Abstract
To enhance the production of isoprene, a volatile 5-carbon hydrocarbon, in the Gram-positive spore-forming rod-shaped bacterium Bacillus subtilis, 1-deoxy-d-xylulose-5-phosphate synthase (Dxs) and 1-deoxy-d-xylulose-5-phosphate reductoisomerase (Dxr) were overexpressed in B. subtilis DSM 10. For the strain that overexpresses Dxs, the yield of isoprene was increased 40% over that by the wild-type strain. In the Dxr overexpression strain, the level of isoprene production was unchanged. Overexpression of Dxr together with Dxs showed an isoprene production level similar to that of the Dxs overproduction strain. The effects of external factors, such as stress factors including heat (48°C), salt (0.3 M NaCl), ethanol (1%), and oxidative (0.005% H2O2) stress, on isoprene production were further examined. Heat, salt, and H2O2 induced isoprene production; ethanol inhibited isoprene production. In addition, induction and repression effects are independent of SigB, which is the general stress-responsive alternative sigma factor of Gram-positive bacteria.
Isoprene, also known as 2-methyl-1,3-butadiene, is a volatile 5-carbon pure hydrocarbon. Derived from biomass through a biochemical process, isoprene can be utilized as a fossil fuel alternative and a platform chemical for the production of high-value biobased chemicals, such as rubber, elastomers, and isoprenoid medicines (17, 39). Isoprene has several advantages over ethanol. First, it is much easier to separate from the fermentation broth, since it is present in the upper gas phase of a fermentor due to its low boiling point (34°C) and low solubility in water. Second, bacteria are more tolerant to isoprene than to ethanol (the MIC of isoprene for Bacillus subtilis strain DSM 10 is more than 10%, which is higher than the MIC of ethanol according to our unpublished data). Third, isoprene is a highly versatile molecule that can be biochemically and thermochemically re-formed into more complicated products.
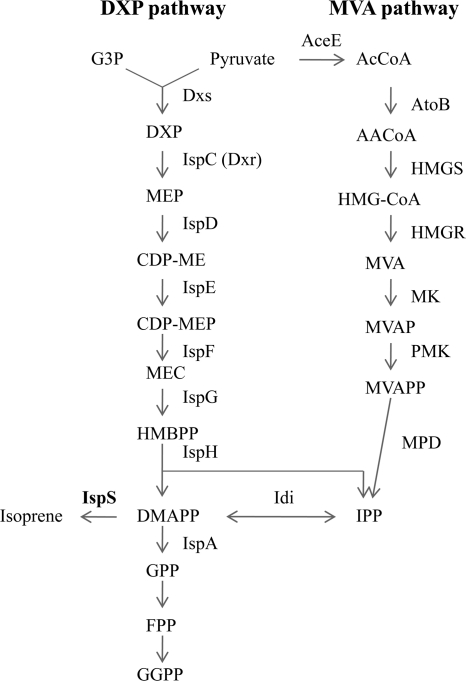
Isoprene is produced both by eukaryotes and by prokaryotes, including humans, plants, yeasts, and bacteria (18). Two pathways in isoprene biosynthesis have been discovered: the mevalonate (MVA) pathway and the nonmevalonate route, or the 1-deoxy-d-xylulose-5-phosphate (DXP) pathway (Fig. 1) (13, 39). The MVA pathway is present in eukaryotes, archaea, and the cytosol of higher plants; the DXP pathway is present in most bacteria, green algae, and the chloroplasts of higher plants (18). B. subtilis, the host in the present study, uses the DXP pathway to produce isoprene (Fig. 1) (37). The DXP pathway initiates with the glycolytic intermediates pyruvate and glyceraldehyde-3-phosphate (G3P). Pyruvate and G3P are condensed to form DXP by 1-deoxy-d-xylulose-5-phosphate synthase (Dxs), and subsequently, 6 enzymes, encoded by the ispC (dxr), ispD, ispE, ispF, ispG, and ispH genes, catalyze sequential reactions converting DXP to isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) (39). IPP and its isomer DMAPP can be interconverted by isopentenyl pyrophosphate isomerase, which is encoded by idi (fni) (14). IPP and DMAPP are universal precursors for the biosynthesis of isoprene and other isoprenoids, such as sterols, carotenoids, and dolichols. DMAPP can be catalyzed by isoprene synthase (IspS) to form isoprene (Fig. 1). No sequences of the ispS gene from prokaryotes are available in any databases at present. Recently, a novel class of oxidoreductases (DXP reductoisomerase-like enzyme [DRL]) catalyzing the conversion of DXP into methylerythritol 4-phosphate (MEP) (the second reaction in the DXP pathway) was discovered in the pathogenic bacterium Brucella abortus (27).
FIG. 1.
Pathways of isoprenoid biosynthesis in E. coli, B. subtilis, and Saccharomyces cerevisiae (14, 38, 39). G3P, glyceraldehyde-3-phosphate; DXP, 1-deoxy-d-xylulose 5-phosphate; MEP, 2-C-methyl-d-erythritol 4-phosphate; CDP-ME, 4-diphosphocytidyl-2-C-methylerythritol; CDP-MEP, CDP-ME 2-phosphate; MEC, 2-C-methyl-d-erythritol-2,4-cyclodiphosphate; HMBPP, (E)-4-hydroxy-3-methylbut-2-enyl-diphosphate; AcCoA, acetyl coenzyme A (acetyl-CoA); AACoA, acetoacetyl-CoA; HMG-CoA, hydroxymethylglutaryl-CoA; MVAP, mevalonate-5-phosphate; MVAPP, mevalonate-5-diphosphate; GPP, geranyl diphosphate; FPP, farnesyl diphosphate; GGPP, geranylgeranyl diphosphate; Dxs, 1-deoxy-d-xylulose-5-phosphate synthase; IspC, 1-deoxy-d-xylulose-5-phosphate reductoisomerase; IspD, 4-diphosphocytidyl-2-C-methyl-d-erythritol synthase; IspE, 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase; IspF, 2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase; IspG, 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate synthase; IspH, 1-hydroxy-2-methyl-butenyl 4-diphosphate reductase; IspA, farnesyl diphosphate synthase; IspS, isoprene synthase; AtoB, acetoacetyl-CoA thiolase; HMGS, hydroxymethylglutaryl-CoA synthase; HMGR, hydroxymethylglutaryl-CoA reductase; MK, mevalonate kinase; PMK, phosphomevalonate kinase; MPD, mevalonate pyrophosphate decarboxylase; Idi, isopentenyl pyrophosphate isomerase.
