Abstract
Exosome-like vesicles containing virulence factors, enzymes, and antigens have recently been characterized in fungal pathogens, such as Cryptococcus neoformans and Histoplasma capsulatum. Here, we describe extracellular vesicles carrying highly immunogenic α-linked galactopyranosyl (α-Gal) epitopes in Paracoccidioides brasiliensis. P. brasiliensis is a dimorphic fungus that causes human paracoccidioidomycosis (PCM). For vesicle preparations, cell-free supernatant fluids from yeast cells cultivated in Ham's defined medium-glucose were concentrated in an Amicon ultrafiltration system and ultracentrifuged at 100,000 × g. P. brasiliensis antigens were present in preparations from phylogenetically distinct isolates Pb18 and Pb3, as observed in immunoblots revealed with sera from PCM patients. In an enzyme-linked immunosorbent assay (ELISA), vesicle components containing α-Gal epitopes reacted strongly with anti-α-Gal antibodies isolated from both Chagas' disease and PCM patients, with Marasmius oreades agglutinin (MOA) (a lectin that recognizes terminal α-Gal), but only faintly with natural anti-α-Gal. Reactivity was inhibited after treatment with α-galactosidase. Vesicle preparations analyzed by electron microscopy showed vesicular structures of 20 to 200 nm that were labeled both on the surface and in the lumen with MOA. In P. brasiliensis cells, components carrying α-Gal epitopes were found distributed on the cell wall, following a punctuated confocal pattern, and inside large intracellular vacuoles. Lipid-free vesicle fractions reacted with anti-α-Gal in ELISA only when not digested with α-galactosidase, while reactivity with glycoproteins was reduced after β-elimination, which is indicative of partial O-linked chain localization. Our findings open new areas to explore in terms of host-parasite relationships in PCM and the role played in vivo by vesicle components and α-galactosyl epitopes.
INTRODUCTION
Paracoccidioides brasiliensis is the fungus responsible for paracoccidioidomycosis (PCM). It is a thermodimorphic fungus that grows in the yeast phase at 37°C and as mycelium at temperatures below 26°C. One of the best-acknowledged molecular features that parallels this morphological change is an alteration in structural cell wall glucans from β-1,3-linked glucan in the mycelium phase to mostly α-1,3-linked glucan in the yeast phase, as well as an increment in chitin content (34). Infection starts with inhalation of P. brasiliensis conidia present in the environment and subsequent transformation into yeasts in the pulmonary alveoli. PCM is a systemic granulomatous mycosis prevalent in Latin America, where roughly 10 million individuals could be infected (34). Active PCM mainly affects the lungs, but the fungus can disseminate to any other organ. Cellular immunity is responsible for protection, while antibody titers tend to reflect disease severity; therefore, detection of antifungal components can be useful in diagnosis, and especially in the prognosis of the disease. On the other hand, mouse protection and immunotherapy with monoclonal antibodies have been achieved using the gp43 and gp70 antigens as targets (11, 15). gp43 is a secreted glycoprotein that elicits both cellular and humoral immune responses; it is the main diagnostic antigen so far characterized in P. brasiliensis (38).
