Abstract
Shiga toxin-producing Escherichia coli (STEC) is a zoonotic pathogen that causes diarrheal disease in humans and is of public health concern because of its ability to cause outbreaks and severe disease such as hemorrhagic colitis or hemolytic-uremic syndrome. More than 400 serotypes of STEC have been implicated in outbreaks and sporadic human disease. The aim of this study was to develop a PCR binary typing (P-BIT) system that could be used to aid in risk assessment and epidemiological studies of STEC by using gene targets that would represent a broad range of STEC virulence genes. We investigated the distribution of 41 gene targets in 75 O157 and non-O157 STEC isolates and found that P-BIT provided 100% typeability for isolates, gave a diversity index of 97.33% (compared with 99.28% for XbaI pulsed-field gel electrophoresis [PFGE] typing), and produced 100% discrimination for non-O157 STEC isolates. We identified 24 gene targets that conferred the same level of discrimination and produced the same cluster dendrogram as the 41 gene targets initially examined. P-BIT clustering identified O157 from non-O157 isolates and identified seropathotypes associated with outbreaks and severe disease. Numerical analysis of the P-BIT data identified several genes associated with human or nonhuman sources as well as high-risk seropathotypes. We conclude that P-BIT is a useful approach for subtyping, offering the advantage of speed, low cost, and potential for strain risk assessment that can be used in tandem with current molecular typing schema for STEC.
Shiga-toxin producing Escherichia coli (STEC) is a zoonotic pathogen that causes diarrheal disease in humans and is of public health concern because of its ability to cause outbreaks and severe disease such as hemorrhagic colitis (HC) or hemolytic-uremic syndrome (HUS) (42). E. coli O157:H7 is the most prevalent STEC serotype associated with STEC outbreaks, HC, and HUS (20, 30, 42); however, more than 400 serotypes of non-O157 STEC have also been implicated in outbreaks and sporadic human disease, with about 50 of these serotypes being associated with bloody diarrhea or HUS in humans (42, 56, 69). Internationally, the number of reported human diarrheal cases associated with non-O157 STEC (including those leading to HUS) is rising rapidly, mainly due to increased surveillance for these pathogens (13, 18, 27, 35, 61). There is currently no standard method for identifying non-O157 STEC of significance to human health from the over 500 STEC serotypes characterized to date; therefore, the World Health Organization has highlighted the development of such a method as a public health priority (68).
To this end, seropathotype (SPT) classification was developed to identify STEC serotypes linked to outbreaks and/or serious disease based on their relative incidences in human illness (31). Serotypes classified as SPT A (O157:H7 and O157:NM [nonmotile]) or SPT B (O26:H11/NM, O103:H2, O111:H8/NM, O121:H19, and O145:NM) have been associated with outbreaks and HUS; however, SPT A is more frequently reported. SPT C comprises serotypes (e.g., O5:NM, O91:H21, O113:H21, O121:NM, and O128:H2) that have been associated with sporadic cases of HUS but not with outbreaks. SPT D includes the remainder of the STEC serotypes that have been reported to cause sporadic disease and have been associated with diarrhea but not HUS, and serotypes classified as SPT E have not been associated with human illness.
Several studies have identified genetic elements associated with particular seropathotypes that appear to play a role in influencing bacterial virulence (7, 10, 19, 31). Bacterial virulence traits of STEC strains are governed by the dynamic exchange and loss of mobile genetic elements such as plasmids, transposons, insertion sequences, integrons, bacteriophages, and genomic islands (32). These genetic loci characterize the pathogenicity of bacterial species by determining what toxins are produced and how the bacterium attaches to and invades host cells, modulates the host cell cycle and immune responses, survives in stressful environments, and produces biofilms. Many virulence loci have been characterized for STEC, including a number of pathogenicity islands (e.g., the locus of enterocyte effacement [LEE], the locus of proteolysis activity [LPA], the high-pathogenicity island [HPI], the E. coli type III secretion apparatus [ETT2], the urease gene cluster, the long polar fimbrial operon, O-island 36 [OI-36], OI-57, OI-71, OI-122, OI-141, and OI-154), two key virulence plasmids (pO157 and pO113), and chromosomal lambdoid bacteriophage insertions that carry genes for two types of Shiga toxin (10, 14, 29, 31, 36, 38, 39, 42, 48, 59, 63). Variants of the genes encoding the Shiga toxins have been reported, and certain variants, including stx2 and stx2c, are more likely to be associated with HC and HUS (3, 15).
A growing body of studies have analyzed the association of many of these virulence genes with severity of disease, as well as the variability of these genes among STEC serotypes (5, 10, 15, 52). PCR-based methods can be used to detect a suite of virulence genes for an E. coli isolate. The presence or absence of these genes (binary typing) produces a genetic fingerprint for each isolate (11). The fingerprints can be used to identify strains that have a greater potential to cause harm using a process called molecular risk assessment (10).
We recently developed a PCR binary typing system (P-BIT) for subtyping Campylobacter jejuni based on 18 gene targets associated with epidemicity (11). The aim of the present study was to develop a similar P-BIT system that could be used to aid in risk assessment and epidemiological studies of STEC by using gene targets that would represent a broad range of STEC virulence genes. We examined the distribution of 41 virulence genes among STEC isolates to produce “virulence bar codes” for each isolate, which were then compared to typing by pulsed-field gel electrophoresis (PFGE), bacteriophage insertion site genotyping (BISG), seropathotype (SPT), and colonization potential as determined in a bovine in vitro organ culture (bIVOC) model infection system (S. M. Brandt and S. Paulin, unpublished data).
MATERIALS AND METHODS
Bacterial isolates.
Seventy-five E. coli isolates (46 O157 and 29 non-O157) from a variety of human and nonhuman sources were used in this study and are summarized in Table 1.
TABLE 1.
STEC isolates used in this study
| Isolate | Serotype | Country | Source | 41-gene P-BIT code | SPT | Reference(s) |
|---|---|---|---|---|---|---|
| ERL05-1904 | O5:NM | New Zealand | Lamb, minced | 611-00000100-1040 | C | 19 |
| ERL04-2204 | O6(rel):NM | New Zealand | Lamb, diced | 651-00000140-1441 | D | 19 |
| ERL05-0344 | O8:H25 | New Zealand | Lamb, diced | 611-00000040-4000 | E | 22 |