Beyond biofuel production, research on isoprene biosynthesis has broader significance (6). The DXP pathway is absent in humans and present in many human pathogens; enzymes of the DXP pathway are good targets for the development of antimicrobial drugs against serious diseases, including malaria, tuberculosis, and sexually transmitted diseases (13, 25).
Reports on the use of genetic modification to enhance isoprene production in bacteria are scarce. A photosynthetic bacterium, the cyanobacterium Synechocystis sp. PCC6803, has been employed to produce isoprene (19). Because the isoprene synthase gene (ispS) has not been identified in bacteria, a codon usage-optimized plant ispS gene is utilized in Synechocystis to produce isoprene. Since the biosynthesis of isoprenoids shares most of the DXP pathway with the biosynthesis of isoprene, previous efforts, which include the overproduction of valuable isoprenoids, such as carotenoids and terpenoids, through genetic modification of either the MVA or the DXP pathway, are informative for the study of isoprene overproduction. “Isoprenoid” is a broad term that includes the entire class of compounds derived from the universal precursors IPP and DMAPP. Various strategies have been utilized to modify the DXP pathway so as to maximize the production of the isoprenoid lycopene (a pigment and antioxidant). For example, the native dxs and dxr genes were introduced into several expression vectors under the control of three different promoters and were transformed into three Escherichia coli strains. E. coli with overexpressed dxs under the control of the arabinose-inducible araBAD promoter produced the highest yield (12.3 mg/liter in 24 h) of lycopene compared to the control and other genetic constructs (16). Based on the results from stoichiometric flux balance analysis, a triple knockout mutant was created to overproduce lycopene in E. coli. After the gdhA, aceE, and fdhF genes, which encode glutamate dehydrogenase, pyruvate dehydrogenase, and formate dehydrogenase, respectively, were deleted, the triple knockout construct showed a ∼40% increase in lycopene biosynthesis (6,600 ppm, or 6,600 μg per g [dry weight] of cells) over that of a parental strain in which the idi, ispFD, and dxs genes were overexpressed (1). The DXP pathway was also optimized to overproduce lycopene in E. coli by using a multiplex automated genome engineering (MAGE) approach (38). The ribosome binding sites of dxs and idi were optimized, and a 5-fold increase (∼9,000 ppm) in lycopene production over that of the parental strain was achieved. The MVA pathway from Saccharomyces cerevisiae and a synthetic amorpha-4,11-diene synthase gene were engineered into E. coli to produce amorphadiene, which is the precursor of artemisinin, an antimalarial drug (2, 21, 35).
B. subtilis is utilized as the host for the overproduction of isoprene due to its unique properties. B. subtilis is a Gram-positive spore-forming rod-shaped soil bacterium. Bacillus species have been the primary bacterial workhorses in microbial fermentation for decades (28). They have a wide substrate range, a broad metabolic potential, and a high growth rate, and they are able to survive under harsh conditions, such as low pH, high temperatures, and high salt concentrations. In addition, B. subtilis is listed by the Food and Drug Administration as GRAS (generally regarded as safe) (26). Thus, after fermentation, the biomass can be dried and used without regulatory restrictions. Furthermore, B. subtilis has the ability to ferment both pentoses and hexoses (24). Lignocellulosic materials contain 5 to 20% pentose sugars such as xylose and arabinose; these pentoses are not fermented by the common industrial fermentation microorganisms, the yeast Saccharomyces cerevisiae and the ethanologenic bacterium Zymomonas mobilis, which in their native form ferment only 6-carbon sugars (34). B. subtilis can also produce cellulases to digest lignocellulosic biomass (20, 23). The first step of lignocellulose conversion into biofuel requires efficient depolymerization of cellulose and hemicelluloses to soluble sugars. This step requires expensive cellulases. Thanks to its innate cellulases, the utilization of B. subtilis will decrease the cost of biomass pretreatment. More importantly, Bacillus spp. produce a higher concentration of isoprene than the other bacterial species tested, such as the Gram-negative bacteria E. coli and Pseudomonas aeruginosa and the Gram-positive bacterium Micrococcus luteus (17). The rate of isoprene production by B. subtilis ATCC 6051 is 7 to 13 nmol per g of cells per h (17).
The present study utilized overexpression mutants to increase isoprene production, because the yield of isoprene by the wild-type strain is unsatisfactory. Until now, the DXP pathway in Gram-positive bacteria has not been modified. Additionally, multiple conditions that affect isoprene biosynthesis were tested. This study shows that overexpression of Dxs can increase isoprene production 40% over that by the wild-type strain B. subtilis DSM 10. The stress factors heat (48°C), salt (0.3 M), and H2O2 (0.005%) induce the production of isoprene; 1% ethanol inhibits isoprene production. These effects are independent of SigB, the general stress-responsive alternative sigma factor, which is a subunit of bacterial RNA polymerase.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. subtilis strain DSM 10 (ATCC 6051) was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ). E. coli strain DH5α (Invitrogen) was used in cloning procedures. B. subtilis strains were grown in Luria-Bertani (LB) medium at 30°C with shaking. E. coli strains were grown in LB medium at 37°C with shaking. The media were supplemented with the antibiotics chloramphenicol (20 μg/ml) and ampicillin (100 μg/ml) as needed.
