice with PBS-2% FBS (PAA Laboratories, Canada) and resuspended at 2 × 106 cells/ml in RPMI 1640 medium (Sigma Aldrich, Ayrshire KA, United Kingdom) supplemented with 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco), 2 mM glutamine (Glutamax; Gibco), 5 × 10−5 M 2-mercaptoethanol (Sigma, St. Louis, MO), and 5% FBS. Finally, 2 × 105 cells/well were cultured in 96-well round-bottom plates with the antigens (3 log10 PFU of DEN-2 antigen or a mock preparation). Concanavalin A (Sigma) was used as a positive control. In all the experiments, three wells were plated for each antigen. After 4 days of culture, culture supernatants were collected and stored at −20°C.
The culture supernatants previously stimulated with each antigen were analyzed in duplicate for the gamma interferon (IFN-γ) concentration by ELISA using monoclonal antibody pairs (Mabtech, Sweden). The ELISA protocol recommended by the manufacturer was used, with slight modifications. The lower limit of detection of cytokine in this assay was 10 pg/ml.
RESULTS
Viremia response after virus inoculation.
A group of 3 green monkeys was infected with 4 log10 PFU of DEN-2, and the viremia was followed by isolation in Vero cells and detection of the virus by ELISA. The virus was efficiently isolated from the sera of the infected animals, with a mean of 4.67 days of viremia. These results are similar to those previously described by Martín et al. in 2009 for this monkey species (19).
Humoral immune response of infected green monkeys after the immunizations.
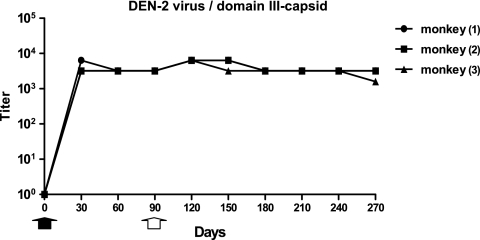
Three months later, animals received one dose of the aggregated form of the domain III-capsid protein adjuvanted on alum. Antiviral antibodies that developed after both immunizations were determined by ELISA. As shown in Fig. 1, the antiviral response after inoculation of the infective viral dose reached maximum values at days 30 and 60 upon infection, with geometric mean titers (GMTs) of 4,031.7 and 3,200, respectively (Fig. 1). Following the administration of the recombinant protein, a slight increase in antiviral antibody titers was detected, with a GMT of 6,400 at 120 days (30 days after the booster dose).
FIG. 1.
Kinetics of IgG antibodies against DEN-2 by ELISA. Black arrow, day of inoculation with DEN-2; white arrow, day of inoculation of a dose of domain III-capsid protein. Points in the graph indicate antibody titers.
The functionality of the antibodies induced by the two immunizations was determined by a PRNT. Table 1 shows the neutralizing antibody titers obtained at each time. In contrast to the antiviral response, titers of neutralizing antibodies following immunization with the recombinant protein domain III-capsid were markedly boosted, reaching a peak 1 month later. The neutralizing antibody GMT at day 90 was 160.9, whereas at day 120 it reached a value of 879.4, indicating a clear boost effect upon administration of the recombinant protein. Even though the neutralizing antibody titers declined slowly over time, at 6 months they were still detectable and higher than those observed at the time of inoculation of the heterologous dose. The humoral immune response measured by ELISA and by PRNT at day 270 showed GMTs of 2,539.8 for antiviral antibodies and 341.1 of neutralizing antibodies, respectively.
TABLE 1.
Neutralizing antibody response induced in vaccinated monkeys, measured by PRNT
| Monkey | Neutralizing antibody titer on daya: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (prime) | 30 | 60 | 90 (boost) | 120 | 150 | 180 | 210 | 270 | |
| 1 | <10 | 68 | 42 | 213 | 850 | 1,000 | 1,000 | 1,000 | 174 |
| 2 | <10 | 35 | 278 | 136 | 1,000 | 1,000 | 677 | 650 | 637 |
| 3 | <10 | 16 | 112 | 144 | 800 | 683 | 916 | 308 | 358 |
| GMT | <10 | 33.6 | 109.4 | 160.9 | 879.4 | 880.7 | 852.7 | 584.9 | 341.1 |
Results are given as antibody titers and are representative of those from three independent experiments. The neutralizing antibody titer is the highest serum dilution that resulted in a 50% reduction in the number of plaques produced by DEN-2. The prime consisted of infection with DEN-2, and the boost consisted of inoculation of the recombinant domain III-capsid protein.
Determination of cell-mediated immune response.
