Abstract
Amino acid- and inosine-induced germination of Bacillus cereus ATCC 14579 spores was reversibly inhibited in the presence of 3 mM undissociated sorbic acid. Exposure to high hydrostatic pressure, Ca-dipicolinic acid (DPA), and bryostatin, an activator of PrkC kinase, negated this inhibition, pointing to specific blockage of signal transduction in germinant receptor-mediated germination.
A wide range of food preservatives, including weak acids such as lactates and sorbic acid (SA), are effective in maintenance of food quality and safety (5, 14) and are therefore widely used in industry. We previously assessed the impact of SA on germination and outgrowth of Bacillus cereus spores (16) and reported that at concentrations of 3 mM undissociated sorbic acid (HSA) and higher, amino acid- and inosine-induced spore germination was blocked, with spores remaining fully refractile. An earlier study on Clostridium botulinum and B. cereus spores suggested that the mode of action of sorbic acid was by competitive inhibition on the l-alanine/inosine receptor (13). In later work performed by Blocher and Busta (3), competitive inhibition was disputed, and evidence was provided that led the authors to propose that inhibition occurred after germinant binding and could involve alteration of the spore's inner membrane permeability or inhibition of cortex lytic enzymes. Based on current knowledge, the B. cereus ATCC 14579 genome encodes seven putative germinant receptors, and spore germination in this organism can be triggered not only by alanine, via the germinant receptor GerR, but also by a range of other germinants, including inosine, adenosine, glutamine, cysteine, threonine, and phenylalanine (7).
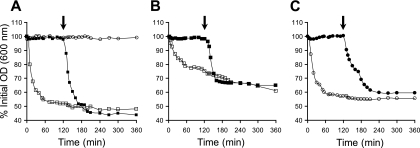
This triggered us to assess the impact of sorbic acid on the different Ger receptors by using inosine and selected amino acids as germinants with both B. cereus ATCC 14579 wild-type spores and gerR deletion mutant spores, which are insensitive to alanine-induced germination (6). Spore preparations of the B. cereus ATCC 14579 wild type and its gerR deletion mutant were prepared for germination assays as described previously (6, 16). In short, heat-activated spores (70°C for 15 min) of both the wild type and gerR mutant were resuspended in morpholineethanesulfonic acid (MES) buffer corresponding to the test conditions prior to addition to a microtiter plate. Both wild-type and gerR mutant spores were exposed to a 1 mM concentration of one of the germinants l-alanine, l-glutamine, or inosine, thereby triggering GerR, GerG and GerQ, and GerI, respectively, as demonstrated by Hornstra et al. (7). The spores were incubated at pH 5.5 with or without 3 mM HSA and germinant (Fig. 1). To test the reversibility of HSA-arrested germination, the spores were incubated for 2 h in the presence of selected germinants before the pH was raised to 7.1 by the addition of NaOH in the HSA-exposed samples. This instantaneously shifted the equilibrium between SA and HSA, resulting in a decrease from 3 mM HSA to less than 0.2 mM, with a concomitant relief of inhibition of germination (Fig. 1).
FIG. 1.
Impact of pH and sorbic acid on amino acid- and inosine-induced germination of spores derived from B. cereus ATCC 14579 and its ΔgerR mutant. Heat-activated spores of B. cereus ATCC 14579 (squares) (A and B) and its ΔgerR mutant (circles) (A and C) were incubated at pH 5.5 (open symbols) or pH 5.5 plus 3 mM HSA (closed symbols). At time zero, the following germinants were used to induce germination: l-alanine (1 mM) (A), l-glutamine (1 mM) (B), and inosine (1 mM) (C). The vertical axis expresses the percentage of OD600 at the indicated time points, relative to that determined at time zero. The drop in optical density signifies germination. After 2 h of incubation, the pH in sorbic acid-stressed spore cultures was raised to pH 7.1 by addition of 2 M NaOH (indicated with the black arrow). Graphs show the results from triplicate experiments.
The data show that not only germination via l-alanine but also that via the germinants inosine and glutamine is inhibited by the presence of 3 mM HSA. Comparable results were obtained for germination induced with cysteine, threonine, and phenylalanine (data not shown). Thus, HSA blocks germination in B. cereus spores via the receptors GerR, GerG, GerQ, and GerI and does not display a receptor-specific effect, as was previously reported by Cortezzo for Bacillus subtilis spores (4). In the latter study, HSA arrested germination only when triggered by l-alanine in B. subtilis, but not when triggered by a mixture of l-asparagine, d-glucose, d-fructose, and potassium ions (AGFK). Notably, the concentration of sorbic acid used in the latter study (5 mg/liter) was far below the range (250 to 2,000 mg/liter) that is applied by industry (14), and given that experiments were performed at pH 6, the amount of undissociated acid was in the micromolar range.
Inhibition of germination by HSA was for all tested nutrients a reversible process; when the pH was raised, spore germination is activated, as reflected in the drop in optical density (OD) indicative of the transition from phase bright to phase dark. Thus, in an extension of the previously reported data (3), inhibition of nutrient-induced germination of B. cereus by HSA does not involve competitive inhibition of (specific) germinant receptors.