Exosomes have been recognized as important structures related to the virulence of microorganisms and modulation of the host's immunity (39). Exosome-like vesicles carrying virulence factors, enzymes, and antigens have recently been characterized in fungal pathogens, such as Cryptococcus neoformans and Histoplasma capsulatum (3, 33). In these microorganisms, membranous vesicular structures could be seen crossing the cell wall and being exported to the extracellular milieu. Extracellular vesicles could then be recovered by ultracentrifugation of culture supernatants. Ergosterol and glucosylceramide have been detected in extracellular vesicles from live (but not heat-killed) C. neoformans, suggesting that they are produced and secreted by viable yeasts as opposed to being a product of cell debris (33). In C. neoformans, membranous vesicles carry glucuronoxylomannan (GXM), the major capsular polysaccharide, to the extracellular space. The polysaccharide is then released and reincorporated into the cell surface as a parallel mechanism of capsule growth (32, 33, 42). Vesicles containing GXM have also been shown to be produced during macrophage infection, suggesting a role in the pathogenesis of the microorganism (26). Indeed, proteomic and lipidomic analyses of vesicles isolated from culture supernatants from both C. neoformans (32) and H. capsulatum (3) have evidenced the presence of virulence factors and enzymes. Microscopic analysis identified a heterogeneous population of extracellular vesicles, not only in C. neoformans (32, 33), but also in H. capsulatum, Saccharomyces cerevisiae, Candida albicans, Candida parapsilosis, and Sporothrix schenckii (3, 27), pointing to the existence of sophisticated mechanisms of vesicle biogenesis in fungi (14). Trocoli Torrecilhas et al. (37) described the role of extracellular vesicles from Trypanosoma cruzi in the pathogenesis of Chagas' disease. Major components of extracellular vesicles from T. cruzi trypomastigotes are glycoproteins of the gp85 trans-sialidase superfamily and glycoconjugates bearing α-linked galactosyl epitopes (α-Gal). Cell components bearing α-Gal epitopes are recognized by anti-α-Gal IgG antibodies that can be isolated from patients with chronic Chagas' disease by affinity chromatography using immobilized Galα1-3Galβ1-4GlcNAc (6). Chagasic (Ch) anti-α-Gal IgG is a lytic antibody, i.e., it can agglutinate and cause parasite lysis by a complement-mediated (6) or -independent (4, 5, 29) mechanism.
In the present work, we characterized extracellular vesicles from P. brasiliensis isolates Pb18 and Pb3. Pb18 represents the major paraphyletic group S1 of the Paracoccidioides complex (23, 36) and has been widely used by researchers due to its virulence (12); Pb3 represents a cryptic PS2 species. We have previously shown that the progression of experimental PCM in B10.A mice differed when infections caused by isolates from S1 (more virulent) and PS2 isolates were compared (13). We show here that vesicles from both isolates carry antigenic molecules that are recognized by total sera and anti-α-Gal antibodies from PCM patients. We also demonstrate that P. brasiliensis is enriched with glycoconjugates containing α-galactosyl epitopes, which are largely expressed on the fungal cell wall and are stored in intracellular vacuoles.
MATERIALS AND METHODS
P. brasiliensis isolates and growth conditions.
P. brasiliensis isolates Pb3 and Pb18 (described in reference 24) were used in this work. The isolates were maintained in the yeast phase at 36°C in slants of modified YPD (mYPD) medium (0.5% yeast extract, 0.5% casein peptone, and 1.5% glucose, pH 6.5). For isolation of extracellular vesicles, yeast cells were transferred from 7-day-old slants to Erlenmeyer flasks containing 200 ml of defined Ham's F-12 medium (Invitrogen) complemented with 1.5% glucose (F-12/glc) and cultivated for 5 days at 36°C with shaking (preinocula). Cell pellets from four preinocula were collected, inoculated in 500 ml of fresh medium, and cultivated for 48 h for vesicle purification. Control cultures for sterol analysis of the vesicles were grown in parallel, and the cell precipitates from preinocula were heat killed at 55°C for 2 h, inoculated in 500 ml of fresh medium, and cultivated for 2 days before processing of the cell-free supernatant.
Isolation of extracellular vesicles.
Vesicles were isolated from P. brasiliensis culture supernatants following subtle modifications of the protocol described by Rodrigues et al. (33). All the steps were carried out in ice or at 4°C to avoid vesicle rupture and/or fusion. Sequential centrifugations of liquid cultures at 4,000 × g (15 min) and 15,000 × g (30 min) were followed to remove whole cells and smaller debris. The pellets were discarded, and the cell/debris-free supernatants were concentrated 20-fold using an Amicon ultrafiltration system (100-kDa cutoff). Concentrated supernatants containing high-molecular-weight components were centrifuged at 15,000 × g (30 min) to remove aggregates, and the resulting supernatant was then ultracentrifuged at 100,000 × g for 1 h to precipitate vesicles. The pellets were either suspended in phosphate-buffered saline (PBS) (250 to 300 μl) for Western blotting and enzyme-linked immunosorbent assay (ELISA) analyses or processed for cryosections and immunogold assays.
