Abstract
In this study, we examined the role of recombination at the telomeres of the yeast Kluyveromyces lactis. We demonstrated that an abnormally long and mutationally tagged telomere was subject to high rates of telomere rapid deletion (TRD) that preferentially truncated the telomere to near-wild-type size. Unlike the case in Saccharomyces cerevisiae, however, there was not a great increase in TRD in meiosis. About half of mitotic TRD events were associated with deep turnover of telomeric repeats, suggesting that telomeres were often cleaved to well below normal length prior to being reextended by telomerase. Despite its high rate of TRD, the long telomere showed no increase in the rate of subtelomeric gene conversion, a highly sensitive test of telomere dysfunction. We also showed that the long telomere was subject to appreciable rates of becoming elongated substantially further through a recombinational mechanism that added additional tagged repeats. Finally, we showed that the deep turnover that occurs within normal-length telomeres was diminished in the absence of RAD52. Taken together, our results suggest that homologous recombination is a significant process acting on both abnormally long and normally sized telomeres in K. lactis.
Telomeres are complexes of DNA and proteins that protect the ends of chromosomes from being recognized as double-strand breaks (9, 28, 50). Due to the inability of replicative polymerases to fully replicate ends, telomeres gradually shorten over time in the absence of a specialized means of maintaining them (48, 75). In the majority of eukaryotes, the ribonucleoprotein telomerase acts to extend telomere ends by adding nucleotides to a short 3′ overhang at telomeres (49, 63). In what is thought to be an anticancer adaptation, most human cells have little or no telomerase, which leads to replicative senescence when telomeres become too short (62). Furthermore, telomeres are normally “capped” by a complex of proteins which protect them from nonhomologous end joining (NHEJ) and homologous recombination (11, 51). In some organisms, including humans, telomere capping may also involve telomeric loops (t-loops), the structures resulting from the intramolecular strand invasion of the 3′ overhang at telomeres into the internal telomeric repeats (14, 37).
While gradual loss and extension by telomerase are the most common modes of telomere length changes, other processes, most notably recombination, have been shown to affect telomere length under certain circumstances. Some DNAs, including the chromosomes of the mosquito Anopheles gambiae and the linear mitochondrial DNAs of certain yeasts and ciliates, appear to use recombination as their normal method of telomere maintenance (36, 47, 59, 66). Telomerase-deficient mutants of a number of yeast species are able to maintain their telomeres by recombination (27, 30, 42, 64). In Kluyveromyces lactis and Saccharomyces cerevisiae, the species that have been studied the most, telomere elongation appears to occur by a “roll-and-spread” model, in which a small circle of telomeric repeats (t-circle) first acts as a template for rolling circle DNA synthesis to elongate a single telomere. The sequence from the first long telomere can then be copied in a break-induced replication (BIR)-like event to elongate the other telomeres in the cell (15, 26, 43, 44, 67). In both species, this recombinational telomere elongation is dependent on the major recombination protein encoded by RAD52 and is initiated by the loss of telomere capping brought on by very short telomere lengths (27, 30, 33). More recent work has demonstrated that recombinational telomere elongation can also occur as a consequence of certain mutations directly affecting telomere capping (2, 12, 13, 18, 22, 53, 78). Interestingly, these mutations frequently create telomeres that are longer and more heterogeneous than telomeres undergoing recombination caused by the loss of telomerase.
Recombination has also been shown to be an important telomere maintenance mechanism in some cancer cells. Telomerase activity is very low or absent in most human somatic cells (20). While the majority of human cancers reactivate telomerase to maintain telomeres and immortalize cells, a significant minority use a recombinational mechanism called alternative lengthening of telomeres (ALT) (3, 20, 58). Telomeres become highly heterogeneous in ALT cancer cells, with some telomeres being very long and others having few or no telomeric repeats. Extrachromosomal telomeric DNA, in both linear and circular forms, is commonly found in these cells (6, 41, 74). Various types of telomeric recombination have been demonstrated to be common in ALT cells. Experiments using a sequence tag introduced on one telomere have shown that recombination events can transfer the sequence to other telomeres as well as duplicate it at the same telomere (10, 38). Also, reciprocal sister chromatid exchanges have been shown to occur at greatly elevated rates in ALT cells (1).
Telomeres can also be subject to dramatic shortening events under a variety of different circumstances, including normal growth and development in some organisms. Examples include the abrupt shortening of all telomeres in newly developed macronuclei of the ciliate Euplotes crassus (71), the rapid trimming back to normal size of telomeres greatly lengthened by continuous mitotic growth in Tetrahymena thermophila (23), and the large truncations and elongations occurring during antigenic variation in Trypanosoma brucei (40). Even the comparatively large telomere attrition per cell division of relatively long telomeres in human fibroblasts and in telomerase mutants of Caenorhabditis elegans has been proposed to involve additional mechanisms besides gradual loss due to the end-replication problem (7, 17). Considerable evidence now suggests that oxidative damage to telomeres can accelerate telomere attrition in human cells (57, 61, 72, 73).
Sudden shortening events are commonly associated with telomeres that have capping defects. Early studies of immortalized human cells showed dramatic shortenings, sometimes losing all telomeric signal, at a single telomere that had become long (39). Human cells containing mutations of the double-stranded telomere binding protein TRF2 showed a depletion of 3′ overhangs and telomere deletions that were the size of t-loops (70, 74). A K. lactis mutant with very long telomeres occasionally displayed better-growing colonies with much shorter telomeres (31). Even in situations where recombinational maintenance is occurring, there can also be concurrent large shortening events. Evidence from a K. lactis mutant lacking telomerase and having telomeric repeats defective for binding of Rap1 suggested that rapid truncations to very short lengths occurred regularly in cells with very long telomeres maintained by recombination (2).
The best-studied example of dramatic telomere shortening is telomere rapid deletion (TRD), which has been shown to truncate abnormally long telomeres in S. cerevisiae (25). These truncations predominantly shorten telomeres down to the size of resident wild-type telomeres and were proposed to represent a trimming mechanism that shortened telomeres that had become too long. TRD in S. cerevisiae is partially dependent on the major recombination protein Rad52 and also shows a dependency on the Mre11p-Rad50p-Xrs2p complex and the Ku70/80 heterodimer (4, 25). The retention of introduced HaeIII sites at relatively more basal positions in telomeres that had undergone TRD led to the proposal that TRD occurred though a terminal deletion initiated by a t-loop intermediate (4). TRD showed a 30- to 70-fold increase in meiosis, which was dependent on the meiotic bouquet protein Ndj1p (19). TRD has also been reported for abnormally long telomeres in Arabidopsis thaliana but does not appear to be dependent on paralogs of RAD51 or on the MRE11-RAD50-NBS1 complex (76). Interestingly, in human cells overexpressing telomerase RNA, telomeres become long and heterogeneous in length and t-circles become abundant, the latter presumably because of trimming by a TRD-like mechanism (54).
In order to determine the fate of a long telomere in K. lactis cells, we introduced an abnormally long telomere made up of phenotypically silent repeats containing a BclI restriction site into cells with otherwise wild-type telomeres. Like the case for S. cerevisiae, we observed abundant TRD events that shortened telomeres to apparent wild-type size. However, about half of these events were associated with deep turnover of telomeric repeats. We also report a lower frequency of further elongation of the Bcl telomere brought about by recombination.
MATERIALS AND METHODS
Strains and culture conditions.
The K. lactis strain ZT-LBT1, containing the long Bcl telomere examined in this study, was a derivative of 7B520 (ura3 his2-2 trp1) (77). It contained a deletion of the K. lactis telomerase RNA gene TER1 (60). TER1 was replaced in these cells by use of the plasmid pJR31, which is a derivative of pKL316 (60). This plasmid contains an S. cerevisiae HIS3 gene, which complements the his2 mutation in 7B520, for selection. The long Bcl telomere with URA3 adjacent to it was constructed and transformed into K. lactis cells to replace a single telomere as described previously (67). To create a rad52Δ strain with a long telomere, cells containing the long telomere were mated to SI-E4 (ade2-202 rad52Δ), a strain of the opposite mating type (Shilpa Iyer, unpublished data).
For the mitotic TRD studies, clones of the strain containing the long Bcl telomere were plated on SD minimal medium lacking histidine in order to retain pJR31 and were grown for 3 to 4 days to allow adequate growth. The rad52Δ and postmeiosis strains were plated on yeast extract-peptone-dextrose (YPD) after ascertaining that they had obtained the wild-type TER1 allele after mating.
Matings were conducted on malt extract, and diploids were grown on YPD after initial selection on an SD plate lacking tryptophan. Sporulation of the diploids took place on minimal sporulation medium, and spore dissections took place on YPD with 1 M sorbitol. DNAs from the resulting spores were subjected to Southern blotting in order to determine which of them had obtained both the rad52Δ allele and the long telomere. Membranes were probed with the sequence of the RAD52 gene from the plasmid pSK(KlRAD52) to determine which had received the rad52Δ allele (35).
TRD was observed during meiosis by mating the ZT-LBT1 strain containing the long telomere to the K. lactis strain GG1958 (ade2-202). After tetrad dissection, DNAs from all four spores were examined by Southern blotting to determine whether TRD had occurred.
Detecting and quantifying length changes in the long Bcl telomere.