Cloning and sequencing of dxs and dxr genes.
The dxs and dxr genes were amplified by PCR using genomic DNA as a template and were cloned into the E. coli-B. subtilis shuttle vector pHT01 (MoBiTec) (22). BamHI and XbaI restriction sites were incorporated into the forward and reverse PCR primers for cloning purposes. Primers dxs-BamHI-F and dxs-XbaI-R were used for amplification of dxs; dxr-BamHI-F and dxr-XbaI-R were used for the amplification of dxr (Table 1). The recombinant plasmids are named pHTdxs and pHTdxr, respectively. Genomic DNA was isolated from B. subtilis strain DSM 10 by using the DNeasy Blood & Tissue kit (Qiagen) according to the manufacturer's instructions. Plasmids were purified using the QIAprep Spin Miniprep kit (Qiagen). To create pHTdxsr, which contains both dxs and dxr, dxs was amplified by PCR using primers dxs-BamHI-F and dxs-SOE-R, and dxr was amplified by PCR using primers dxr-SOE-F and dxr-XbaI-R (Table 1). dxs and dxr were linked by splice-by-overlap extension (SOE) PCR (12) using primers dxs-BamHI-F and dxr-XbaI-R; they were then cloned into pHT01. In the newly constructed plasmids, the expression of inserted genes is under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter. Newly constructed plasmids were verified by DNA sequencing and were introduced into DSM 10 by electroporation. The electrotransformation procedure used is based on previous publications with some modifications (36, 40). Briefly, Bacillus cultures were grown in brain heart infusion (BHI) medium supplemented with 0.5 M sucrose at 37°C to an optical density at 600 nm (OD600) of 0.7 to 1.0, and the cell wall-weakening agent glycine was added to a final concentration of 1.3%. After the addition of glycine, cultures were allowed to grow for 1 h; then cells were harvested and washed in 0.5 M ice-cold sucrose. Finally, cells were resuspended in 0.5 M sucrose. For electroporation, 100 μl of cells was mixed with 10 μl of plasmid (∼1 μg), and the mixture was transferred to 0.2-mm-gap electroporation cuvettes. After a single electroporation pulse (2,480 V) using an Eppendorf electroporator, model 2510, cells were recovered in BHI supplemented with 0.5 M sucrose for 2 h at 30°C and were then spread onto BHI plates containing 0.5 M sucrose and 5 μg ml−1 chloramphenicol.
TABLE 1.
Primers used for the construction of overexpression strains and the sigB deletion strain
| Primera | Sequenceb |
|---|---|
| dxs-BamHI-F | CGGGATCCTTGGATCTTTTATCAATACAGGAC |
| dxs-XbaI-R | GCTCTAGATCATGATCCAATTCCTTTGTG |
| dxr-BamHI-F | CGGGATCCTTGAAAAATATTTGTCTTTTAGGAGCA |
| dxr-XbaI-R | GCTCTAGA TCA CGA ACA TAC CAC CTT ATG |
| dxs-SOE-R | TCA TGA TCC AAT TCC TTT GTG |
| dxr-SOE-F | CAC AAA GGA ATT GGA TCA TGA TTG AAA AAT ATT TGT CTT TTA GGA GCA |
| sigB-BamHI-A1-F | CGGGATCCGCGGTTCAGCACGCTTACAAAGAA |
| sigB-B1-R | GCGTTTCCTGCGCTTGTTCATCTT |
| sigB-C1-F | AAGATGAACAAGCGCAGGAAACGCGGGACATTCTCGGTATATCTCAAATGCACG |
| sigB-XbaI-D1-R | GCTCTAGATTTCAGGATAGATGCTGTAGCGCC |
F, forward primer; R, reverse primer.
Primers were designed using published sequences available at the GenoList server (http://genodb.pasteur.fr/cgi-bin/WebObjects/GenoList). The restriction sites incorporated into primers for cloning purposes are shown in boldface and underlined. The overhangs complementary to the dxs-SOE-R primer and the sigB-B1-R primer are underlined in the sequences of primers dxr-SOE-F and sigB-C1-F, respectively.
Construction of a sigB in-frame deletion mutant.
A sigB in-frame deletion strain was constructed using B. subtilis DSM 10 according to the allelic exchange mutagenesis approach described previously (4, 32). The chromosomal copy of the sigB gene, which encodes 262 amino acids, was replaced by homologous recombination with a truncated sigB gene, which encodes the N-terminal 33 amino acids and the C-terminal 34 amino acids. The in-frame deletion gene was created by SOE PCR in plasmid pKSV7 (a temperature-sensitive suicide shuttle vector) (4, 32) using PCR primers shown in Table 1. Briefly, the AB fragment, located at the 5′ end of sigB, was amplified using PCR primers sigB-BamHI-A1 and sigB-B1, and the CD fragment, located at the 3′ end of sigB, was amplified using PCR primers sigB-C1 and sigB-XbaI-D1. The AD fragment, which contains the truncated gene, was amplified by SOE PCR using primers sigB-BamHI-A1 and sigB-XbaI-D1. The BamHI- and XbaI-digested fragment AD was ligated into pKSV7 and was transformed into E. coli DH5α (Invitrogen). The newly constructed plasmid was transformed into B. subtilis DSM 10 by electroporation and was integrated into the chromosome by growing the bacteria at 41°C (a nonpermissive temperature for pKSV7 replication) in LB medium with chloramphenicol (10 μg/ml). Plasmid pKSV7 was excised and cured by growing the bacteria at 30°C in LB medium without chloramphenicol. Cells were reverted to wild type or were turned into an in-frame deletion mutant after excision of pKSV7 resulting from homologous recombination. Colonies of in-frame deletion mutants, which are chloramphenicol sensitive, were screened by colony PCR using primers sigB-BamHI-A1 and sigB-XbaI-D1. The deletion mutant shows a shorter band (∼780 bp); the wild-type strain shows a longer band (∼1,400 bp). The truncated gene was confirmed by DNA sequencing. The deletion in DSM 10 was also verified by real-time reverse transcription-PCR (RT-PCR) (data not shown). No antibiotic marker was introduced into the mutant.
