Abstract
Listeria monocytogenes has been exploited as a vaccine carrier based upon its ability to induce a strong cell-mediated immune response. At present, the safety of live, attenuated L. monocytogenes vaccines in patients is being studied in clinical trials. L. monocytogenes is also an attractive vaccine vector for use in poultry; however, the pathogenicity and immunogenicity of this organism in poultry remain to be fully elucidated. In this study, we investigated the pathogenicity and immunogenicity of an actA- and plcB-deficient L. monocytogenes strain, yzuLM4ΔactA/plcB, and its wild-type parent strain, yzuLM4, in an avian infection model. The results showed that the wild-type strain could infect ISA brown chickens, causing serious tissue disruptions, including various degrees of degeneration, necrotic lesions, and inflammatory cell infiltration in the liver, spleen, heart, and kidney. However, the mutant strain showed reduced virulence in embryonated eggs compared with that of the parent strain (the 50% lethal dose [LD50] was 3 logs higher). The mutant strain also showed low virulence in chickens and was rapidly eliminated by the host. There were no obvious pathological changes in tissue sections, but the mutant strain still retained the ability to stimulate high levels of antibody against the protein listeriolysin O (LLO). Booster immunization with the mutant strain led to rapid bacterial clearance from the livers and spleens of chickens challenged by the intramuscular route or the oral route. Collectively, our data suggest that the wild-type serotype 1/2a L. monocytogenes strain can cause serious disease in chickens but the mutant strain with a deletion of the actA and plcB genes is less virulent but induces a strong immune response. This mutant strain of L. monocytogenes is therefore a promising candidate as a safe and effective vector for the delivery of heterologous antigens to prevent zoonosis and infectious disease in poultry.
Listeria monocytogenes is an important food-borne intracellular pathogen which causes serious disease in humans, with a very high mortality rate. The average case-fatality rate for L. monocytogenes infection is 20% to 30% despite adequate antimicrobial treatment. Outbreaks of listeriosis have been reported in many areas of the world, including North America, Europe, and Japan (2, 6, 12, 13). As well as infecting humans, L. monocytogenes can also infect and cause listeriosis in animals, including birds, goats, sheep, horses, dogs, cats, and fish. More than 17 avian species have been verified to be susceptible to L. monocytogenes, with cases of listeriosis being reported in chickens, turkeys, pigeons, and water fowl (3, 18). Birds can be infected via the airborne route by inhalation, uptake, or debeaking. Young birds appear to be more susceptible to Listeria infection than older birds; the same principle is true for mammals, and listeriosis is most commonly manifested as septicemia (18).
Listeriosis in humans has been recognized as an important food-borne disease only since the 1980s, when a number of outbreaks were attributed to the contamination of poultry, particularly processed, ready-to-eat poultry products (19, 21). In recent years, dozens of recalls due to listeriae in poultry products have been reported by the news media. For example, the outbreaks of listeriosis caused by infected delicatessen turkeys in multiple states in the United States in 2000 and 2002 were caused by L. monocytogenes serotypes 1/2a and 4b, respectively (24). Like many animal species, poultry may excrete Listeria in feces, and this bacterium has been detected in 4% to 33% of the individual or pooled intestinal contents of broilers (4, 5, 22). Contact of poultry with fecal materials during slaughter processing has been suggested to be an important means of transmitting L. monocytogenes to poultry meat (22). Therefore, L. monocytogenes is generally considered a bacterial pathogen of food safety importance to both the poultry industry and public health.
Understanding both the L. monocytogenes infection process and the host immune response against L. monocytogenes is important for the development of preventive and therapeutic strategies. L. monocytogenes has also been proposed as a potential carrier for the delivery of heterologous antigens. For this reason, it has been extensively studied in mice (1, 20, 23); however, few studies using the chicken infection model have been reported. There is a need for novel effective avian vaccines against Listeria infection, and recombinant Listeria vaccines are an attractive alternative to traditional avian vaccines, which are costly and carry the risk of toxin leakage. Hence, demonstrating its pathogenicity and immunogenicity is especially necessary for its development as a vector for delivery of heterologous antigens of avian pathogens.
