Abstract
The serogroup A meningococcal conjugate vaccine MenAfriVac has the potential to confer herd immunity by reducing carriage prevalence of epidemic strains. To better understand this phenomenon, we initiated a meningococcal carriage study to determine the baseline carriage rate and serogroup distribution before vaccine introduction in the 1- to 29-year old population in Burkina Faso, the group chosen for the first introduction of the vaccine. A multiple cross-sectional carriage study was conducted in one urban and two rural districts in Burkina Faso in 2009. Every 3 months, oropharyngeal samples were collected from >5,000 randomly selected individuals within a 4-week period. Isolation and identification of the meningococci from 20,326 samples were performed by national laboratories in Burkina Faso. Confirmation and further strain characterization, including genogrouping, multilocus sequence typing, and porA-fetA sequencing, were performed in Norway. The overall carriage prevalence for meningococci was 3.98%; the highest prevalence was among the 15- to 19-year-olds for males and among the 10- to 14-year-olds for females. Serogroup Y dominated (2.28%), followed by serogroups X (0.44%), A (0.39%), and W135 (0.34%). Carriage prevalence was the highest in the rural districts and in the dry season, but serogroup distribution also varied by district. A total of 29 sequence types (STs) and 51 porA-fetA combinations were identified. The dominant clone was serogroup Y, ST-4375, P1.5-1,2-2/F5-8, belonging to the ST-23 complex (47%). All serogroup A isolates were ST-2859 of the ST-5 complex with P1.20,9/F3-1. This study forms a solid basis for evaluating the impact of MenAfriVac introduction on serogroup A carriage.
After the introduction of vaccines against Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae became the dominant causes of bacterial meningitis. N. meningitidis is a Gram-negative diplococcus classified into 12 serogroups on the basis of its capsular polysaccharide composition, with serogroups A, B, C, W135, X, and Y being the main disease-causing serogroups. Meningococci are transmitted between individuals by airborne droplets of respiratory or throat secretions. Healthy humans colonized by meningococci in the upper respiratory tract are the principal source of spread of the bacterium in the population (17, 41).
Epidemic meningitis is a significant cause of morbidity and mortality in sub-Saharan Africa, in an area stretching from Senegal to Ethiopia. This area is designated the meningitis belt (15), where epidemic meningitis is primarily caused by serogroup A meningococci (NmA), with disease incidence peaking in the dry season every year. In addition to the yearly cycles, large, multicountry epidemics recur unpredictably every 2 to 10 years (11). The West African country Burkina Faso, with a population of about 15 million, is located in the middle of the meningitis belt and has repeatedly been affected by epidemics of meningitis. The latest epidemic year was 2007, with 26,878 recorded cases and 1,923 deaths. In 2008, 10,401 cases and 1,067 deaths were recorded, and there were 4,723 cases and 629 deaths in 2009 (MDSC Meningitis Weekly Bulletin, http://www.who.int/csr/disease/meningococcal). Moreover, Burkina Faso was the first country to experience a major serogroup W135 outbreak in 2002 (24), with 13,000 reported cases, of whom 1,400 died (www.who.int/csr/disease/meningococcal/w135). The World Health Organization (WHO) currently recommends reactive mass vaccination to halt ongoing epidemics (37), and countries can apply for an emergency stockpile of the A/C or A/C/W polysaccharide vaccine (38). However, the protective immunity of the polysaccharide vaccines is relatively brief, and immunogenicity in young children is considered suboptimal (29, 32).
Conjugate vaccines are safe and effective not only in preventing disease among those vaccinated but also in preventing carriage of the disease-causing organisms, leading to reduced transmission of the disease (10, 19, 20, 28, 35). The Meningitis Vaccine Project (MVP), a public-private partnership between WHO and the Program for Appropriate Technology in Health (PATH), has developed MenAfriVac, an effective monovalent serogroup A conjugate meningococcal vaccine, at an affordable price (14). Clinical trials have been performed in India and Africa, with very promising results (2, 3, 12, 13, 26). MenAfriVac has the potential to prevent significant morbidity and mortality, especially among those aged below 30 years, in sub-Saharan Africa and to eliminate the devastating serogroup A meningococcal epidemics that regularly occur in these countries. A country-wide mass vaccination campaign for the first introduction of the vaccine was carried out in Burkina Faso in 2010, where the vaccine was offered to all individuals aged 1 to 29 years.
To assess the indirect effect of the vaccine on protection from acquisition, carriage studies must be conducted in the populations that will be targeted by the vaccine. Such an assessment would be easier using population groups with a high prevalence of carriage. However, risk factors associated with carriage in other parts of the world might not necessarily represent the situation in West Africa, since strain distribution and socioeconomic conditions may be quite different. Previous carriage studies conducted in Africa have provided variable information on prevalence and affected age groups (34). To accurately determine the ability of MenAfriVac to reduce carriage of serogroup A N. meningitidis, an initial milestone consists of securing good baseline data on serogroup A carriage prevalence before the introduction of the vaccine. Therefore, we conducted a series of carriage studies in Burkina Faso between October 2008 and December 2009. Carriage prevalence of different serogroups of N. meningitidis and molecular characterization of the retrieved strains are presented here, together with seasonal and geographical variations, as well as the age and gender distributions of the asymptomatic carriers.
MATERIALS AND METHODS
Ethics.
The study obtained ethical clearance from the Norwegian Regional Committee for Medical Research Ethics, Southern Norway; the Ethical Committee for Health Research in Burkina Faso; and the Internal Review Board at the Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Study population.
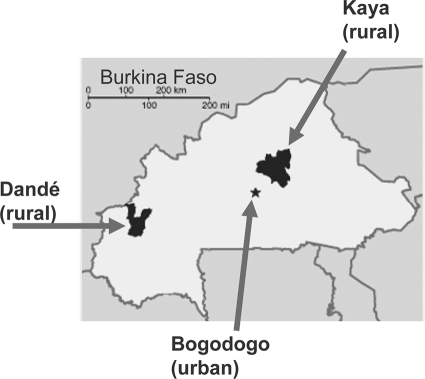
The study was implemented in three health districts in Burkina Faso: Bogodogo, an urban district in the capital, Ouagadougou, with roughly 615,478 inhabitants; Dandé, a rural district in the west with 214,470 inhabitants; and Kaya, a rural district in the east with 500,207 inhabitants (Fig. 1). Each study site was supported by a national microbiological laboratory: the Centre National Pédiatrique Charles de Gaulle, Ouagadougou, for Bogodogo; the Centre Hospitalier Universitaire Souro Sanou, Bobo-Dioulasso, for Dandé; and the Centre Hospitalier Universitaire Yalgado, Ouagadougou, for Kaya.