| ERL97-0595 | O8:H25 | New Zealand | Meat | 670-00000000-4040 | E | 22 |
| NZRM4160 | O26:H11 | New Zealand | Human | 673-17645330-6362 | B | 31 |
| NZRM4155 | O26:NM | New Zealand | Human | 663-17645330-2362 | B | 19 |
| ERL05-1307 | O38:H26 | New Zealand | Beef, minced | 673-00100100-1040 | E | 22 |
| ERL05-0411 | O68:H4 | New Zealand | Lamb, minced | 711-04122100-1340 | D | 15 |
| ERL05-1850 | O75:H8 | New Zealand | Beef, minced | 433-04100140-5341 | E | 22 |
| ERL02-2853 | O84:H2 | New Zealand | Human | 673-55645160-7042 | C | 15 |
| ERL03-0954 | O84:H2 | New Zealand | Bovine | 653-44644120-7062 | C | 15 |
| NZRM4163 | O84:NM | New Zealand | Human | 673-55244160-7240 | C | 22 |
| NZRM4153 | O91:H21 | New Zealand | Human | 673-00010160-6010 | C | 31 |
| ERL97-3923 | O91:NM | New Zealand | Ovine meat | 453-00010050-7043 | C | 19 |
| ERL04-0388 | O107:H51 | New Zealand | Human | 023-00010000-2342 | D | This study |
| ERL05-1848 | O113:H21 | New Zealand | Beef, minced | 673-65746770-5453 | C | 31 |
| NZRM3616 | O113:H21 | New Zealand | Human | 673-00004160-4410 | C | 31 |
| ERL04-2941 | O117:H7 | New Zealand | Human | 043-00000000-2362 | D | 33, 71 |
| ERL05-1308 | O123:H10 | New Zealand | Lamb, minced | 613-00000140-5341 | E | 2 |
| ERL05-0622 | O128:H2 | New Zealand | Lamb, minced | 611-00000120-7042 | C | 19 |
| ERL06-2014 | O128:H2 | New Zealand | Human | 673-00010040-7003 | C | 19 |
| NZRM4157 | O128:NM | New Zealand | Human | 673-00010140-7543 | D | 15 |
| NZRM4162 | O130:H11 | New Zealand | Human | 672-00000140-6000 | C | 13 |
| ERL99-1671 | O145:NM | New Zealand | Human | 010-55605340-5000 | B | 33 |
| NZRM4166 | O153:H25 | New Zealand | Human | 601-04004040-4043 | C | 22 |
| 96/2998 | O157:[H7] | Australia | Human | 762-77737751-0040 | A | 31 |
| ACC 3634 | O157:H7 | Australia | Human | 772-77737751-0040 | A | 31 |
| CDC 16-98 | O157 | USA | Human | 773-77777770-0000 | A | 31 |
| CDC 20-98 | O157 | USA | Human | 773-77777770-0040 | A | 31 |
| E27 | O157:H7 | New Zealand | Animal | 771-77777770-0000 | A | 31 |
| E47 | O157:H7 | New Zealand | Animal | 773-77777770-0000 | A | 31 |
| E56 | O157:H7 | New Zealand | Human | 773-77777770-0040 | A | 31 |
| ERL01-4131 | O157:H7 | New Zealand | Milk | 713-77777770-0000 | A | 31 |
| ERL02-0927 | O157:H7 | New Zealand | Human | 773-77777370-0000 | A | 31 |
| ERL02-1097 | O157:H7 | New Zealand | Food | 773-77776770-0000 | A | 31 |
| ERL02-2190 | O157:H7 | New Zealand | Human | 773-77777770-0000 | A | 31 |
| ERL02-2447 | O157:H7 | New Zealand | Human | 773-77777770-0000 | A | 31 |
| ERL02-2841 | O157:H7 | New Zealand | Human | 773-77777770-0000 | A | 31 |
| ERL02-3939 | O157:H7 | New Zealand | Human | 773-77777770-0000 | A | 31 |
| ERL02-4897 | O157:H7 | New Zealand | Human | 763-77777770-0000 | A | 31 |
| ERL03-1416 | O157:H7 | New Zealand | Water | 711-76556770-0000 | A | 31 |
| ERL03-4262 | O157:H7 | New Zealand | Venison, minced | 773-77777751-0000 | A | 31 |
| ERL04-3476 | O157:H7 | New Zealand | New Zealand pork | 773-77777751-0002 | A | 31 |
| ERL05-0623 | O157:H7 | New Zealand | Bobby veal | 771-73777770-0041 | A | 31 |
| ERL05-1306 | O157:[H7] | New Zealand | Lamb, diced | 771-77777771-0040 | A | 31 |
| ERL05-1784 | O157:H7 | New Zealand | Australia pork | 733-77777751-0040 | A | 31 |
| ERL06-2084 | O157:[H7] | New Zealand | Water | 753-77777750-0000 | A | 31 |
| ERL06-2442 | O157:H7 | New Zealand | Bovine | 713-77777770-0020 | A | 31 |
| ERL06-2448 | O157:[H7] | New Zealand | Bovine | 713-77777770-0040 | A | 31 |
| ERL06-2456 | O157:H7 | New Zealand | Bovine | 733-77777770-0040 | A | 31 |
| ERL06-2495 | O157:H7 | New Zealand | Bovine | 713-77777770-0000 | A | 31 |
| ERL06-2497 | O157:H7 | New Zealand | Bovine | 773-77577760-0040 | A | 31 |
| ERL06-2503 | O157:[H7] | New Zealand | Bovine | 773-77777770-0000 | A | 31 |
| ERL06-2505 | O157:H7 | New Zealand | Bovine | 733-77777770-0000 | A | 31 |
| ERL06-2517 | O157:H7 | New Zealand | Bovine | 733-77777770-0000 | A | 31 |
| ERL06-2532 | O157:[H7] | New Zealand | Bovine | 733-77777770-0000 | A | 31 |
| ERL08-1225 | O157:H7 | New Zealand | Spring water | 753-77777771-0000 | A | 31 |
| ERL99-3094 | O157:H7 | New Zealand | Water | 713-77777750-0000 | A | 31 |
| ERL99-3231 | O157:H7 | New Zealand | Spring water | 773-77777770-0000 | A | 31 |
| ERL99-4475 | O157:H7 | New Zealand | Bovine | 773-77777770-0000 | A | 31 |
| NZRM3614 | O157:H7 | Australia | Human | 773-73277730-0000 | A | 31 |
| NZRM3647 | O157:H7 | Australia | Human | 773-77777730-0000 | A | 31 |
| NZRM4156 | O157:H7 | New Zealand | Spring water | 773-77777730-0000 | A | 31 |
| NZRM4159 | O157:H7 | New Zealand | Bovine | 773-77777770-0000 | A | 31 |
| NZRM4164 | O157:H7 | New Zealand | Human | 773-77777770-0040 | A | 31 |
| NZRM4168 | O157:H7 | New Zealand | Human | 773-77777770-0000 | A | 31 |
| NZRM4169 | O157:H7 | New Zealand | Human | 773-77377771-0000 | A | 31 |
| ERL02-3185 | O157:NM | New Zealand | Human | 773-77777770-0000 | A | 31 |
| NZRM4150 | O157:NM | New Zealand | Human | 773-77777771-0000 | A | 31 |
| NZRM4165 | O157:NM | New Zealand | Human | 771-77777370-6641 | A | 31 |
| ERL04-0301 | O157:H16 | New Zealand | Human | 651-10100040-0000 | E | 7 |
| ERL05-1845 | O176:H4 | New Zealand | Pork, minced | 713-00022000-1340 | E | This study |
| ERL05-0346 | O176:NM | New Zealand | Human | 771-00022100-1340 | D | This study |
| ERL04-2759 | O177:NM | New Zealand | Human | 473-55245360-5042 | C | 22 |
| ERL98-3865 | Ont:H18a | New Zealand | Human | 611-00002041-4302 | D | 15 |
Ont, not O-serotypeable.
DNA extraction.
Isolates were grown on Luria broth (LB) (Invitrogen, Carlsbad, CA) agar for 18 to 24 h at 37°C. Three colonies were transferred into 10 ml LB and grown for 18 to 24 h at 37°C in a shaking water bath. DNA was extracted from 1 ml of broth culture using the DNeasy blood and tissue kit as per the manufacturer's instructions for Gram-negative bacteria (Qiagen, Hilden, Germany). DNA quantification was performed using a Nanodrop 1000 (Fisher Thermo Scientific, Waltham, MA). DNA was diluted to 50 ng μl−1 in sterile 1× Tris-EDTA (TE) buffer (pH 7.0) (Invitrogen) and stored at −20°C.
Binary typing of isolates.
Forty-one gene targets for the P-BIT system were chosen based on published information regarding their role in STEC pathogenesis (Table 2). These included genes with an established role in facilitating infection and genes shown to be associated with human illness or severity of clinical infections. In many cases where a cluster of genes (e.g., a pathogenicity island or plasmid) had been implicated in disease, a single representative gene target was chosen. In cases where the variability of genes within a gene cluster had been associated with degrees of bacterial virulence, multiple gene targets from a particular loci were chosen. Detection of gene targets was performed using previously published PCR methods (Table 2).
TABLE 2.