MATERIALS AND METHODS
Bacterial strains.
The virulent L. monocytogenes strain yzuLM4, serotype 1/2a, was isolated and preserved by the Jiangsu Key Laboratory of Zoonosis (Yangzhou, China). The L. monocytogenes mutant strain yzuLM4ΔactA/plcB, with in-frame deletions within the actA and plcB genes achieved by allelic replacement, as described previously (26), was used. The bacteria were grown in 10 ml Bacto brain heart infusion (BHI) medium (Becton Dickinson Co., Franklin Lakes, NJ) per 100-ml flask by shaking at 37°C for 14 h, and then they were inoculated (1:50) in another 10 ml BHI per 100-ml flask and grown for 3 h under the same culturing conditions.
Animals.
Specific-pathogen-free (SPF) chicken embryos and female chickens were procured from the Shandong Institute of Poultry Science (Jinan, China). Animals were housed, handled, and immunized following approval by the institutional animal experimental committee.
Preparation of Listeria strains.
For immunization and protection studies, fresh bacterial cultures, prepared from an overnight culture, were used. Briefly, the mutant strain yzuLM4ΔactA/plcB and the wild-type parent strain were grown in BHI media, harvested in the exponential growth phase (optical density at 600 nm [OD600] = 1.0), and washed twice with phosphate-buffered saline (PBS; pH 7.2). The pellet was resuspended in PBS, and the bacterial concentration was calibrated by optical absorption and CFU counting. Further dilutions were prepared in PBS to obtain the required number of bacteria for immunizations or challenge.
Virulence in chicken embryos.
The 50% lethal doses (LD50) of the two strains were estimated by using the trimmed Spearman-Karber method (11). In a separate experiment, 0.1 ml of an appropriate 10-fold dilution of the mutant or parent strain in PBS was injected via the chorioallantoic membrane into 14-day-old embryonated eggs by using a technique described previously (14). Five eggs were used for each inoculum. Eggs receiving PBS were included as a control. The inoculum concentration was confirmed by enumeration of the viable count on BHI plates. Inoculated eggs were incubated in a horizontal position at 37.5°C, and embryo death was monitored daily over 8 days by transillumination for LD50 calculation.
Infection and bacterial enumeration.
Two groups of 9-day-old chickens were used. yzuLM4ΔactA/plcB and yzuLM4 at doses of 1 × 109 CFU were injected into the right pectoralis muscles of 25 chickens in each group by using a 1-ml syringe. On days 1, 2, 3, 4, and 6 postinoculation, chickens were sacrificed, and the spleens and livers were taken out and homogenized by glass homogenizers; after 10-fold serial dilutions, they were plated on BHI plates (37 g BHI medium and 18 g agar in 1 liter distilled water, pH 7.4, with sterilization at 121°C for 15 min and pouring onto the sterile plates). Colonies were counted and identified after 24 h of aerobic incubation at 37°C. The detection limit of this procedure was 102 CFU per organ.
Histopathology.
Two groups of chickens were immunized with the same route and dose as described in “Infection and bacterial enumeration” (above), and a third group was included as a control and received intramuscular inoculation with PBS. On day seven postimmunization, sections of livers, hearts, spleens, duodenums, and kidneys were fixed in 13% neutral buffered formalin. Paraffin-embedded sections were cut at 5 μm, stained with hematoxylin and eosin, and examined for histological lesions with a 400× microscope (Leica Microsystems Wetzlar GmbH).
Effect on chicken body weight.
Two groups of chickens were inoculated as described in “Infection and bacterial enumeration” (above). The chickens were then boosted at 23 days of age. On days 7 and 14 postpriming and on days 7, 14, 21, and 28 postboosting, the chickens were weighed.
Titer of serum antibody to LLO protein, as measured by ELISA.
