Abstract
Unmarked gene deletions facilitate studies of Legionella pneumophila multicomponent processes, such as motility and exonuclease activity. For this purpose, FRT-flanked alleles constructed in Escherichia coli using λ-Red recombinase were transferred to L. pneumophila by natural transformation. Resistance cassettes were then efficiently excised using the Flp site-specific recombinase encoded on a plasmid that is readily lost.
Virulence strategies of Legionella pneumophila can be studied by exploiting its genome sequence, natural competence, and growth in artificial media (18, 20). However, research has been complicated by limited selectable markers and the microbe's functional redundancy, including numerous secretion substrates (7, 14, 15). To facilitate construction of strains with multiple unmarked nonpolar deletions, we coupled phage-mediated recombination in Escherichia coli with Flp-mediated excision in L. pneumophila. By exploiting a λ phage enzyme to mediate homologous recombination between DNA substrates with as few as 35 nucleotides of homology, so-called recombineering offers several advantages over restriction enzyme-based cloning, including increased efficiency (8, 10, 27, 28, 31). The Saccharomyces cerevisiae Flp site-specific recombinase excises DNA flanked by directly repeated 34-bp FRT sites (9, 12, 13, 19, 21, 23, 24). Here we efficiently generated unmarked deletions in L. pneumophila using Flp induced from plasmids, which were then cured from the strain. Compared to traditional methods, this approach generated unmarked deletions at a higher frequency and with greater consistency.
To construct null alleles with FRT-flanked antibiotic resistance cassettes, 500 to 1,000 bp flanking each gene to be deleted were amplified by PCR from the chromosome of wild-type L. pneumophila and cloned into pGEM-T Easy (Promega) using E. coli DH5α as the host (Table 1). The gene of interest was replaced by an FRT-flanked cat or kan cassette from pKD3 or pKD4, respectively (10), using recombineering and E. coli DY330 (Table 1) (27, 28). The recombinant allele from pGEM was then transferred to the Lp02 chromosome by natural transformation (25, 26). An FRT-flanked gentamicin resistance cassette can be amplified with the same primers used for the cat and kan genes (Table 1).
TABLE 1.
Abbreviated list of bacterial strains and plasmids used in this studya
| Bacterium and strain | Genotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | supE44 ΔlacU169 (80lacZΔM15)hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Laboratory collection |
| DY330 | W3110 ΔlacU169 gal490 λcI857 Δ(cro-bioA) | 31 |
| pGEM-T Easy | Promega | |
| pKD3 (FRT-cat-FRT allele) | 10 | |
| pKD4 (FRT-kan-FRT allele) | 10 | |
| MB838 | DH5αλpir pR6KγFRT-gent-FRT | This work |
| MB790 | DH5α pMMBFlp | This work |
| MB791 | DH5α pBSFlp | This work |
| MB750 | DH5α pGEMfliA | This work |
| MB751 | DH5α pGEMΔfliA::FRT-cat-FRT | This work |
| L. pneumophila strains | ||
| MB110 | Lp02 wild type; thyA hsdR rpsL (Strr) | 1 |
| MB811 | Lp02 ΔfliA::FRT-cat-FRT | This work |
| MB818 | Lp02 ΔfliA::FRT | This work |
| MB758 | Lp02 recJ::FRT-cat-FRT | 1a |
| MB819 | Lp02 ΔrecJ::FRT | This work |
| MB759 | Lp02 xseA::FRT-kan-FRT | 1a |
| MB820 | Lp02 ΔxseA::FRT | This work |
| MB760 | Lp02 recJ::FRT-cat-FRT xseA::FRT-kan-FRT | 1a |
| MB821 | Lp02 recJ::FRT xseA::FRT | This work |
See Table S1 for a complete list of strains.
FRT-flanked cassettes excised using vectors that express Flp.