RNA isolation and transcript analysis by quantitative real-time RT-PCR.
Strains were grown at 30°C in LB medium supplemented or not with 20 μg/ml chloramphenicol to an OD600 of ∼1.0. After the addition of 1 mM IPTG, cultures continued to grow for 3 more hours. RNA from 3-ml cultures was stabilized using the RNAprotect bacterial reagent (Qiagen) and was purified with the RNeasy minikit (Qiagen). The mRNA quantitation method used has been described previously (41). The RT-PCR primers used for analysis of dxs, dxr, sigB, and a 16S rRNA endogenous control gene are listed in Table 2. Amplification steps were performed using a MiniOpticon system with Opticon Monitor software, version 3.1 (Bio-Rad). The data were analyzed by using the comparative CT method, where the parameter CT (threshold cycle) is the cycle number at which the fluorescence emission due to PCR products exceeds the fixed threshold (7).
TABLE 2.
Primers used for real-time RT-PCR
| Gene targeted | Primer orientationa | Primer sequenceb |
|---|---|---|
| 16S rRNA gene | F | ACGGTCGCAAGACTGAAACT |
| R | TAAGGTTCTTCGCGTTGCTT | |
| dxs | F | GAAAATGAAGGCCAGCACAT |
| R | CATCCATTTTTACGCCGAGT | |
| dxr | F | GCGCTTGCAAATAAGGAAAC |
| R | GGCTGAATGCTCACTGTCAA | |
| sigB | F | GCCGAAAGTCGAAGAGATTG |
| R | GGTCAACGGATAAGGCTTGA |
F, forward primer; R, reverse primer.
Primers were designed using published sequences available at the GenoList server (http://genodb.pasteur.fr/cgi-bin/WebObjects/GenoList).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
Bacterial cells were harvested by centrifugation and were then resuspended in Tris-EDTA (TE) buffer (pH 8.0) with protease inhibitor cocktail (Thermo Scientific). After the cells were mechanically disrupted by glass beads (diameter, 0.1 mm) using a Precellys 24 homogenizer (Mo Bio Laboratories), glass beads and insoluble materials were removed by centrifugation. The protein concentration of the supernatant was determined by the Pierce bicinchoninic acid (BCA) protein assay (Thermo Scientific) using a microtiter plate reader (Dynex) according to the manufacturer's instructions. Equal amounts of protein samples from various strains were loaded onto 4 to 12% precast gels (ClearPAGE; C.B.S. Scientific).
Detection of isoprene production by GC-MS.
To determine the concentration of isoprene in the headspace of liquid cultures, a gas chromatography-mass spectrometry (GC-MS) method was established (17). An Agilent 7890A GC and 5975 mass selective detector (MSD) with autosamplers were used. An autosampler (CTC PAL system) with a solid-phase microextraction (SPME) syringe (Supelco) was used to absorb the headspace volatiles (33). The fiber material was 50/30 divinylbenzene-carburen on polydimethylsiloxane on a stable flex fiber. A DB-5ms column (length, 30 m; inner diameter, 0.25 mm; film thickness, 0.25 μm) with helium as the carrier gas at a flow rate of 0.9 ml min−1 was used. The oven temperature was initiated at 30°C for 3.5 min and was then increased to 120°C at a rate of 20°C per min. The headspace of LB medium was used as a negative control. To identify bacterial isoprene production, peak retention times and mass spectra were compared with those of the standard. Isoprene eluted at 1.9 min. The concentrations of isoprene produced by bacterial cells were calculated by converting the GC-MS peak area to nanograms of isoprene via a calibration curve. An isoprene standard (Sigma-Aldrich) of various concentrations was added to 10 ml LB medium to make a calibration curve.
Growth curve experiments.
Growth curve experiments with DSM 10, DSM 10/pHTdxs, DSM 10/pHTdxr, and DSM 10/pHTdxsr were performed using a microtiter plate reader with Revelation Software (Dynex). A starter culture of 5 ml LB medium was inoculated and grown overnight (16 to 18 h) at 30°C with shaking; then the starter culture was diluted into LB medium to an OD600 of 0.025. Aliquots of 100 μl were taken from the diluted cultures and were added to each well of microtiter plates. Chloramphenicol (20 μg/ml) was added to the LB medium of the overexpression strains. Bacterial cells were grown in microtiter plates at 30°C with shaking. Microtiter plates were read every 30 min at a wavelength of 595 nm. When the OD595 reached about 0.15, 1 mM IPTG was added to the wells of the overexpression strains. Each sample was measured in triplicate.
Isoprene production with the stress factor heat, salt, ethanol, or oxidative stress.
Cultures of the wild-type strain and the sigB deletion mutant were grown in LB at 30°C with shaking until mid-log phase (OD600, ∼0.5). Aliquots of cultures were transferred to 20-ml headspace vials (10 ml in each vial), and then NaCl, ethanol, or H2O2 was added to the cultures to a final concentration of 0.3 M, 1%, or 0.005%, respectively. The cultures continued to grow for 4 h at either 30°C or 48°C. The concentration of isoprene released from the bacterial cultures to the headspace was determined by GC-MS, and the OD600 values of the cultures in vials were taken using a spectrophotometer.
RESULTS
Isoprene production is increased by overexpression of dxs but not by overexpression of dxr.