Two groups of chickens were immunized as described in “Infection and bacterial enumeration” (above). Chickens were boosted at 30 days of age. On day 21 postpriming and day 21 postboosting, blood from veins of the chicken wings was taken, the sample was incubated at 4°C for 30 min, and then the serum was harvested by centrifugation at 1,000 × g for 5 min. Serum antibody against listeriolysin O (LLO) protein was determined by indirect enzyme-linked immunosorbent assay (ELISA). The LLO protein expressed by recombinant Escherichia coli was purified and used as a coating antigen in the ELISA plate (0.34 μg per well). All serum samples were serially diluted for analysis. The secondary antibody horseradish peroxidase (HRP)-conjugated rabbit anti-chicken IgG (Pierce Biotechnology, Rockford, IL) was used at a concentration of 1:10,000. Serum from nonimmunized SPF chickens and the monoclonal antibody against LLO were used as the negative and positive controls, respectively, for validation of the ELISA. The A490 data from the negative-control serum were used to calculate the positive/negative (P/N) values. The experiment was carried out in triplicate.
Protection efficacy.
Two groups of 10 9-day-old chickens were intramuscularly injected with yzuLM4ΔactA/plcB at a dose of 1 × 109 CFU, and another two groups served as controls and received intramuscular inoculation with PBS. Chickens were boosted at 23 days of age. On day 14 postboosting, oral gavage was performed for one group of immunized chickens and one control group with 500 μl of a 4 × 1010-CFU inoculum of yzuLM4 in PBS through a gavage needle on a 1-ml syringe. Another group of immunized chickens and another control group were challenged via the intramuscular injection route with yzuLM4 at doses of 1 × 109 CFU. The birds were euthanized by carbon dioxide asphyxiation 48 h postchallenge, and the liver and spleen were removed immediately. Each tissue was weighed, homogenized by glass homogenizers, and suspended (1:10) in PBS; 10-fold dilutions were prepared for cell counting on BHI agar plates. Then, the plates were incubated at 37°C for 24 h. Round, smooth, and milky colonies typical of Listeria were counted.
Statistical analysis.
Differences between groups were analyzed by using the Statistical Package for the Social Sciences (SPSS version 15.0; SPSS, Chicago, IL). P values less than 0.05 were considered to be significant, and those less than 0.01 were considered to be highly significant.
RESULTS
Reduced virulence of the L. monocytogenes mutant strain in embryonated eggs.
The mutant strain showed reduced virulence compared to its parent strain (LD50 3 logs higher) in the chicken embryo model test (Table 1). More than 80% of chicken embryos infected with the wild-type strain died at 72 h postinoculation, and 86.7% of the embryos died at 7 days postinoculation. However, none of the chicken embryos inoculated with the mutant strain died at 72 h postinoculation, and only 25% of inoculated embryos died at 7 days postinoculation. These results showed that the virulence of the mutant strain was significantly reduced.
TABLE 1.
LD50 of L. monocytogenes for embryonated chicken eggs
| Treatment | No. of chicken embryos that died/total at infection dose (CFU) |
Log LD50a | |||||
|---|---|---|---|---|---|---|---|
| 1.33 × 107 | 1.33 × 106 | 1.33 × 105 | 1.33 × 104 | 1.33 × 103 | 1.33 × 102 | ||
| yzuLM4ΔactA/plcB | 3/5 | 3/5 | 2/5 | 2/5 | 1/5 | 1/5 | 5.223 |
| yzuLM4 | 5/5 | 5/5 | 5/5 | 5/5 | 3/5 | 3/5 | 2.423 |
| PBS | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
The logarithm of median lethal dose to base 10.
In vivo survival and persistence of the L. monocytogenes mutant strain and its wild type.