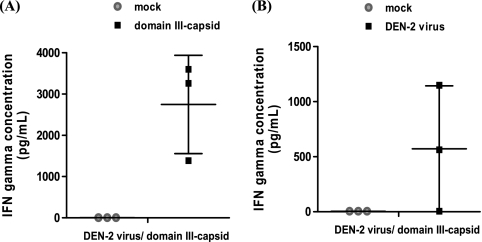
In the present study, the ability of the immunized monkeys to develop a cell-mediated immune response after receiving the two heterologous doses was also determined. The secretion of IFN-γ was measured in the supernatants of PBMCs extracted from the blood of these monkeys upon stimulation with two antigens: recombinant protein and infective DEN-2. Cytokine secretion was measured 6 months after the booster dose, corresponding to day 270 of the study. As shown in Fig. 2A, upon stimulation with domain III-capsid protein, high levels of IFN-γ were detected in the supernatants of cultured PBMCs of the 3 animals that received the booster dose, with the individual values being 3,259.3 pg/ml, 3,601.3 pg/ml, and 1,385.7 pg/ml. When the PBMCs were stimulated with a negative-control preparation, the levels of IFN-γ were below 10 pg/ml.
FIG. 2.
IFN-γ concentration determined in culture supernatants of stimulated PBMCs. Culture supernatants of the peripheral blood mononuclear cells from immunized animals were tested by ELISA. Data represent means ± standard deviations. (A) Culture supernatants of protein-stimulated or mock-stimulated PBMCs; (B) culture supernatants of DEN-2-stimulated PBMCs or mock-stimulated PBMCs. These experiments were repeated three times, with the same results achieved each time (n = 3).
A second experiment was performed using infective DEN-2 as antigen. As shown in Fig. 2B, two of the monkeys tested were positive, exhibiting values of IFN-γ secretion of 562.8 pg/ml and 1,149.3 pg/ml. Similar to the previous experiment, when the PBMCs were stimulated with a mock preparation, no cytokine secretion was detected.
Three nonimmunized animals were included as negative controls. As expected, secretion of IFN-γ was not detected when PMBCs were stimulated either with recombinant protein or with the DEN-2 preparation (data not shown).
DISCUSSION
Among the viral structural proteins of DEN, we have selected two as targets to develop DEN vaccine candidates: the envelope protein (domain III) and the capsid protein. Domain III of the E protein has been proposed for involvement in receptor recognition (3), as supported by several studies, demonstrating that both recombinant domain III proteins and antibodies generated against domain III of flaviviral E antigens can inhibit entry into target cells (4, 7, 17). We have also described the functionality of fusion proteins containing domain III of the E protein from DEN to induce neutralizing antibodies and protection in mice and monkeys (1, 14-16, 26, 28). Therefore, this region, properly folded and presented to the immune system, is an inducer of neutralizing antibodies against DEN.
Cell-mediated immunity has also been recognized to be an important factor in protection against DEN in mice (9, 29). It has been shown that the immunization of mice with four CD8+ T cell epitopes from DEN that are immunodominant in this species (31) enhances viral clearance in animals, which developed viremia similar to that described for monkeys. On the other hand, we have demonstrated the protective capacity of the capsid protein, expressed in Escherichia coli and as nucleocapsid particles, in the mouse encephalitis model (10).
Based on these pieces of evidence, we designed and obtained a chimeric recombinant protein, domain III-capsid, which contains viral fragments that are potentially inducers of neutralizing antibodies and CMI. The molecule was efficiently produced in E. coli and was properly folded and partially purified in this expression system. When it was presented as a particulate aggregate with random ODNs, it induced antiviral and neutralizing antibodies and CMI and conferred a significant level of protection in mice (26). Based on these features, we selected this molecule, in a particulate form with ODNs, to combine it with infective DEN-2 in a heterologous prime-boost regime of immunization in monkeys.
Due to the lack of attenuated dengue virus strains in our laboratory (Center for Genetic Engineering and Biotechnology, Havana, Cuba), in this work we used the replicative virus as a model of attenuated virus to evaluate the capacity of the domain III-capsid protein to recall the immune response generated upon infection of monkeys with infective DEN-2. After administration of the viral infective dose to monkeys, a consistent viremia was detected, indicating a successful infection. The day of mean viremia was similar to that previously reported by our group in different green monkey assays (19, 20, 28). The humoral immune response measured by ELISA reached maximal values at day 30 after infection, whereas titers of neutralizing antibodies had maximal values between days 60 and 90 postinfection. Three months later, upon administration of the recombinant protein formulation, antiviral antibodies slightly increased, while neutralizing antibody titers were notably augmented (5.4-fold compared to the values on the day of the booster dose). It was also worth noting that even 6 months after the last dose, levels of neutralizing antibodies were still 3-fold higher than those detected on the day of the booster dose, indicating the durability of the recalled functional response. The origin of the neutralizing antibody recall was probably due to the presence of the domain III region in the chimeric protein, since the recombinant capsid protein of DEN-2 was not able to induce either antiviral antibodies or neutralizing antibodies in mice (10). In addition, after natural infection, owing to the envelope nature of the virus, antibodies against the capsid protein are not favored. In fact, recent studies with human sera revealed that upon natural infection, antibodies did not recognize the capsid protein, at least in a Western blot assay (8).