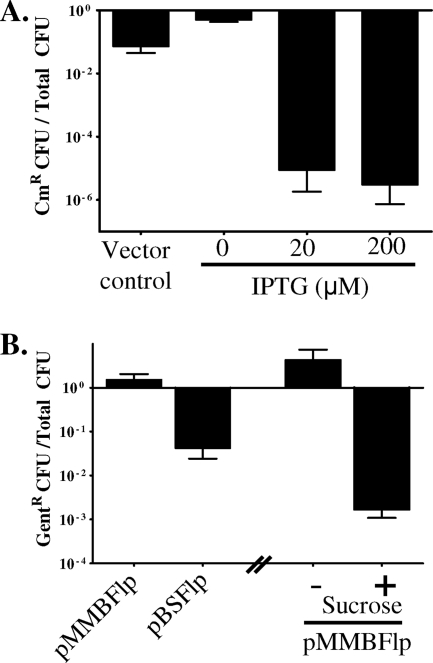
For construction of a shuttle vector for efficient expression of the Flp recombinase by L. pneumophila, flp was cloned into a gentamicin-resistant and sucrose-sensitive derivative of the broad-host-range plasmid pMMB206, yielding pMMBFlp (see Table S1 in the supplemental material). Vector pMMBFlp encodes an inducible and functional Flp recombinase (Fig. 1 A; see Table S1). However, since this plasmid was difficult to cure from the host strain (Fig. 1B), it is suitable for stable flp expression in L. pneumophila or for development of tools in other species (see Table S1). For both transient Flp expression and ready segregation from L. pneumophila, pBSFlp was constructed by replacing the RSF1010 origin of replication of pMMBFlp with the ColE1 ori from pBluescript KS− (Table 1).
FIG. 1.
After antibiotic resistance cassettes are efficiently cured from the L. pneumophila chromosome, plasmids expressing the Flp recombinase can be segregated from the host strain. (A) Flp-mediated excision of a cat cassette was examined in a letA::FRT-cat-FRT strain using pMMBFlp and IPTG induction in broth and then plating on medium with and without chloramphenicol (see Table S1 in the supplemental material). Data represent means ± standard errors of the means (SEMs) of 3 experiments. (B) Loss of Flp-encoding plasmids with either an RSF1010 (pMMBFlp) or ColE1 (pBSFlp) origin of replication. For cultures that were selected for plasmid loss with sucrose, overnight cultures were first grown in the absence of sucrose and then exponential-phase cultures were normalized, resuspended, and grown overnight in ACES-buffered yeast extract broth with thymidine plus 5% sucrose without antibiotics. After cultures were plated on media with and without gentamicin, the presence of the resistance cassette carried on the vector was scored. Data represent means ± SEMs of ≥3 experiments.
Flp-mediated excision of cassettes and removal of vector.
pBSFlp was transferred by electroporation (1a, 5, 16) to L. pneumophila strains harboring FRT-flanked deletion constructs, and transformants were selected on ACES [N-(2-acetamido)-2-aminoethanesulfonic acid]-buffered charcoal yeast extract (22) supplemented with 100 μg/ml of thymidine (CYET), gentamicin (10 μg/ml), and IPTG (isopropyl-β-d-thiogalactopyranoside) (200 μM). After being incubated for 5 to 6 days, individual colonies from the transformation were patched onto medium without antibiotics and IPTG and incubated overnight. Next, clones were isolated by streaking them onto CYET containing 5% sucrose, and their phenotypes and genotypes were determined. To verify the versatility of the method, deletions were constructed in lpg1782 (fliA), lpg2217, lpg0826 (xseA), and lpg1461 (recJ); an xseA recJ double mutant was also generated (Table 1).
For every locus examined, the vast majority of clones had excised the resistance cassette and lost the plasmid. When mutants were generated in fliA, lpg2217, xseA, and recJ, 100% of the total clones (n = 96) lost the desired resistance cassette when plated on medium containing IPTG, whereas 89% (n = 44) of the isolates plated on medium without IPTG did so. Loss of vector was also highly efficient; 100% (n = 96) of clones lacked the vector after being restreaked on sucrose-containing medium.