Because all of the chickens survived over a number of days with infection, they were sacrificed before bacteria were recovered from their organs. The first and crucial step in the in vivo characterization of the mutant and wild-type strains was the determination of their growth kinetics in chicken spleens and livers. As indicated in Fig. 1, for the mutant strain group, no bacteria were isolated from the liver and the number of bacteria in spleen dropped rapidly from day 2 postinoculation, leveling off and persisting at a low number up to day 5 postinfection. However, for the wild-type strain infection group, the number of bacteria in the liver was high (>4 logs) on day 1, reached a peak on day 2, and then gradually decreased, and the bacteria were cleared by day 8. The number of L. monocytogenes organisms in the spleen was highest on day 1 and then gradually decreased, and the bacteria were finally cleared on day 8. This result showed that there were fewer bacteria in the spleen and liver after infection with the mutant strain and that the mutant strain cleared quicker than the wild-type strain. The bacterial growth kinetics in vivo therefore indicated that the translocation ability of the mutant strain was reduced, and there was attenuated virulence.
FIG. 1.
The CFU counts of L. monocytogenes in the organs (livers [A] and spleens [B]) of commercial ISA brown chickens after intramuscular infection. Twenty-five chickens in each group were intramuscularly injected with yzuLM4ΔactA/plcB or yzuLM4 at a dose of 1 × 109 CFU. On days (d) 1, 2, 3, 4, and 6 after the infection, chickens were sacrificed, and the numbers of viable bacteria in the organs were enumerated as described in the text.
Effect of infection on chicken body weight.
As shown in Table 2, the body weights of chickens infected with the wild-type strain were significantly decreased in the intramuscular challenge group compared with the weights of the unchallenged controls and those infected with the mutant strain (P < 0.05). However, the body weights of chickens infected with the mutant strain showed no significant difference from those of the unchallenged controls, except at week 1 postboosting (P > 0.05; Table 2). There was a significant association between infection with the wild-type strain and body weight, while the mutant strain did not affect body weight, suggesting that the mutant had highly attenuated virulence.
TABLE 2.
Effect of injection with L. monocytogenes on chicken weight
| Time | Chicken weight (g) after treatmenta |
||
|---|---|---|---|
| Control | yzuLM4ΔactA/plcB | yzuLM4 | |
| 1 wk postpriming | 176.47 ± 11.58 a | 170.45 ± 14.73 a | 143.90 ± 14.21 b |
| 2 wks postpriming | 260.95 ± 22.93 a | 250.72 ± 21.13 a | 217.91 ± 19.36 c |
| 1 wk postboosting | 357.93 ± 25.12 a | 338.77 ± 23.42 b | 305.53 ± 24.84 c |
| 2 wks postboosting | 508.12 ± 25.66 a | 497.43 ± 21.08 a | 458.22 ± 23.34 b |
| 3 wks postboosting | 578.45 ± 27.44 a | 563.24 ± 24.27 a | 521.46 ± 28.50 b |
| 4 wks postboosting | 645.62 ± 27.44 a | 630.81 ± 24.27 a | 588.70 ± 28.49 b |
Within a row, data followed by the same lowercase letters had no significant difference (P) at the 0.05 level by comparison of different treatments.
Gross pathology and histopathological studies of L. monocytogenes-infected chickens. (i) Gross pathology.
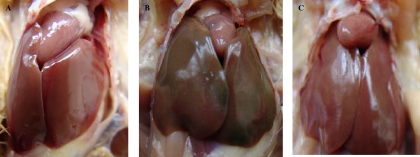
After intramuscular infection of chickens with the virulent L. monocytogenes strain yzuLM4, the gross pathological symptoms included loss of appetite, reduced intake, flagging spirit, and diarrhea. Systemic abnormalities, such as splenomegaly, mild hepatomegaly, myocarditis, and white miliary spot necrosis at the site of injection, were observed in these chickens. No gross symptoms were observed in either the mutant-inoculated group or the control group (Fig. 2).
FIG. 2.
Gross lesions in the visceral organs of chickens. (A) Control group. (B) yzuLM4-receiving group. (C) yzuLM4ΔactA/plcB-receiving group.
(ii) Histopathological lesions.