The booster effect of the domain III-capsid protein in terms of neutralizing antibodies is similar to that previously published by our group with the fusion protein PD5 (27), but in the present work, this effect was attained with only a 3-month interval between the heterologous doses, an important advantage that would allow condensed schedules for human vaccination.
It should be highlighted that in 2009 Wahala et al. concluded in their work that the domain III region is not a target of neutralizing antibodies upon primary infection in humans, as they were unable to show a decrease in the neutralizing activity of human sera after their incubation with domain III and, consequently, a potential depletion of antibodies directed to this region (30). On the contrary, in this work and in the previous one with PD5 protein (27), we demonstrated that, at least in monkeys, primary infection with DEN-2 induced a neutralizing immune response which is recalled by the administration of domain III of the E protein from the same serotype presented in two different fusion proteins. Taking together all this evidence, we can conclude that the domain III region, properly presented as a fusion protein to the immune system, is able to recall the neutralizing antibody response generated after homologous virus infection in monkeys. Our results are in accordance with those described by Crill et al. in 2009, who state that domain III is indeed a target of neutralizing antibodies after natural infection in humans (6).
In the present work, another arm of the immune system was also measured: the cell-mediated immunity. After 6 months of receiving the two heterologous doses, monkeys developed PBMCs capable of producing IFN-γ upon in vitro stimulation of DEN-2. We cannot assert that such a response was recalled after the booster dose with the recombinant protein, since the determination was not performed at day 0 of the booster dose. Nevertheless, the fact that high levels of IFN-γ were obtained 6 months after the last dose indicates that functional and long-lasting CMI is present. High levels of IFN-γ were also obtained after in vitro stimulation with the chimeric protein, demonstrating that epitopes of the viral regions present in the protein are able to stimulate the PBMCs of the vaccinated monkeys. The capsid protein as well as the domain III-capsid protein were also able to induce high levels of IFN-γ in mice after in vitro stimulation of the splenocytes with the infective virus (10, 26).
As a conclusion, we can affirm that domain III-capsid protein, aggregated with random ODNs, is able to boost the neutralizing antibodies induced by infection of monkeys with DEN-2 and, in turn, that only 3 months was required to obtain such an effect. These results then constitute a proof of concept of the heterologous prime-boost approach strategy combining attenuated dengue viruses and a subunit vaccine candidate in the same immunization schedule. In general, immunization with the attenuated viruses currently under development provokes a nonequivalent immune response against the four serotypes (13, 23, 24); therefore, multiple doses spaced over time (about 6 months) are required to induce a broad response (13, 22-24). In addition, the duration of the functional immune response should be strictly guaranteed, since the antibody-dependent enhancement phenomenon by waning immunity, at least against the heterologous serotype, against which immunity decreases, may occur because of the presence of multiple cross-reactive regions in the antigens contained in the vaccines. On the basis of the previous disadvantages and the results obtained in the present work, use of the combination of these strains with recombinant chimeric proteins as domain III-capsid in a heterologous prime-boost regime of immunization can be an attractive approach. This immunization method leads to development of a safe immunization schedule (only one dose of the infective virus and the rest with subunit vaccine) that is condensed and able to induce a durable memory immune response against dengue virus without any viral interference.
Acknowledgments
We are very grateful to Ricardo Silva (CIGB) and Harold Curiel (CQB) for their critical reading and useful comments in the revision of the manuscript.
This investigation received financial support from the Cuban Program for Dengue Vaccine Development.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print on 5 January 2011.