ZT-LBT1 cells from a freezer stock were plated onto SD plates lacking histidine. After one additional streak on SD lacking histidine, several parent colonies from this plate were serially diluted and replated onto fresh SD plates lacking histidine to obtain independent colonies. Colony subclones from these plates were chosen for genomic DNA preparations and were subjected to XhoI digestion for visualization by Southern blotting. All subclones used for detection of TRD were grown for a similar number of divisions prior to their analysis.
To calculate TRD rates, we obtained 518 subclones from 15 different parent colonies. For our analysis, we considered any subclone containing a Bcl telomere that was at least 200 bp shorter than the long Bcl telomere precursor as having undergone TRD. We then obtained the frequency of colonies that had undergone TRD for each parent colony. In order to determine the rate of TRD, we used the method of the median (24). In parent colonies 2 and 3, there was a small amount of degraded DNA in the lanes running below ∼0.5 kb which obscured our ability to see TRD events that occurred in a fraction of the cell population in the samples examined. Therefore, the number of TRD events occurring in fractions of samples is likely to be higher than the number listed.
Gel electrophoresis and Southern blotting.
Restriction digestion of yeast genomic DNA preps was conducted in the presence of RNase, and products were run in 0.8% agarose gels in Tris-borate buffer unless otherwise specified in the text. The gels were subsequently blotted onto Hybond N+ membranes in 0.4 M NaOH. They were transferred for approximately 1 day, and the membranes then were cross-linked using UV light from an electronic cross-linker.
The membranes were probed using the telomeric G-stranded telomeric probe Klac1-25 (ACGGATTTGATTAGGTATGTGGTGT), which was labeled with [γ-32P]ATP. They were hybridized for at least 4 h at 48°C in 500 mM Na2HPO4 and 7% sodium dodecyl sulfate and washed three times for 5 min in 100 mM Na2HPO4 and 2% sodium dodecyl sulfate. The Bcl-specific probe was the oligonucleotide GATCAGGTATGTGG, with the underlined base indicating the Bcl-specific mutation (68). It was labeled with [γ-32P]ATP and then hybridized for 4 h at 40°C in 500 mM Na2HPO4 and 7% sodium dodecyl sulfate and washed two times for 5 min in 100 mM Na2HPO4 and 2% sodium dodecyl sulfate at 35°C. Filters were visualized using a General Electric (Sunnyvale, CA) Storm phosphorimager.
Subtelomeric gene conversion assay.
In order to measure the subtelomeric gene conversion rate near the long Bcl telomere, we used a previously described assay (33). Briefly, we used the loss of the subtelomeric URA3 marker beside the long Bcl telomere as a measure of telomere stability. K. lactis cells were serially diluted, and spots of these dilutions were plated onto SD plates lacking histidine, SD plates lacking both uracil and histidine, and SD plates lacking histidine but containing the drug 5-fluoroorotic acid (5-FOA), which selects for cells lacking URA3. Colony counts from 5-FOA plates lacking histidine and SD plates simply lacking histidine were made to determine the frequency of ura3 cells. 5-FOA-resistant clones derived from cells containing a URA3-tagged telomere were shown to have replaced the tagged telomere and URA3 gene with a sequence from another telomere (33, 45). The method of the median was used to determine the subtelomeric gene conversion rate (24).
Turnover experiment.
A TER1-7C(Bcl) strain (34) at a stage soon after introduction of the template mutation was mated to SI-E4. The diploids were sporulated, tetrads were dissected, and individual spores were subjected to genomic DNA preparation. Spores were identified that obtained the TER1-7C(Bcl) allele and either the RAD52 allele or the rad52Δ allele by Southern blots hybridized to probes prepared from K. lactis RAD52 and TER1 genes.
Four TER1-7C(Bcl) RAD52 strains and five TER1-7C(Bcl) rad52Δ strains that were identified were then restreaked every 3.5 days. Whole colonies grown from representative streaks (streak 8 and streak 20) of each isolate were serially diluted in order to determine how many cells were present in a colony. Genomic DNA preps of colonies grown for ∼400 cell divisions were conducted and subjected to digestion with BsrBI to determine whole telomere length and with BsrBI plus BclI to determine the number of wild-type repeats present at the base of the telomere.
To measure telomeric turnover, the signals from BsrBI-BclI-digested telomeric fragments from RAD52 and rad52Δ cells passaged for ∼400 cell divisions were quantified on a phosphorimager and their relative abundance determined by correcting for length (signal is directly proportional to length). The statistical significance of differences in mean fragment lengths was determined using Student's t test.
RESULTS
A long telomere undergoes frequent rapid deletion in K. lactis.
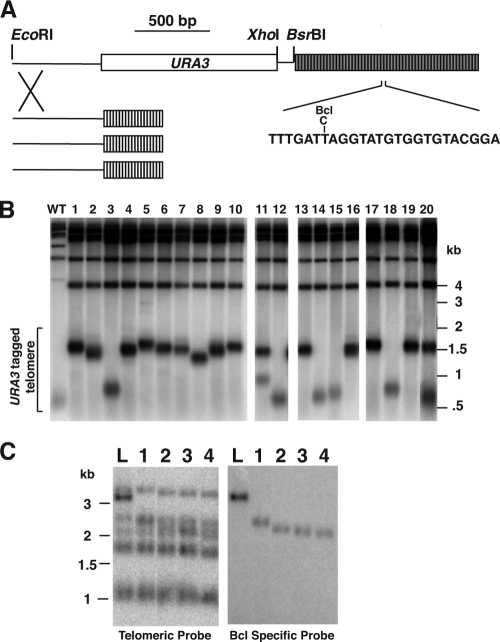
In order to look for the occurrence of TRD at telomeres in K. lactis, we took advantage of a previously constructed strain that contains a single ∼1.5-kb telomere in cells that otherwise have telomeres of the normal 400- to 600-bp length (67). The long telomere has a URA3 gene located next to it, and each of its repeats contains the phenotypically silent Bcl mutation that creates a BclI restriction site (Fig. 1 A) (34). Although the long Bcl telomere would gradually shorten to normal length upon long-term passaging of cells containing it, short-term passaging permitted testing for TRD events. To do this, we prepared genomic DNAs from 518 subclones of 15 parent colonies containing the long Bcl telomere and digested them with XhoI, which cleaves the Bcl telomere next to the URA3 gene and allows separation of it from all other telomeric fragments in gels. As shown in Fig. 1B, most subclones retained a Bcl telomeric fragment of ∼1.5 kb, the original size of the telomere. However, some subclones were found to have part or all of their Bcl telomere shortened by large increments, including many that were reduced to near the normal (∼500-bp) length. The altered size of the fragment was due to shortening of the Bcl telomere, as indicated by the decrease in intensity of hybridization to the telomeric probe (Fig. 1B) as well as by mapping with other restriction enzymes (data not shown). Clones containing a mixture of a long and a shortened Bcl telomere (Fig. 1B, lanes 11 and 20, and data not shown) likely represent cases where the shortening event occurred in a cell division which took place shortly after plating for single cells and consequently resulted in only a fraction of the Bcl telomeres being short in the cell population examined. For the sake of our calculations, we considered Bcl telomeres that were >200 bp shorter than the initial telomere to have undergone TRD events. The size of 200 bp represents twice the average amount of sequence loss expected at a K. lactis telomere that is not lengthened by telomerase in the estimated number of divisions required to form a colony (20 cell divisions times ∼5 bp of loss per cell division). We cannot rule out completely the possibility that some clones in which the Bcl telomere was shortened by less than 200 bp might also represent TRD events. In total, in 62 of 518 samples (12%), all cells in the colony had apparently undergone TRD, as indicated by the absence of the original long band and the appearance of a new, shorter band (Table 1). Additionally, samples from at least 6 colonies were observed to have both a shortened band and the original long telomeric band. These likely represent instances where TRD events occurred in one or more cells at a very early state of colony formation. Due to a particularly high TRD frequency from precursor colony 3 and because not all TRD events from each precursor were necessarily independent, the median frequency of 5.2% likely represents a more accurate measure of TRD frequency. This corresponds to a TRD rate of ∼4 × 10−3 per cell division.
Fig. 1.
Frequent deletions occur at a long telomere in K. lactis. (A) Diagram of the single long Bcl telomere introduced into K. lactis cells (shown to scale). This telomere contains a URA3 selectable marker gene (white box) inserted into the subtelomeric sequence. The unique XhoI site allows the Bcl telomere to be separated from other telomeres in gels. A native BsrBI site 3 bp internal to the telomere is present in 10 of the 12 wild-type telomeres in the cell. The long telomere is made up completely of Bcl repeats containing a single base pair change that makes a BclI restriction site (gray blocks). After transformation into K. lactis cells with wild-type telomeric repeats (white blocks), the long Bcl telomere recombines via subtelomeric homology and replaces a single native telomere. (B) Southern blot hybridized to a telomeric probe, showing an XhoI digest of genomic DNAs from subclones of cells containing the long Bcl telomere. The wild-type (WT) control is an equivalent telomeric fragment containing a subtelomeric URA3 gene, but of wild-type length and composed of wild-type telomeric repeats. After introduction into K. lactis cells, the XhoI fragment containing the long Bcl telomere measures ∼1.5 kb and contains ∼55 telomeric repeats, indicating that it is ∼3 times longer than a wild-type telomere. The position of the introduced telomere is indicated by the bracket. (C) Southern blot showing an EcoRI digest of telomeres hybridized to the Klac1-25 probe (which hybridizes to both wild-type and Bcl telomeric repeats) and a probe specific for Bcl repeats. Four clones that had undergone TRD are shown (lanes 1 to 4) as well as a clone with the original long telomere (lane L). Markers are shown in kilobases in both panels B and C.