Primers and PCR conditions for gene targets of the STEC binary typing system
| Genesa |
PCR conditions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene name | Locus | Virulence function | Associated with diarrhea | Associated with HUS and/or outbreaks | Variable among STEC isolates | Reference(s) | Primers (forward/reverse) | Annealing temp (°C) | Product size (bp) | Reference(s) |
| agn43 | Chromosome | Biofilms | × | 53 | CTGGAAACCGGTCTGCCCTT/CCTGAACGCCCAGGGTGATA | 55 | 433 | 53 | ||
| agn43EDL933 | Chromosome | Biofilms | × | 53 | CGTATCGCTGTGCCCGATAAC/CCGTATACGAGTTGTCAGAATCA | 55 | 707 | 46, 53 | ||
| chuA | Chromosome | Iron uptake | × | 44 | GACGAACCAACGGTCAGGAT/TGCCGCCAGTACCAAAGACA | 55 | 279 | 9 | ||
| cif | Chromosome | Cell cycle control | 37 | AACAGATGGCAACAGACTGG/AGTCAATGCTTTATGCGTCAT | 50 | 383 | 4 | |||
| espC | Chromosome | Hemolysis | × | × | 41 | TAGTGCAGTGCAGAAAGCAGTT/AGTTTTCCTGTTGCTGTATGCC | 55 | 301 | 53 | |
| iutA | Chromosome | Iron uptake | × | 34 | GGCTGGACATCATGGGAACTGG/CGTCGGGAACGGGTAGAATCG | 60 | 301 | 43 | ||
| paa | Chromosome | Colonization | × | 1, 4 | ATGAGGAACATAATGGCAGG/TCTGGTCAGGTCGTCAATAC | 50 | 350 | 4 | ||
| pic | Chromosome | Hemolysis | × | 50 | ACTGGATCTTAAGGCTCAGGAT/GACTTAATGTCACTGTTCAGCG | 48 | 572 | 53 | ||
| stx1 | Chromosome | Toxin | × | 15 | ATAAATCGCCATTCGTTGACTAC/AGAACGCCCACTGAGATCATC | 65→60 | 180 | 47 | ||
| stx2 | Chromosome | Toxin | × | × | 15 | GGCACTGTCTGAAACTGCTCC/TCGCCAGTTATCTGACATTCTG | 65→60 | 255 | 47 | |
| stx2c | Chromosome | Toxin | × | × | 15 | GCGGTTTTATTTGCATTAGT/AGTACTCTTTTCCGGCCACT | 50 | 124 | 65 | |
| stx2d | Chromosome | Toxin | × | 15 | GGTAAAATTGAGTTCTCTAAGTAT/CAGCAAATCCTGAACCTGACG | 52 | 175 | 65 | ||
| ECs3737 | ETT2 | TTSA | × | 52 | GGAAATCTGCATTAACTCTTCTGC/CGGGAATACCATCCAGTCG | 50 | 530 | 51 | ||
| eivF | ETT2 | TTSA | × | 52 | ATTACTGCTCATTGACCGAAGC/GATTTCCAACACGGCTCTGG | 60 | 457 | 51 | ||
| etrA | ETT2 | TTSA | × | 52 | CTTCTTCCTAACGAAACATCATTA/TGACATATCAACTTTCTCTTACGC | 52 | 914 | 51 | ||
| yqeH | ETT2 | TTSA | × | 52 | CATGCAATAGTTGCTCAATGC/CCCATTCTCTTTTCGATTCG | 55 | 553 | 51 | ||
| fyuA | HPI | Iron uptake | × | 29 | TGATTAACCCCGCGACGGGAA/CGCAGTAGGCACGATGTTGTA | 60 | 784 | 26 | ||
| irp2 | HPI | Iron uptake | × | 29 | AAGGATTCGCTGTTACCGGAC/TCGTCGGGCAGCGTTTCTTCT | 60 | 280 | 29 | ||
| eaeA | LEE | Attachment | × | 25 | GACCCGGCACAAGCATAAGC/CCACCTGCAGCAACAAGAGG | 65→60 | 384 | 47 | ||
| espA-γ1 | LEE | Unknown | × | 17 | GCGAGTTCTTCGACATC/ACGAATACCAGTTACACTT | 55 | 333 | 17 | ||
| espIb | LPA | Mucosal | × | 59 | ATGGACAGAGTGGAGACAG/GCCACCTTTATTCTCACCA | 50 | 560 | 59 | ||
| iha | LPA | Attachment | × | 59 | CAGTTCAGTTTCGCATTCACC/GTATGGCTCTGATGCGATG | 50 | 1,305 | 59 | ||
| lpfAO26 | LPF operon | Attachment | × | 62 | GTTCTGTTTGCCTTATCTGC/TAAGTCAGGTTGAAGTCGAC | 55 | 509 | 62 | ||
| lpfAO113 | LPF operon | Attachment | × | 62 | ATGAAGCGTAATATTATAG/TTATTTCTTATATTCGAC | 50 | 573 | 12 | ||
| nleC | OI-36 | Unknown | × | × | 10 | ACAGTCCAACTTCAACTTTTCC/ATCGTACCCAGCCTTTCG | 55 | 777 | 10 | |
| nleG2-3 | OI-57 | Unknown | × | × | 10 | GGATGGAACCATACCTGG/CGCAATCAATTGCTAATGC | 55 | 551 | 10 | |
| nleG | OI-71 | Unknown | × | × | 10 | ATGTTATCGCCCTCTTCTATAAAT/ACTTAATACTACACTAATAAGATCCA | 55 | 902 | 10 | |
| efa1a | OI-112 | Colonization | × | × | × | 7, 31, 70 | TATCAGGCCAATCAAAACAG/AGACACTGGTAAATTTCGC | 48 | 974 | 24 |
| nleB | OI-112 | Colonization | × | × | × | 7, 31, 70 | GGAAGTTTGTTTACAGAGACG/AAAATGCCGCTTGATACC | 55 | 297 | 10 |
| pagC | OI-112 | Immune evasion | × | × | × | 7, 31, 70 | ATGAGTGGTTCAAGACTGG/CCAACTCCAACAGTAAATCC | 55 | 521 | 31 |
| sen | OI-112 | Enterotoxin | × | × | × | 7, 31, 70 | GGATGGAACCATACCTGG/CGCAATCAATTGCTAATGC | 55 | 551 | 31 |
| lpfAO157/OI-141 | OI-141 | Attachment | × | 62 | CTGCGCATTGCCGTAAC/ATTTACAGGCGAGATCGTG | 55 | 412 | 62 | ||
| lpfAO157/OI-154 | OI-154 | Attachment | × | 62 | GCAGGTCACCTACAGGCGGC/CTGCGAGTCGGCGTTAGCTG | 55 | 525 | 62 | ||
| saa | pO113 | Hemolysis | × | 8, 49 | CGTGATGAACAGGCTATTGC/ATGGACATGCCTGTGGCAAC | 60 | 119 | 8 | ||
| subA | pO113 | Toxin | × | 8, 49 | TATGGCTTCCCTCATTGC/TATAGCTGTTGCTTCTGACG | 50 | 556 | 49 | ||
| ehxA | pO157 | Hemolysis | × | × | 5, 6, 41, 57, 58 | GCATCATCAAGCGTACGTTCC/AATGAGCCAAGCTGGTTAAGCT | 65→60 | 534 | 47 | |
| espP | pO157 | Mucosal damage | × | × | 5, 6, 41, 57, 58 | AAACAGCAGGCACTTGAACG/GGAGTCGTCAGTCAGTAGAT | 50 | 1,830 | 5 | |
| etpD | pO157 | Type II secretion | × | 5, 6, 57, 58 | CGTCAGGAGGATGTTCAG/CGACTGCACCTGTTCCTGATTA | 48 | 1,062 | 58 | ||
| katP | pO157 | Catalase peroxidase | × | 5, 6, 57, 58 | TGCATCCGTTGATGATGTTT/TTTCAGGAACGGTGAGATCC | 55 | 720 | 5 | ||
| toxB | pO157 | Toxin | × | 5, 6, 57, 58 | ATACCTACCTGCTCTGGATTGA/TTCTTACCTGATCTGATGCAGC | 55 | 599 | 64 | ||
| ureC | Urease cluster | Ureolysis | × | 16, 45 | TCTAACGCCACAACCTGTAC/GAGGAAGGCAGAATATTGGG | 50 | 397 | 41 | ||
ETT2, E. coli type III secretion apparatus; HPI, high-pathogenicity island; LEE, locus of enterocyte effacement; LPA, locus of proteolysis activity; LPF, long polar fimbriae; OI, O island; TTSA, type III secretion apparatus.
Also known as nleA.
One PCR per gene target was performed in a 25-μl volume containing 2.5 mM MgCl2, 1× PCR buffer II (50 mM KCl, 10 mM Tris, pH 8.3) (Applied Biosystems, Foster City, CA), 250 μM each deoxynucleoside triphosphate (dNTP), 12.5 pmol each primer, 1.25 U AmpliTaq DNA polymerase (Applied Biosystems), and 50 ng extracted DNA. Thermocycling was performed in either an ABI 9700 or ABI Veriti PCR machine (Applied Biosystems) using the following conditions: 5 min at 95°C followed by 30 cycles of denaturation at 95°C for 30 s, annealing at various temperatures specific to the gene target (listed in Table 2) for 30 s, extension at 72°C for 30 s per 500 bp of amplicon size (listed in Table 2), and then a final extension at 72°C for 10 min.
PCR products were separated by electrophoresis on 2% SeaKem LE agarose gels (Lonza, Rockland, ME) with 1× Tris-borate-EDTA (TBE) (USB Corporation, Cleveland, OH) running buffer for 70 min at 110 V, and amplicons were visualized using 0.5 μg ml−1 ethidium bromide. The presence or absence of amplicons of the expected size (Table 2) was loaded into a BioNumerics version 5.10 (Applied Maths, Ghent, Belgium) database. Interstrain relationships were assessed by numerical analysis of the P-BIT data using the simple matching coefficient and Ward's clustering.
P-BIT bar codes were generated as previously published (11). Briefly, genes were divided into groups of three in the order in which they are shown in Table 3. The PCR result for each gene was recorded as 1 if an amplicon of correct size was present and as 0 for a negative result. The result for the first gene in the group was multiplied by 1, the second by 2, and the third by 4. The results were then added together to yield a number from 0 to 7 representing the results from the three genes. The results were sequentially combined by concatenation to form a bar code.
TABLE 3.