REFERENCES
- 1.Bernardo, L., et al. 2008. Immunogenicity and protective efficacy of a recombinant fusion protein containing the domain III of the dengue 1 envelope protein in non-human primates. Antiviral Res. 80:194-199. [DOI] [PubMed] [Google Scholar]
- 2.Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1125. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 3.Chen, Y., T. Maguire, and R. M. Marks. 1996. Demonstration of binding of dengue virus envelope protein to target cells. J. Virol. 70:8765-8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin, J. F., J. J. Chu, and M. L. Ng. 2007. The envelope glycoprotein domain III of dengue virus serotypes 1 and 2 inhibit virus entry. Microbes Infect. 9:1-6. [DOI] [PubMed] [Google Scholar]
- 5.Churdboonchart, V., N. Bhamarapravati, and S. Peampramprecha. 1991. Antibodies against dengue viral proteins in primary and secondary dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 44:481-493. [DOI] [PubMed] [Google Scholar]
- 6.Crill, W. D., H. R. Hughes, M. J. Delorey, and G. J. Chang. 2009. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 4:4991-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crill, W. D., and J. T. Roehrig. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:7769-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejnirattisai, W., et al. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil, L., et al. 2009. The cellular immune response plays an important role in protecting against dengue virus in the mouse encephalitis model. Viral Immunol. 22:23-29. [DOI] [PubMed] [Google Scholar]
- 10.Gil, L., et al. 2009. Recombinant nucleocapsid-like particles from dengue-2 virus induce protective CD4+ and CD8+ cells against viral encephalitis in mice. Int. Immunol. 21:1175-1183. [DOI] [PubMed] [Google Scholar]
- 11.Gubler, D. J. 2002. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 10:100-103. [DOI] [PubMed] [Google Scholar]
- 12.Guirakhoo, F., et al. 2006. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum. Vaccine 2:60-67. [DOI] [PubMed] [Google Scholar]
- 13.Guy, B., et al. 2008. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 26:5712-5721. [DOI] [PubMed] [Google Scholar]
- 14.Hermida, L., et al. 2006. A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine 24:3165-3171. [DOI] [PubMed] [Google Scholar]
- 15.Hermida, L., et al. 2004. A dengue-2 envelope fragment inserted within the structure of the P64k meningococcal protein carrier enables a functional immune response against the virus in mice. J. Virol. Methods 115:41-49. [DOI] [PubMed] [Google Scholar]
- 16.Hermida, L., et al. 2004. A fragment of the envelope protein from dengue-1 virus, fused in two different sites of the meningococcal P64k protein carrier, induces a functional immune response in mice. Biotechnol. Appl. Biochem. 39:107-114. [DOI] [PubMed] [Google Scholar]
- 17.Hung, J. J., et al. 2004. An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J. Virol. 78:378-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitchener, S., et al. 2006. Immunogenicity and safety of two live-attenuated tetravalent dengue vaccine formulations in healthy Australian adults. Vaccine 24:1238-1241. [DOI] [PubMed] [Google Scholar]
- 19.Martín, J., et al. 2009. Viremia and antibody response in green monkeys (Chlorocebus aethiops sabaeus) infected with dengue virus type 2: a potential model for vaccine testing. Microbiol. Immunol. 53:216-223. [DOI] [PubMed] [Google Scholar]
- 20.Martín, J., et al. 2009. Viremia and the magnitude of the immune response upon infection of green monkeys with dengue virus type 2 are strain-dependent. Curr. Microbiol. 59:579-583. [DOI] [PubMed] [Google Scholar]
- 21.Morens, D. M., S. B. Halstead, and P. M. Repik. 1985. Simplified plaque reduction assay for dengue viruses by semimicro methods in BHK 21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J. Clin. Microbiol. 22:250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison, D., et al. 2010. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J. Infect. Dis. 201:370-377. [DOI] [PubMed] [Google Scholar]
- 23.Sabchareon, A., et al. 2004. Safety and immunogenicity of a three dose regimen of two tetravalent live-attenuated dengue vaccines in five- to twelve-year-old Thai children. Pediatr. Infect. Dis. J. 23:99-109. [DOI] [PubMed] [Google Scholar]
- 24.Simasathien, S., et al. 2008. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus naive children. Am. J. Trop. Med. Hyg. 78:426-433. [PubMed] [Google Scholar]
- 25.Simmons, M., T. Burgess, J. Lynch, and R. Putnak. 2010. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology 396:280-288. [DOI] [PubMed] [Google Scholar]
- 26.Valdés, I., et al. 2009. A novel fusion protein domain III-capsid from dengue-2, in a highly aggregated form, induces a functional immune response and protection in mice. Virology 394:249-258. [DOI] [PubMed] [Google Scholar]
- 27.Valdés, I., et al. 2010. Heterologous prime-boost strategy in non-human primates combining the infective dengue virus and a recombinant protein in a formulation suitable for human use. Int. J. Infect. Dis. 14:377-383. [DOI] [PubMed] [Google Scholar]
- 28.Valdés, I., et al. 2009. Immunological evaluation in nonhuman primates of formulations based on the chimeric protein P64k-domain III of dengue 2 and two components of Neisseria meningitides. Vaccine 27:995-1001. [DOI] [PubMed] [Google Scholar]
- 29.Van der Most, R. G., K. Murali-Krishna, R. Ahmed, and J. H. Strauss. 2000. Chimeric yellow fever/ dengue virus as a candidate dengue vaccine: quantitation of the dengue virus-specific CD8 T-cell response. J. Virol. 4:8094-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahala, W. M., A. A. Kraus, L. B. Haymore, M. A. Accavitti-Loper, and A. M. de Silva. 2009. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 392:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yauch, L. E., et al. 2009. A protective role for dengue virus-specific CD8+ T cells. J. Immunol. 182:4865-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]