Table 1.
Summary of shortening and lengthening events associated with the long Bcl telomere
| Parent colonya | No. of TRD events |
No. of elongation events |
Total no. of subclones examined | ||
|---|---|---|---|---|---|
| All of sampleb | Subset of samplec | All of sampled | Subset of samplee | ||
| 1 | 3 | 0 | 0 | 1 | 57 |
| 2 | 0 | 0 | 0 | 0 | 40 |
| 3 | 38 | 1 | 0 | 1 | 98 |
| 4 | 12 | 3 | 1 | 12 | 99 |
| 5 | 4 | 1 | 1 | 4 | 125 |
| 6 | 0 | 0 | 0 | 0 | 10 |
| 7 | 0 | 1 | 1 | 0 | 10 |
| 8 | 1 | 0 | 0 | 0 | 10 |
| 9 | 1 | 0 | 0 | 0 | 10 |
| 10 | 1 | 0 | 0 | 0 | 10 |
| 11 | 1 | 0 | 0 | 2 | 10 |
| 12 | 0 | 0 | 0 | 1 | 10 |
| 13 | 0 | 0 | 0 | 0 | 10 |
| 14 | 1 | 0 | 0 | 0 | 9 |
| 15 | 0 | 0 | 0 | 0 | 10 |
| Total | 62 | 6 | 3 | 21 | 518 |
The parent colonies were taken from 15 subclones of the long telomere transformant.
TRD events where telomere shortening occurred in all cells of the colony examined. This was indicated by the loss of the original long telomere band.
TRD events where telomere shortening occurred in only a percentage of the cells of the colony examined. This was indicated by the retention of a signal at the position of the original long telomere band.
Telomere elongation events where telomere elongation occurred in all cells of the colony examined.
Telomere elongation events where telomere elongation occurred in only a percentage of the colony examined, as indicated by retention of some signal at the position of the original Bcl telomere.
If the TRD events observed were the result of reciprocal exchanges between wild-type telomeres and the long telomere, then Bcl repeats would be transferred to other telomeres after TRD. To test this, DNAs from 17 clones that had undergone TRD events were hybridized to a probe specific to Bcl repeats (Fig. 1C and data not shown). While this hybridization readily detected the shortened original Bcl telomere, it did not detect any Bcl repeats at any other telomeres. We concluded that the TRD events were not due to reciprocal events between different telomeres.
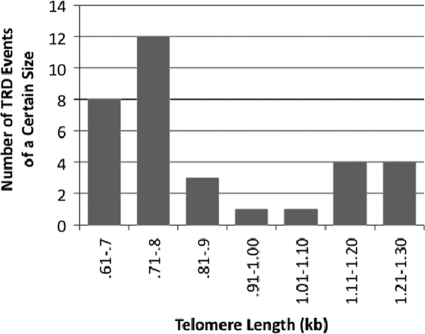
Figure 2 shows a summary of the lengths of telomeres for a representative group of 33 of the TRD events that were observed. Some TRD events were excluded from this analysis because of the presence of small DNA degradation products that hampered accurate measurement of TRD products (without affecting visualization of the long Bcl telomere) (data not shown). Twenty of these TRD events produced telomeres that had shortened to lengths of 0.6 to 0.8 kb, which was within 200 bp of the length of the wild-type control URA3-tagged telomere shown in Fig. 1A. The remaining 13 TRD events produced shortening to intermediate lengths. Because we used a conservative cutoff of 200 bp shorter than the precursor telomere to define a TRD event, it remains possible that the number of intermediate events may actually be underestimated to some extent. Nonetheless, our results suggest that TRD in K. lactis, like that reported for S. cerevisiae (25), preferentially shortens long telomeres to near-wild-type length.
Fig. 2.
Summary of telomere lengths after TRD. The bar graph shows the length distribution of 33 of the TRD events observed. The telomere length was measured as the size of the XhoI Bcl telomeric fragment after TRD, and the vertical axis shows the number of samples which were shortened to within a given size range.
TRD events are frequently associated with turnover deep into the telomere.
While the majority of the TRD events we observed shortened the long Bcl telomere to approximately wild-type size, a question that remained was whether the telomere was actually first shortened to below wild-type size and then reextended to normal size by the resident telomerase. Because any addition to a shortened Bcl telomere by telomerase would add wild-type repeats, we were able to address this question. Figure 3 A shows two potential outcomes after TRD shortens a telomere to approximately wild-type size. The first (Fig. 3A, left diagrams) is that the telomere is shortened to near-wild-type size. In this case, cleavage with XhoI plus BclI will result in cleavage of the telomere into monomeric repeats that will not show up in a Southern blot. In the second possible outcome (Fig. 3A, right diagrams), TRD shortens the telomere to well below wild-type size, and the telomere then becomes reelongated to normal length by the resident telomerase. In this alternative, XhoI-plus-BclI digestion will cleave any basal Bcl repeats but will leave a visible smeared band representing the terminal array of wild-type repeats.
Fig. 3.
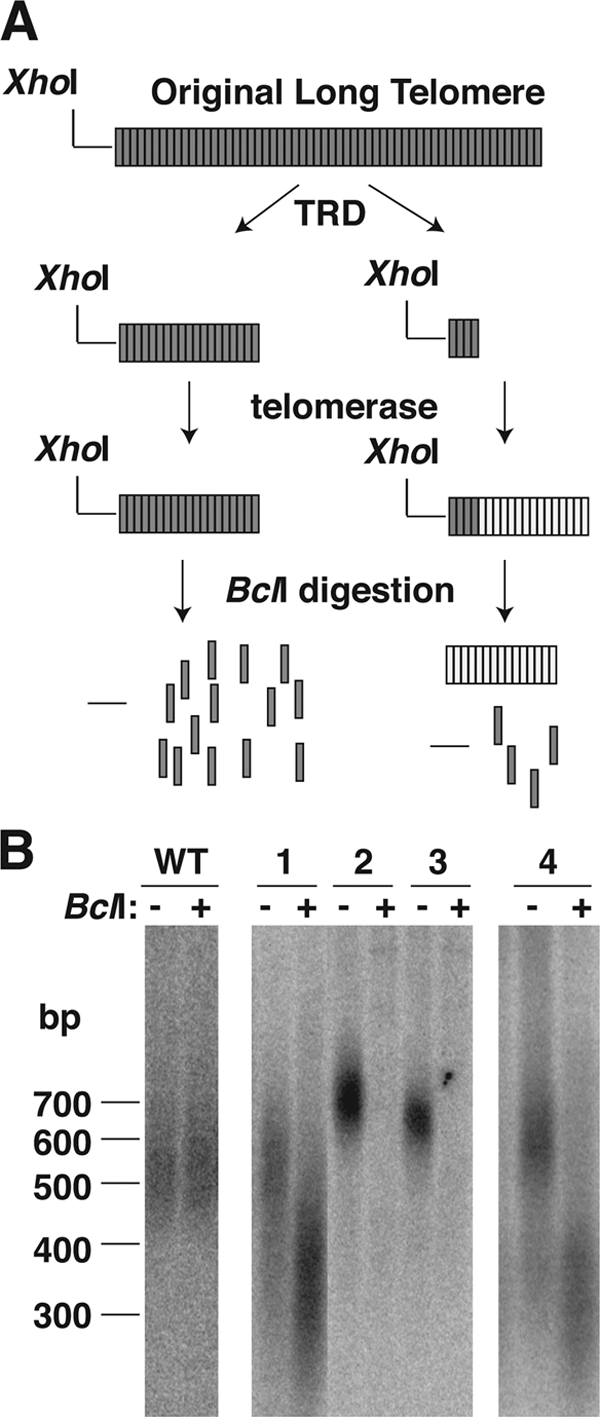
TRD events can result in turnover deep into the shortened telomere. (A) Two possible outcomes for the long Bcl telomere after undergoing TRD. In the first, on the left, the long telomere is shortened to wild-type size, and all of the remaining repeats are Bcl repeats. After cleavage with BclI, the telomeric repeats are cleaved into individual repeats, and a small subtelomeric segment is liberated. In the outcome on the right, the long telomere is shortened to well below wild-type size and then reextended by the wild-type telomerase. After cleavage with BclI, a block of wild-type repeats will be left over, the Bcl repeats will be cleaved into individual repeats, and a small subtelomeric fragment will again be liberated. (B) Southern blots of 2% agarose gels hybridized to the Klac1-25 telomeric probe (which hybridizes to both wild-type and Bcl repeats), showing XhoI and XhoI-plus-BclI digests of four subclones that underwent TRD. The WT control described in the legend to Fig. 1 is shown on the left.
We cleaved DNAs from 15 subclones that had undergone TRD events to near-wild-type telomere length, using XhoI and XhoI plus BclI, in order to test for the presence of wild-type repeats in the shortened telomere. For nine of the subclones (two of which are shown in Fig. 3B), BclI digestion eliminated all hybridization signals produced by the Bcl telomere, consistent with the shortened telomeres in these subclones being composed entirely, or almost entirely, of Bcl repeats. Notably, in these clones, the length of the shortened telomere was slightly longer than the wild-type size. However, in 7 of the 16 subclones, those where the shortened telomere was closest to wild type in length (including cases 1 and 4 in Fig. 3B), we found that wild-type repeat arrays estimated to be ∼100 to 300 bp were present on the end of the shortened Bcl telomere. By taking the length of the telomere in the XhoI digest and subtracting the size of both the 133-bp subtelomeric region and the wild-type repeat array left over after BclI cleavage, we estimated that subclone 4 had ∼7 Bcl repeats remaining, while subclone 1 had just 3 or 4 Bcl repeats remaining. Among the other 5 events, 4 were estimated to result in only ∼7 Bcl repeats remaining after TRD, and 1 resulted in ∼9 repeats (data not shown). Our results suggest that some TRD events in the long Bcl telomere may shorten the telomere to well below normal length prior to a reextension of the telomere to normal size, presumably mediated by telomerase.