Preparation of whole-cell lysates.
P. brasiliensis yeast cell pellets were washed twice in PBS containing proteinase inhibitors {1 mM phenylmethanesulfonylfluoride, 1 μM pepstatin A, 1 mM o-phenanthroline, and 10 μM E-64 [trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane]}, mixed with similar volumes of glass beads (425 to 600 μm; Sigma) and double the volume of PBS, and then vigorously agitated in a vortex (15 times for 30 s, with 30-s intervals in ice). The cell debris was pelleted by centrifugation (5,600 × g), and the supernatant was stored in aliquots at −20°C for further analysis.
Vesicle fractionation and analysis by HPTLC.
For sterol analysis by high-performance thin-layer chromatography (HPTLC), extracellular vesicle pellets from P. brasiliensis Pb18 were processed as described by Rodrigues et al. (33). The pellets were suspended in methanol, and two volumes of chloroform were added. The mixture was vortexed and centrifuged to discard precipitates, and the supernatants were dried by vacuum centrifugation and then partitioned according to the method of Folch et al. (16). The lower phase, containing neutral lipids, was loaded into HPTLC silica plates (Si 60F254s; LiChrospher, Germany) and separated using a solvent system containing hexane-ether-acetic acid (80:40:2 [vol:vol:vol]). The sterol spots were identified after the plate was sprayed with a solution of 50 mg ferric chloride (FeCl3) dissolved in a mixture of 90 ml H2O, 5 ml acetic acid, and 5 ml sulfuric acid. The sprayed plates were incubated at 100°C for 5 min. For ELISA analysis of lipid and lipid-free vesicle fractions, vesicle preparations were extracted with methanol-chloroform, as described previously; both the pellet (lipid free) and the dried supernatant (lipids) were then analyzed.
Isolation of anti-α-galactosyl IgG from patients' sera.
Ch and PCM anti-α-Gal were isolated through affinity chromatography on Synsorb 115 resin containing the trisaccharide Galαl-3Galβ1-4GlcNAc (Chembiomed, Edmonton, Canada) and subsequently on protein A-Sepharose (Amersham Biociences), as described by Almeida et al. (6). Anti-α-Gal antibodies from healthy individuals (normal human serum [NHS] anti-α-Gal) were isolated similarly. IgG was quantified by optical density readings (1.0 A280 unit = 750 μg/ml IgG).
TEM and immunogold labeling.
For transmission electron microscopy (TEM) analysis of P. brasiliensis yeasts, cells from logarithmic-phase cultures grown in liquid mYPD medium were processed in fixative solutions and embedded in hydrophobic Spurr resin exactly as described by Batista et al. (9). For immunogold labeling, both P. brasiliensis Pb18 cells and vesicle pellets obtained after centrifugation at 100,000 × g were processed as described by Rodrigues et al. (33). Briefly, cryosections were obtained in a temperature range from −70°C to −90°C using an Ultracut cryoultramicrotome (Reichert). After being blocked in acetylated bovine serum albumin (cBSA) (Aurion)-PBS and 50 mM NH4Cl, the cryosections were incubated overnight at 4°C with 20 μg/ml of gold-labeled Marasmius oreades agglutinin (MOA) lectin (EY Laboratories; 5 nm) or Ch anti-α-Gal IgG (50 μg/ml in 1% cBSA-PBS), followed by a 1-h incubation with 12-nm colloidal-gold–goat anti-human IgG (Jackson ImmunoResearch; 1:20 in 1% cBSA-PBS). Control grids incubated with MOA-gold particles were treated with green coffee bean α-galactosidase (Sigma Aldrich), and Ch anti-αGal control grids were processed in the absence of primary antibodies. Samples were examined in a JEOL 1200 EX-II electron microscope. Quantification of immunogold labeling was done by counting gold particles per nm2 in the regions of interest.