Association of P-BIT gene targets with O157, non-O157, human, and nonhuman STEC isolates
| Isolates in which gene is frequently founda | Locus | Gene | Ο157/non-Ο157 isolate comparison |
Human/nonhuman isolate comparison |
||||
|---|---|---|---|---|---|---|---|---|
| Prevalence |
χ2P value | Prevalence |
χ2P value | |||||
| No. (%) O157 (n = 46) | No. (%) non-O157 (n = 29) | No. (%) human (n = 36) | No. (%) nonhuman (n = 39) | |||||
| O157 and non-O157 | ETT2 | eivF | 45 (98) | 3 (10) | <0.001 | 20 (56) | 28 (72) | NSb |
| etrA | 46 (100) | 23 (79) | <0.01 | 32 (89) | 37 (95) | NS | ||
| yqeH | 46 (100) | 26 (90) | NS | 33 (92) | 39 (100) | NS | ||
| ECs3737 | 44 (96) | 25 (86) | NS | 30 (83) | 39 (100) | <0.05 | ||
| Chromosome | pic | 37 (80) | 16 (55) | <0.05 | 31 (86) | 22 (56) | <0.025 | |
| Chromosome | espC | 35 (76) | 18 (62) | NS | 32 (89) | 21 (54) | <0.005 | |
| Chromosome | agn43 | 44 (96) | 26 (90) | NS | 32 (89) | 38 (97) | NS | |
| LPA | iha | 40 (87) | 19 (61) | NS | 30 (83) | 29 (74) | NS | |
| O157 | LEE | eaeA | 46 (100) | 6 (21) | <0.001 | 26 (72) | 26 (67) | NS |
| espA-g1 | 45 (98) | 1 (3) | <0.001 | 19 (53) | 27 (69) | NS | ||
| OI-122 | pagC | 45 (98) | 6 (21) | <0.001 | 23 (64) | 28 (72) | NS | |
| sen | 44 (96) | 7 (21) | <0.001 | 25 (69) | 26 (67) | NS | ||
| efa1a | 45 (98) | 2 (7) | <0.001 | 21 (58) | 26 (67) | NS | ||
| nleB | 43 (93) | 11 (38) | <0.001 | 25 (69) | 29 (74) | NS | ||
| OI-36 | nleC | 45 (98) | 4 (17) | <0.001 | 19 (53) | 30 (77) | NS | |
| OI-57 | nleG2-3 | 43 (93) | 8 (25) | <0.001 | 25 (69) | 26 (67) | NS | |
| OI-71 | nleG | 43 (93) | 6 (31) | <0.001 | 21 (58) | 28 (72) | NS | |
| OI-154 | lpfAO157/OI-154 | 45 (98) | 5 (17) | <0.001 | 23 (64) | 27 (69) | NS | |
| OI-141 | lpfAO157/OI-141 | 44 (96) | 3 (10) | <0.001 | 20 (56) | 27 (69) | NS | |
| Chromosome | agn43EDL933 | 43 (93) | 7 (24) | <0.001 | 22 (61) | 28 (72) | NS | |
| Chromosome | paa | 43 (93) | 5 (17) | <0.001 | 24 (67) | 24 (62) | NS | |
| Chromosome | chuA | 45 (98) | 5 (17) | <0.001 | 21 (58) | 29 (74) | NS | |
| Chromosome | ureC | 45 (98) | 10 (34) | <0.001 | 27 (75) | 28 (72) | NS | |
| pO157 | ehxA | 45 (98) | 20 (69) | <0.005 | 30 (83) | 35 (90) | NS | |
| toxB | 45 (98) | 5 (17) | <0.001 | 23 (64) | 27 (69) | NS | ||
| etpD | 43 (93) | 1 (3) | <0.001 | 17 (47) | 27 (69) | NS | ||
| katP | 44 (96) | 4 (14) | <0.001 | 21 (58) | 27 (69) | NS | ||
| espP | 38 (83) | 10 (34) | <0.001 | 24 (67) | 24 (62) | NS | ||
| Chromosomal | stx2 | 43 (93) | 17 (59) | <0.001 | 29 (81) | 31 (79) | NS | |
| stx2c | 9 (20) | 1 (3) | NS | 5 (14) | 5 (13) | NS | ||
| Non-O157 | LPA | espI | 0 (0) | 18 (62) | <0.001 | 7 (19) | 11 (28) | NS |
| LPF | lpfAO26 | 1 (2) | 13 (45) | <0.001 | 11 (31) | 3 (8) | <0.025 | |
| LPF | lpfAO113 | 1 (2) | 20 (69) | <0.001 | 13 (36) | 8 (21) | NS | |
| HPI | irp2 | 0 (0) | 11 (38) | <0.001 | 7 (19) | 4 (10) | NS | |
| fyuA | 1 (2) | 11 (38) | <0.001 | 8 (22) | 4 (10) | NS | ||
| pO113 | subA | 1 (2) | 4 (14) | NS | 3 (83) | 2 (5) | NS | |
| saa | 0 (0) | 3 (10) | NS | 2 (6) | 1 (3) | NS | ||
| Chromosome | cif | 1 (2) | 4 (14) | NS | 3 (8) | 2 (5) | NS | |
| Chromosome | stx1 | 12 (26) | 22 (76) | <0.001 | 16 (44) | 18 (46) | NS | |
| Chromosome | stx2d | 2 (4) | 8 (28) | <0.025 | 4 (11) | 6 (15) | NS | |
| Chromosome | iutA | 1 (2) | 14 (48) | <0.001 | 10 (28) | 5 (13) | NS | |
Based on previously published studies outlined in Table 2.
NS, not significant.
Statistical analysis of P-BIT data.
Based on the frequency of detection of genes by PCR in isolates, an association of each P-BIT target gene with O157 or non-O157 STEC isolates, human or nonhuman sources, or dendrogram clusters was determined using the chi-square (χ2) test. A P value of <0.05 was considered significant. Our results were then compared to expected associations of genes with O157, non-O157, or both O157 and non-O157 STEC isolates based on previously published studies (Table 2). The total numbers of 24-gene P-BIT targets present in isolates from each SPT group, with HUS- or non-HUS-associated serotypes, and with outbreak- or non-outbreak-associated serotypes were compared using a Mann-Whitney U test; this statistical test was chosen because of the variable number of isolates contained in the groups being tested and because the distribution of the data was not always normal.
PFGE typing of isolates.
All isolates were analyzed by PFGE using the standardized PulseNet protocol with Salmonella enterica serovar Braenderup H9812 digested with XbaI as a sizing standard (21, 54). DNA samples were digested with XbaI and separated by electrophoresis at 6.0 V cm−1 for 20 h on a 1% (wt/vol) SeaKem Gold agarose gel (Lonza, Rockland, ME) using initial and final switch times of 2.2 and 54.2 s, respectively. PFGE profiles were analyzed and compared using BioNumerics version 5.10 and submitted to the PulseNet Aotearoa (New Zealand) E. coli database, where XbaI pattern designations were assigned.
Shiga toxin-encoding bacteriophage insertion site genotyping.
A subset of 25 isolates were analyzed by a genotyping system based on the diversity of insertion sites of the stx-carrying bacteriophages (60). A multiplex PCR was performed as previously published using DNA extracted by the method described above (67).
RESULTS
Binary typing.
Forty-one gene targets were chosen from published methods to create a PCR-based typing system that would distinguish between O157 and non-O157 STEC isolates and provide information based on virulence gene content (Table 2). Gene targets were chosen based on published information regarding a role in STEC pathogenesis or an association with human disease or severity of disease sequelae. The system included gene targets from a range of known pathogenicity islands, virulence plasmids, and gene clusters so as to provide a broad overview of the virulence gene content of an isolate of interest. Additional gene targets shown to be variable among STEC isolates were also included to provide the system with discriminatory power.
The STEC P-BIT system produced 58 types from 75 STEC isolates, including 29 O157 types from 46 isolates and 29 non-O157 types from 29 isolates. The system had an overall diversity index of 97.33% ± 2.4%, with 100% discriminatory power for non-O157 isolates. The diversity index for O157 isolates was slightly lower, at 92.85% ± 5.87%.
Numerical analysis of P-BIT data.