The long Bcl telomere is susceptible to further lengthening by the addition of more Bcl repeats.
Although an abnormally long telomere should be resistant to elongation by both telomerase and recombination, we observed 24 events, mostly events that had occurred in only a fraction of the cells in the populations tested (Table 1), where the long Bcl telomere was observed to have become elongated further. Five of these events are indicated with black arrows in Fig. 4 A. For subclone 1, the lengthened telomere is visible as a faint band present above the original long telomere band. For subclone 2, the great majority of signal from the Bcl telomere is present in a band of lengthened size, with the rest present as two shortened bands. The latter may represent TRD events that occurred to the longer Bcl telomere. For subclone 4, two lengthened telomeric bands are present along with the original band. The largest appears to be about twice the size of the original long telomere, or ∼3 kb. For subclone 6, the lengthening event added ∼750 bp to the length of the original Bcl telomere, which appears to be present in the entire sample.
Fig. 4.
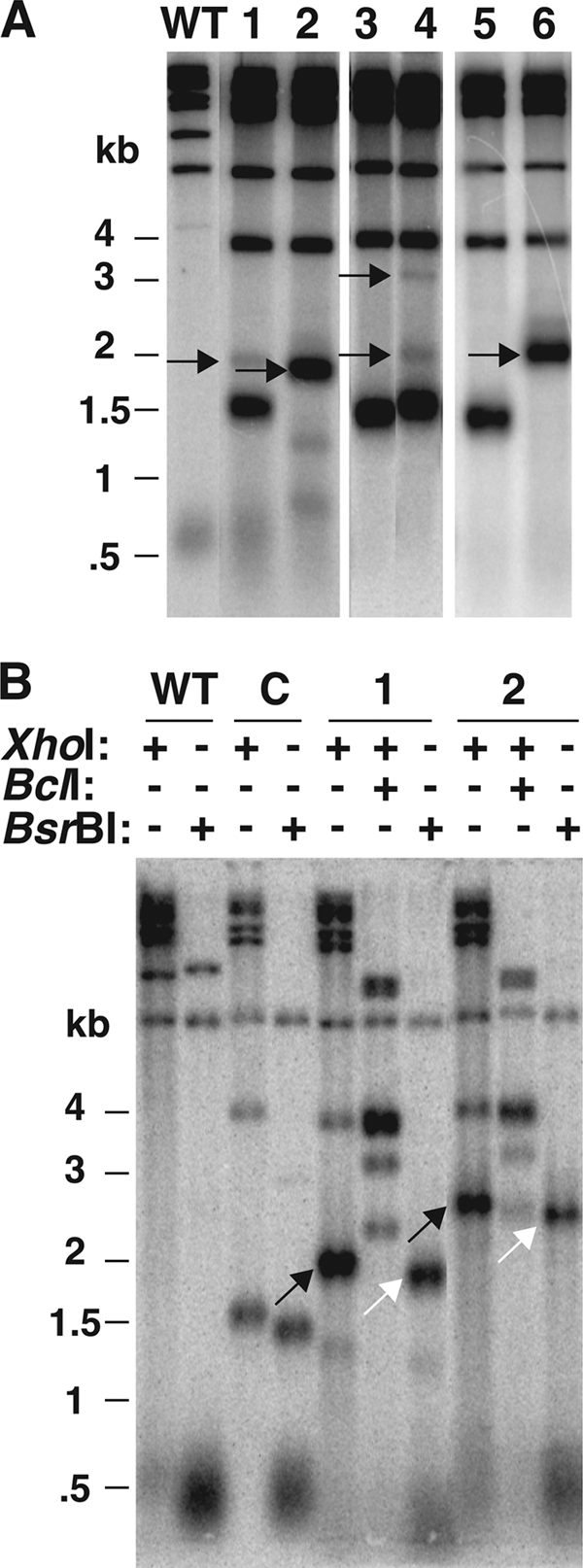
Further elongation of the long Bcl telomere can occur, and these elongations are made up of Bcl repeats. (A) Southern blot showing an XhoI digest of several long Bcl telomeres that have undergone elongation events. A wild-type control, as described in the legend to Fig. 1, is shown on the left. Black arrows show the positions of elongated telomeres in the sample. (B) Southern blot showing cleavage of DNAs from two subclones that had undergone elongation events with XhoI, XhoI plus BclI, and BsrBI, as indicated. A wild-type control is shown on the left, and “C” represents a control long telomere that has not undergone TRD or elongation. Positions of elongated telomeres in samples 1 and 2 are shown with slanted black arrows, and positions of the elongated telomeres in samples 1 and 2 after cleavage with BsrBI are shown with white arrows. Note that subclones 1 and 2 are not the same subclones as those shown in panel A. Markers for both panels are shown in kilobases.
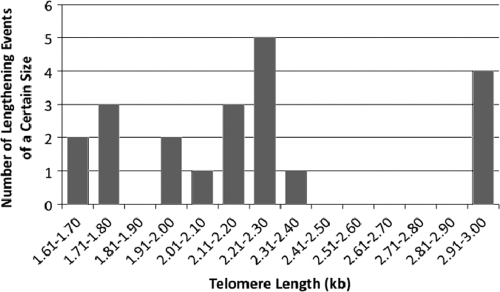
DNAs from 20 of the samples showing lengthening events were cleaved with BsrBI, which cleaves 3 bp away from the telomeric repeats and/or at restriction sites within the URA3 gene (Fig. 4B and data not shown). Each of the BsrBI digests shifted the elongated telomere to a position representing an ∼130-bp shorter product than that for a digest by XhoI. Additionally, double digestion with the 4-base cutters AluI and Tsp509I also retained long telomeric fragments of similar size while cleaving all of the other telomeres to very short lengths (data not shown). These data are consistent with each of the longer fragments being telomeres that were elongated by the addition or insertion of a sequence lacking sites for the very frequently cutting AluI and Tsp509I enzymes. Among the six lengthening events we studied (three from the original TRD analysis and three for telomeres that were later purified from clones containing mixtures of lengthened and unlengthened telomeres), two that had increased the telomere size more than 0.5 kb were seen to have clearly increased signal intensities in a Southern blot (Fig. 4A and B). Since the Klac1-25 probe hybridizes to each telomeric repeat (and to both wild-type and Bcl repeats), this signal increase represents an increase in the number of telomeric repeats present on the telomere. These data taken together argue that the elongation events of the Bcl telomere are in fact due to the acquisition of additional telomeric repeats. A summary of the sizes of 21 of the lengthened telomeres we observed is shown in Fig. 5. While the majority of these lengthening events appeared to add less than 1 kb of telomere repeats to the long Bcl telomere, four of the lengthening events approximately doubled the length of the original long telomere. None of the lengthening events observed to occur in just a fraction of the sample examined were found to also contain a corresponding shortening event of the same size (Fig. 4A and data not shown). We concluded from this that the mechanism of lengthening did not involve reciprocal sister chromatid exchanges.
Fig. 5.
Summary of lengths of Bcl telomeres after further elongation. The bar graph shows the length distribution of 21 elongation events, including those detected as all of the Bcl telomeres in the samples examined or as a subset of the total. The telomere length was measured as the size of the XhoI Bcl telomeric fragment after elongation, and the vertical axis shows the number of samples which were lengthened to within a given size range.
DNAs from four samples representing the largest elongation events (with estimated additions of 650 bp, 750 bp, 1,100 bp, and 1,500 bp) were also cleaved with BclI in order to observe whether the elongated telomeres were composed of the Bcl repeats or of wild-type repeats. Additions composed of wild-type repeats (as would be expected by either sequence addition by telomerase or recombinational copying of sequence from another telomere) would produce sizable bands of predicted sizes that were resistant to BclI digestion. With all four samples, including the two clones shown in Fig. 4B, the addition of the BclI enzyme resulted in the apparent complete digestion of the telomere (Fig. 4B and data not shown). We concluded that the lengthening in these clones occurred by a mechanism that largely or entirely adds additional Bcl repeats. This indicates that the mechanism involves recombination of the Bcl telomere either with itself or with a sister Bcl telomere. Since we did not see any evidence of reciprocal exchange with a sister Bcl telomere, we hypothesized that recombination with a sister Bcl telomere might occur by a nonreciprocal BIR event.
The rates of subtelomeric gene conversion are similar near both long and normal-length Bcl telomeres.
The high rates of both TRD events and lengthening events at the long Bcl telomere suggest that long length might destabilize telomere function. To address this possibility, we took advantage of an existing assay that can measure rates of subtelomeric gene conversion in K. lactis. Eleven of the 12 K. lactis telomeres share subtelomeric homology immediately adjacent to the telomeric repeats (46). These sequences can undergo highly elevated rates of homologous recombination when telomere function is compromised (5, 18, 33, 69). This recombination can be measured by quantifying the loss, through gene conversion, of a URA3 gene inserted next to a single telomere (33). These gene conversions were found to replace both telomeric and subtelomeric sequences from one telomere with sequences from another, in what mechanistically are likely to be BIR events (45).