Although the exact mechanisms following nutrient binding are not fully understood, several subsequent events are triggered upon binding of the nutrient to the receptor. After being committed to germinate, cations are released from the spores, followed by the release of Ca2+ and dipicolinic acid (DPA), which subsequently activates the cortex lytic system (8), allowing for expansion of the spore core by the uptake of water. Ca-DPA release involves the presence of SpoVA proteins that have been suggested to act as functional components of a specific channel (17). In this scenario, sorbic acid may display its inhibiting effect either by (i) directly blocking the Ca-DPA channel, (ii) inactivating the cortex lytic system, or (iii) preventing the signal transduction between nutrient-activated receptor(s) and the Ca-DPA channel.
To assess whether sorbic acid inhibits germination by directly blocking the Ca-DPA channel or by inactivating the cortex lytic system, dormant spores were exposed to either hydrostatic pressure (HP) or to exogenous Ca-DPA in the presence or absence of HSA. Ca-DPA is known to trigger spore cortex degradation by directly activating cortex lytic enzyme Cw1J (9, 10). Exposure of dormant spores to relatively high pressure (500 to 600 MPa) can induce germination either by the activation of channels in the inner membrane or by creating pores, with both possibilities resulting in the release of Ca-DPA (2). The triggering of germination by 100 to 200 MPa is assumed to involve activation of the nutrient receptors, even in the absence of nutrients, followed by activation of Ca-DPA channels (18, 19).
Heat-activated spores in MES buffer (pH 5.5) with or without 3 mM HSA were exposed to temperature-controlled pressure as previously described by Hornstra et al. (6) at 150 or 500 MPa or Ca-DPA at a final concentration of 50 mM. After exposure to HP (30 min) or to Ca-DPA (60 min), part of the samples was plated directly on brain heart infusion (BHI) plates (total number of spores), while a second part was heat treated for 10 min at 80°C before plating (only nongerminated spores survive) to determine the extent of germination. CFU were counted after overnight incubation at 30°C. Figure 2 A and B show that spores were able to germinate when exposed to 50 mM Ca-DPA or 500 MPa, regardless of whether HSA was present or not, thereby excluding an effect of sorbic acid on the cortex lytic system or Ca-DPA release. Only germination of B. cereus spores exposed to 150 MPa is affected by HSA, indicating that receptor-mediated signaling is inhibited. The fact that pressure-induced germination at 150 MPa without nutrients was still inhibited in the presence of sorbic acid supports the earlier assumption by Blocher and Busta (3) that a role for sorbic acid in competitive inhibition at the nutrient receptors is unlikely.
FIG. 2.
Impact of sorbic acid on B. cereus ATCC 14579 spore germination induced by chemical and physical triggers. Heat-activated spores of B. cereus ATCC 14579 were incubated in MES buffer at pH 5.5 with 3 mM HSA (gray bars) or without HSA (white bars). The following germination triggers were used: a 1:1 chelate of 50 mM calcium and dipicolonic acid (Ca-DPA) (A), high hydrostatic pressure at either 150 or 500 MPa (B), and 10 μM bryostatin (C). The germination percentages were determined by comparing the number of germinated spores with the total number of spores present in each sample. The percentage bars show the results from duplicate experiments for high hydrostatic pressure and triplicate experiments for Ca-DPA and bryostatin, respectively.
Recently, a novel mechanism for initiating spore germination was reported, in which germination is triggered by breakdown products of peptidoglycan (12). This route depends on the presence of the eukaryote-like Ser/Thr protein kinase PrkC, which is activated upon binding of cell wall peptidoglycan fragments or muropeptides. This newly identified germination pathway appears to act independently from the germinant receptor pathway. To test whether the PrkC pathway is sensitive to sorbic acid, both HSA- and non-HSA-exposed spores were induced to germinate by the addition of the cyclic macrolide bryostatin, a potent activator of the kinase-induced germination pathway (12). Heat-activated spores were incubated in MES buffer at pH 5.5 without or with HSA while being exposed to 10 μM bryostatin. After incubation for 120 min, an aliquot of the sample was plated directly on BHI plates, while a second aliquot was heat treated for 10 min at 80°C prior to plating (30°C overnight) to determine the proportion of (germinated) spores.
Both control and HSA-exposed spores displayed a high percentage of germination when triggered with 10 μM bryostatin (Fig. 2C). The observed efficiency in the control (non-HSA-exposed spores) is comparable to that of bryostatin-induced germination of B. subtilis spores (12). B. cereus spore germination in the presence of 3 mM HSA appeared to be only slightly reduced compared to that in non-HSA-exposed spores, which shows that activation and signaling of germination via the membrane-bound PrkC kinase is not prevented by HSA.