Statistical analysis of the P-BIT results using the χ2 test was performed to identify genes associated with O157 STEC, with non-O157 STEC, or with both O157 and non-O157 STEC (Table 3). All of the genes that would be expected to be associated with O157 STEC, based on previous studies (Table 2), were significantly associated with O157 STEC isolates (P < 0.05), except for stx2c due to the low prevalence of this gene among the O157 isolates that were used in this study. However, stx2c was present mainly in O157 isolates (n = 9) in comparison to non-O157 STEC isolates (n = 1). Eight of 11 genes that would be expected to be associated with non-O157 STEC, based on previous studies (Table 2), were associated with non-O157 STEC isolates (P < 0.05), except for the genes subA, saa, and cif due to the low prevalence of these genes in the non-O157 isolates that were chosen for this study; this was not unexpected for the genes subA and saa, as they are located on a virulence plasmid associated with certain non-O157 serotypes (8, 49). Seven of the eight genes that would be expected to be found frequently in both O157 and non-O157 STEC were prevalent (>50%) in both O157 and non-O157 STEC isolates; however, three of these genes (eivF, etrA, and pic) were significantly associated with the O157 STEC isolates used in this study.
Statistical analysis of the P-BIT results using the χ2 test was also performed to identify genes associated with human versus nonhuman sources. Three genes (pic, espC, and lpfAO26) were associated with human isolates. pic and espC were associated with both O157 and non-O157 human isolates; lpfAO26 was associated with non-O157 human isolates. ECs3737 was the only gene associated with nonhuman isolates.
Cluster analysis of P-BIT data.
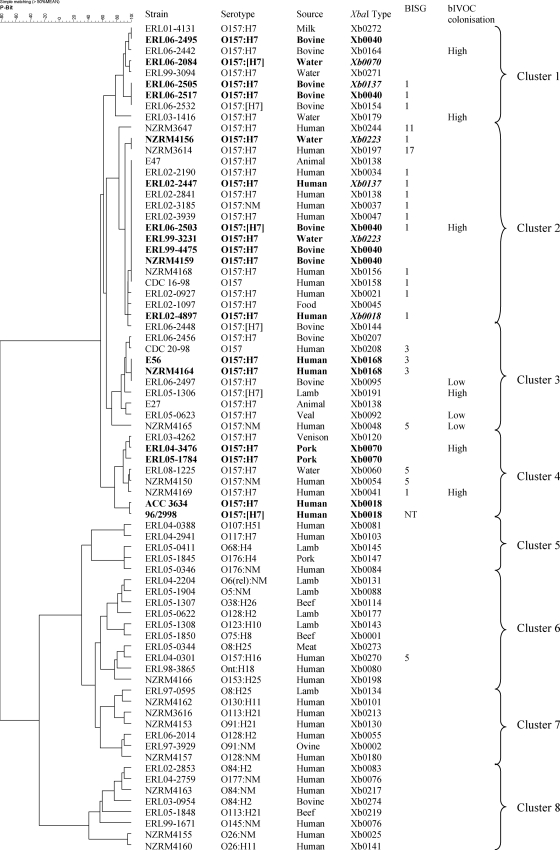
Interstrain relationships were assessed by preparing a cluster dendrogram of P-BIT data using the simple matching coefficient and Ward's clustering (Fig. 1). The 75 isolates were separated into two main branches; branch 1 comprised O157:H7 and O157:NM isolates, and branch 2 comprised all other serotypes. The O157 branch was further segregated into four clusters (clusters 1 to 4) at the 72% similarity level. The non-O157 branch was also segregated into four clusters (clusters 5 to 8) at the 42% similarity level.
FIG. 1.
Cluster dendrogram of 41-gene P-BIT data in comparison with PFGE typing using XbaI macrorestriction of genomic DNA (54), bacteriophage insertion site genotyping (BISG) (67), and colonization potential (high or low) of bovine in vitro organ culture (Brandt and Paulin, unpublished data). Strains that share the same PFGE XbaI profile with another strain and that also cluster closely based on P-BIT type are highlighted in bold. Strains that share the same PFGE XbaI profile with another strain but do not cluster closely based on P-BIT type are highlighted in bold and italic. Ont, not O-serotypeable.
Non-O157 serotypes that have been associated with severe disease and outbreaks were found in clusters 7 and 8. These included serotypes commonly associated with HUS (e.g., O26:H11 and O145:NM) and less commonly associated with HUS (e.g., O84:H2, O91:H21, O113:H21, O128:H2, O130:H11, and O177:NM) (13, 19, 22, 31, 33). These isolates were PCR positive for many of the genes previously shown to be associated with diarrheal disease, including many associated with HUS and/or outbreaks (Table 2). The gene targets associated with clusters 7 and 8, in comparison to clusters 5 and 6, by the χ2 test (P < 0.05) included pic, espC, iha (LPA), eaeA (LEE), pagC (OI-122), sen (OI-122), nleG2-3 (OI-57), nleG (OI-71), agn43EDL933, paa, ureC, toxB (pO157), espP (pO157), lpfAO26, and lpfAO113.
Human and nonhuman isolates were distributed among all of the dendrogram clusters except cluster 1, which contained only nonhuman O157:H7 STEC isolates (n = 9) from water, bovine, and milk sources. Cluster 6 also contained a subcluster comprised of nonhuman non-O157 STEC isolates (n = 6) from beef and lamb sources. All but one isolate in both of these clusters was negative for one or both of the pic and espC genes. Statistical analysis by the χ2 test confirmed that these two genes were not associated with isolates in these clusters (P < 0.005).
Comparison with other typing methods.
Pulsed-field gel electrophoresis (PFGE) typing of isolates using XbaI macrorestriction of genomic DNA was more discriminatory than P-BIT typing (Fig. 1). PFGE typing produced diversity indexes of 99.28% ± 0.66% (in comparison to 97.33% for P-BIT) when comparing all 75 STEC isolates and 98.07% ± 1.63% (in comparison to 92.85% for P-BIT) when comparing O157 STEC isolates only. Both P-BIT and PFGE XbaI typing were completely discriminatory for non-O157 STEC. There was good correspondence between isolates that shared PFGE XbaI types (shown in bold in Fig. 1) and clustering produced by P-BIT. Although PFGE XbaI typing was more discriminatory overall, P-BIT could discriminate most isolates that PFGE XbaI typing could not, except for two isolates that shared type Xb0168 and three isolates that shared type Xb0040.
Bacteriophage insertion site genotyping (BISG) also corresponded well with clusters formed by PBIT typing (Fig. 1). BISG type 1 was mostly present in clusters 1 and 2. BISG types 3 and 5 were contained in clusters 3 and 4.
The colonization potentials of nine O157 STEC isolates had been previously characterized using a bovine in vitro organ culture (bIVOC) system (Brandt and Paulin, unpublished data). The bIVOC model infection system identified low- and high-colonizing strains using bovine colonic tissue. High-colonizing strains identified by this study were present in P-BIT clusters 1, 2, and 4 (Fig. 1). All of the low-colonizing strains were present in cluster 3. One high-colonizing strain was present in cluster 3; however, this strain was PCR positive for stx2c, a defining genetic feature of isolates contained in cluster 4 and a gene that has been shown to be associated with HUS (15).
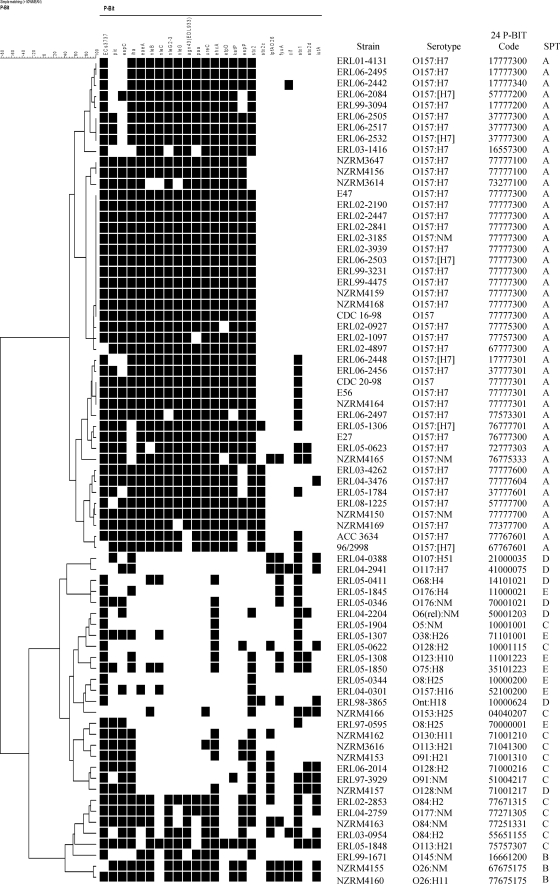
Minimum STEC P-BIT set.
A subset of 24 gene targets could discriminate O157 from non-O157 isolates and generated the same cluster groups as the full set of 41 gene targets (Fig. 2). These cluster groupings correlated well with seropathotype classification, with SPT A located in clusters 1 to 4, SPT B and C mainly in clusters 7 and 8, and SPT D and E mainly in clusters 5 and 6.
FIG. 2.