The ability of Flp recombinase to excise two cassettes in one step was also examined. A double recJ::FRT xseA::FRT unmarked deletion mutant was constructed in three steps: one step for each cassette insertion and then Flp-mediated excision of both cassettes in a single final step (Table 1). In contrast, traditional allelic exchange methods utilizing counter selection would require four selection steps, costing the researcher more time while increasing the chances of second-site mutations. Minimizing strain passage was particularly important for the nuclease mutants, whose DNA repair is predicted to be defective.
Mutations in multiple genes with redundant functions reveal phenotypes.
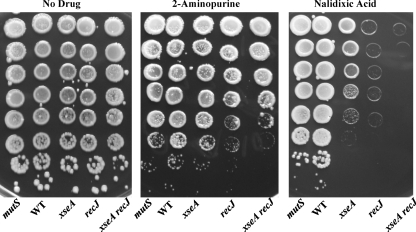
To examine whether functionally redundant genes can be studied by constructing multiple unmarked deletions in a single strain, we investigated the individual and combined effects of the RecJ and ExoVII (XseA) nucleases. E. coli has four canonical single-stranded nucleases (ssExos) with a great deal of redundancy (2, 11, 29, 30), whereas only two of these enzymes are annotated in the L. pneumophila genome (1a, 6). In E. coli, increased sensitivity to the base analog 2-aminopurine (2-AP) is not revealed until three or four of its ssExos are mutated (29). Nuclease mutants also exhibit increased sensitivity to nalidixic acid (Fig. 2) (4, 29). To test genetically if the L. pneumophila RecJ and ExoVII nucleases are redundant and together provide the majority of ssExo activity, we examined the susceptibilities of single and double mutants to 2-AP and nalidixic acid. When the mutants were treated with either DNA-damaging agent, the plating efficiency of the double mutant was less than that of either single mutant, suggesting overlapping function (Fig. 2). It is notable that the L. pneumophila double mutant was more susceptible to 2-AP than the corresponding E. coli double mutant, yet it was more resistant than the E. coli quadruple mutant (2). Since in E. coli nucleases are necessary for methyl-directed mismatch repair (MDMR), L. pneumophila may have a less responsive MDMR system than E. coli or recruit other mechanisms to repair base mismatches; alternatively, this pathogen may have unrecognized ssExos. However, the extreme sensitivity of the double mutant to nalidixic acid suggests that RecJ and ExoVII are L. pneumophila's main ssExos.
FIG. 2.
Exonuclease double mutants are sensitive to 2-aminopurine and nalidixic acid. The indicated strains were grown to the postexponential phase, as assessed by motility, cultures were normalized to an OD600 of 1.0, and 10-fold serial dilutions were plated on CYET without a drug or on CYET supplemented with 350 μg/ml of 2-AP or 2 μg/ml of nalidixic acid. A mutS::cat strain (1a) is shown as a specificity control.
Unmarked fliA deletion alleles are not polar on motAB.
Unmarked mutations pose a lower risk of polar effects on downstream genes than insertion mutations. For example, a transposon mutation in the gene encoding the flagellar sigma factor, fliA, completely eliminates flagella and motility, and these defects are only partially complemented (17). Positioned 9 bp 3′ of fliA is the motAB locus, which encodes ion channels critical for flagellar motion, as <5% of motAB mutant L. pneumophila exhibit motility (17).
To test the polarities of fliA mutations generated with Flp, we compared the resulting phenotypes to those of the fliA transposon mutant (17). Motility was scored microscopically, since soft-agar assays are not applicable for the nonchemotactic L. pneumophila (3). Wet mounts were observed through an inverted-phase microscope at least three times as the broth cultures achieved optical densities at 600 nm (OD600s) of 3.70 to 4.70. Plasmid-borne fliA restored full motility only to the fliA unmarked-mutant strain (Table 2). Since the partial motility of both the fliA transposon and the FRT-cat-FRT mutant strains carrying fliA in trans resembled that of the motAB mutant, both of the insertion mutations are likely polar.