Analysis of histopathological lesions in chickens infected by wild-type L. monocytogenes revealed serious disruption (Fig. 3). The pathological characteristics of the visceral organs were as follows. The liver sections showed inflammatory cell infiltration in the hepatic lobule. In the spleen section, hyalinosis and fibrinous exudates were observed in the reticular fiber of the spleen, and homogeneous red staining material had accumulated among lymphocytes. The kidney section revealed that not only had the connective tissue of the kidney hyperplasia proliferated and the mesenchyme broadened but also numerous inflammatory cells had appeared in the venule. In the heart section, unequal-sized focal necrotic lesions had appeared in the disintegrated muscle fibers. In the intestine section, cast-off intestinal epithelial cells were evident. However, it is clear from Fig. 3 that no obvious pathological changes appeared in the sections taken from chickens inoculated with the mutant strain. The mutant strain induced only a mild inflammatory reaction, as evidenced by infiltration of inflammatory cells into the peripheral and central veins of the liver and the tissue of the kidney.
FIG. 3.
Histopathology of chickens infected with L. monocytogenes via intramuscular routes. Magnifications, ×400. (A) The liver of a chicken infected with the wild-type strain. (B) The liver of a chicken infected with mutant strain yzuLM4ΔactA/plcB. (C) Healthy control liver of a chicken. (D) The spleen of a chicken infected with wild-type strain yzuLM4. (E) The spleen of a chicken infected with mutant strain yzuLM4ΔactA/plcB. (F) Healthy control spleen of a chicken.
L. monocytogenes-specific serum antibody response.
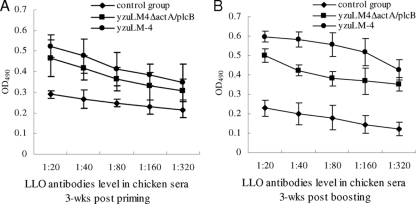
To analyze the humoral immune response induced by L. monocytogenes, we chose the highly antigenic LLO protein as an indicator and determined the level of anti-LLO antibody in chicken sera. Although the level of antibody induced by the mutant strain was significantly reduced compared with that induced by the wild-type strain (Fig. 4A and B), the attenuated mutant still retained the ability to stimulate a high level of antibody against LLO. Combined with the results of kinetic studies of L. monocytogenes infection in chickens, these findings suggested that the level of antibody induced by Listeria correlated with the number of bacteria entering the host.
FIG. 4.
Anti-LLO antibody levels in sera after inoculation of chickens with L. monocytogenes, on day 21 postpriming (A) or day 21 postboosting (B). Data presented are representative of three independent experiments.
Protection acquired after immunization with the mutant strain against wild-type strain challenge.
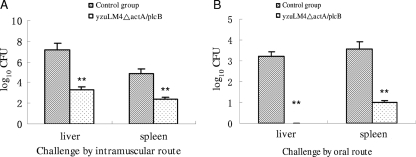
To further analyze the ability of the mutant strain to induce protective immunity, chickens were immunized and boosted with L. monocytogenes mutant strain yzuLM4ΔactA/plcB. Based upon the fact that the wild-type strain yzuLM4 could infect ISA brown chickens and cause disease but was not fatal to chickens at 1 × 109 CFU per chicken by the intramuscular route, we evaluated the protection efficacy by comparing the CFU counts of L. monocytogenes in the organs. CFU numbers in spleens and livers of chickens were determined at day 2 after challenge infections by the intramuscular and oral routes (Fig. 5A and B, respectively). The results showed that the immunized chickens cleared L. monocytogenes bacteria quicker than the unimmunized group at day 2 after challenge by either the oral route or the intramuscular route. This difference was statistically significant (P < 0.01). The vaccination regime resulting in quicker clearance suggested that immunization of chickens with L. monocytogenes strain yzuLM4ΔactA/plcB could induce protective immune responses.
FIG. 5.