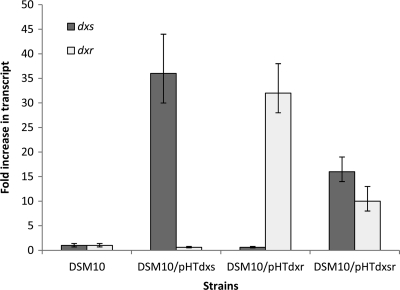
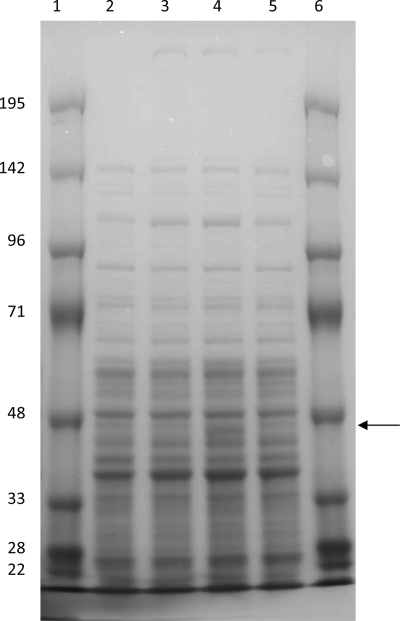
Previous work has shown that overexpression of Dxs alone or with Dxr can increase lycopene production in E. coli (16). Synthesis of lycopene and synthesis of isoprene use the same universal precursors, IPP and DMAPP. However, it has not yet been demonstrated directly in B. subtilis whether overexpression of genes in the DXP pathway can increase the production of isoprene. To test if genetic modification of the DXP pathway can enhance the formation of isoprene in B. subtilis, wild-type dxs (the first gene in the DXP pathway), dxr (the second gene in the DXP pathway), or both were introduced into the wild-type strain DSM 10. The constructs are designated DSM 10/pHTdxs, DSM 10/pHTdxr, and DSM 10/pHTdxsr, respectively. To confirm that dxs and dxr are overexpressed at the transcriptional level, mRNA levels of dxs and dxr in DSM 10 and the overexpression strains were analyzed by real-time RT-PCR (Fig. 2). In strain DSM 10/pHTdxs, the dxs mRNA level increased to 36-fold the level in DSM 10; in strain DSM 10/pdxr, the dxr mRNA level increased to 32-fold the level in DSM 10; in strain DSM 10/pHTdxsr, the dxs and dxr mRNA levels increased to 16- and 10-fold the corresponding levels in DSM 10, respectively. Overexpression of the Dxs and Dxr proteins was also analyzed by SDS-PAGE (Fig. 3). No band corresponding to the size of Dxs (70 kDa) could be identified in the SDS-PAGE gel, probably because of a low protein expression level. It is highly likely that overexpressed Dxs still has a low expression level relative to those of other proteins; therefore, it could not be shown by SDS-PAGE. The band corresponding to the size of Dxr (43 kDa) is visible in DSM 10/pHTdxr but not in DSM 10/ pHTdxsr. The reason might be a lower Dxr level in DSM 10/pHTdxsr, in which both dxs and dxr are controlled by one promoter. The results of RT-PCR and SDS-PAGE analysis confirmed the overexpression of dxr; therefore, the unchanged isoprene production level in strain DSM 10/pHTdxr is not caused by a lack of Dxr overproduction (Fig. 3). Overexpression of Dxs can increase isoprene production 40% over that by the wild-type strain DSM 10. However, overexpression of Dxs and Dxr together in DSM 10/pHTdxsr showed a level of isoprene production similar to that of DSM 10/pHTdxs (Table 3). According to Table 3, in the wild-type and overexpression strains, isoprene production is higher in the early-exponential phase than in the mid- and late-exponential phases, a finding that is not consistent with the previous finding (17).
FIG. 2.
Real-time RT-PCR analysis of the relative levels of dxs and dxr mRNAs in DSM 10 and three overexpression strains. The averages of results obtained from three independent RNA preparations are shown. All transcript levels were measured in triplicate for each RNA preparation. Error bars represent standard deviations from the means.
FIG. 3.
SDS-PAGE analysis of B. subtilis DSM 10, DSM 10/pHTdxs, DSM 10/pHTdxr, and DSM 10/pHTdxsr. Lanes: 1 and 6, protein markers; 2, wild type; 3, DSM 10/pHTdxs; 4, DSM 10/pHTdxr; 5, DSM 10/pHTdxsr. The arrow indicates the expected size for Dxr (43 kDa). The expected band for Dxs (70 kDa) could not be identified from SDS-PAGE.
TABLE 3.
Isoprene production in different growth phases and in various strainsa
| Growth phase | Concn (ng/ml/OD600) of isopreneb produced by the following strain: |
|||
|---|---|---|---|---|
| DSM 10 | DSM 10/pHTdxs | DSM 10/pHTdxr | DSM 10/pHTdxsr | |
| Early log | 2.67 ± 0.16 | 3.73 ± 0.36 | 2.61 ± 0.22 | 3.45 ± 0.37 |
| Mid-log | 2.33 ± 0.24 | 3.29 ± 0.15 | 2.47 ± 0.16 | 3.27 ± 0.53 |
| Late log | 1.72 ± 0.09 | 1.92 ± 0.09 | 1.62 ± 0.01 | 2.31 ± 0.15 |
Strains were grown at 30°C in LB medium with shaking to OD600 values of ∼0.2 for the early-log phase, ∼0.5 for the mid-log phase, and ∼1.0 for the late-log phase. Twenty micrograms of chloramphenicol per milliliter was added to the LB medium of the overexpression strains. During the course of the experiment, 10-ml aliquots were taken from the growing cultures and were transferred to 20-ml airtight vials. IPTG (1 mM) was added to the 10-ml cultures of the overexpression strains. These vials were incubated for one more hour with shaking at 30°C. Finally, the isoprene concentrations in the headspace were determined by GC-MS; then the OD600 values of the cultures in the vials were taken using a spectrophotometer.
Values are averages ± standard deviations for three experiments.
Growth curve experiment.