Serial dilutions of cell suspensions made from freshly grown colonies of the long Bcl telomere strain and a control subclone that had previously undergone TRD to produce a Bcl telomere of wild-type length were spotted onto 5-FOA medium, which selects for ura3 cells, as well as onto control medium without 5-FOA. The results from our analysis showed that long and normal-length Bcl telomeres exhibited similar levels of subtelomeric gene conversion (rate of loss of URA3, through gene conversion or BIR events [mean mutation rate ± standard error], 1.8 × 10−5 ± 1.4 × 10−5 [assay performed 31 times] for wild-type Bcl telomeres and 1.3 × 10−5 ± 7.1 × 10−6 [assay performed 35 times] for long Bcl telomeres). Thus, by this measure at least, the long telomere is not inherently more unstable than a telomere with a wild-type length. The rate of subtelomeric gene conversion in both telomeres was slightly higher than that originally reported for a wild-type K. lactis telomere (33). This was not likely due to the mutant Bcl repeats, as a Bcl telomere was previously shown to display a similar rate to that of a wild-type telomere (34). A more likely explanation is that the strains used in this study have the telomerase RNA gene carried on a plasmid rather than at its normal chromosomal locus.
Testing whether the long Bcl telomere can undergo TRD in a rad52Δ strain.
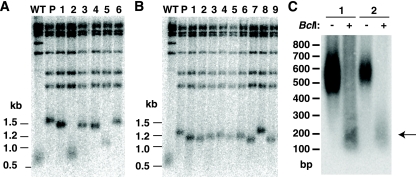
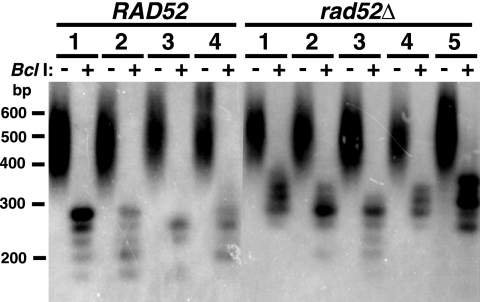
Rad52 is the protein that is involved most broadly in various types of homologous recombination in yeast, and TRD in S. cerevisiae has been shown to be partially dependent on its presence (25). Because telomeric repeats in K. lactis are much more homogeneous in size and sequence than those of S. cerevisiae, it might be expected that telomeric recombination would be highly Rad52 dependent. To test this, we monitored the stability of the long Bcl telomere in strains constructed to lack RAD52, which were generated through mating and sporulation. As shown in Fig. 6 and Table 2, TRD was found to occur in 9 of the 178 (5.1%) rad52Δ subclones examined. This decreased frequency relative to that for RAD52 cells may suggest that most TRD in K. lactis is RAD52 dependent. However, because of our small sample sizes, we were not able to conclude this for certain. Our results do clearly demonstrate that there is an appreciable rate of RAD52-independent TRD in K. lactis. Our data also suggest that TRD in rad52Δ cells is similar to TRD in RAD52 cells in primarily producing shortened telomeres of wild-type or near-wild-type length. This was the case for six of the nine TRD events that were identified in rad52 strains. While we did not see any elongation events that lengthened telomeres more than ∼100 to 300 bp longer than the length in the precursor strain among the rad52Δ strains, we did observe two apparent slight elongation events, one of which is shown in lane 8 of Fig. 6B.
Fig. 6.
rad52Δ cells undergo TRD that often has appreciable turnover associated with it. (A) Southern blot showing XhoI digest of two TRD events in a rad52Δ strain containing the long Bcl telomere. Lane WT, URA3-tagged telomere that is wild type in length and sequence; lane P, precursor URA3-tagged long Bcl telomere from immediately prior to isolation of the subclones in the other lanes. The long Bcl telomere in the precursor was slightly longer than the same telomere in the subclones because of gradual sequence attrition in the latter from undergoing more cell divisions. (B) Southern blot showing XhoI digest of an apparent slight elongation event in a rad52Δ strain. Lanes marked WT and P indicate wild-type and precursor Bcl telomeres, as described for panel A. (C) Southern blot of a 1.5% agarose gel showing XhoI and XhoI-plus-BclI digests of two subclones that had undergone TRD to near-wild-type size in a rad52Δ strain. The position of the leftover wild-type block of repeats is shown with an arrow. Markers in panels A and B are shown in kilobases, and markers in panel C are shown in base pairs.
Table 2.
Summary of shortening and lengthening events associated with the long Bcl telomere in a rad52Δ strain
| Parent colonya | No. of TRD events |
No. of elongation events |
Total no. of subclones examined | ||
|---|---|---|---|---|---|
| All of sampleb | Subset of samplec | All cellsd | Some cellse | ||
| 1.1 | 0 | 0 | 0 | 0 | 10 |
| 1.2 | 0 | 0 | 0 | 0 | 10 |
| 1.3 | 0 | 0 | 0 | 0 | 10 |
| 1.4 | 0 | 0 | 0 | 0 | 10 |
| 1.5 | 0 | 0 | 0 | 0 | 10 |
| 1.6 | 1 | 0 | 1 | 0 | 10 |
| 2.1 | 0 | 0 | 0 | 0 | 10 |
| 2.2 | 1 | 0 | 0 | 0 | 10 |
| 2.3 | 0 | 0 | 0 | 0 | 10 |
| 3.1 | 2 | 0 | 0 | 0 | 10 |
| 3.2 | 0 | 0 | 1 | 0 | 10 |
| 3.3 | 1 | 0 | 0 | 0 | 10 |
| 3.4 | 1 | 0 | 0 | 0 | 9 |
| 3.5 | 0 | 0 | 0 | 0 | 10 |
| 3.6 | 0 | 0 | 0 | 0 | 10 |
| 3.7 | 1 | 0 | 0 | 0 | 10 |
| 3.8 | 0 | 0 | 0 | 0 | 9 |
| 3.9 | 2 | 0 | 0 | 1 | 10 |
| Total | 9 | 0 | 1 | 1 | 178 |
The parent colonies were taken from three different spore colonies.
TRD events where telomere shortening occurred in all cells of the colony examined. This was indicated by the loss of the original long telomere band.
TRD events where telomere shortening occurred in only a percentage of the cells of the colony examined. This was indicated by the retention of a signal at the position of the original long telomere band.
Telomere elongation events where telomere elongation occurred in all cells of the colony examined.
Telomere elongation events where telomere elongation occurred in only a percentage of the colony examined, as indicated by retention of some signal at the position of the original Bcl telomere.
We next investigated whether the TRD events that shortened the Bcl telomere to wild-type size produced deep turnover within the telomere. Among the three TRD events that shortened telomeres to close to wild-type size that we tested in XhoI and XhoI-plus-BclI digests, two were found to be associated with turnover events that left ∼200 bp of wild-type repeats at their termini (Fig. 6B). The other subclone was completely cleaved by BclI, indicating that it had not undergone detectable turnover (data not shown).
TRD does not exhibit a large increase in frequency during meiosis in K. lactis.
Studies of S. cerevisiae showed a 30- to 70-fold increase of TRD during meiosis, which was dependent on the meiotic bouquet formation protein Ndj1p (19). In order to determine if the TRD frequency is increased similarly in K. lactis, we mated the strain containing the long telomere with a wild-type strain of the opposite mating type, sporulated the diploids, and dissected tetrads. Because the long Bcl telomere was present in one of the two parent cells, we observed that the URA3-tagged Bcl telomere segregated 2:2, as expected. After examining this telomere in spores from 42 tetrads (84 spore cells), we observed only 1 TRD event (data not shown). The other spore that had obtained the long telomere in this tetrad had not shortened, indicating that the TRD event had occurred postreplication. Although the 1.2% TRD frequency we observed during meiosis is slightly higher than the per-cell division rate of TRD in mitotic cells, it is much lower than the meiotic TRD frequency of 11 to 23% per meiosis previously reported for S. cerevisiae (19). We concluded that TRD does not have a comparatively large increase during meiosis in K. lactis.
Turnover of repeats within normal-length telomeres is affected by absence of RAD52.
If TRD occurs at normal-length K. lactis telomeres, it should manifest itself by influencing the kinetics of turnover of telomeric repeats, perhaps particularly at positions relatively basal within telomeres. To test this, we constructed RAD52 and rad52Δ strains whose only telomerase was provided by a recently introduced TER1-7C(Bcl) allele which synthesizes Bcl repeats. Several independent isolates of each strain type were then passaged for ∼400 cell divisions to allow turnover of the original wild-type repeats with Bcl repeats to occur within most of the outer part of the telomeres. The numbers of passages on solid medium were slightly greater for rad52Δ strains to control for the slightly lower average growth rate of the mutant cells (see Materials and Methods).