The CFU counts of L. monocytogenes in the organs of commercial ISA brown chickens immunized with yzuLM4ΔactA/plcB and challenged with virulent yzuLM4 at 2 weeks postboosting. Chickens were sacrificed on day 2 after challenge by the intramuscular route (A) or the oral route (B), and the numbers of viable bacteria in spleens and livers were enumerated as described in the text. Data presented are representative of three independent experiments. **, P value of <0.01 relative to the corresponding control group.
DISCUSSION
Recombinant L. monocytogenes strains have been designed and verified as effective delivery systems for a variety of heterologous antigens inducing profound and effective antipathogen or antitumor immunity in a mouse model (9, 10, 17). However, the pathogenic mechanism and the host immune response to L. monocytogenes still remain to be systematically investigated in an avian model. L. monocytogenes serotypes 1/2a and 4b are responsible for the majority of cases of human listeriosis worldwide, and serotype 1/2a belongs to lineage II. Lineage II strains are common in foods; they seem to be widespread in natural and farm environments and have also been isolated from animal listeriosis cases and sporadic human clinical cases (15). Furthermore, many countries have seen a shift in the L. monocytogenes serotypes causing human infections from predominantly serotype 4b to serotypes 1/2a and 1/2b (2, 16). Taking this into consideration, we used the serovar 1/2a mutant strain yzuLM4ΔactA/plcB and its parent strain, yzuLM4, in this study to infect chickens via muscular injection and investigated pathogenicity, immunogenicity, and efficacy of immunization.
L. monocytogenes is an important food-borne pathogen, and listeriosis is a zoonotic disease. L. monocytogenes is highly effective at crossing the intestinal barrier, the blood-brain barrier, and the fetoplacental barrier, causing meningoencephalitis, mastitis, abortion, metritis, and septicemia. In this study, we found that the serotype 1/2a wild-type strain yzuLM4, which is highly virulent in mice (LD50 of 1.47 × 104 in BALB/c mice), is also highly pathogenic in SPF chicken embryos (LD50 of 2.65 × 102) and ISA brown hens. This bacterium could cross the natural barriers in chickens and reach the liver and spleen at about 104 and 107 CFU, respectively, 24 h after infection via the intramuscular route. The bacteria were finally cleared by day 8. Analysis of tissue sections showed that serious pathological changes in the liver and spleen were caused by the virulent strain. The pathology seen in this study is consistent with the findings of Huff et al. (8). The high-level virulence of the wild-type strain resulted in significantly decreased body weight compared with those of chickens infected with the attenuated strain, indicating that the wild-type strain seriously affected the growth of chickens. However, the invasive ability of the attenuated strain yzuM4ΔactA/plcB was clearly reduced. L. monocytogenes was not isolated from the liver, and the number of bacteria isolated from the spleen was reduced about 103-fold on day 1; as well, the mutant bacteria were cleared 3 days earlier than the parent strain bacteria. Pathological analysis of tissue sections also showed only minimal inflammation in the visceral organs due to the immune response caused by the attenuated strain. This mutant strain did not affect body weight, and there was no detectable difference between body weights of the mutant group and the control group. Hence, these results suggest that the ability of the mutant strain of L. monocytogenes to diffuse and translocate is reduced, thereby significantly reducing the virulence.
To evaluate the ability of L. monocytogenes to elicit an immune response in ISA brown hens, we tested for production of antibodies against protein LLO in an indirect ELISA. Although the results showed a significant difference between antibody titers in the attenuated and virulent strains, the attenuated strain could still induce a high anti-LLO titer after inoculation via injection. LLO protein is a major immunodominant listerial antigen playing an important role in the presentation of passenger antigens to the class I pathway of the major histocompatibility complex (MHC) and therefore elicits strong CD8+ T-cell-mediated immune responses (7, 25). Therefore, the central role of LLO in the listerial infection cycle, coupled with the significant antigenicity of this protein, suggested that the mutant strain retains the ability to induce strong cellular immunity. In this study, we found that chickens vaccinated with the attenuated strain acquired sufficient immune protection against the wild-type strain yzuLM4 to clear L. monocytogenes infection more rapidly than unvaccinated chickens. This result confirmed that the attenuated strain retains the ability to induce an immune response against L. monocytogenes. It also indicated that the attenuated strain yzuLM4ΔactA/plcB is effective at delivering pathogenic antigens and inducing an immune response, a property that could be applied to the prevention of infection by pathogens.