Cluster dendrogram of 24-gene P-BIT data in comparison with seropathotype (SPT). Serotypes classified as SPT A and B have been associated with HUS and outbreaks. Serotypes classified as SPT C have been associated with sporadic HUS but not outbreaks. Serotypes classified as SPT D have been associated with diarrhea. Serotypes classified as SPT E have not been associated with human disease. P-BIT bar codes were generated as previously published (11).
The 24-gene target P-BIT system produced an 8-digit bar code that could be used for strain typing and risk assessment. High numbers, with 7 being the maximum, indicated PCR-positive results for many target virulence genes. Isolates classified as SPT A to C contained bar codes with high numbers at most positions, indicating potential to cause severe disease, whereas isolates classified as SPT D or SPT E had low numbers at most positions, indicating low potential to cause severe disease.
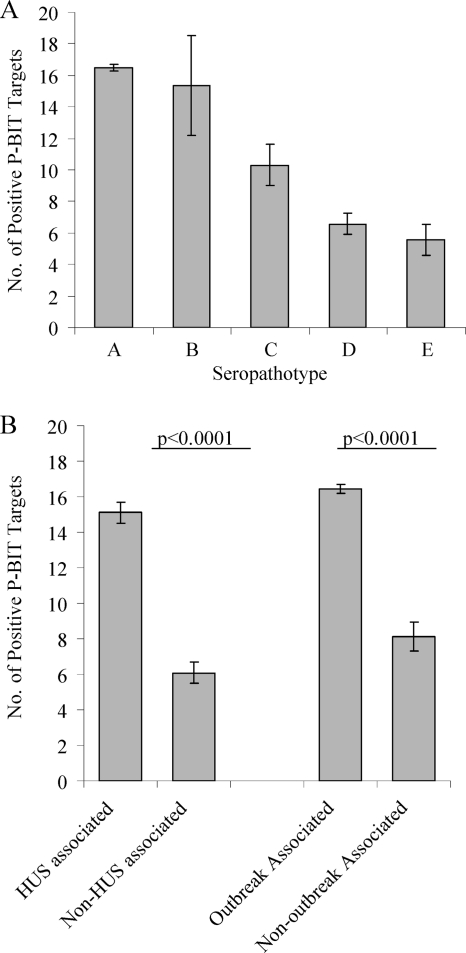
The total number of positive PCR results using the 24-gene target P-BIT system correlated with predicted STEC virulence based on the seropathotyping scheme (Fig. 3). Serotypes classified as SPT A or SPT B had the most P-BIT targets present (averages of 16.5 and 15.3 genes, respectively), followed by SPT C (average of 10.3 genes), then SPT D (average of 6.6 genes), and lastly SPT E (average of 5.6 genes). There was no significant difference between the number of P-BIT targets present in serotypes classified as SPT A or SPT B or between serotypes classified as SPT D or SPT E using a two-tailed Mann-Whitney U test; however, there were significantly greater numbers of P-BIT targets present in serotypes associated with HUS or outbreaks (averages of 15.1 and 16.4 genes, respectively) in comparison to serotypes not associated with HUS or outbreaks (averages of 6.1 and 8.1 genes, respectively). These results established that that the STEC P-BIT system can identify high-risk serotypes of importance to public health; however, it does not discriminate between serotypes that cause sporadic cases of diarrhea and serotypes that are not associated with disease in humans.
FIG. 3.
Correlation between the presence of P-BIT gene targets (based on the 24-gene scheme), seropathotype classification, and a serotype's reported association with HUS or outbreaks. (A) The greatest number of P-BIT gene targets were present in serotypes associated with outbreaks and HUS (SPT A and SPT B), followed by strains associated with HUS only (SPT C), strains associated with diarrhea (SPT D), and lastly strains not associated with human disease (SPT E). (B) HUS (SPT A-C)- and outbreak (SPT A and B)-associated serotypes were positive for a significantly higher number of P-BIT gene targets than were non-HUS (SPT D and E)- and non-outbreak (SPT C-E)-associated serotypes. Bars indicate the average number of positive PCR results, and error bars indicate the standard error of the mean. Statistical significance was determined by a two-tailed Mann-Whitney U test.
DISCUSSION
The aim of this study was to develop a novel subtyping method for STEC that could also provide information about the potential for a given isolate to cause disease in humans. We have chosen to develop a PCR-based approach because this platform is commonly available to research and reference laboratories, does not require expensive equipment or consumables, and is not labor-intensive and because methods can easily be standardized across laboratories. By producing a simple bar code derived from the presence or absence of virulence genes as detected by PCR, P-BIT results can easily be exchanged via e-mail, collected by commonly used database programs, and compared without a need for specialized software or training. In addition, the STEC P-BIT system was built with gene targets from an array of known genomic and extrachromosomal virulence loci that are known to be distributed among O157 and non-O157 STEC isolates so as to offer complete typeability.
Phenotypic methods for typing STEC, such as serotyping and phage typing, offer limited applicability, as few laboratories are equipped to carry out the full range of O- and H-antigen serotyping and phage typing has not yet been developed for non-O157 STEC (28). Octamer-based genome scanning (OBGS), lineage-specific polymorphism assay (LSPA), single nucleotide polymorphisms (SNPs), and microarray analysis are molecular methods with the potential for high discriminatory power; however, protocols have not been developed for application of these methods to non-O157 STEC, and expensive sequencing or microarray scanning equipment is required (28). Random amplification of polymorphic DNA (RAPD) typing is a simple and inexpensive PCR-based method that has been developed for both O157 and non-O157 STEC; its main disadvantage lies in difficulties with method standardization and reproducibility (28). Pulsed-field gel electrophoresis (PFGE), and amplified fragment length polymorphism (AFLP) are highly discriminatory typing methods that are suitable for subtyping O157 and non-O157 STEC; however, these methods are labor-intensive and require expensive equipment and software to facilitate comparisons of strain types (28). Multiple-locus variable-number tandem-repeats analysis (MLVA) is highly discriminatory, rapid, and low-cost; however, standard protocols for O157 STEC have not yet been agreed upon, and only one protocol has been developed for a non-O157 serotype, O26 (28).
Of these methods, PFGE is the most commonly used STEC subtyping method and has provided essential strain identification for disease surveillance and outbreak investigations (28). Our results indicate that PFGE typing using XbaI macrorestriction of genomic DNA is more discriminatory for O157 STEC than P-BIT; however, the two typing methods had equal discriminatory power for non-O157 STEC. Some correspondence was observed between PFGE XbaI type relatedness and P-BIT profile relatedness, which is not unexpected as both methods are dependent on the genomic content of strains. P-BIT was able to discriminate some strains that PFGE was not and vice versa, suggesting that the two subtyping methods may be suitable to use in tandem, especially given that although PFGE is more discriminatory, P-BIT results can be obtained faster and indicate seropathotype along with virulence potential.
The strength of P-BIT is the ability to subtype in conjunction with a broad assessment of STEC pathogenicity that can be used for all STEC isolates. P-BIT had a high discriminatory power for STEC, including O157 STEC, which is a challenging organism to subtype due to the clonal relatedness of strains (66). Cluster analysis of P-BIT results identified clusters of strains that based on SPT classification have been more frequently associated with outbreaks and severe disease (clusters 1 to 4, 7, and 8) and clusters of strains that have been reported less frequently or have not been involved in human disease (clusters 5 and 6). In addition, statistical analysis of P-BIT results identified genes associated with human or nonhuman isolates. This information was used to build a minimum P-BIT system comprised of 24 gene targets that was as discriminatory as the full gene system and generated the same cluster dendrogram.
The 24-gene target P-BIT system produced a genetic fingerprint of STEC virulence by detecting genes that have been associated with outbreaks and severe disease, including the LEE, OI-36, OI-57, OI-71, OI-122, pO157, the long polar fimbrial operon, the urease gene cluster, stx1, and stx2. We recognize that P-BIT data provide indicative but not absolute data regarding the presence or absence of a gene, as sequence variation of target genes at the site of primer annealing might result in a false-negative PCR result. This is a compromise to build a rapid, low-cost system for assessing virulence and strain type.