Confocal microscopy.
P. brasiliensis yeast cells from isolate Pb18 growing in F-12/glc aerated liquid cultures in logarithmic phase were collected, washed twice in PBS, adjusted to 3 × 106 viable cells/ml, and fixed for 30 min in cold methanol (80%), 4% formamide, 1% glutaraldehyde, which also made the cells permeable. Cell pellets were incubated with 3% (wt/vol) BSA in PBS (blocking buffer) for 4 h at room temperature, washed three times with PBS, and then incubated with 100 μg/ml of Ch, PCM, or NHS anti-α-Gal IgG for 16 h at 4°C. The cells were then incubated for 2 h in the dark with Alexa Fluor 488 goat anti-human IgG (Invitrogen) in blocking buffer at 1:200 dilution. Between incubation steps with antibodies, the fungal cells were washed six times with PBS. Each suspension (10 μl) was prepared on glass slides with Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA) and analyzed using a laser scanning confocal microscope (LSM-510 Axiovert; Carl Zeiss, Jena, Germany).
ELISA and immunoblotting.
To analyze the reactivity of both biotinylated MOA and Ch anti-α-Gal IgG with vesicle preparations and whole-cell extracts from P. brasiliensis Pb3 and Pb18, 96-well microplates (Nunc) were coated with 1 nM vesicle sterol contents (estimated with the Amplex Red Cholesterol Assay kit) or cell extracts expressed as protein contents (estimated with Bradford's protein assay [10]). When applied, treatment with α-galactosidase was carried out with substrate immobilized to the microplates. The microplates were incubated overnight at 4°C, and nonspecific interactions were blocked with 5% skim milk in PBS for 4 h at 36°C or with 0.1% BSA-PBS overnight at 4°C. A concentration of 70 μg/ml of biotinylated MOA or 2.5 to 10 μg/ml Ch anti-α-Gal diluted in 0.1% BSA-PBS was used to react with vesicle preparations upon incubation for 4 h at 36°C or overnight at 4°C. This step was followed by a 1-h incubation with peroxidase-conjugated sheep anti-human IgG or peroxidase-conjugated streptavidin (1:1,000 in 0.1% BSA-PBS). The reactions were visualized using a chemiluminescent substrate (Pierce or Millipore ECL substrate), and the results were recorded in a microplate luminometer. ELISA with lipid fractions extracted from vesicles was carried out as described previously (25), with solvent-washed 96-well plates coated with lipids diluted in methanol and evaporated to dryness at room temperature. ELISA data were recorded (in relative luminescence units [RLU]) as mean values from triplicates, with standard deviations. The values of negative controls in the absence of either antibodies or substrate were subtracted from the values obtained for test samples.
For immunoblotting, about 6% of one vesicle preparation batch (500 ml culture) from P. brasiliensis was subjected to electrophoresis in a 10% SDS-polyacrylamide gel (21) and electrotransferred to nitrocellulose membranes. The incubation conditions with antibodies and conjugates were as described above for ELISA and elsewhere (17). For silver staining (8), about half of a vesicle preparation (500 ml culture) was necessary for band visualization.
Deglycosylation with endoglycosidase H and β-elimination.
Vesicle and whole-cell extract preparations of P. brasiliensis Pb18 were treated with endoglycosidase H (Endo H; Sigma Aldrich) to removed high-mannose N-linked oligosaccharides in 50 mM sodium citrate buffer, pH 5.0, for 48 h at 36°C. Control samples were incubated in the absence of enzyme. Chemical treatment with 1 M NH4OH for 48 h at 36°C was used to remove O-linked glycan chains (20). The proteinase inhibitors E-64 (15 μM) and pepstatin (1 μM) were added in all reactions.