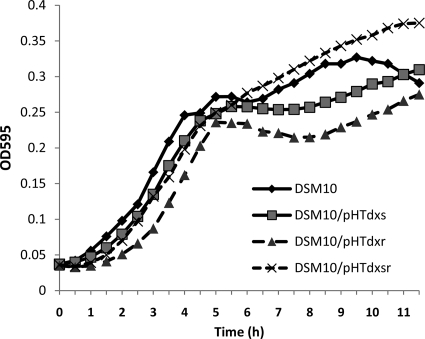
To test if any growth defect is caused by overexpression of dxs, dxr, or both in the overexpression strains, a growth curve experiment was performed using a microtiter plate reader (Fig. 4). Although none of the three strains showed any significant growth defect, there are slight differences between the overexpression strains and the wild-type strain. The wild-type strain showed the typical diauxic growth, with two distinct growth phases. Strain DSM 10/pHTdxsr showed continued growth instead of diauxic growth, and there was no transitional phase from the preferred carbon source to other sources. DSM 10/pHTdxs and DSM 10/pHTdxr showed a slower transitional phase than the wild-type strain.
FIG. 4.
Growth curve analysis of the wild-type strain DSM 10 and overexpression strains in LB medium at 30°C. The curves indicate the average OD595 of each culture over time. Each sample was measured in triplicate.
Heat, salt, and oxidative stress induce the production of isoprene, while ethanol inhibits isoprene production.
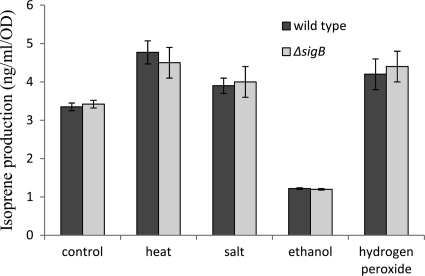
Previous work has shown that isoprene production in bacterial cultures responds to changes in temperature: isoprene production increases in the temperature range from 25°C to about 45°C and then decreases to almost zero at 65°C (17). In the present work, the level of isoprene production, determined under the widely used testing condition of heat stress (48°C), increased 40% over that for the control (in LB medium at 30°C). Therefore, the finding that isoprene production is induced under heat conditions is consistent with the previous report. To test if other stress factors could affect the formation of isoprene in B. subtilis, the wild-type strain was exposed to salt (0.3 M NaCl), 1% ethanol, or oxidative stress (0.005% H2O2). Surprisingly, these stressors showed various effects on isoprene formation. Salt and oxidative stress can enhance isoprene production 16% and 25%, respectively, over that by the control. In contrast, after ethanol addition, the level of isoprene production was lowered 60%.
Previous work has shown that overexpression of rpoS can increase lycopene production in E. coli (15). rpoS encodes the sigma S subunit of RNA polymerase in E. coli. RpoS (σS) and RpoS-dependent genes respond to many stress conditions. RpoS has been called “the master regulator of the general stress response in E. coli” (11). The gene corresponding to rpoS in Gram-positive bacteria is sigB (σB) (5). B. subtilis cells respond to a variety of stresses by induction of about 150 SigB-dependent general stress genes (9, 10). It has been reported that heat, salt, ethanol, and oxidative stress can induce sigB in B. subtilis (3). In addition, biosynthesis of lycopene and biosynthesis of isoprene share most of the DXP pathway. Increased lycopene production by overexpression of RpoS might suggest a role for rpoS in the DXP pathway. Therefore, we tested whether isoprene production is under the control of SigB. According to Fig. 5, when bacterial cells were grown in LB medium at 30°C (control), the sigB deletion strain and the wild-type strain showed similar isoprene production levels. In addition, the sigB deletion mutant showed induction and repression effects similar to those of the wild-type strain with each of these four stress factors. Therefore, the production of isoprene via the DXP pathway is not controlled by SigB, and the induction and repression effects with these four stress factors are SigB independent.
FIG. 5.
Isoprene production is induced by heat (48°C), salt (0.3 M NaCl), and oxidative stress (0.005% H2O2) and is repressed by 1% ethanol in the B. subtilis strain DSM 10 and the sigB in-frame deletion mutant. The average concentrations (ng/ml/OD600) obtained from three independent cultures are shown. Error bars represent standard deviations from the means.
DISCUSSION
The production of biofuels and chemicals from cellulosic biomass through microbial conversion using saccharolytic fermentative microorganisms is an attractive approach to low-cost biomass processing. Due to its cellulase enzymes, B. subtilis appears to be a promising candidate for bioconversion. B. subtilis is also a good candidate for isoprene production, because it produces about 18-fold the level of isoprene produced by E. coli (17), which has frequently been used as the host for genetic modification during the microbial conversion process. We optimized the expression of some native genes in B. subtilis to overproduce isoprene. We have further demonstrated that overexpression of Dxs enhances isoprene production; overexpression of Dxr does not have a significant effect on isoprene production in B. subtilis DSM 10. The limiting factors in a biochemical reaction could be limited substrate supplies, low enzyme availability, fast degradation of the enzyme, and the unavailability of cofactors, etc. After Dxr is overproduced, unchanged isoprene production could not be caused by limited enzyme availability. Moreover, the isoprene production level of strain DSM 10/pHTdxsr is similar to that of the Dxs overexpression strain (there is no additive effect), suggesting that neither the enzyme level nor the availability of the substrate is a limiting factor for Dxr. Therefore, based on the current understanding of the DXP pathway, during the genetic modification of B. subtilis, the key regulating point—Dxs rather than Dxr—should be emphasized.
Interestingly, when Dxs and Dxr were overexpressed together, the overexpression strain lost diauxic growth. This finding might suggest that the DXP pathway is related to carbon catabolite repression. Because LB medium contains little carbohydrate, and peptides and free amino acids are primary carbon sources for bacteria grown in LB medium (30), the diauxie-like growth behavior of DSM 10 probably reflects the transition from easier-to-use amino acids to harder-to-use amino acids. However, in strain DSM 10/pHTdxsr, overproduction of Dxs and Dxr led to nonpreferential utilization of amino acids. The question of how the DXP pathway interacts with carbon catabolite repression needs further investigation.