FIG. 1.
Geographical distribution of the study sites in Burkina Faso (map source, The World Factbook, Central Intelligence Agency web page).
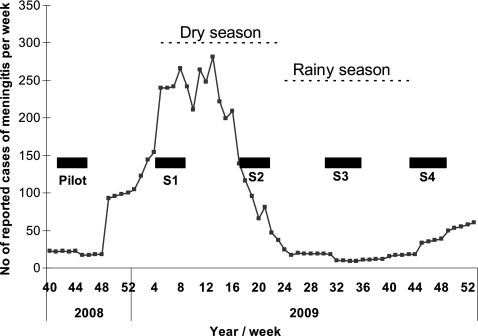
The study was designed as a multiple cross-sectional survey. To account for seasonal fluctuations in carriage prevalence (1, 5, 16, 22, 27, 34), sampling campaigns were executed every 3 months, starting in October 2008 (Fig. 2). The campaigns were conducted simultaneously in all three districts within a 4-week period. Representative sampling of persons aged 1 to 29 years was accomplished by multistage cluster design. Eight villages in each rural district were selected by probability proportional to size. Two additional villages per rural district were selected as alternates in case the former villages were inaccessible due to rain. All housing compounds in selected villages were mapped with Global Positioning System (GPS) coordinates before the study launch. During each sampling campaign, we selected 42 compounds per village by simple random sample and navigated to them using GPS coordinates. All persons within the target age range living in the selected compound were asked to participate.
FIG. 2.
Timelines for sampling campaigns and epidemic curve in Burkina Faso, 2008 and 2009.
In the urban district, all residential blocks were identified using existing maps. Sixteen blocks and five alternates per study round were selected by simple random sample and mapped with GPS coordinates. During each sampling campaign, all the households in the selected blocks were visited. New selections were done for each sampling campaign.
Inclusion of participants and administration of questionnaires.
The village population was informed about the project through the local health workers and community leaders. Each randomly selected household was visited by study personnel, and the purpose of the study was explained. A first questionnaire with general questions about the compound was administered to the head of the compound after his written consent. Then, a second questionnaire was administered to each of the 1- to 29-year-old members of the household after their individual written consent or, in the case of children below 18 years of age, the consent of their parent or guardian. Each participant was given a paper wristband with a barcode corresponding to a unique identifier number linked to the questionnaire.
Sample collection.
Oropharyngeal samples were obtained by sweeping the posterior pharyngeal wall behind the uvula and one tonsil with a sterile cotton swab (Copan, Italy). The swab was immediately plated onto modified Thayer-Martin VCNT agar, containing 3 mg/liter vancomycin, 7.5 mg/liter colistin, 12.5 U/liter nystatin, 5 mg/liter trimethoprim lactate, and Vitox supplement (manufactured by WHO/Multi Disease Surveillance Centre, Burkina Faso). In the field, inoculated plates were rapidly incubated in humidified and CO2-rich air using 7.0-liter jars (Remel, GA) with a CO2-generating system (CO2 GEN; Oxoid, United Kingdom). Personal digital assistants (PDAs) were used to register the barcode on the participant's wristband as well as the barcode label used on the inoculated plate, creating a link between the person identifier number and the laboratory specimen number. As soon as possible and within 6 h after sampling, the jars with the plates were incubated at 37°C in the laboratory. Between 100 and 110 samples were collected daily at each site, 4 days per week, during the 4 weeks of each campaign.
Bacterial identification and characterization.
Primary identification was performed after 24 and 48 h of incubation. Colonies with a typical morphology of N. meningitidis were subcultured on blood agar plates and subjected to further laboratory analysis. Oxidase-positive, Gram-negative diplococci with β-galactosidase (o-nitrophenyl-β-d-galactopyranoside)-negative activity and γ-glutamyltransferase (GGT)-positive activity were considered probable N. meningitidis isolates and serogrouped by slide agglutination using commercial antisera (Remel). Purified isolates were inoculated into two cryovials containing 0.5 to 1 ml Greaves solution (8) and stored at −70°C. After each campaign, one of the vials was sent to the Norwegian Institute of Public Health (NIPH), Oslo, Norway, on dry ice for confirmation and further analyses.
The identification tests described above were repeated at NIPH, and sugar fermentation tests were performed, if necessary. Strains confirmed to be N. meningitidis were further characterized using molecular methods.
Genotypic characterization.
DNA from each strain was isolated by suspending 1 loop of bacteria in 200 μl Tris-EDTA (TE) buffer, pH 8.0, heating at 95°C for 10 min, and centrifugation at 16,000 × g for 5 min, and the supernatant was stored at −20°C until use. Each strain was assigned to a specific sequence type (ST), using multilocus sequence typing (MLST), which identifies allelic variation within seven housekeeping genes (18). Variation in the porA and fetA genes, coding for the outer membrane proteins PorA and FetA, respectively, was determined by DNA sequencing, as described previously (30, 33a). New MLST alleles and STs were submitted to the MLST database (http://pubmlst.org/neisseria/), together with the strains' serogroups and porA and fetA sequences. PCR analysis of the genes coding for the polysaccharide capsule (33) was performed for genogroup determination of nonserogroupable isolates or for confirmation of questionable slide agglutination test results.
Amplification of the porA and fetA genes, as well as the genes used in MLST, was done with 35 cycles of denaturation at 95°C for 1 min, annealing at 52°C for 1 min, and elongation at 72°C for 1 min. Genes used for genogrouping were amplified with 30 cycles of denaturation at 95°C for 40 s, annealing at 58°C for 1 min, and elongation at 72°C for 40 s. Sequencing reactions were performed with an ABI Prism BigDye Terminator cycle sequencing ready reaction kit (ABI, Foster City, CA), according to the manufacturer's recommendations, using a epMotion 5070 robot (Eppendorf, Bergman, Oslo, Norway). Sequencing was performed using an ABI 3730 DNA analyzer (Applied Biosystems).
Data collection and analyses.