Statistical analysis.
We used Student's t test to analyze the significance of our results.
RESULTS
Characterization of extracellular vesicles.
The presence of extracellular vesicles produced by P. brasiliensis was evidenced by TEM. In rare images, vesicles could be seen at the surface of the cell wall in preparations of yeast cells growing in both mYPD medium (Fig. 1A) and F-12/glc (not shown). In order to isolate extracellular vesicles from culture supernatants, yeast cells were cultivated for 48 h in F-12/glc medium starting from heavy preinocula constituted of cell precipitates from 5-day-old logarithmic cultures. The estimated average sterol yields from two different preparations were 38 ng sterol/ml of cells (wet pellet) for Pb3 and 65 ng sterol/ml of cells for Pb18. Preparations from 500-ml cultures resulted in wet cell pellets of about 6 ml for Pb3 and 8 ml for Pb18. Figure 1B shows the presence of vesicles smaller than 100 nm in a representative 100,000 × g pellet. The lipid bilayer membrane can be seen in a representative vesicle (Fig. 1C and D, labeled with MOA-gold particles).
Fig. 1.
(A to C) TEM images of a P. brasiliensis yeast cell (A) and vesicle pellets from two different preparations (B and C). In panel A, vesicles are seen at the surface (arrows) outside of the cell wall (CW). (D) Detail of the boxed area of the vesicle shown in panel C allowing visualization of a double-layer membrane structure. The dark dots in panel C are MOA-labeled gold particles, as explained in the text. Scale bars of 100 nm (A and B) and 50 nm (C) are included.
To confirm that the vesicles originated from exosomal bilayer formation and not from membranes released from dead cells, we analyzed a control 100,000 × g pellet preparation obtained from culture supernatants of heat-killed P. brasiliensis yeast cells, in parallel with live cells. HPTLC detected sterols migrating next to ergosterol only in preparations from live-cell cultures (Fig. 2), suggesting that membranes did not result from cell debris. Similar results have previously been obtained for C. neoformans (33).
Fig. 2.

HPTLC of extracellular 100,000 × g pellets obtained from supernatant cultures of live or heat-killed (HK) yeast cells. Ergosterol (arrow) was used as a standard. #, origin of the chromatogram. For this experiment, one batch of vesicle preparation (500 ml culture) was used for sterol extraction and visualization in the chromatogram. Our best results are shown.
Preliminary evidence of extracellular vesicles in P. brasiliensis cultures has been obtained by our group from a series of studies with the Pb339 isolate (A. L. Matsuo, L. Ganiko, I. C. Almeida, and R. Puccia, unpublished data). In the present work, we used Pb18 as a model but compared some antigenic characteristics with Pb3 vesicle preparations. Silver-stained SDS-PAGE profiles of vesicle preparations from Pb18 and Pb3 were similar, with visible components migrating within molecular masses between 98 and 50 kDa (Fig. 3). The stacking gel did not stain, suggesting that most components had indeed been resolved in the running gel. Some vesicle components were recognized specifically by sera from a pool of PCM patients in immunoblotting (Fig. 3), as opposed to the complete lack of reactivity with a pool of sera from healthy individuals (NHS). A reactive broad smear was clearly seen in both Pb18 and Pb3 preparations, as well as three other bands with masses estimated as 49 kDa, 64 kDa, and 75 kDa. A high-molecular-mass antigenic band was observed in Pb3 and only faintly in Pb18; a 47-kDa component was revealed only in Pb3, whereas two weak and thin bands slower than 82 kDa were observed only in Pb18 preparations.
Fig. 3.
Silver-stained components and antigenic profiles of Pb3 and Pb18 vesicle preparations in reducing SDS-PAGE 10% gels. Antigenic profiles are seen in immunoblots revealed with a pool of PCM patients' sera, compared with a pool of sera from healthy individuals (NHS), both tested at 1:1,000. The asterisks indicate migrations of differentially reactive components. The positions of mass markers (kDa) are indicated on the left. sg, stacking gel.