It has been reported that enhanced isoprene production occurs in the late-log phase, a phenomenon that is likely caused by rapid physiological changes during the transition from log phase to stationary phase (17). In contrast to the previous report, we propose that the decline in isoprene production during log phase might be caused by depletion of some essential nutrients. The previous report has shown that bacteria grown in rich medium produce more isoprene than bacteria grown in minimal medium (17), a result that somewhat supports our finding.
According to our results, some external factors, such as heat (48°C), salt (0.3 M or 1.8% NaCl), and H2O2 (0.005%), induce isoprene production, while 1% ethanol inhibits isoprene production. Previous research has shown that these stress factors induce sigB expression (3). SigB (σB) is the general stress-responsive alternative sigma factor in Gram-positive bacteria; RpoS (σS) is the counterpart in Gram-negative bacteria (5, 10). It has also been reported that overexpression of rpoS can enhance lycopene production in E. coli (15). Additionally, biosynthesis of lycopene and biosynthesis of isoprene shared most of the DXP pathway. These facts suggest that the DXP pathway might be under the control of SigB in B. subtilis. According to Fig. 5, the DXP pathway is not regulated by SigB; the sigB deletion strain showed an isoprene production level similar to that of the wild-type strain under both control and stress conditions. The increased lycopene production caused by RpoS overexpression in E. coli might be a result of increased resistance to the challenge of lycopene accumulation (15). Heat, salt, oxidative stress, and ethanol stress factors could either directly regulate isoprene synthase or indirectly affect some regulators (such as transcriptional regulators), which could subsequently control isoprene synthase and thus govern isoprene production. However, these hypotheses await further research. It will also be interesting to compare and contrast the effects of stresses on the production of isoprene and other isoprenoids in a future study.
Because isoprene production is induced under heat stress, it is possible that the isoprene biosynthesis pathway is under the control of members of the heat shock stimulon. The heat shock stimulon is grouped into 6 classes of heat shock genes in B. subtilis (29). Class I, the HrcA regulon, encodes the molecular chaperones; class II, the SigB regulon, controls the general stress response; class III, the CtsR regulon, includes ATP-dependent proteases; class IV, the htpG gene, codes for a molecular chaperone; class V, the CssRS regulon, comprises membrane-anchored proteases; class VI, a group of heat shock genes with diverse functions, is controlled by unknown mechanisms. Additionally, it is possible that the isoprene biosynthesis pathway is regulated by members of the osmotic stress regulons. Previous research has shown that under osmotic stress (6% NaCl), about 500 B. subtilis genes are upregulated (8). The upregulated genes include large parts of the SigB, SigW, SigM, and SigX regulons. Moreover, there might be some overlap between heat shock genes and osmotic stress genes. Because isoprene production is induced under heat, salt, and oxidative stresses and is repressed by ethanol addition, it seems likely that if genes that are coordinated under both induction conditions and the repression condition could be identified, these genes would be involved in isoprene production. These unknown genes could be utilized as the targets for further genetic modification to overproduce isoprene in B. subtilis.
Although isoprene synthase was partially purified, the isoprene synthase gene has not been identified in bacteria (31). It is not even clear whether IspS is a unique enzyme or a phosphatase with multiple catalytic activities. The only choice for metabolic engineering is the utilization of plant isoprene synthase, which requires codon optimization (19). In addition, most bacterial pathways are tightly regulated by transcriptional regulators; however, no transcriptional activator or repressors that are either directly or indirectly related to the DXP pathway have been discovered. Therefore, the regulation of the DXP pathway in bacteria awaits detailed characterization. Elucidation of the regulation of the DXP pathway and identification of the bacterial isoprene synthase will facilitate the metabolic engineering process for isoprenoid production.
Acknowledgments
We acknowledge financial support from the Washington States STAR researcher program given to B. K. Ahring (2008-10).
We thank Kurt Miller for kindly providing the pKSV7 plasmid. We are very grateful to Weiqun Zhong for technical assistance.
Footnotes
The authors have paid a fee to allow immediate free access to this article.
Published ahead of print on 4 February 2011.
REFERENCES
- 1.Alper, H., Y. S. Jin, J. F. Moxley, and G. Stephanopoulos. 2005. Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab. Eng. 7:155-164. [DOI] [PubMed] [Google Scholar]
- 2.Anthony, J. R., et al. 2009. Optimization of the mevalonate-based isoprenoid biosynthetic pathway in Escherichia coli for production of the anti-malarial drug precursor amorpha-4,11-diene. Metab. Eng. 11:13-19. [DOI] [PubMed] [Google Scholar]
- 3.Boylan, S. A., A. R. Redfield, M. S. Brody, and C. W. Price. 1993. Stress-induced activation of the sigma B transcription factor of Bacillus subtilis. J. Bacteriol. 175:7931-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira, A., C. P. O'Byrne, and K. J. Boor. 2001. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl. Environ. Microbiol. 67:4454-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortman, J. L., et al. 2008. Biofuel alternatives to ethanol: pumping the microbial well. Trends Biotechnol. 26:375-381. [DOI] [PubMed] [Google Scholar]
- 7.Freeman, W. M., S. J. Walker, and K. E. Vrana. 1999. Quantitative RT-PCR: pitfalls and potential. Biotechniques 26:112-122. [DOI] [PubMed] [Google Scholar]