The results of this study allow us to draw several important conclusions about the action of HSA on spore germination. We demonstrated that HSA does not target specific germinant receptors, and in agreement with that finding, a role for sorbic acid in competitive inhibition of nutrients can thus be excluded. This is also supported by the fact that inhibition is instantaneously released when pH is upshifted. Additionally, the results show that inhibition of germination by sorbic acid can be bypassed by triggering later events of the germination pathway or by activating Ger protein receptor-independent germination pathways, suggesting that sorbic acid specifically interferes in the signaling between Ger receptors and putative ion/Ca-DPA channels. Insights provided by the present study may contribute to more efficient application of sorbic acid in food preservation, especially in those cases that involve mild thermal treatments. Here, it may be advisable to aim at intermediate HSA concentrations that prevent outgrowth, but do not completely inhibit germination, thereby maximizing spore inactivation at the thermal treatment step. Additionally, the data illustrate the importance of pH control in food preservation since small pH upshifts strongly affect HSA concentrations, which could lead to outgrowth opportunities for spores.
The mode of action of sorbic acid remains to be elucidated; however, two possible effects can be envisioned: (i) undissociated sorbic acid enters the spore and dissociates because of the higher core pH (11), acidifying the spore's core and interfering with signaling; or (ii) the highly lipophilic undissociated sorbic acid accumulates in the core membrane and in this way interferes with signaling. The low mobility and reduced availability of water in the spore's core (15), the effects described for other nonacid lipophilic compounds (4), and the fact that a high concentration (15 mM) of undissociated acetic acid (pKa of 4.76, similar to that of sorbic acid, i.e., pKa 4.75) does not inhibit germination (although outgrowth is blocked) (1), combined with the immediate germination after pH upshift, suggest that the second mode of action is the most likely.
Footnotes
Published ahead of print on 28 January 2011.
REFERENCES
- 1.Abee, T., et al. 2011. Germination and outgrowth of spores of Bacillus cereus group members: diversity and role of germinant receptors. Food Microbiol. 28:199-208. [DOI] [PubMed] [Google Scholar]
- 2.Black, E. P., et al. 2007. Analysis of factors influencing the rate of germination of spores of Bacillus subtilis by very high pressure. J. Appl. Microbiol. 102:65-76. [DOI] [PubMed] [Google Scholar]
- 3.Blocher, J. C., and F. F. Busta. 1985. Multiple modes of inhibition of spore germination and outgrowth by reduced pH and sorbate. J. Appl. Bacteriol. 59:469-478. [DOI] [PubMed] [Google Scholar]
- 4.Cortezzo, D. E., B. Setlow, and P. Setlow. 2004. Analysis of the action of compounds that inhibit the germination of spores of Bacillus species. J. Appl. Microbiol. 96:725-741. [DOI] [PubMed] [Google Scholar]
- 5.Doores, S. 2005. Organic acids, p. 116-120. In P. M. Davidson, J. N. Sofos, and A. L. Branen (ed.), Antimicrobials in food, 3rd ed. Taylor & Francis Group, Boca Raton, FL.
- 6.Hornstra, L. M., Y. P. de Vries, W. M. de Vos, T. Abee, and M. H. Wells-Bennik. 2005. gerR, a novel ger operon involved in l-alanine- and inosine-initiated germination of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 71:774-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornstra, L. M., Y. P. de Vries, M. H. Wells-Bennik, W. M. de Vos, and T. Abee. 2006. Characterization of germination receptors of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 72:44-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moir, A. 2006. How do spores germinate? J. Appl. Microbiol. 101:526-530. [DOI] [PubMed] [Google Scholar]
- 9.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Setlow, B., and P. Setlow. 1980. Measurements of the pH within dormant and germinated bacterial spores. Proc. Natl. Acad. Sci. U. S. A. 77:2474-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah, I. M., M. H. Laaberki, D. L. Popham, and J. Dworkin. 2008. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135:486-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smoot, L. A., and M. D. Pierson. 1981. Mechanisms of sorbate inhibition of Bacillus cereus T and Clostridium botulinum 62A spore germination. Appl. Environ. Microbiol. 42:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stopforth, J. D., J. N. Sofos, and F. F. Busta. 2005. Sorbic acid and sorbates, p. 61-68. In P. M. Davidson, J. N. Sofos, and A. L. Branen (ed.), Antimicrobials in food, 3rd ed. Taylor & Francis Group, Boca Raton, FL.
- 15.Sunde, E. P., P. Setlow, L. Hederstedt, and B. Halle. 2009. The physical state of water in bacterial spores. Proc. Natl. Acad. Sci. U. S. A. 106:19334-19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Melis, C. C. J., M. N. Nierop Groot, M. H. Tempelaars, R. Moezelaar, and T. Abee. 2011. Characterization of germination and outgrowth of sorbic acid-stressed Bacillus cereus ATCC 14579 spores: phenotype and transcriptome analysis. Food Microbiol. 28:275-283. [DOI] [PubMed] [Google Scholar]
- 17.Vepachedu, V. R., and P. Setlow. 2007. Role of SpoVA proteins in release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J. Bacteriol. 189:1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wuytack, E. Y., S. Bovens, and C. W. Michiels. 1998. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl. Environ. Microbiol. 64:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wuytack, E. Y., J. Soons, F. Poschet, and C. W. Michiels. 2000. Comparative study of pressure- and nutrient-induced germination of Bacillus subtilis spores. Appl. Environ. Microbiol. 66:257-261. [DOI] [PMC free article] [PubMed] [Google Scholar]