Finding α-Gal epitopes in P. brasiliensis vesicles.
While looking for vesicle markers, we surprisingly detected strong reactivity in ELISA of P. brasiliensis preparations from both Pb3 and Pb18 with anti-α-galactosyl IgG isolated from patients with chronic Chagas' disease (Fig. 4, left). Immunoreactivity with Pb3 and Pb18 preparations decreased about 45% upon treatment with green coffee bean α-galactosidase in two sets of experiments. Figure 4 shows one of these experiments, where the enzyme was used to compete with binding of the anti-α-galactosyl IgG. These results suggested the involvement of terminal α-galactosyl epitopes in the reaction and were corroborated by intense reactivity in ELISA with MOA lectin (Fig. 4, right), which specifically binds to terminal nonreducing Galα1,3Gal moieties (41). The reactivity with MOA lectin decreased about 75% upon treatment of P. brasiliensis vesicles from both Pb3 and Pb18 with α-galactosidase (Fig. 4, right) in single experiments, supporting similar results previously obtained with vesicles from Pb339.
Fig. 4.
Reactivity in ELISA of Pb18 and Pb3 vesicle preparations with Ch anti-α-Gal antibodies (left) or MOA lectin (right). The preparations were previously treated (+) or not (−) with α-galactosidase. The percentages of inhibition after treatment with α-galactosidase are indicated. The asterisks denote statistical significance (P < 0.05). The error bars indicate standard deviations.
TEM images confirmed the ELISA data and revealed immunogold labeling with MOA lectin on the surfaces and in the lumen of Pb18 vesicles (Fig. 5A, B, and C). Negative controls—vesicles treated with α-galactosidase—had decreased reactivity (Fig. 5D), as indicated by particle counts (Fig. 5E), once again suggesting the presence of vesicular components carrying α-galactosyl residues that are involved in lectin binding.
Fig. 5.
TEM images of two distinct vesicle preparations (panel A and panels B, C, and D) incubated with MOA-labeled gold before (A, B, and C) and after (D) treatment with α-galactosidase. (E) Quantification of the gold particles showed decreased labeling after treatment with the enzyme. The error bars indicate standard deviations. Scale bars of 100 nm (A) and 50 nm (B, C, and D) are included.
Isolation of PCM α-Gal IgG and confocal and immunogold microscopy.
We managed to isolate anti-α-Gal IgG from a pool of PCM patients' sera using the same methodology employed to purify Ch anti-α-Gal (6). Since we did not have access to large volumes of PCM patients' sera, only a few experiments were carried out with PCM anti-α-Gal. In Fig. 6, left, note that it reacted in ELISA with Pb18 vesicle preparations in a dose-dependent manner, while at comparable antibody concentrations, NHS anti-αGal purified from healthy individuals reacted poorly. For both Pb3 and Pb18 vesicles, the reactivity with PCM anti-α-Gal was significantly inhibited by α-galactosidase between 34% and 38%, pointing to the involvement of terminal α-galactosyl epitopes in the reaction.
Fig. 6.
(A) Reactivity in ELISA of a Pb18 vesicle preparation with different concentrations of either PCM or natural (NHS) anti-α-Gal. (B) Reactivity in ELISA of 2.5 μg/ml of PCM anti-α-Gal with Pb3 and Pb18 vesicle preparations treated (+) or not (−) with α-galactosidase. Similar percentages of inhibition were obtained in replicates. The asterisks indicate statistical significance (P < 0.05). The error bars indicate standard deviations.