- 8.Hahne, H., et al. 2010. A comprehensive proteomics and transcriptomics analysis of Bacillus subtilis salt stress adaptation. J. Bacteriol. 192:870-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hecker, M., J. Pane-Farre, and U. Volker. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61:215-236. [DOI] [PubMed] [Google Scholar]
- 11.Hengge, R. 2009. Proteolysis of σS (RpoS) and the general stress response in Escherichia coli. Res. Microbiol. 160:667-676. [DOI] [PubMed] [Google Scholar]
- 12.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8:528-535. [PubMed] [Google Scholar]
- 13.Hunter, W. N. 2007. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 282:21573-21577. [DOI] [PubMed] [Google Scholar]
- 14.Julsing, M. K., M. Rijpkema, H. J. Woerdenbag, W. J. Quax, and O. Kayser. 2007. Functional analysis of genes involved in the biosynthesis of isoprene in Bacillus subtilis. Appl. Microbiol. Biotechnol. 75:1377-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang, M. J., et al. 2005. Identification of genes affecting lycopene accumulation in Escherichia coli using a shot-gun method. Biotechnol. Bioeng. 91:636-642. [DOI] [PubMed] [Google Scholar]
- 16.Kim, S. W., and J. D. Keasling. 2001. Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol. Bioeng. 72:408-415. [DOI] [PubMed] [Google Scholar]
- 17.Kuzma, J., M. Nemecek-Marshall, W. H. Pollock, and R. Fall. 1995. Bacteria produce the volatile hydrocarbon isoprene. Curr. Microbiol. 30:97-103. [DOI] [PubMed] [Google Scholar]
- 18.Kuzuyama, T. 2002. Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci. Biotechnol. Biochem. 66:1619-1627. [DOI] [PubMed] [Google Scholar]
- 19.Lindberg, P., S. Park, and A. Melis. 2010. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 12:70-79. [DOI] [PubMed] [Google Scholar]
- 20.Maki, M., K. T. Leung, and W. Qin. 2009. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int. J. Biol. Sci. 5:500-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin, V. J., D. J. Pitera, S. T. Withers, J. D. Newman, and J. D. Keasling. 2003. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 21:796-802. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen, H. D., T. T. Phan, and W. Schumann. 2007. Expression vectors for the rapid purification of recombinant proteins in Bacillus subtilis. Curr. Microbiol. 55:89-93. [DOI] [PubMed] [Google Scholar]
- 23.Ou, M. S., N. Mohammed, L. O. Ingram, and K. T. Shanmugam. 2009. Thermophilic Bacillus coagulans requires less cellulases for simultaneous saccharification and fermentation of cellulose to products than mesophilic microbial biocatalysts. Appl. Biochem. Biotechnol. 155:379-385. [DOI] [PubMed] [Google Scholar]
- 24.Patel, M. A., M. S. Ou, L. O. Ingram, and K. T. Shanmugam. 2005. Simultaneous saccharification and co-fermentation of crystalline cellulose and sugar cane bagasse hemicellulose hydrolysate to lactate by a thermotolerant acidophilic Bacillus sp. Biotechnol. Prog. 21:1453-1460. [DOI] [PubMed] [Google Scholar]
- 25.Rohdich, F., A. Bacher, and W. Eisenreich. 2005. Isoprenoid biosynthetic pathways as anti-infective drug targets. Biochem. Soc Trans. 33:785-791. [DOI] [PubMed] [Google Scholar]
- 26.Romero, S., E. Merino, F. Bolivar, G. Gosset, and A. Martinez. 2007. Metabolic engineering of Bacillus subtilis for ethanol production: lactate dehydrogenase plays a key role in fermentative metabolism. Appl. Environ. Microbiol. 73:5190-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sangari, F. J., J. Perez-Gil, L. Carretero-Paulet, J. M. Garcia-Lobo, and M. Rodriguez-Concepcion. 2010. A new family of enzymes catalyzing the first committed step of the methylerythritol 4-phosphate (MEP) pathway for isoprenoid biosynthesis in bacteria. Proc. Natl. Acad. Sci. U. S. A. 107:14081-14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schallmey, M., A. Singh, and O. P. Ward. 2004. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 50:1-17. [DOI] [PubMed] [Google Scholar]
- 29.Schumann, W. 2003. The Bacillus subtilis heat shock stimulon. Cell Stress Chaperones 8:207-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sezonov, G., D. Joseleau-Petit, and R. D'Ari. 2007. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 189:8746-8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivy, T. L., M. C. Shirk, and R. Fall. 2002. Isoprene synthase activity parallels fluctuations of isoprene release during growth of Bacillus subtilis. Biochem. Biophys. Res. Commun. 294:71-75. [DOI] [PubMed] [Google Scholar]
- 32.Smith, K., and P. Youngman. 1992. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 74:705-711. [DOI] [PubMed] [Google Scholar]
- 33.Strobel, G. A., E. Dirkse, J. Sears, and C. Markworth. 2001. Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147:2943-2950. [DOI] [PubMed] [Google Scholar]
- 34.Taylor, M. P., et al. 2009. Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends Biotechnol. 27:398-405. [DOI] [PubMed] [Google Scholar]
- 35.Tsuruta, H., et al. 2009. High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS One 4:e4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turgeon, N., C. Laflamme, J. Ho, and C. Duchaine. 2006. Elaboration of an electroporation protocol for Bacillus cereus ATCC 14579. J. Microbiol. Methods 67:543-548. [DOI] [PubMed] [Google Scholar]
- 37.Wagner, W. P., D. Helmig, and R. Fall. 2000. Isoprene biosynthesis in Bacillus subtilis via the methylerythritol phosphate pathway. J. Nat. Prod. 63:37-40. [DOI] [PubMed] [Google Scholar]
- 38.Wang, H. H., et al. 2009. Programming cells by multiplex genome engineering and accelerated evolution. Nature 460:894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Withers, S. T., and J. D. Keasling. 2007. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Biotechnol. 73:980-990. [DOI] [PubMed] [Google Scholar]
- 40.Xue, G. P., J. S. Johnson, and B. P. Dalrymple. 1999. High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformis. J. Microbiol. Methods 34:183-191. [Google Scholar]
- 41.Xue, J., et al. 2005. Novel activator of mannose-specific phosphotransferase system permease expression in Listeria innocua, identified by screening for pediocin AcH resistance. Appl. Environ. Microbiol. 71:1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]