The databases containing data collected in the field were uploaded to a computer on a daily basis. In the laboratories in Burkina Faso and in Norway, results were registered on paper before computerized data entry into Microsoft Access and Excel databases (Microsoft Corporation, Redmond, WA). A combined database with data from all the computer files was used for the evaluation of the results and the statistical analysis. Some samples were excluded due to missing or duplicate links between the person and the laboratory identification or between the person and his or her household. Only samples with data available in all the databases were included in the analysis. For all the isolates confirmed to be N. meningitidis at NIPH, all genes described could be sequenced. Thus, none were excluded at this level. The characteristics of the retrieved isolates are based on confirmed results obtained at NIPH. Statistical analyses were performed using the chi-square test with 1 degree of freedom. P values of <0.05 were considered significant.
RESULTS
Exclusion of the first sampling campaign.
Altogether, five sampling campaigns were performed at 3-month intervals, starting in October 2008 (Fig. 2). Results from the first campaign (October and November 2008) yielded an N. meningitidis carriage prevalence of only 0.87% in the 1- to 29-year-old Burkinabes (0.75%, 0.51%, and 1.35% in the districts of Bogodogo, Dandé, and Kaya, respectively). Evaluation of the initial study concluded that the unexpectedly low carriage prevalence was due to poor selectivity of the commercial modified Thayer-Martin agar plates purchased for that campaign, leading to contamination and difficulties in selecting colonies from the plates. Improvements of the study standard operating procedures and quality control procedures were undertaken, and local production of selective agar plates was successfully established in Ouagadougou. The first campaign was therefore considered a pilot study.
Samples and overall carriage prevalence.
The remaining four sampling campaigns were conducted in 2009 and were designated S1 through S4. Data collection for each round occurred in January-February, April-May, July-August, and October-November for the respective four campaigns. They yielded a total of 20,326 oropharyngeal samples (range, 5,024 to 5,121 samples per campaign). After isolation and primary identification in Burkina Faso, 1,049 isolates were sent to NIPH for confirmation and molecular characterization (range, 191 to 357 isolates per campaign). Of these, 811 (77.3%) isolates were confirmed to be N. meningitidis; 809 were registered in all the data sets and could be included in the data analysis, giving an overall meningococcal carriage rate of 3.98% (Table 1).
TABLE 1.
Carriage rate and serogroup prevalence of N. meningitidis in Burkina Faso at four sampling times in 2009
| Parameter | Sampling campaign |
Total | |||
|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||
| Total no. of samples | 5,024 | 5,121 | 5,074 | 5,107 | 20,326 |
| No. (%) of N. meningitidis isolates | 206 (4.10) | 270 (5.27) | 171 (3.37) | 162 (3.17) | 809 (3.98) |
| No. (%) of NmA isolates | 21 (0.42) | 32 (0.62) | 12 (0.24) | 15 (0.29) | 80 (0.39) |
| No. (%) of NmC isolates | 0 | 4 (0.08) | 0 | 0 | 4 (0.02) |
| No. (%) of NmW135 isolates | 27 (0.54) | 11 (0.21) | 20 (0.39) | 12 (0.23) | 70 (0.34) |
| No. (%) of NmX isolates | 8 (0.16) | 40 (0.78) | 25 (0.49) | 17 (0.33) | 90 (0.44) |
| No. (%) of NmY isolates | 116 (2.31) | 158 (3.09) | 83 (1.64) | 100 (1.96) | 457 (2.25) |
| No. (%) of nongroupable isolates | 34 (0.68) | 25 (0.49) | 31 (0.61) | 18 (0.35) | 108 (0.53) |
Regional and seasonal variations in carriage prevalence.
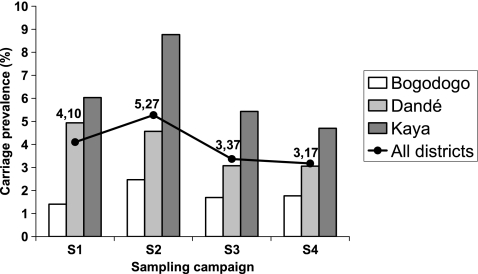
The N. meningitidis carriage prevalence rates were 4.10%, 5.27%, 3.37%, and 3.17% at the time points of S1, S2, S3, and S4, respectively (Fig. 3). Overall carriage prevalence was higher during the dry season (S1 and S2) than during the rest of the year (S3 and S4) (P < 0.001), although the difference was not significant in the Bogodogo District. Seasonal variation was also statistically significant between S1 and S2 (P < 0.01), S1 and S4 (P < 0.05), S2 and S3 (P < 0.001), and S2 and S4 (P < 0.001) but not between S1 and S3 or between S3 and S4.
FIG. 3.
Carriage prevalence of N. meningitidis in three districts in Burkina Faso at four sampling times in 2009.
We found a significant difference in carriage rates when the rate for the urban district (average, 1.82%) was compared to the rates for the two rural districts (average, 5.07%) (P < 0.001). The results also showed a geographic variation among the two rural districts, with the carriage prevalence in Kaya (average, 6.23%; range, 4.71 to 8.77%) being significantly higher than that in Dandé (average, 3.91%; range, 3.06 to 4.94%) (P < 0.001) (Fig. 3). This district ranking was the same for all the campaigns.
Determination of serogroup.
Of the 809 isolates, 574 were assigned to a serogroup on the basis of the result from slide agglutination, while 235 isolates were nonserogroupable and further analyzed by capsule gene PCR. After PCR, 102 isolates remained nonserogroupable, while 16 isolates were NmA, 2 isolates NmC, 18 isolates NmX, 60 isolates NmY, and 37 isolates NmW135. Thus, over half of the isolates that were nonserogroupable by serum agglutination harbored a capsule gene that was not expressed phenotypically. In addition, some isolates for which agglutination did not match the other molecular characteristics were checked by capsule gene PCR. The serogroup of 14 isolates was then changed according to the PCR result.
NmA carriage prevalence.
NmA carriage prevalence rates at the time points of S1, S2, S3, and S4 were 0.42%, 0.62%, 0.24%, and 0.29%, respectively (Fig. 4), corresponding to an average NmA carriage prevalence of 0.39%. Seasonal variation was statistically significant (P < 0.01) when the rates during the dry season (S1 and S2) and the rest of the year (S3 and S4) were compared. The overall NmA carriage prevalence rates in each of the districts were 0.06%, 0.21%, and 0.94%, for Bogodogo, Dandé, and Kaya, respectively. NmA carriage prevalence follows the same pattern of variation described for the overall carriage prevalence, with a higher level in the rural districts than in the urban district (P < 0.001) and with significant differences between the rural districts (P < 0.05). Interestingly, the NmA carriage prevalence in S4 was the same as that in the pilot study, executed almost exactly 1 year earlier (0.29% in both).