DNA from each of the strains was digested with BsrBI and BsrBI plus BclI and run in a high-percent-agarose gel. As shown in Fig. 7, our results indicated that the passaged TER1-7C(Bcl) RAD52 cells exhibited telomeres that were cleaved, on average, to shorter lengths with BclI, indicative of their having fewer remaining basal wild-type repeats than TER1-7C(Bcl) rad52Δ cells. The TER1-7C(Bcl) RAD52 strains produced telomeric fragments with BclI digestion that averaged 9.6 ± 1.4 repeats in length, while those of the five TER1-7C(Bcl) rad52Δ strains averaged 11.6 ± 1.4 repeats. This difference was statistically significant (P < 0.001) by Student's t test. Notably, fragments of 300 bp or longer were essentially present only in the TER1-7C(Bcl) rad52Δ samples. These results argue that homologous recombination contributes to turnover of repeats deep within telomeres of wild-type K. lactis cells.
Fig. 7.
RAD52 contributes to turnover at K. lactis telomeres. The Southern blot, hybridized to a telomeric probe, shows BsrBI and BsrBI-plus-BclI digests of DNAs from five independent clones of rad52Δ TER1-7C(Bcl) cells and four independent clones of RAD52 TER1-7C(Bcl) cells. All clones were grown for ∼400 cell divisions prior to analysis. Positions of DNA size markers are indicated.
DISCUSSION
It is becoming increasingly clear that telomere maintenance can involve processes in addition to elongation mediated by telomerase and gradual shortening from incomplete replication. Homologous recombination is now well established as a mechanism for maintaining telomeres in certain circumstances where telomeres are dysfunctional (32). Even telomeres without obvious functional defects can undergo truncations of sizes too large to be accounted for by gradual sequence loss. Examples of this include the accelerated telomere shortening from oxidative damage in cultured human cells (52), the truncations of ciliate macronuclear telomeres by an unknown mechanism (23), and the TRD of artificially elongated telomeres in S. cerevisiae (25). In our studies here, we used a single long telomere in K. lactis to look for sudden changes in its size.
TRD has been characterized best for S. cerevisiae, where it is primarily a phenomenon that shortens abnormally long telomeres that exist in the presence of normal-length telomeres (25). The TRD we observed occurring at a long Bcl telomere in K. lactis has several similarities to TRD in S. cerevisiae. First, TRD in both organisms was very abundant. We estimated that it occurred at a frequency of 4 × 10−3 per cell division in mitotically growing K. lactis cells, quite close to the frequency of 1.2 × 10−3 reported for mitotically growing S. cerevisiae cells. Second, like that in S. cerevisiae, TRD in K. lactis appears to shorten telomeres mostly to within 200 bp of wild-type size. This occurs in spite of the Bcl telomere initially being ∼1,000 bp longer than the wild-type telomeres. Third, for both species, a significant fraction of TRD events can occur in cells lacking the major recombination gene RAD52. In S. cerevisiae, an estimated one-third of TRD events occurred in the absence of RAD52. In our work, which required more labor-intensive screening using Southern blots, we found that TRD occurred in a rad52 mutant at a frequency that at least approached that seen in RAD52 cells. However, our experiments were not sensitive enough to tell whether some, or perhaps most, of the TRD events we saw were RAD52 dependent. Although RAD52 is required for the bulk of homologous recombination in yeast, some recombination, including some telomeric recombination, can be observed in its absence (30, 44, 55, 56). We favor the possibility that TRD in K. lactis is at least partially RAD52 dependent. This would be most consistent with the RAD52-dependent effect on deep turnover of telomeres (Fig. 7). One significant difference that was found between TRD in K. lactis and S. cerevisiae was its frequency in meiosis. In S. cerevisiae, TRD increases 30- to 70-fold during meiosis relative to its rate during mitosis (19). However, we did not see a corresponding increase in the meiotic rate of TRD in K. lactis. The basis of this difference is not known.
The mechanism by which TRD occurs also remains unclear. It is entirely possible, and perhaps likely, that more than one mechanism is involved. DNA damage or nucleolytic cleavage could potentially explain some RAD52-independent events. Lustig and coworkers proposed that most TRD in S. cerevisiae occurs through intramolecular strand invasion of a telomeric end into its more internal repeats, followed by cleavage of the t-loop structure (Fig. 8 A) (4). In support of this, they observed that TRD events involved deletion of the more terminal parts of the telomere and were not associated with reciprocal exchanges. Our experiments similarly argue against reciprocal exchange. Reciprocal exchange between different telomeres can be ruled out, as Bcl repeats did not move to other telomeres when TRD events occurred (Fig. 1C). Additionally, reciprocal sister chromatid exchanges would have led to events of sudden lengthening of the Bcl telomere that were equal in number to the TRD events that shortened it. While some events lengthening the Bcl telomere were observed in our experiments, these were observed much less commonly than the TRD events. Our results thus support the idea that TRD in K. lactis occurs through a nonreciprocal terminal loss such as occurs in the t-loop deletion model (Fig. 8A) or through the structurally similar possibility of deletion occurring following strand invasion in trans (Fig. 8B).
Fig. 8.
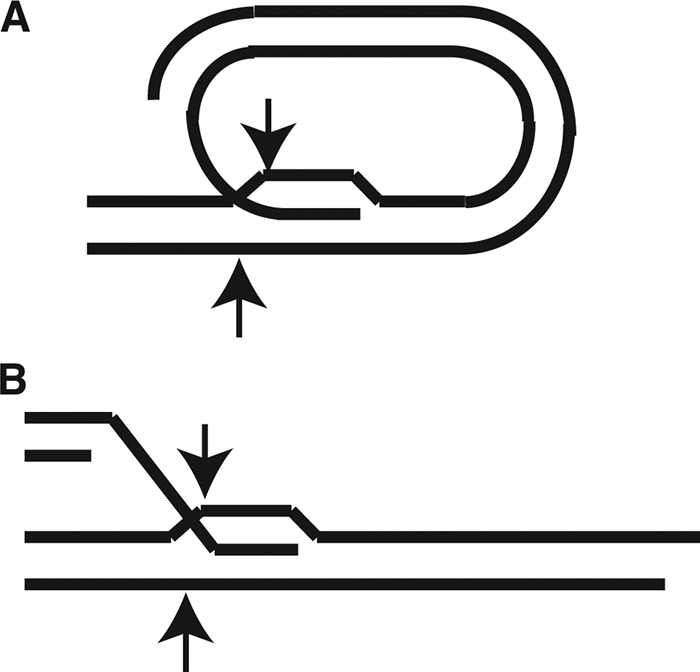
Two potential mechanisms of TRD. (A) Model showing intramolecular strand invasion of a telomeric end into its own telomeric repeats, forming a t-loop. (B) Model showing strand invasion of a wild-type telomere into an abnormally long telomere. Nucleolytic cleavage positions that might produce a TRD event are shown with arrows in each model.
A particularly notable result was that the long Bcl telomere could sometimes undergo sudden elongation events. Our results suggest that further lengthening of the long Bcl telomere occurs by copying of more Bcl repeats. This indicates that elongation occurs by recombination, not by telomerase, which in our experiments could synthesize only wild-type repeats. There are a number of possibilities for how elongation that added Bcl repeats could occur. We believe that unequal reciprocal exchange between sister chromatids is unlikely to be the explanation. This model would predict that in those subclones where elongation by, for example, 300 bp, occurred in only a fraction of the Bcl telomere, there would be a corresponding equal fraction that was shortened by 300 bp. Such simultaneous reciprocal losses were not observed (Fig. 4 and unpublished data). Another potential model is that the long telomere was generated by copying a telomeric circle. This model cannot be ruled out but seems unlikely given that it requires a second, earlier recombination event (to create a t-circle) and that the elongated telomeres we observed were never more than double the size of Bcl telomeres and mostly produced lengthening of less than 900 bp. The latter observation suggests instead that lengthening may occur through a BIR event that follows strand invasion of the end of a Bcl telomere either into its own, more internal repeats or into repeats of its sister chromatid (Fig. 8A and B). Extension of the invaded 3′ end by a DNA polymerase would then allow copying to the end of that telomeric sequence. Such a mechanism is predicted to no more than double the size of the original telomere.
TRD has been proposed to function as a mechanism that trims abnormally long telomeres to normal size (25). Consistent with this, our data show that TRD in K. lactis preferentially shortens telomeres to wild-type length. This demonstrates that this feature of TRD is not limited to S. cerevisiae and may be more generally conserved. Other data of ours, however, suggest that recombination involving the long Bcl telomere may be more stochastic than orderly. The appreciable incidence of telomere elongation strongly suggests that telomeric recombination occurring at an abnormally long telomere can work in both directions and cause either shortening or lengthening. Furthermore, the deep turnover into the telomere that is associated with a significant fraction of TRD events suggests that many shortening events initially reduce the Bcl telomere to a length much shorter than wild-type length. While we cannot rule out completely that the deep turnover occurred slightly after, and independently of, the TRD events, we consider this possibility unlikely. Instead, we favor the idea that TRD in K. lactis frequently shortens telomeres to sizes substantially shorter than normal length and that telomerase, or perhaps in some cases break-induced replication events, lengthens the telomere back to normal size. Whether the events that shorten telomeres to near normal size and those that shorten telomeres to below normal size are caused by the same mechanism is not known.