When PCM anti-α-Gal was used to localize the corresponding epitopes in P. brasiliensis yeast cells by confocal microscopy, we observed intense labeling that was comparable to that obtained with Ch anti-α-Gal (Fig. 7). In both cases, fluorescence was distributed in a punctuated pattern, which is compatible with vesicular distribution. The same pattern was observed on the cell surface and inside the cell (Fig. 7). NHS anti-α-Gal stained the P. brasiliensis cells poorly and diffusely, following a background pattern. Intracellular localization of α-galactosyl epitopes was also suggested by ELISA carried out with cell lysates, for which the patterns of dose-response reactivity with PCM and NHS anti-α-Gal were similar to those observed in Fig. 6 (not shown). When Pb18 yeast cells were incubated with Ch anti-α-Gal and analyzed by TEM, immunogold particles were abundantly observed on the cell wall (Fig. 8A) and inside vacuoles (Fig. 8B), but not in negative controls (Fig. 8C and D). Labeling in these compartments was specific, as indicated by particle counting (Fig. 8E). Labeling in vesicle-like structures inside the vacuoles is suggested in Fig. 8B.
Fig. 7.
Confocal microscopy of P. brasiliensis yeast cells comparatively labeled with natural (NHS), PCM, and Ch anti-α-Gal, as indicated. Both immunofluorescence and merged images with phase-contrast are shown. Scale bars are included.
Fig. 8.
(A and B) Cell wall (A) and vacuolar (B) localization of components bearing α-Gal epitopes in P. brasiliensis yeast cells incubated with Ch anti-α-Gal. (C and D) Negative controls lacked the primary Ch anti-α-Gal antibody. (E) Quantification of gold particles was negligible in the Formvar grids and in the cytoplasm. Higher labeling was detected on the cell wall (CW) and vacuole. The error bars indicate standard deviations. Scale bars of 500 nm (A and C), 250 nm (B), and 200 nm (D) are included.
Nature of α-Gal-containing components.
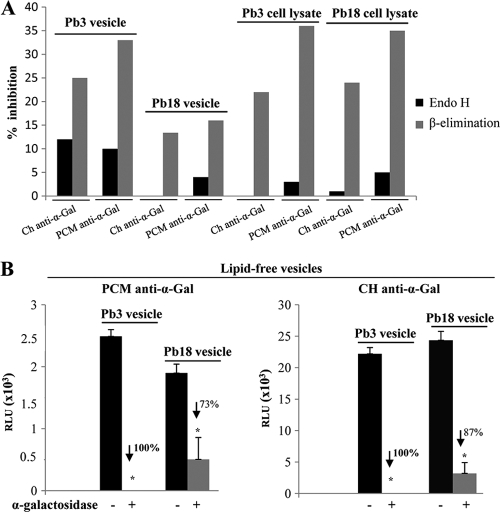
In T. cruzi, α-galactosyl epitopes are part of O-linked glycans found in mucins (5). In P. brasiliensis, we investigated the nature of protein moieties carrying α-Gal epitopes by treating vesicle and cell lysate preparations of P. brasiliensis Pb18 either with Endo H, to remove N-linked mannose chains, or mildly with NH4OH (β-elimination), to remove O-linked glycans. The treated samples were compared with controls by ELISA reactivity with both PCM and Ch anti-α-Gal. Figure 9A shows percentages of inhibition up to 35% after β-elimination, suggesting that at least part of the P. brasiliensis epitopes recognized by PCM and Ch anti-α-Gal are found in oligosaccharides O linked to proteins. Smaller percentages of inhibition were obtained with Pb18 vesicles, and differences in the nature of α-Gal epitopes between Pb18 and Pb3 have to be better investigated. Our results with Endo H Pb3 vesicles also suggest that some α-Gal epitopes could be expressed in N-linked chains, since inhibitions of about 10% were seen.
Fig. 9.