FIG. 4.
Carriage prevalence of serogroup A N. meningitidis in three districts in Burkina Faso at four sampling times in 2009.
Prevalence of other serogroups.
The carriage prevalence of the different serogroups, presented by district and campaign, showed a predominance of NmY in all three districts at most time points (Fig. 5). The increase in NmX prevalence seen between S1 and S2 (Table 1) was significant (P < 0.01) and was essentially due to an increase in the district of Kaya. In all the districts, carriage prevalence of NmA, NmY, and NmX increased from S1 to S2 (significantly for NmY [P < 0.05] and NmX [P < 0.01]) and decreased from S2 to S3 (significantly for NmA [P < 0.01] and NmY [P < 0.01]), while Nm W135 and nonserogroupable strains had changes in the opposite direction (significant decrease of NmW135 between S1 and S2 [P < 0.01]).
FIG. 5.
Serogroup distribution of meningococcal isolates in three districts in Burkina Faso at four sampling times in 2009.
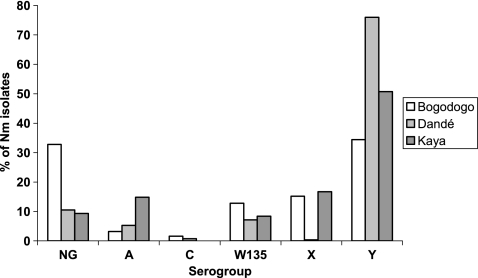
Overall, nonserogroupable isolates were significantly more dominant in the urban district of Bogodogo (33% of the isolates) than in the rural districts of Dandé and Kaya (11% and 9%, respectively) (P < 0.001) (Fig. 6). Kaya had the highest proportion of serogroup A isolates, as they represented 15% of all isolates, while the proportions of NmA in Dandé and Bogodogo were lower and comparable (5% and 3%, respectively). Although NmY dominated in all three districts, it represented 76% of the isolates in Dandé but only 51% in Kaya and 34% in Bogodogo. The proportions of NmX isolates were comparable in Bogodogo and Kaya (15% and 17%, respectively), but NmX was almost nonexistent in Dandé (<1%, corresponding to a single carrier).
FIG. 6.
Overall serogroup distribution of meningococcal isolates in three districts in Burkina Faso in 2009.
Molecular characterization.
The 809 isolates confirmed to be N. meningitidis were assigned to 29 different STs, of which 15 STs belonged to 5 different clonal complexes. A total of 51 different porA-fetA combinations were identified, and the isolates were assigned to 65 different combinations of serogroup, ST, and porA-fetA. During the pilot and the carriage studies, we found 17 new STs, 5 new PorA variants, and 1 new FetA variant; all have been submitted to the MLST database (http://pubmlst.org/neisseria/).
All 80 NmA isolates were ST-2859 (ST-5 complex), and all had the porA-fetA combination P1.20,9/F3-1 (Table 2). This single clone of serogroup A has been present for the whole period from October 2008 to November 2009, as all 14 NmA strains retrieved from the pilot study also had this characteristic. In addition, two isolates with ST-2859, P1.20,9/F3-1, were nongroupable both by serogrouping and by PCR.
TABLE 2.
Characteristics of N. meningitidis retrieved from the carriers in Burkina Faso according to serogroup
| Serogroup | MLST |
PorA-FetA variant |
||||
|---|---|---|---|---|---|---|
| ST complex | ST no. | No. of isolates | PorA | FetA | No. of isolates | |
| A | 5 | 2859 | 80 | P1.20,9 | F3-1 | 80 |
| C | 41/44 | 206 | 3 | P1.5-2,10-2 | F5-2 | 2 |
| P1.7,30-8 | F5-2 | 1 | ||||
| 7929a | 1 | P1.7,30-11 | F5-2 | 1 | ||
| W135 | 175 | 2881 | 69 | P1.5-1,2-36 | F5-1 | 67 |
| F5-8 | 1 | |||||
| P1.22,14-6 | F5-1 | 1 | ||||
| 7928a | 1 | P1.5-1,2-36 | F5-1 | 1 | ||
| X | UAb | 181 | 67 | P1.5-1,10-1 | F1-31 | 53 |
| F1-35 | 1 | |||||
| F1-38 | 1 | |||||
| F3-1 | 1 | |||||
| F5-8 | 1 | |||||
| F5-69 | 2 | |||||
| F5-94a | 4 | |||||
| P1.5-1,2-2 | F3-1 | 4 | ||||
| 751 | 11 | P1.5-1,10-1 | F4-23 | 11 | ||
| 5789 | 12 | P1.5-1,10-1 | F4-23 | 12 | ||
| Y | 23 | 4375 | 396 | P1.5-1,2-2 | F4-23 | 1 |
| F5-8 | 383 | |||||
| —c | 4 | |||||
| P1.5-1,2-36 | F5-8 | 1 | ||||
| P1.5-1,2-56 | F5-8 | 1 | ||||
| P1.5-1,2-62a | F5-8 | 2 | ||||
| P1.5-8,2-2 | F5-8 | 1 | ||||
| —c | F5-8 | 3 | ||||
| 7784a | 1 | P1.5-1,2-2 | F5-8 | 1 | ||
| 7872a | 1 | P1.5-1,2-2 | F5-8 | 1 | ||
| 8219a | 5 | P1.5-1,2-2 | F5-8 | 5 | ||
| 167 | 767 | 15 | P1.5-1,10-8 | F1-3 | 9 | |
| F5-8 | 1 | |||||
| — | 5 | |||||
| 2880 | 10 | P1.5-1,10-8 | F1-3 | 7 | ||
| F1-35 | 1 | |||||
| F3-9 | 1 | |||||
| — | 1 | |||||
| 7375a | 28 | P1.5-1,10-1 | F5-12 | 2 | ||
| P1.5-1,10-8 | F1-3 | 26 | ||||
| 7949a | 1 | P1.5-1,10-8 | F1-3 | 1 | ||
| NGd | 5 | 2859 | 2 | P1.20,9 | F3-1 | 2 |
| 23 | 4375 | 2 | P1.5-1,2-2 | F5-8 | 2 | |
| 167 | 767 | 1 | P1.5-1,10-6 | — | 1 | |
| 175 | 2881 | 12 | P1.5-1,2-36 | F1-3 | 11 | |
| P1.19,5 | F5-1 | 1 | ||||
| 198 | 198 | 10 | P1.18,25-7 | F5-5 | 8 | |
| P1.18,25 | F5-5 | 2 | ||||
| 865 | 865 | 1 | P1.5-1,2-2 | F6-1 | 1 | |
| UA | 181 | 1 | P1.5-1,10-1 | F1-31 | 1 | |
| 188 | 5 | P1.19,5 | F1-18 | 3 | ||
| F1-15 | 1 | |||||
| F3-9 | 1 | |||||
| 192 | 56 | P1.18,42-7a | — | 1 | ||
| P1.18-1,45 | F2-23 | 5 | ||||
| P1.18-11,42 | — | 7 | ||||
| P1.18-11,42-1 | — | 39 | ||||
| P1.18-11,42-5a | — | 1 | ||||
| P1.18-11,42-6a | — | 1 | ||||
| P1.18-11,42-8a | — | 2 | ||||
| 4899 | 1 | P1.21-14,28-3 | F5-66 | 1 | ||
| 6918 | 1 | P1.22-11,15-34 | F1-31 | 1 | ||
| NG | UA | 7697a | 3 | P1.18-11,42-1 | — | 3 |
| 7698a | 1 | P1.18-11,42-1 | — | 1 | ||
| 7873a | 1 | P1.19,15 | F1-18 | 1 | ||
| 7925a | 5 | P1.22-11,15-34 | F5-69 | 5 | ||
| 8083a | 2 | P1.19,15 | — | 2 | ||
| 8248a | 3 | P1.21-14,28-3 | F5-66 | 2 | ||
| P1.7,30 | — | 1 | ||||
| 8268a | 1 | P1.7,16 | F5-66 | 1 | ||
New ST, new PorA variant or new FetA variant.