A major conclusion of ours is that homologous recombination plays a role in telomeric repeat turnover in K. lactis cells with normal-length telomeres. TER1-7C(Bcl) cells, which recently replaced the wild-type telomerase with one that synthesizes only Bcl repeats, showed more turnover of internal repeats in the presence of RAD52 than in its absence. It was shown previously that over the course of hundreds of cell divisions the TER1-7C(Bcl) mutant Bcl repeats eventually replaced all but the innermost 1 to 4 repeats of the telomeres (34). This deep turnover cannot be explained readily by gradual replicative sequence loss and was postulated to occur via terminal truncations of the telomeres that would typically be repaired by the resident TER1-7C(Bcl) telomerase. We suggest that TRD is not limited to abnormally long telomeres but also occurs at normal-length telomeres and is at least partly responsible for turnover of repeats deep within telomeres. This view is consistent with the possibility that occasional uncapping of a telomere that leads to a TRD event might occur not only at abnormally long telomeres but also at normal-length telomeres in K. lactis. The fact that normal-length and long Bcl telomeres display similar rates of subtelomeric BIR events adds further weight to this possibility. It could be pointed out that some of the TRD observed in S. cerevisiae occurred at telomeres that were at the extreme end of normal telomere size (25). We also note that the absence of RAD52 was previously shown to lead to telomere lengthening and heterogeneity in the pathogenic yeast Candida albicans (8). One possible interpretation of this is that TRD events are important contributors to normal telomere length control in that organism. Recombination at telomeres might be predicted to be difficult to prevent completely. The simple repetitive structures of telomeres, along with their 3′ overhangs, might serve as features that could greatly promote the likelihood of recombination even if multiple other features of telomeres act to repress it.
TRD occurring via a t-loop structure (Fig. 8A) has been postulated to be the mechanism by which t-circles form (4, 15, 74). TRD may thus be critical to recombinational telomere elongation, which appears, at least in yeast telomerase deletion mutants, to maintain telomeres through a process dependent upon rolling circle copying of a t-circle (26, 43, 44). Although mitochondrial DNA in certain yeast species, such as Candida parapsilosis, might normally utilize this process (47), no information to date has suggested that t-circle formation is of importance to normal yeast chromosomal telomere maintenance.
Most models for recombinational repair of DNA suggest that a strand-invaded 3′ end will be used as a primer for at least limited DNA synthesis. This might seem to suggest that strand invasion of a telomeric end into either itself (Fig. 8A) or another telomere (Fig. 8B) would lead to telomere elongation being more frequent than telomere shortening. Yet the reverse is observed in both K. lactis and S. cerevisiae. We suggest that it may be more important for telomeric capping function to block recombinational elongation than recombinational shortening. One reason to favor this possibility is that in most circumstances, shortening of a normal-length telomere is likely to be corrected easily and rapidly by sequence addition by telomerase, which is known to be favored at shorter telomeres (65). Furthermore, recombinational elongation by self copying or sister copying (models favored by our data) has the potential to cause telomeres to increase exponentially in length if the process ever becomes even moderately frequent.
It is interesting to speculate that TRD might influence not just telomeres but also subtelomeric genes. One route for this might be the epigenetic silencing that is common near telomeres and known to be relaxed when telomeres are short (21). Additionally, by cleaving deep within telomeres and perhaps occasionally rendering them subject to further DNA repair reactions, TRD could conceivably be a factor influencing subtelomeric stability. Subtelomeric recombination triggered by partial failure of telomere function has been postulated to be an adaptive mechanism that can permit rapid evolution of contingency genes located near chromosome ends (29).
How significant TRD might be to other organisms remains largely unknown. We suggest that TRD may be particularly important to human cells. In part, this would stem simply from the long length (relative to yeast telomeres) of human telomeres, which would make them vulnerable to losing much more sequence in a single event. More significantly, though, the very low telomerase levels in most human somatic tissues would leave TRD-shortened telomeres without a means of becoming reextended. Because human cells arrest their growth when a small number of telomeres become too short (16), a small number of TRD events could potentially have dramatic effects on the replicative capacity of a cell. Gaining a better understanding of TRD is therefore clearly a goal of considerable significance.
ACKNOWLEDGMENTS
We thank Matthew Schultz, Nicole Umberger, and Yogin Patel for technical assistance in the early stages of this work. We also thank Sidney Kushner, Michelle Momany, Walter Schmidt, and Michael Terns for critical readings of the manuscript.
This work was supported by a grant to M.J.M. from the National Institutes of Health (GM 61645).
Footnotes
Published ahead of print on 10 December 2010.
REFERENCES
- 1. Bailey S. M., Brenneman M. A., Goodwin E. H. 2004. Frequent recombination in telomeric DNA may extend the proliferative life of telomerase-negative cells. Nucleic Acids Res. 32:3743–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bechard L. H., et al. 2009. Mutant telomeric repeats in yeast can disrupt the negative regulation of recombination-mediated telomere maintenance and create an alternative lengthening of telomeres-like phenotype. Mol. Cell. Biol. 29:626–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bryan T. M., Englezou A., Gupta J., Bacchetti S., Reddel R. R. 1995. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 14:4240–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bucholc M., Park Y., Lustig A. J. 2001. Intrachromatid excision of telomeric DNA as a mechanism for telomere size control in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:6559–6573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter S. D., Iyer S., Xu J., McEachern M. J., Astrom S. U. 2007. The role of nonhomologous end-joining components in telomere metabolism in Kluyveromyces lactis. Genetics 175:1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cesare A. J., Griffith J. D. 2004. Telomeric DNA in ALT cells is characterized by free telomeric circles and heterogeneous t-loops. Mol. Cell. Biol. 24:9948–9957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung I., Schertzer M., Rose A., Lansdorp P. M. 2006. High incidence of rapid telomere loss in telomerase-deficient Caenorhabditis elegans. Nucleic Acids Res. 34:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ciudad T., et al. 2004. Homologous recombination in Candida albicans: role of CaRad52p in DNA repair, integration of linear DNA fragments and telomere length. Mol. Microbiol. 53:1177–1194 [DOI] [PubMed] [Google Scholar]
- 9. Denchi E. L. 2009. Give me a break: how telomeres suppress the DNA damage response. DNA Repair (Amsterdam) 8:1118–1126 [DOI] [PubMed] [Google Scholar]
- 10. Dunham M. A., Neumann A. A., Fasching C. L., Reddel R. R. 2000. Telomere maintenance by recombination in human cells. Nat. Genet. 26:447–450 [DOI] [PubMed] [Google Scholar]
- 11. Ferreira M. G., Miller K. M., Cooper J. P. 2004. Indecent exposure: when telomeres become uncapped. Mol. Cell 13:7–18 [DOI] [PubMed] [Google Scholar]
- 12. Grandin N., Charbonneau M. 2003. The Rad51 pathway of telomerase-independent maintenance of telomeres can amplify TG1-3 sequences in yku and cdc13 mutants of Saccharomyces cerevisiae. Mol. Cell. Biol. 23:3721–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grandin N., Damon C., Charbonneau M. 2001. Cdc13 prevents telomere uncapping and Rad50-dependent homologous recombination. EMBO J. 20:6127–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffith J. D., et al. 1999. Mammalian telomeres end in a large duplex loop. Cell 97:503–514 [DOI] [PubMed] [Google Scholar]
- 15. Groff-Vindman C., Natarajan S., Cesare A., Griffith J. D., McEachern M. J. 2005. Recombination at dysfunctional long telomeres forms tiny double and single stranded t-circles. Mol. Cell. Biol. 25:4406–4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hemann M. T., et al. 2001. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol. Biol. Cell 12:2023–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huffman K. E., Levene S. D., Tesmer V. M., Shay J. W., Wright W. E. 2000. Telomere shortening is proportional to the size of the G-rich telomeric 3′-overhang. J. Biol. Chem. 275:19719–19722 [DOI] [PubMed] [Google Scholar]
- 18. Iyer S., Chadha A., McEachern M. J. 2005. A mutation in the STN1 gene triggers an alternative lengthening of telomere-like runaway recombinational telomere elongation and rapid deletion in yeast. Mol. Cell. Biol. 25:8064–8073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joseph I., Jia D., Lustig A. J. 2005. Ndj1p-dependent epigenetic resetting of telomere size in yeast meiosis. Curr. Biol. 15:231–237 [DOI] [PubMed] [Google Scholar]
- 20. Kim N. W., et al. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015 [DOI] [PubMed] [Google Scholar]
- 21. Kyrion G., Liu K., Liu C., Lustig A. J. 1993. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 7:1146–1159 [DOI] [PubMed] [Google Scholar]
- 22. Larrivee M., Wellinger R. J. 2006. Telomerase- and capping-independent yeast survivors with alternate telomere states. Nat. Cell Biol. 8:741–747 [DOI] [PubMed] [Google Scholar]
- 23. Larson D. D., Spangler E. A., Blackburn E. H. 1987. Dynamics of telomere length variation in Tetrahymena thermophila. Cell 50:477–483 [DOI] [PubMed] [Google Scholar]
- 24. Lea D., Coulson C. 1948. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49:226–284 [DOI] [PubMed] [Google Scholar]