In this study, we found that the wild-type strain yzuLM4 could infect ISA brown chickens and cause disease, but it was not fatal to chickens at 1 × 109 CFU per chicken via the intramuscular infection route. However, in another study, we showed that serotype 4b strain yzuF36 was fatal to chickens by the same infection route and dosage (data not published), indicating that the virulence of strain yzuLM4 is less than that of strain yzuF36 in the chicken infection model. Furthermore, the wild-type strain yzuLM4 is virulent in mice (LD50 = 1.47 × 104) but less virulent in chickens. These results confirmed that differences between the pathogenic tropisms of L. monocytogenes strains exist. Our results are concordant with the opinions of Orsi et al.; their study suggested that four L. monocytogenes lineages (I, II, III, and IV) identified so far represent distinct ecologic, genetic, and phenotypic characteristics which appear to affect their ability to be transmitted through foods and to cause human disease (15).
In summary, we investigated the characteristics of L. monocytogenes infection and immunogenicity and analyzed the safety and efficacy of L. monocytogenes immunization. The results confirmed that chickens were susceptible to the strains used in these experiments. The mutant strain yzuLM4ΔactA/plcB showed decreased invasion and virulence but was able to elicit a strong immune response and provide immune protection against the wild-type homologous strain. Further studies are now required to determine the most effective dosage and vaccination procedure. By the results of this study taken together, the attenuated strain of L. monocytogenes described in this study is a promising vaccine candidate for preventing listeriosis, and this L. monocytogenes strain may be further exploited as a potential candidate to express heterologous antigens for the prevention of avian infectious diseases and zoonoses.
Acknowledgments
This work was supported by the National Key Technology R&D Program (grants 2009BADB9B01 and 2007BAD40B01), Program for Changjiang Scholars and Innovative Research Team in University (grant PCSIRT), and Natural Science Foundation of Jiangsu Higher Education Institutions of China (grant 2009KJA230007).
Footnotes
Published ahead of print on 12 January 2011.
REFERENCES
- 1.Busch, D. H., S. Vijh, and E. G. Pamer. 2001. Animal model for infection with Listeria monocytogenes. Curr. Protoc. Immunol. 19:19.9. [DOI] [PubMed] [Google Scholar]
- 2.Clark, C. G., et al. 2009. Surveillance for Listeria monocytogenes and listeriosis, 1995-2004. Epidemiol. Infect. 12:1-14. [DOI] [PubMed] [Google Scholar]
- 3.Cooper, G., et al. 1992. Listeriosis in California broiler chickens. J. Vet. Diagn. Invest. 4:343-345. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra, R. G. 1976. Listeria-encephalitis in cows through litter from a broiler farm. Zentalbl. Bakteriol. 161:383-385. [PubMed] [Google Scholar]
- 5.Dijkstra, R. G. 1978. The incidence of Listeria monocytogenes in the intestinal contents of broilers on different farms. Tijdschr. Diergeneeskd. 15:229-231. [PubMed] [Google Scholar]
- 6.Gottlieb, S. L., et al. 2006. Multistate outbreak of listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin. Infect. Dis. 42:29-36. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton, S. E., V. P. Badovinac, A. Khanolkar, and J. T. Harty. 2006. Listeriolysin O-deficient Listeria monocytogenes as a vaccine delivery vehicle: antigen-specific CD8 T cell priming and protective immunity. J. Immunol. 177:4012-4020. [DOI] [PubMed] [Google Scholar]