UA, unassigned.
—, no gene detected.
NG, nonserogroupable.
The four serogroup C isolates originated from the districts of Bogodogo and Dandé at the time point of S2, belonged to the ST-41/44 clonal complex, and contained the FetA variant F5-2 (Table 2). Three of these isolates were ST-206, and one was a new ST (ST-7929), a single-locus variant of ST-206. During the pilot study, a single NmC isolate was found and attributed to another new ST (ST-7376) of the ST-41/44 clonal complex, also a single-locus variant of ST-206.
NmW135 (n = 70) was represented in all three districts by the ST-175 complex, almost exclusively by ST-2881 (n = 69), but also by a single isolate of a new ST, ST-7928, which is a single-locus variant (adk) of ST-2881. The porA-fetA combination P1.5-1,2-36/F5-1 was found for 97% of the NmW135 isolates.
The serogroup X isolates (n = 90) belonged to STs not assigned to any clonal complex: ST-181, ST-751, and ST-5789. ST-5789 is a single-locus variant (adk) of ST-181, while ST-751 is a double-locus variant from ST-181 (gdh and phdC). Carriage of NmX was mainly observed in the districts of Kaya (n = 70) and Bogodogo (n = 19). NmX ST-181 (n = 67) was present in both Kaya and Bogodogo, while ST-5789 was present only in Bogodogo (n = 12). ST-751 was present only in Kaya (n = 10) and Dandé (n = 1). Of the NmX isolates, 96% had the PorA variant P1.5-1,10-1, of which 63% harbored the F1-31 FetA variant and 27% harbored F4-23 (Table 2).
The NmY (n = 457) isolates belonged to the ST-23 complex (n = 403) and the ST-167 complex (n = 54). The ST-23 complex was mainly represented by ST-4375 (n = 396), the dominant ST in our study, but 3 new STs were identified, all of them single-locus variants of ST-4375. Among the isolates assigned to ST-4375, eight different porA-fetA combinations were identified, but 97% of the ST-4375 isolates had the porA-fetA combination P1.5-1,2-2/F5-8, making this clone the dominant strain circulating in Burkina Faso during the study period (47% of all isolates). The ST-167 complex was more diverse, represented by four STs and 10 porA-fetA combinations. However, 96% had PorA variant P1.5-1,10-8 and 77% had FetA variant F1-3.
Age and gender distribution.
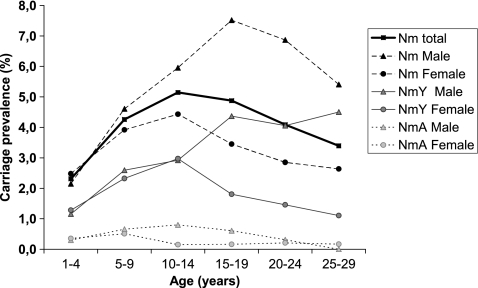
Among the 20,326 participants, 43.7% were males and 56.3% were females. Overall carriage prevalence in males was higher than that in females for NmA (P < 0.05), NmY (P < 0.01), and nonserogroupable meningococci (P < 0.05). The only STs with significant differences in prevalence between genders were ST-2859 (P < 0.05) and ST-4375; both were more common among males (P < 0.01). Carriage of N. meningitidis was the highest among the 10- to 14-year-olds when both male and female participants were considered. Among male participants, the 15- to 19-year-olds had the highest carriage prevalence (Fig. 7). For the female participants, the carriage prevalence was the highest among the 10- to 14-year-olds. The difference in age distribution between genders was mostly due to serogroup Y, since this serogroup dominated, but it was also substantial for serogroup A and nonserogroupable meningococci.
FIG. 7.
Carriage prevalence of N. meningitidis, NmA, and NmY by age group and gender.
The highest prevalence of NmA was found among 5- to 9-year-olds (overall, 0.58%; male, 0.66%; female, 0.51%). Among the 10- to 14-year-olds, males had an even higher prevalence (0.80%), while the prevalence among females in the same age group dropped (0.15%). The oldest recorded person carrying NmA was 26 years of age, although a 29-year-old NmA carrier was identified during the pilot campaign.
Except for NmC, all the detected serogroups were represented in all age groups, but the variation by age group was high for NmY (range, 1.22 to 2.95%) compared to that for the other serogroups (Fig. 8). The increase in prevalence of nonserogroupable isolates with age, peaking in the 15- to 19-year-olds, was observed for both male and female participants.
FIG. 8.
Carriage prevalence of each meningococcal serogroup by age group.
By investigating the carriage rates in relation to the age, in years, of the participants, we found a local peak of NmA and NmY prevalence among 5-year-olds (0.70% and 3.33%, respectively), as well as a local peak of NmA and NmX prevalence among 9-year-olds (1.22% and 0.82%, respectively).