- 25. Li B., Lustig A. J. 1996. A novel mechanism for telomere size control in Saccharomyces cerevisiae. Genes Dev. 10:1310–1326 [DOI] [PubMed] [Google Scholar]
- 26. Lin C. Y., et al. 2005. Extrachromosomal telomeric circles contribute to Rad52-, Rad50-, and polymerase δ-mediated telomere-telomere recombination in Saccharomyces cerevisiae. Eukaryot. Cell 4:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lundblad V., Blackburn E. H. 1993. An alternative pathway for yeast telomere maintenance rescues est1− senescence. Cell 73:347–360 [DOI] [PubMed] [Google Scholar]
- 28. Lydall D. 2009. Taming the tiger by the tail: modulation of DNA damage responses by telomeres. EMBO J. 28:2174–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McEachern M. J. 2008. Telomeres: guardians of genomic integrity or double agents of evolution?, p. 100–113 In Nosek J., Tomaska L. (ed.), Origin and evolution of telomeres. Landes Bioscience, Austin, TX [Google Scholar]
- 30. McEachern M. J., Blackburn E. H. 1996. Cap-prevented recombination between terminal telomeric repeat arrays (telomere CPR) maintains telomeres in Kluyveromyces lactis lacking telomerase. Genes Dev. 10:1822–1834 [DOI] [PubMed] [Google Scholar]
- 31. McEachern M. J., Blackburn E. H. 1995. Runaway telomere elongation caused by telomerase RNA gene mutations. Nature 376:403–409 [DOI] [PubMed] [Google Scholar]
- 32. McEachern M. J., Haber J. E. 2006. Break-induced replication and recombinational telomere elongation in yeast. Annu. Rev. Biochem. 75:111–135 [DOI] [PubMed] [Google Scholar]
- 33. McEachern M. J., Iyer S. 2001. Short telomeres in yeast are highly recombinogenic. Mol. Cell 7:695–704 [DOI] [PubMed] [Google Scholar]
- 34. McEachern M. J., Underwood D. H., Blackburn E. H. 2002. Dynamics of telomeric DNA turnover in yeast. Genetics 160:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Milne G. T., Weaver D. T. 1993. Dominant negative alleles of RAD52 reveal a DNA repair/recombination complex including Rad51 and Rad52. Genes Dev. 7:1755–1765 [DOI] [PubMed] [Google Scholar]
- 36. Morin G. B., Cech T. R. 1988. Mitochondrial telomeres: surprising diversity of repeated telomeric DNA sequences among six species of Tetrahymena. Cell 52:367–374 [DOI] [PubMed] [Google Scholar]
- 37. Munoz-Jordan J. L., Cross G. A., de Lange T., Griffith J. D. 2001. t-loops at trypanosome telomeres. EMBO J. 20:579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muntoni A., Neumann A. A., Hills M., Reddel R. R. 2009. Telomere elongation involves intra-molecular DNA replication in cells utilizing alternative lengthening of telomeres. Hum. Mol. Genet. 18:1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murnane J. P., Sabatier L., Marder B. A., Morgan W. F. 1994. Telomere dynamics in an immortal human cell line. EMBO J. 13:4953–4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Myler P. J., Aline R. F., Scholler J. K., Jr., Stuart K. D. 1988. Changes in telomere length associated with antigenic variation in Trypanosoma brucei. Mol. Biochem. Parasitol. 29:243–250 [DOI] [PubMed] [Google Scholar]
- 41. Nabetani A., Ishikawa F. 2009. Unusual telomeric DNAs in human telomerase-negative immortalized cells. Mol. Cell. Biol. 29:703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura T. M., Cooper J. P., Cech T. R. 1998. Two modes of survival of fission yeast without telomerase. Science 282:493–496 [DOI] [PubMed] [Google Scholar]
- 43. Natarajan S., Groff-Vindman C., McEachern M. J. 2003. Factors influencing the recombinational expansion and spread of telomeric tandem arrays in Kluyveromyces lactis. Eukaryot. Cell 2:1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Natarajan S., McEachern M. J. 2002. Recombinational telomere elongation promoted by DNA circles. Mol. Cell. Biol. 22:4512–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Natarajan S., Nickles K., McEachern M. J. 2006. Screening for telomeric recombination in wild-type Kluyveromyces lactis. FEMS Yeast Res. 6:442–448 [DOI] [PubMed] [Google Scholar]
- 46. Nickles K., McEachern M. J. 2004. Characterization of Kluyveromyces lactis subtelomeric sequences including a distal element with strong purine/pyrimidine strand bias. Yeast 21:813–830 [DOI] [PubMed] [Google Scholar]
- 47. Nosek J., Rycovska A., Makhov A. M., Griffith J. D., Tomaska L. 2005. Amplification of telomeric arrays via rolling-circle mechanism. J. Biol. Chem. 280:10840–10845 [DOI] [PubMed] [Google Scholar]
- 48. Olovnikov A. M. 1973. A theory of marginotomy. J. Theor. Biol. 41:181–190 [DOI] [PubMed] [Google Scholar]
- 49. Osterhage J. L., Friedman K. L. 2009. Chromosome end maintenance by telomerase. J. Biol. Chem. 284:16061–16065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. O'Sullivan R. J., Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat. Rev. Mol. Cell. Biol. 11:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palm W., de Lange T. 2008. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42:301–334 [DOI] [PubMed] [Google Scholar]
- 52. Passos J. F., Saretzki G., von Zglinicki T. 2007. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 35:7505–7513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Petreaca R. C., Chiu H. C., Nugent C. I. 2007. The role of Stn1p in Saccharomyces cerevisiae telomere capping can be separated from its interaction with Cdc13p. Genetics 177:1459–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pickett H. A., Cesare A. J., Johnston R. L., Neumann A. A., Reddel R. R. 2009. Control of telomere length by a trimming mechanism that involves generation of t-circles. EMBO J. 28:799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pluta A. F., Zakian V. A. 1989. Recombination occurs during telomere formation in yeast. Nature 337:429–433 [DOI] [PubMed] [Google Scholar]
- 56. Prado F., Aguilera A. 1995. Role of reciprocal exchange, one-ended invasion crossover and single-strand annealing on inverted and direct repeat recombination in yeast: different requirements for the RAD1, RAD10, and RAD52 genes. Genetics 139:109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Richter T., von Zglinicki T. 2007. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp. Gerontol. 42:1039–1042 [DOI] [PubMed] [Google Scholar]
- 58. Rogan E. M., et al. 1995. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol. Cell. Biol. 15:4745–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roth C. W., Kobeski F., Walter M. F., Biessmann H. 1997. Chromosome end elongation by recombination in the mosquito Anopheles gambiae. Mol. Cell. Biol. 17:5176–5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roy J., Fulton T. B., Blackburn E. H. 1998. Specific telomerase RNA residues distant from the template are essential for telomerase function. Genes Dev. 12:3286–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Serra V., Grune T., Sitte N., Saretzki G., von Zglinicki T. 2000. Telomere length as a marker of oxidative stress in primary human fibroblast cultures. Ann. N. Y. Acad. Sci. 908:327–330 [DOI] [PubMed] [Google Scholar]
- 62. Shay J. W., Wright W. E. 2007. Hallmarks of telomeres in ageing research. J. Pathol. 211:114–123 [DOI] [PubMed] [Google Scholar]
- 63. Shore D., Bianchi A. 2009. Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J. 28:2309–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steinberg-Neifach O., Lue N. F. 2006. Modulation of telomere terminal structure by telomerase components in Candida albicans. Nucleic Acids Res. 34:2710–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Teixeira M. T., Arneric M., Sperisen P., Lingner J. 2004. Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117:323–335 [DOI] [PubMed] [Google Scholar]
- 66. Tomaska L., Nosek J., Makhov A. M., Pastorakova A., Griffith J. D. 2000. Extragenomic double-stranded DNA circles in yeast with linear mitochondrial genomes: potential involvement in telomere maintenance. Nucleic Acids Res. 28:4479–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Topcu Z., Nickles K., Davis C., McEachern M. J. 2005. Abrupt disruption of capping and a single source for recombinationally elongated telomeres in Kluyveromyces lactis. Proc. Natl. Acad. Sci. U. S. A. 102:3348–3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tzfati Y., Knight Z., Roy J., Blackburn E. H. 2003. A novel pseudoknot element is essential for the action of a yeast telomerase. Genes Dev. 17:1779–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Underwood D. H., Carroll C., McEachern M. J. 2004. Genetic dissection of the Kluyveromyces lactis telomere and evidence for telomere capping defects in TER1 mutants with long telomeres. Eukaryot. Cell 3:369–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van Steensel B., Smogorzewska A., de Lange T. 1998. TRF2 protects human telomeres from end-to-end fusions. Cell 92:401–413 [DOI] [PubMed] [Google Scholar]
- 71. Vermeesch J. R., Williams D., Price C. M. 1993. Telomere processing in Euplotes. Nucleic Acids Res. 21:5366–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27:339–344 [DOI] [PubMed] [Google Scholar]
- 73. von Zglinicki T., Saretzki G., Docke W., Lotze C. 1995. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 220:186–193 [DOI] [PubMed] [Google Scholar]
- 74. Wang R. C., Smogorzewska A., de Lange T. 2004. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119:355–368 [DOI] [PubMed] [Google Scholar]
- 75. Watson J. D. 1972. Origin of concatemeric T7 DNA. Nat. New Biol. 239:197–201 [DOI] [PubMed] [Google Scholar]
- 76. Watson J. M., Shippen D. E. 2007. Telomere rapid deletion regulates telomere length in Arabidopsis thaliana. Mol. Cell. Biol. 27:1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wray L. V., Jr., Witte M. M., Dickson R. C., Riley M. I. 1987. Characterization of a positive regulatory gene, LAC9, that controls induction of the lactose-galactose regulon of Kluyveromyces lactis: structural and functional relationships to GAL4 of Saccharomyces cerevisiae. Mol. Cell. Biol. 7:1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zubko M. K., Lydall D. 2006. Linear chromosome maintenance in the absence of essential telomere-capping proteins. Nat. Cell Biol. 8:734–740 [DOI] [PubMed] [Google Scholar]