Molecular risk assessment for non-O157 STEC has greatly advanced due to a number of recent studies that found a strong association between the presence of non-LEE effectors (nle) located in STEC O islands with serotypes classified as SPT A to C, and thereby also associated with outbreaks and HUS (7, 10, 19, 23, 31). Our results supported these findings, and therefore gene targets to detect OI-36, OI-57, OI-71, and OI-122 were included in the minimum P-BIT system. These gene targets were necessary for generating the clusters that corresponded well with SPT classification but were not sufficient; our work also identified other gene targets (pic, espC, and iha) that were important for separating serotypes classified as SPT C from serotypes classified as SPT D and SPT E. Previous studies have shown that other methods used to form E. coli phylogenetic groupings, such as multilocus sequence typing (MLST) and multilocus enzyme electrophoresis (MLEE), do not correlate with SPT classification and are not useful for assessing the public health risk of isolates (71). Because P-BIT analyzes the virulence gene content of isolates, it is a much better predictor of SPT classification and thereby the potential for a strain to cause serious illness. P-BIT clustering and SPT classification were not in complete agreement; however, serotyping is not an absolute predictor of strain virulence, and we would argue that examining the virulence gene content of a strain and how this compares to those of other known highly pathogenic strains is more objective and potentially a more accurate predictor of risk to human health. This is supported by Scheutz, who argued that a classification system based on a virulence profile, which could be fluid as more information regarding pathogenic mechanisms became available, would be better suited for grouping isolates into those capable of causing severe disease (outbreaks/HUS), those that are likely to cause sporadic diarrheal disease, and those that are associated only with animals [F. Scheutz, presented at the Public Health Significance of Non-O157 Shiga Toxin-Producing Escherichia coli (STEC) Public Meeting, Arlington, VA, 17 October 2007].
To this end, P-BIT bar codes used in combination with P-BIT clustering can, in principle, be used for risk assessment of isolates based on P-BIT type and on whether the isolate clusters with other isolate types known to be associated with outbreaks or severe disease. We have demonstrated that 24-gene P-BIT clustering corresponds well with SPT classification. The true test for the P-BIT typing system as a risk assessment tool will come when it is routinely used side by side with other established typing methods, such as PFGE, to analyze clinical isolates, investigate outbreaks, and attempt to establish attribution.
In addition to comparison with epidemiological data, molecular risk assessment will be improved through more work comparing genetic fingerprints with phenotypic characteristics of isolates, especially traits involved in bacterial pathogenesis. P-BIT clustering was compared with a recent study characterizing the colonization potentials of a small number of O157 STEC isolates in a model bovine infection system (Brandt and Paulin, unpublished data). Isolates with low potential for colonizing bovine tissue were all located in cluster 3. This suggests that genetic determinants specific to cluster 3 may be associated with reduced colonization of cattle tissues. Interestingly, isolates in cluster 3 were of BISG types 3 (n = 3) and 5 (n = 1). These BISG types are frequently found in cattle around the world, apart from Australia and Germany (67). The prevalence of these BISG types in New Zealand cattle is unknown. Also, since certain O157 phage types have been found more frequently in animals than in humans (40, 55), further evaluation of P-BIT clustering with O157 phage typing could reveal that cluster 3 and the nonhuman cluster 1 correspond to particular O157 phage types associated with animals.
In conclusion, the STEC P-BIT typing system offers subtyping in a rapid, low-cost format and enables STEC strains to be assessed for the potential risk to public health. Molecular risk assessment is a useful tool to aid in public health surveillance and outbreak investigations; such uses could include providing information to help public health officials gauge the level of mobilization of resources required to address a potential threat, as well as a tool to monitor over time the emergence of new potentially dangerous STEC strains. Molecular risk assessment can also be used for source attribution and can provide important information for targeted intervention along the farm-to-fork continuum. Such tools are of paramount importance to address the increasing public health concern about non-O157 STEC.
Acknowledgments
We thank Carolyn Nicol for use of Enteric Reference Laboratory strains and Beverley Horn for advice regarding statistical analysis. We also thank Nigel French for technical assistance and helpful comments on the manuscript.
This work was funded in part by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract N01-A1-30055 and by capability funding provided by the New Zealand Ministry of Research Science and Technology, administered by the Institute of Environmental Science and Research, Ltd.
Footnotes
Published ahead of print on 4 February 2011.
REFERENCES
- 1.An, H., J. M. Fairbrother, C. Desautels, and J. Harel. 1999. Distribution of a novel locus called Paa (porcine attaching and effacing associated) among enteric Escherichia coli. Adv. Exp. Med. Biol. 473:179-184. [DOI] [PubMed] [Google Scholar]
- 2.Beutin, L., D. Geier, H. Steinruck, S. Zimmermann, and F. Scheutz. 1993. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J. Clin. Microbiol. 31:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutin, L., S. Jahn, and P. Fach. 2008. Direct and reliable detection of classical enterohaemorrhagic E. coli (EHEC) from single colonies with the “GeneDisc” real-time PCR system. Bundesinstitut fur Risikobewertung, Berlin, Germany.
- 4.Beutin, L., S. Kaulfuss, S. Herold, E. Oswald, and H. Schmidt. 2005. Genetic analysis of enteropathogenic and enterohemorrhagic Escherichia coli serogroup O103 strains by molecular typing of virulence and housekeeping genes and pulsed-field gel electrophoresis. J. Clin. Microbiol. 43:1552-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunder, W., H. Schmidt, M. Frosch, and H. Karch. 1999. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 145:1005-1014. [DOI] [PubMed] [Google Scholar]
- 6.Brunder, W., H. Schmidt, and H. Karch. 1996. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:3305-3315. [DOI] [PubMed] [Google Scholar]
- 7.Bugarel, M., L. Beutin, and P. Fach. 2010. Low-density macroarray targeting non-locus of enterocyte effacement effectors (nle genes) and major virulence factors of Shiga toxin-producing Escherichia coli (STEC): a new approach for molecular risk assessment of STEC isolates. Appl. Environ. Microbiol. 76:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman, T. A., et al. 2006. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 72:4782-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coombes, B. K., et al. 2008. Molecular analysis as an aid to assess the public health risk of non-O157 Shiga toxin-producing Escherichia coli strains. Appl. Environ. Microbiol. 74:2153-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelius, A. J., B. Gilpin, P. Carter, C. Nicol, and S. L. On. 2010. Comparison of PCR binary typing (P-BIT), a new approach to epidemiological subtyping of Campylobacter jejuni, with serotyping, pulsed-field gel electrophoresis, and multilocus sequence typing methods. Appl. Environ. Microbiol. 76:1533-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doughty, S., et al. 2002. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect. Immun. 70:6761-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott, E. J., et al. 2001. Nationwide study of haemolytic uraemic syndrome: clinical, microbiological, and epidemiological features. Arch. Dis. Child. 85:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fratamico, P., J. Smith, and R. Buchanan. 2002. Escherichia coli, p. 79-101. In D. Clier and H. Riemann (ed.), Foodborne diseases. Academic Press, Amsterdam, Netherlands.
- 15.Friedrich, A. W., et al. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74-84. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich, A. W., et al. 2006. Urease genes in non-O157 Shiga toxin-producing Escherichia coli: mostly silent but valuable markers for pathogenicity. Clin. Microbiol. Infect. 12:483-486. [DOI] [PubMed] [Google Scholar]
- 17.Garrido, P., et al. 2006. STEC-EPEC oligonucleotide microarray: a new tool for typing genetic variants of the LEE pathogenicity island of human and animal Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) strains. Clin. Chem. 52:192-201. [DOI] [PubMed] [Google Scholar]
- 18.Gerber, A., H. Karch, F. Allerberger, H. M. Verweyen, and L. B. Zimmerhackl. 2002. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997-2000, in Germany and Austria: a prospective study. J. Infect. Dis. 186:493-500. [DOI] [PubMed] [Google Scholar]
- 19.Girardeau, J. P., et al. 2005. Association of virulence genotype with phylogenetic background in comparison to different seropathotypes of Shiga toxin-producing Escherichia coli isolates. J. Clin. Microbiol. 43:6098-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 21.Hunter, S. B., et al. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J. Clin. Microbiol. 43:1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussein, H. S. 2007. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J. Anim. Sci. 85:E63-E72. [DOI] [PubMed] [Google Scholar]
- 23.Imamovic, L., et al. 2010. OI-57, a genomic island of Escherichia coli O157, is present in other seropathotypes of Shiga toxin-producing E. coli associated with severe human disease. Infect. Immun. 78:4697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janka, A., M. Bielaszewska, U. Dobrindt, and H. Karch. 2002. Identification and distribution of the enterohemorrhagic Escherichia coli factor for adherence (efa1) gene in sorbitol-fermenting Escherichia coli O157: H. Int. J. Med. Microbiol. 292:207-214. [DOI] [PubMed] [Google Scholar]
- 25.Jelacic, J. K., et al. 2003. Shiga toxin-producing Escherichia coli in Montana: bacterial genotypes and clinical profiles. J. Infect. Dis. 188:719-729. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, K. E., C. M. Thorpe, and C. L. Sears. 2006. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 43:1587-1595. [DOI] [PubMed] [Google Scholar]
- 28.Karama, M., and C. L. Gyles. 2010. Methods for genotyping verotoxin-producing Escherichia coli. Zoonoses Public Health 57:447-462 [DOI] [PubMed] [Google Scholar]
- 29.Karch, H., et al. 1999. A genomic island, termed high-pathogenicity island, is present in certain non-O157 Shiga toxin-producing Escherichia coli clonal lineages. Infect. Immun. 67:5994-6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karmali, M. A., et al. 2003. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 41:4930-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly, B. G., A. Vespermann, and D. J. Bolton. 2009. The role of horizontal gene transfer in the evolution of selected foodborne bacterial pathogens. Food Chem. Toxicol. 47:951-968. [DOI] [PubMed] [Google Scholar]
- 33.Konczy, P., et al. 2008. Genomic O island 122, locus for enterocyte effacement, and the evolution of virulent verocytotoxin-producing Escherichia coli. J. Bacteriol. 190:5832-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kresse, A. U., et al. 2007. Enterohaemorrhagic Escherichia coli O157 and non-O157 serovars differ in their mechanisms for iron supply. Int. J. Med. Microbiol. 297:9-15. [DOI] [PubMed] [Google Scholar]
- 35.Lopez, E. L., M. M. Contrini, and M. F. de Rosa. 1998. Epidemiology of Shia toxin-producing Escherichia coli in South America, p. 30-37. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, DC.