DISCUSSION
Meningococcal carriage.
The overall carriage prevalence rates of meningococci and NmA were low compared to those found in other African studies, which have reported overall carriage prevalence rates of between 3 and 30% and NmA carriage rates of between 0 and 31% (34). Nonetheless, our results are comparable to those of other longitudinal or cross-sectional studies performed in West Africa, such as in northern Ghana, where 6.1% carried N. meningitidis (range, 0.6 to 19.8%) and 1.1% carried NmA (range, 0.3 to 4.3%) over an 8-year period (16), or in northern Nigeria, where 6.2% carried N. meningitidis (range, 1 to 10%) and 1.1% carried NmA over 1.5 years (4, 9).
It is not clear how the carriage prevalence of N. meningitidis is linked to epidemic occurrence, but it has been shown that carriage of outbreak strains can increase significantly during an epidemic (23, 34). The low carriage prevalence rate of NmA in our study is comparable to the rates in other reports of carriage prevalence of virulent strains of between <1% and 5% during endemic or hyperendemic periods (23). During the study period, the epidemic situation in Burkina Faso was calm compared to that in earlier years and other countries of the African meningitis belt, with only 4,723 suspected cases of meningitis in 2009 (39). Only 30% of the cerebrospinal fluid-positive samples collected from suspected cases contained NmA and 3% contained NmW135, whereas 66% contained S. pneumoniae (39).
Standardization of operational practices.
One of the major challenges in a large multicenter study is the harmonization of operational procedures while ensuring a high quality of the work over a long period of time. Several measures were implemented, including training, pilot studies, wrap-up and briefing meetings between each campaign, the use of written standard operating procedures, and close supervision by local and, occasionally, by external supervisors. Laboratory quality control was also implemented to ensure and document correct laboratory practices. The sampling technique and the method of directly plating of the samples that we used are well-known and recommended to obtain a good yield (7).
Selection bias.
Young female participants were overrepresented in the sampled population, a reflection of the society in general, where women often work at home while the men are working away from the household. The study included only the persons physically present at the household at the time of the visit, with the exception of selected schoolchildren, who were included during a normal break. This selection bias might lead to an underestimation of total carriage prevalence, since males were more frequently carriers and because carriage prevalence in the youngest age groups was lower. However, the overall study goal of comparing prevalence before and after vaccination should not be compromised, as the sampling methods remained the same throughout the study.
Age and gender distribution.
Our results are consistent with other data showing higher carriage prevalence in males over females, but with an age distribution different from that seen in Europe and North America (6). Age-specific carriage prevalence likely reflects differences in social behavior, as this has been hypothesized to be more important than age or sex (17). Multivariate analysis of risk factors affecting meningococcal carriage is being done and will be presented in a future publication.
Geographic variation.
The variations of carriage prevalence and serogroup distribution by district can be attributed to differences in climate and living conditions. The difference between rural and urban areas is consistent with previous findings (9). The district of Kaya is the harshest district in the study and notably drier than the more agricultural district of Dandé, but the living conditions are similar between these rural districts and the selected villages in each district were not very different. The district of Bogodogo has a completely different sociological context, with smaller families and a higher standard of living, factors that can affect meningococcal carriage. Easy access to medicines, such as antibiotics, in the capital might also be a contributing factor.
Seasonality.
A review of longitudinal carriage studies conducted in the meningitis belt has concluded that there is no association between carriage prevalence and season (34); in contrast, our data show a significant association with a higher prevalence of carriage in the dry season. This difference may be explained by the large sample size in our study (range, 5,024 to 5,121 per time point) compared to the sizes in prior studies (range, 79 to 525 per time point).
Diversity of strains.
The genetic diversity of the isolates in our material was not very high, with 96% of the isolates belonging to 1 of 11 STs. This is consistent with other carriage studies conducted in Africa (5, 21, 25) but differs from the genotypic diversity of carrier isolates circulating in Europe (7). In contrast to other serogroups, all the NmA carrier isolates were identical: ST-2859, P1.20,9/F3-1. The same molecular characteristics were also found in all the NmA isolates from the pilot study. After a large W135 outbreak in Burkina Faso in 2002-2003 and the subsequent use of a trivalent ACW135 vaccine in 2003 (42), ST-2859 has been the major disease-causing clone circulating in Burkina Faso (11, 31). All the ST-2859 isolates reported in the MLST database are P1.20,9 (http://pubmlst.org/neisseria/). Since PorA and FetA are proteins exposed on the surface of the bacteria, they are targeted by the immune system and have a tendency to adapt by genetic variation more frequently than the housekeeping genes. The nonexistent variation of the PorA and FetA composition in the NmA carrier strains might indicate that this clone is well adapted or that these proteins are less exposed on the bacterial surface than they are in other serogroups.
The other serogroup known to cause disease in Burkina Faso is NmW135. It was also present and very homogeneous among the carriage isolates, as 99% of them belonged to ST-2881. Sporadic cases of meningitis in Burkina Faso from 2008, as well as 24.4% of genotyped isolates from a carriage study in Niger in 2003, have been assigned to NmW135 ST-2881 (25; http://pubmlst.org/neisseria/). This clone is different from the ST-11 clone, which was associated with outbreaks among Hajj pilgrims returning from Saudi Arabia in 2000-2001 and which caused the W135 epidemic in Burkina Faso in 2003 (40). None of the isolates in our study were ST-11.
Meningococcal carriage was dominated by NmY of the ST-23 clonal complex, represented by ST-4375, a single-locus variant (aroE) of ST-23. NmY ST-4375 has previously been found to be the dominant clone in areas of Burkina Faso with little disease, and it has been suggested that NmY carriage might halt the spread of NmA (31). This clone was associated with sporadic cases of meningitis in Burkina Faso in 2006 and 2007 (http://pubmlst.org/neisseria/). For the past 2 decades, ST-23 has been increasingly associated with serogroup Y meningococcal disease in the United States, Canada, Israel, and, to some extent, South Africa, although ST-175 is the predominant NmY clone in South Africa (11, 36). NmY has not yet caused any epidemic in the meningitis belt.