TABLE 2.
Motility phenotypes
| Strain | Chromosome | Plasmid | % motilea |
|---|---|---|---|
| Lp02 | Wild-type postexponential phase | None | >95 |
| Lp02 | Wild-type exponential phase | None | 0 |
| MB560 | motAB::gent | None | <5 |
| MB808 | fliA::kan transposon insertion | pMMBGent-empty | 0 |
| MB510 | fliA::kan transposon insertion | pMMBGent-FliA | <5 |
| MB814 | ΔfliA::FRT-cat-FRT | pMMBGent-FliA | <5 |
| MB816 | ΔfliA::FRT | pMMBGent-empty | 0 |
| MB817 | ΔfliA::FRT | pMMBGent-FliA | >95 |
Motility of >104 cells scored as a population in two independent experiments, as observed periodically from OD600s of 3.70 to 4.70, except for the wild-type exponential phase, which was observed at OD600s of 0.90 to 1.00.
Conclusions.
A powerful approach to study fundamental bacterial processes is the construction and analysis of unmarked nonpolar mutations. Certain experimental questions, including whether factors are functionally redundant, require that multiple mutations be constructed in a single strain. To facilitate the genetic manipulation, we developed an efficient tandem approach in which recombinant alleles are first constructed in E. coli using the λ-Red recombinase system and then Flp-mediated excision is induced in L. pneumophila.
Supplementary Material
Acknowledgments
We thank David Friedman for sharing equipment, Rachel L. Edwards for the pGEMletA plasmid, and Zachary D. Abbott for critical reading of the manuscript.
Our research was supported by NIH grants NIGMS T32 GM07863 and T32 AI07528-10, a University of Michigan Rackham Graduate Student Research Grant, a University of Michigan Department of Microbiology Novy Fellowship to A.B. and NIH grants AI044212-09S1 to K.H. and RO1 AI044212 to M.S.S.
Footnotes
Published ahead of print on 4 February 2011.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7-19. [DOI] [PubMed] [Google Scholar]
- 1a.Bryan, A., and M. S. Swanson. 2011. Oligonucleotides stimulate genomic alterations of Legionella pneumophila. Mol. Microbiol. [Epub ahead of print.] doi: 10.1111/j.1365-2958.2011.07573.x. [DOI] [PubMed]
- 2.Burdett, V., C. Baitinger, M. Viswanathan, S. T. Lovett, and P. Modrich. 2001. In vivo requirement for RecJ, ExoVII, ExoI, and ExoX in methyl-directed mismatch repair. Proc. Natl. Acad. Sci. U. S. A. 98:6765-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chase, J. W., and C. C. Richardson. 1977. Escherichia coli mutants deficient in exonuclease VII. J. Bacteriol. 129:934-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D. Q., S. S. Huang, and Y. J. Lu. 2006. Efficient transformation of Legionella pneumophila by high-voltage electroporation. Microbiol. Res. 161:246-251. [DOI] [PubMed] [Google Scholar]
- 6.Chien, M., et al. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966-1968. [DOI] [PubMed] [Google Scholar]
- 7.Cianciotto, N. P. 2009. Many substrates and functions of type II secretion: lessons learned from Legionella pneumophila. Future Microbiol. 4:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Court, D. L., J. A. Sawitzke, and L. C. Thomason. 2002. Genetic engineering using homologous recombination. Annu. Rev. Genet. 36:361-388. [DOI] [PubMed] [Google Scholar]
- 9.Cox, M. M. 1983. The FLP protein of the yeast 2-microns plasmid: expression of a eukaryotic genetic recombination system in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 80:4223-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutra, B. E., V. A. Sutera, Jr., and S. T. Lovett. 2007. RecA-independent recombination is efficient but limited by exonucleases. Proc. Natl. Acad. Sci. U. S. A. 104:216-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falco, S. C., Y. Li, J. R. Broach, and D. Botstein. 1982. Genetic properties of chromosomally integrated 2 mu plasmid DNA in yeast. Cell 29:573-584. [DOI] [PubMed] [Google Scholar]