- 36.Makino, S., et al. 2003. Distribution of the secondary type III secretion system locus found in enterohemorrhagic Escherichia coli O157:H7 isolates among Shiga toxin-producing E. coli strains. J. Clin. Microbiol. 41:2341-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marches, O., et al. 2003. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol. Microbiol. 50:1553-1567. [DOI] [PubMed] [Google Scholar]
- 38.Meng, J., M. Doyle, T. Zhao, and S. Zhao. 2001. Enterohemorrhagic Escherichia coli, p. 193-213. In M. Doyle, l. Behuchat, and T. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. ASM Press, Washington, DC.
- 39.Mobley, H. L., M. D. Island, and R. P. Hausinger. 1995. Molecular biology of microbial ureases. Microbiol. Rev. 59:451-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mora, A., et al. 2004. Phage types and genotypes of shiga toxin-producing Escherichia coli O157:H7 isolates from humans and animals in spain: identification and characterization of two predominating phage types (PT2 and PT8). J. Clin. Microbiol. 42:4007-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakano, M., et al. 2001. Association of the urease gene with enterohemorrhagic Escherichia coli strains irrespective of their serogroups. J. Clin. Microbiol. 39:4541-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowrouzian, F. L., A. E. Wold, and I. Adlerberth. 2005. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. J. Infect. Dis. 191:1078-1083. [DOI] [PubMed] [Google Scholar]
- 44.Okeke, I. N., I. C. Scaletsky, E. H. Soars, L. R. Macfarlane, and A. G. Torres. 2004. Molecular epidemiology of the iron utilization genes of enteroaggregative Escherichia coli. J. Clin. Microbiol. 42:36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orth, D., K. Grif, M. P. Dierich, and R. Wurzner. 2006. Prevalence, structure and expression of urease genes in Shiga toxin-producing Escherichia coli from humans and the environment. Int. J. Hyg. Environ. Health 209:513-520. [DOI] [PubMed] [Google Scholar]
- 46.Pass, M. A., R. Odedra, and R. M. Batt. 2000. Multiplex PCRs for identification of Escherichia coli virulence genes. J. Clin. Microbiol. 38:2001-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paton, A. W., and J. C. Paton. 1999. Molecular characterization of the locus encoding biosynthesis of the lipopolysaccharide O antigen of Escherichia coli serotype O113. Infect. Immun. 67:5930-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paton, A. W., and J. C. Paton. 2005. Multiplex PCR for direct detection of Shiga toxigenic Escherichia coli strains producing the novel subtilase cytotoxin. J. Clin. Microbiol. 43:2944-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira, A. L., L. R. Ferraz, R. S. Silva, and L. G. Giugliano. 2007. Enteroaggregative Escherichia coli virulence markers: positive association with distinct clinical characteristics and segregation into 3 enteropathogenic E. coli serogroups. J. Infect. Dis. 195:366-374. [DOI] [PubMed] [Google Scholar]
- 51.Prager, R., et al. 2004. Prevalence and deletion types of the pathogenicity island ETT2 among Escherichia coli strains from oedema disease and colibacillosis in pigs. Vet. Microbiol. 99:287-294. [DOI] [PubMed] [Google Scholar]
- 52.Ren, C. P., et al. 2004. The ETT2 gene cluster, encoding a second type III secretion system from Escherichia coli, is present in the majority of strains but has undergone widespread mutational attrition. J. Bacteriol. 186:3547-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Restieri, C., G. Garriss, M. C. Locas, and C. M. Dozois. 2007. Autotransporter-encoding sequences are phylogenetically distributed among Escherichia coli clinical isolates and reference strains. Appl. Environ. Microbiol. 73:1553-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roldgaard, B. B., et al. 2004. VTEC O157 subtypes associated with the most severe clinical symptoms in humans constitute a minor part of VTEC O157 isolates from Danish cattle. Int. J. Med. Microbiol. 294:255-259. [DOI] [PubMed] [Google Scholar]
- 56.Scheutz, F., and N. A. Strockbine. 2005. Genus I. Escherichia, p. 607-624. In G. M. Garrity, D. J. Brenner, N. R. Kreig, and J. T. Staley (ed.), Bergey's Manual of Systematic Bacteriology, 2nd ed. Springer-Verlag, New York, NY.
- 57.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt, H., B. Henkel, and H. Karch. 1997. A gene cluster closely related to type II secretion pathway operons of gram-negative bacteria is located on the large plasmid of enterohemorrhagic Escherichia coli O157 strains. FEMS Microbiol. Lett. 148:265-272. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt, H., et al. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69:6863-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaikh, N., and P. I. Tarr. 2003. Escherichia coli O157:H7 Shiga toxin-encoding bacteriophages: integrations, excisions, truncations, and evolutionary implications. J. Bacteriol. 185:3596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarr, P. I., and M. A. Neill. 1996. The problem of non-O157:H7 Shiga toxin (Verocytotoxin)-producing Escherichia coli. J. Infect. Dis. 174:1136-1139. [DOI] [PubMed] [Google Scholar]
- 62.Toma, C., N. Higa, S. Iyoda, M. Rivas, and M. Iwanaga. 2006. The long polar fimbriae genes identified in Shiga toxin-producing Escherichia coli are present in other diarrheagenic E. coli and in the standard E. coli collection of reference (ECOR) strains. Res. Microbiol. 157:153-161. [DOI] [PubMed] [Google Scholar]
- 63.Torres, A. G., et al. 2002. Identification and characterization of lpfABCC′DE, a fimbrial operon of enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 70:5416-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tozzoli, R., A. Caprioli, and S. Morabito. 2005. Detection of toxB, a plasmid virulence gene of Escherichia coli O157, in enterohemorrhagic and enteropathogenic E. coli. J. Clin. Microbiol. 43:4052-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, G., C. G. Clark, and F. G. Rodgers. 2002. Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. J. Clin. Microbiol. 40:3613-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whittam, T. S., I. K. Wachsmuth, and R. A. Wilson. 1988. Genetic evidence of clonal descent of Escherichia coli O157:H7 associated with hemorrhagic colitis and hemolytic uremic syndrome. J. Infect. Dis. 157:1124-1133. [DOI] [PubMed] [Google Scholar]
- 67.Whitworth, J. H., et al. 2008. International comparison of clinical, bovine, and environmental Escherichia coli O157 isolates on the basis of Shiga toxin-encoding bacteriophage insertion site genotypes. Appl. Environ. Microbiol. 74:7447-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.WHO. 1998. Zoonotic non-O157 Shiga toxin-producing Escherichia coli (STEC), p. 1-35. In Report of a WHO Scientific Working Group Meeting, Berlin, Germany, 23 to 26 June 1998. World Health Organization, Geneva, Switzerland.
- 69.WHO. 1999. Zoonotic non-O157 Shiga toxin-producing Escherichia coli (STEC), p. 1-30. In Report of a WHO Scientific Working Group Meeting, Berlin, Germany, 22 to 23 June 1990. World Health Organization, Geneva, Switzerland.
- 70.Wickham, M. E., et al. 2006. Bacterial genetic determinants of non-O157 STEC outbreaks and hemolytic-uremic syndrome after infection. J. Infect. Dis. 194:819-827. [DOI] [PubMed] [Google Scholar]
- 71.Ziebell, K., et al. 2008. Applicability of phylogenetic methods for characterizing the public health significance of verocytotoxin-producing Escherichia coli strains. Appl. Environ. Microbiol. 74:1671-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]