The dominant serogroup X clone in this study, ST-181, had been isolated from cases of meningitis in Burkina Faso in 2007 (http://pubmlst.org/neisseria/). This is the same clone responsible for the serogroup X outbreak in Niger in 2006, where a particularly high seroprevalence of NmX isolates was found in the southwestern part of the country, close to Burkina Faso. The relative proximity of the districts of Kaya and Bogodogo to the borders of Niger could explain why ST-181 was found only in these two districts. The molecular diversity within the NmX carrier strains was higher than that among the other serogroups, as the dominant clone, ST-181, P1.5-1,10-1/F1-31, represented only 59% of the NmX isolates.
The ST-41/44 complex is a highly diverse clonal complex which has been, among other things, responsible for a serogroup B epidemic in New Zealand. ST-206, the dominant ST belonging to the ST-41/44 in this study, has previously been reported with serogroups B, C, and Z (http://pubmlst.org/neisseria/) but expressed only the serogroup C capsule in our isolates.
Approximately 50% of strains from carriage studies in Europe are nonserogroupable, while only 29% of those recovered in Burkina Faso were nonserogroupable, if we consider the results from slide agglutination (7). Carriage studies conducted in the meningitis belt have reported variable proportions of nonserogroupable strains, many of them lower than 50% (34). Usually, 16 to 20% of carriage isolates are lacking the gene coding for the synthesis of the capsule, while we found an average of 13%, with the differences between urban and rural areas being significant (6). Most of the new ST and PorA variants were identified from the pool of nonserogroupable strains. The diversity of the nonserogroupable strains is likely an expression of environmental adaptation where the bacteria lack the protective capsule.
Conclusions.
To our knowledge this is the largest meningococcal carriage study ever conducted in Africa. The aggregate data will form the basis for the assessment of the impact of MenAfriVac introduction on meningococcal carriage rates. With a prevaccination NmA carriage prevalence of 0.39%, all districts and all rounds confounded, and a sample size of 20,326 subjects, we should obtain significant results if the NmA carriage prevalence is reduced by 50% following vaccine introduction. Due to the low carriage prevalence of NmA, serogroup replacement is unlikely to occur as a direct result of MenAfriVac vaccination, in the similar way that replacement did not occur in the United Kingdom after introduction of a monovalent serogroup C conjugate vaccine.
Acknowledgments
We thank the residents in the districts of Bogodogo, Dandé, and Kaya for their participation in this study and the public health personnel from each district working in the field with mapping, recruitment, and survey. Special thanks go to the leading laboratory technicians, Manoudou Tamboura, Maxime Kienou, and Siakka Traoré, as well as to the many other laboratory technicians involved in this study. The technicians at the bacteriology laboratory of MDSC, Eric Sankara and Idrissa Kamaté, did a fabulous job making the selective agar plates for the study. We thank the technicians at the NIPH, Ida Andreasson, Martha Bjørnstad, Berit Nyland, Torill Alvestad, and Anne-Marie Klem, for training, supervision in Burkina Faso, and laboratory analysis. We thank Kader Kondé, Laurent Toé, Stacey Martin, and Lara Misegades for their contribution and support. We also thank Sylvestre Tiendrebeogo and Bokar Touré for their support and advice. Special acknowledgments are given to Flavien Aké for his technical expertise and engagement in the training, supervision, and technical assistance to the PDA operators, as well as his role in the data management.
This publication made use of the Neisseria Multi Locus Sequence Typing website (http://pubmlst.org/neisseria/), sited at the University of Oxford and funded by the Wellcome Trust and the European Union.
This project was supported by the Norwegian Research Council, grant no. 185784, to D.A.C.
Footnotes
Published ahead of print on 12 January 2011.
REFERENCES
- 1.Amadou, H. A., et al. 2006. Prospective survey on carriage of Neisseria meningitidis and protective immunity to meningococci in schoolchildren in Niamey (Niger): focus on serogroup W135. Microbes Infect. 8:2098-2104. [DOI] [PubMed] [Google Scholar]
- 2.Bash, M. C., et al. 2008. Human complement serum bactericidal activity following vaccination in a phase 2 safety and immunogenicity study of a new meningococcal group A conjugate vaccine in healthy African toddlers residing in the meningitis belt, abstr. 223, p. 281-282. Abstr. 16th Int. Pathog. Neisseria Conf.
- 3.Bash, M. C., et al. 2008. Safety and immunogenicity of a new meningococcal A conjugate vaccine (MenAfriVac™) in a healthy African population 2-29 years of age, abstr. 134, p. 202. Abstr. 16th Int. Pathog. Neisseria Conf.
- 4.Blakebrough, I. S., B. M. Greenwood, and H. C. Whittle. 1982. The epidemiology of infections due to Neisseria meningitidis and Neisseria lactamica in a northern Nigerian community. J. Infect. Dis. 146:626-637. [DOI] [PubMed] [Google Scholar]
- 5.Caugant, D. A., et al. 2006. Pharyngeal carriage of Neisseria meningitidis in 2-19-year-old individuals in Uganda. Trans. R. Soc. Trop. Med. Hyg. 100:1159-1163. [DOI] [PubMed] [Google Scholar]
- 6.Caugant, D. A., and M. C. Maiden. 2009. Meningococcal carriage and disease—population biology and evolution. Vaccine 27(Suppl. 2):B64-B70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caugant, D. A., G. Tzanakaki, and P. Kriz. 2007. Lessons from meningococcal carriage studies. FEMS Microbiol. Rev. 31:52-63. [DOI] [PubMed] [Google Scholar]
- 8.Craven, D. E., C. E. Frasch, J. B. Robbins, and H. A. Feldman. 1978. Serogroup identification of Neisseria meningitidis: comparison of an antiserum agar method with bacterial slide agglutination. J. Clin. Microbiol. 7:410-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emele, F. E., C. N. Ahanotu, and C. E. Anyiwo. 1999. Nasopharyngeal carriage of meningococcus and meningococcal meningitis in Sokoto, Nigeria. Acta Paediatr. 88:265-269. [DOI] [PubMed] [Google Scholar]
- 10.Forleo-Neto, E., et al. 1999. Decreased point prevalence of Haemophilus influenzae type b (Hib) oropharyngeal colonization by mass immunization of Brazilian children less than 5 years old with Hib polyribosylribitol phosphate polysaccharide-tetanus toxoid conjugate vaccine in combination with diphtheria-tetanus toxoids-pertussis vaccine. J. Infect. Dis. 4:1153-1158. [DOI] [PubMed] [Google Scholar]
- 11.Harrison, L. H., C. L. Trotter, and M. E. Ramsay. 2009. Global epidemiology of meningococcal disease. Vaccine 27(Suppl. 2):B51-B63. [DOI] [PubMed] [Google Scholar]
- 12.Hirve, S. S., et al. 2008. Safety and immunogenicity of a new meningococcal A conjugate vaccine in healthy Indian children aged 2-10 years, abstr. 227, p. 285-286. Abstr. 16th Int. Pathog. Neisseria Conf.