- 8.Huff, G. R., W. E. Huff, V. Dutta, M. G. Johnson, and R. Nannapaneni. 2008. Pathogenicity of Listeria monocytogenes Scott A after oral and oculonasal challenges of day-old turkey poults. Avian Dis. 52:444-450. [DOI] [PubMed] [Google Scholar]
- 9.Kim, S. H., et al. 2008. Mage-b vaccine delivered by recombinant Listeria monocytogenes highly effective against breast cancer metastases. Br. J. Cancer 99:741-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, S. H., F. Castro, Y. Paterson, and C. Gravekamp. 2009. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 69:5860-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kline, E. R., A. W. Jarvinen, and M. L. Knuth. 1989. Acute toxicity of triphenyltin hydroxide to three cladoceran species. Environ. Pollut. 56(1):11-17. [DOI] [PubMed] [Google Scholar]
- 12.Little, C. L., N. J. Barrett, K. Grant, and J. McLauchlin. 2008. Microbiological safety of sandwiches from hospitals and other health care establishments in the United Kingdom with a focus on Listeria monocytogenes and other Listeria species. J. Food Prot. 71:309-318. [DOI] [PubMed] [Google Scholar]
- 13.Makino, S. I., et al. 2005. An outbreak of food-borne listeriosis due to cheese in Japan, during 2001. Int. J. Food Microbiol. 104:189-196. [DOI] [PubMed] [Google Scholar]
- 14.Nørrung, B., and J. K. Andersen. 2000. Variations in virulence between different electrophoretic types of Listeria monocytogenes. Lett. Appl. Microbiol. 30:228-232. [DOI] [PubMed] [Google Scholar]
- 15.Orsi, R. H., H. C. Bakker, and M. Wiedmann. 2010. Listeria monocytogenes lineages: genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. [Epub ahead of print.] doi: 10.1016/j.ijmm.2010.05.002. [DOI] [PubMed]
- 16.Pan, Y., F. Breidt, Jr., and S. Kathariou. 2009. Competition of Listeria monocytogenes serotype 1/2a and 4b strains in mixed-culture biofilms. Appl. Environ. Microbiol. 75:5846-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radosevic, K., et al. 2007. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect. Immun. 75:4105-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saif, Y. M. 2005. Diseases of poultry, 11th ed., p. 968-969. Iowa State Press, Ames, IA.
- 19.Schlech, W. F., III, et al. 1983. Epidemic listeriosis—evidence for transmission by foods. N. Engl. J. Med. 308:203-206. [DOI] [PubMed] [Google Scholar]
- 20.Schoen, C., et al. 2008. Listeria monocytogenes as novel carrier system for the development of live vaccines. Int. J. Med. Microbiol. 298:45-58. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz, B., et al. 1988. Association of sporadic listeriosis with consumption of uncooked hot dogs and undercooked chicken. Lancet ii:779-782. [DOI] [PubMed] [Google Scholar]
- 22.Skovgaard, N., and C. A. Morgen. 1988. Detection of Listeria spp. in faeces from animals, in feeds, and in raw food of animal origin. Int. J. Food Microbiol. 6:229-242. [DOI] [PubMed] [Google Scholar]
- 23.Spears, P. A., et al. 2008. A Listeria monocytogenes mutant defective in bacteriophage attachment is attenuated in orally inoculated mice and impaired in enterocyte intracellular growth. Infect. Immun. 76:4046-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swaminathan, B., and P. Gerner-Smidt. 2007. The epidemiology of human listeriosis. Microbes Infect. 9:1236-1243. [DOI] [PubMed] [Google Scholar]
- 25.Yin, Y., et al. 2010. Protective immunity induced by a LLO-deficient Listeria monocytogenes. Microbiol. Immunol. 54:175-183. [DOI] [PubMed] [Google Scholar]
- 26.Yin, Y., G. Zhu, S. Geng, M. Hu, and X. Jiao. 2008. Construction and characterization of a mutant strain of Listeria monocytogenes with a deletion of actA and plcB. Wei Sheng Wu Xue Bao 48:299-303. [PubMed] [Google Scholar]