- 13.Golic, K. G., and S. Lindquist. 1989. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59:499-509. [DOI] [PubMed] [Google Scholar]
- 14.Hubber, A., and C. R. Roy. 2010. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu. Rev. Cell Dev. Biol. 26:261-283. [DOI] [PubMed] [Google Scholar]
- 15.Isberg, R. R., T. J. O'Connor, and M. Heidtman. 2009. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7:13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marra, A., S. J. Blander, M. A. Horwitz, and H. A. Shuman. 1992. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc. Natl. Acad. Sci. U. S. A. 89:9607-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molofsky, A. B., L. M. Shetron-Rama, and M. S. Swanson. 2005. Components of the Legionella pneumophila flagellar regulon contribute to multiple virulence traits, including lysosome avoidance and macrophage death. Infect. Immun. 73:5720-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molofsky, A. B., and M. S. Swanson. 2004. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53:29-40. [DOI] [PubMed] [Google Scholar]
- 19.Morschhäuser, J., S. Michel, and P. Staib. 1999. Sequential gene disruption in Candida albicans by FLP-mediated site-specific recombination. Mol. Microbiol. 32:547-556. [DOI] [PubMed] [Google Scholar]
- 20.Newton, H. J., D. K. Ang, I. R. van Driel, and E. L. Hartland. 2010. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin. Microbiol. Rev. 23:274-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Gorman, S., D. T. Fox, and G. M. Wahl. 1991. Recombinase-mediated gene activation and site-specific integration in mammalian cells. Science 251:1351-1355. [DOI] [PubMed] [Google Scholar]
- 22.Pasculle, A. W., et al. 1980. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J. Infect. Dis. 141:727-732. [DOI] [PubMed] [Google Scholar]
- 23.Schweizer, H. P. 2003. Applications of the Saccharomyces cerevisiae Flp-FRT system in bacterial genetics. J. Mol. Microbiol. Biotechnol. 5:67-77. [DOI] [PubMed] [Google Scholar]
- 24.Senecoff, J. F., R. C. Bruckner, and M. M. Cox. 1985. The FLP recombinase of the yeast 2-micron plasmid: characterization of its recombination site. Proc. Natl. Acad. Sci. U. S. A. 82:7270-7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sexton, J. A., and J. P. Vogel. 2004. Regulation of hypercompetence in Legionella pneumophila. J. Bacteriol. 186:3814-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone, B. J., and Y. A. Kwaik. 1999. Natural competence for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J. Bacteriol. 181:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomason, L., et al. 2007. Recombineering: genetic engineering in bacteria using homologous recombination. Curr. Protoc. Mol. Biol. 1:1.16. [DOI] [PubMed] [Google Scholar]
- 28.Thomason, L. C., N. Costantino, D. V. Shaw, and D. L. Court. 2007. Multicopy plasmid modification with phage lambda Red recombineering. Plasmid 58:148-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viswanathan, M., V. Burdett, C. Baitinger, P. Modrich, and S. T. Lovett. 2001. Redundant exonuclease involvement in Escherichia coli methyl-directed mismatch repair. J. Biol. Chem. 276:31053-31058. [DOI] [PubMed] [Google Scholar]
- 30.Viswanathan, M., and S. T. Lovett. 1998. Single-strand DNA-specific exonucleases in Escherichia coli. Roles in repair and mutation avoidance. Genetics 149:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu, D., et al. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.