- 13.Kshirsagar, N., et al. 2007. Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine 25(Suppl. 1):A101-A107. [DOI] [PubMed] [Google Scholar]
- 14.LaForce, F. M., K. Konde, S. Viviani, and M. P. Preziosi. 2007. The Meningitis Vaccine Project. Vaccine 25(Suppl. 1):A97-A100. [DOI] [PubMed] [Google Scholar]
- 15.Lapeyssonie, L. 1963. La meningite cerebrospinale en Afrique. Bull. World Health Organ. 28(Suppl.):3-114. (In French.) [Google Scholar]
- 16.Leimkugel, J., et al. 2007. Clonal waves of Neisseria colonisation and disease in the African meningitis belt: eight-year longitudinal study in northern Ghana. PLoS Med. 4:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLennan, J., et al. 2006. Social behavior and meningococcal carriage in British teenagers. Emerg. Infect. Dis. 12:950-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiden, M. C., et al. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiden, M. C. J., and J. M. Stuart. 2002. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 359:1829-1830. [DOI] [PubMed] [Google Scholar]
- 20.Moore, M. R., et al. 2004. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J. Infect. Dis. 190:2031-2038. [DOI] [PubMed] [Google Scholar]
- 21.Mueller, J. E., et al. 2007. Molecular characteristics and epidemiology of meningococcal carriage, Burkina Faso, 2003. Emerg. Infect. Dis. 13:847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller, J. E., et al. 2006. Neisseria meningitidis serogroups A and W-135: carriage and immunity in Burkina Faso, 2003. J. Infect. Dis. 193:812-820. [DOI] [PubMed] [Google Scholar]
- 23.Mueller, J. E., and B. D. Gessner. 2010. A hypothetical explanatory model for meningococcal meningitis in the African meningitis belt. Int. J. Infect. Dis. 14:553-559. [DOI] [PubMed] [Google Scholar]
- 24.Nathan, N., et al. 2007. Meningitis serogroup W135 outbreak, Burkina Faso, 2002. Emerg. Infect. Dis. 13:920-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolas, P., et al. 2007. Populations of pharyngeal meningococci in Niger. Vaccine 25(Suppl. 1):A53-A57. [DOI] [PubMed] [Google Scholar]
- 26.Okoko, B. J., et al. 2008. A phase II, observer-blind, randomized, controlled study to evaluate the safety, immunogenicity, and memory of a booster dose of a meningococcal a conjugate vaccine (MenAfriVac™) in healthy African children, abstr. 211, p. 269-270. Abstr. 16th Int. Pathog. Neisseria Conf.
- 27.Raghunathan, P. L., et al. 2006. Predictors of immunity after a major serogroup W-135 meningococcal disease epidemic, Burkina Faso, 2002. J. Infect. Dis. 193:607-616. [DOI] [PubMed] [Google Scholar]
- 28.Ramsay, M. E., N. J. Andrews, C. L. Trotter, E. B. Kaczmarski, and E. Miller. 2003. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. Br. Med. J. 326:365-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reingold, A. L., C. V. Broome, and A. W. Hightower. 1985. Age-specific differences in duration of clinical protection after vaccination with meningococcal polysaccharide A vaccine. Lancet ii:114-118. [DOI] [PubMed] [Google Scholar]
- 30.Russell, J. E., K. A. Jolley, I. M. Feavers, M. C. J. Maiden, and J. Suker. 2004. PorA variable regions of Neisseria meningitidis. Emerg. Infect. Dis. 10:674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sie, A., et al. 2008. ST2859 serogroup A meningococcal meningitis outbreak in Nouna Health District, Burkina Faso: a prospective study. Trop. Med. Int. Health 13:861-868. [DOI] [PubMed] [Google Scholar]
- 32.Soriano-Gabarro, M., J. M. Stuart, and N. E. Rosenstein. 2002. Vaccines for the prevention of meningococcal disease in children. Semin. Pediatr. Infect. Dis. 13:182-189. [DOI] [PubMed] [Google Scholar]
- 33.Taha, M. K. 2000. Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. J. Clin. Microbiol. 38:855-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Thompson, E. A., I. M. Feavers, and M. C. Maiden. 2003. Antigenic diversity of meningococcal enterobactin receptor FerA, a vaccine component. Microbiology 149:1849-1858. [DOI] [PubMed] [Google Scholar]
- 34.Trotter, C. L., and B. M. Greenwood. 2007. Meningococcal carriage in the African meningitis belt. Lancet Infect. Dis. 7:797-803. [DOI] [PubMed] [Google Scholar]
- 35.Vestrheim, D. F., E. A. Hoiby, I. S. Aaberge, and D. A. Caugant. 2010. Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin. Vaccine Immunol. 17:325-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitney, A. M., et al. 2009. Genotypic comparison of invasive Neisseria meningitidis serogroup Y isolates from the United States, South Africa, and Israel, isolated from 1999 through 2002. J. Clin. Microbiol. 47:2787-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. 1998. Control of epidemic meningococcal disease. WHO practical guidelines, 2nd ed. Report WHO/EMC/BAC/98.3. World Health Organization, Geneva, Switzerland.
- 38.World Health Organization. 2008. International coordinating group on vaccine provision for epidemic meningitis control (ICG). Guidelines for applying to the emergency stockpile. WHO Offset Publishing, World Health Organization, Geneva, Switzerland.
- 39.World Health Organization. 2010. MDSC meningitis weekly bulletin. Week 49-53 2009. World Health Organization, Geneva, Switzerland.
- 40.Yaro, S., et al. 2007. Meningococcal carriage and immunity in western Burkina Faso, 2003. Vaccine 25(Suppl. 1):A42-A46. [DOI] [PubMed] [Google Scholar]
- 41.Yazdankhah, S. P., and D. A. Caugant. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53:9-32. [DOI] [PubMed] [Google Scholar]
- 42.Zombré, S., et al. 2007. The outbreak of meningitis due to Neisseria meningitidis W135 in 2003 in Burkina Faso and the national response: main lessons learnt. Vaccine 25(Suppl. 1):A69-A71. [DOI] [PubMed] [Google Scholar]