(A) Effect of β-elimination or Endo H treatment on reactivity with anti-α-Gal (5 μg/ml). Pb3 and Pb18 vesicle or cell lysate preparations were compared for reactivity with PCM and Ch anti-α-Gal before and after treatment. The percent inhibition is shown for representative results from replicate or triplicate experiments. (B) Lipid-free fractions (200 ng of protein/well) from Pb3 and Pb18 vesicles reacted with both PCM and Ch anti-α-Gal (2.5 μg/ml); the percent inhibition after treatment with α-galactosidase (+) is indicated. Similar results were obtained in replicates for Pb3, carried out with both 200 ng and 500 ng of protein/well. The error bars indicate standard deviations.
To better investigate the localization of α-Gal epitopes, we fractionated Pb3 and Pb18 vesicle preparations into lipid and lipid-free fractions and tested the products by ELISA with anti-α-Gal before and after treatment with α-galactosidase. Figure 9B shows that lipid-free fractions reacted with both PCM and Ch anti-α-Gal, and this reaction was highly inhibited by α-galactosidase. Lipid fractions were also tested, but the results were not reproducible, probably because of the small amounts of substrate used in the reactions. We did, however, observe reactivity with lipid fractions at least once, which was completely abolished after α-galactosidase treatment. Therefore, α-Gal epitopes in P. brasiliensis glycolipids are likely to occur, but this has to be better investigated.
DISCUSSION
The present work described the presence of extracellular vesicles in the yeast phase of P. brasiliensis for the first time by using isolate Pb18 as a model and comparing some antigenic characteristics of vesicle preparations with the phylogenetically distinct isolate Pb3. We showed that P. brasiliensis extracellular vesicles carry antigenic components bearing highly immunogenic α-linked galactosyl epitopes. In the cells, components bearing α-Gal epitopes were found abundantly distributed on the cell wall, following a confocal punctuated pattern, but they were also stored inside large intracellular vacuoles. The α-galactosyl-containing components from P. brasiliensis reacted strongly in ELISA with anti-α-Gal antibodies isolated from both Chagasic and PCM patients and with MOA lectin, but only weakly with natural anti-α-Gal from healthy individuals.
Natural immunoglobulins specifically recognizing nonreducing terminal α-galactosyl epitopes are prevalent in sera from healthy individuals and account for about 1% of the total IgG. These antibodies preferentially bind to terminal Galα1,3Galβ1,4GlcNAc-R trisaccharide (Galili epitope), which is absent in humans, apes, and Old World monkeys (reviewed in reference 22). By eliminating self α-Gal epitope through mutation in the α-1,3-galactosyltransferase gene, protective antibodies would be produced against microorganisms. Indeed, viruses, bacteria, and protozoa can express a variety of α-Gal epitopes; reactivity with the host's anti-α-Gal might cause elimination of the infectious agent (22). In T. cruzi, the causative agent of Chagas' disease, α-galactosyl epitopes are part of long and complex sugar chains that are O linked mainly to threonine residues of glycosylphosphatidylinositol (GPI)-anchored mucin-like glycoproteins (4, 5). Chagasic anti-α-Gal IgG from patients with chronic Chagas' disease purified by affinity chromatography through immobilized Galα1,3Galβ1,4GlcNAc is able to recognize epitopes terminating in Galα1,2Gal, Galα1,3Gal, Galα1,6Gal, and Galα1,3-galactofuranose (Galf) (5). Chagasic anti-α-Gal IgG can agglutinate and cause parasite lysis by a complement-mediated (6) or -independent (4, 5, 29) mechanism, corroborating the aforementioned hypothesis.
In P. brasiliensis, we found that both PCM and Chagasic anti-α-Gal IgG reacted intensely with extracellular vesicles, in contrast with the modest reaction evoked by natural anti-α-Gal antibodies. These observations are similar to those reported by Almeida et al. (4) for trypomastigote surface mucins, which showed enhanced affinity for antibodies from patients with chronic Chagas' disease compared to natural anti-α-Gal. Our results therefore suggest the presence in P. brasiliensis of a variety of nonreducing terminal α-linked galactopyranosyl epitopes that are probably similar to those found in T. cruzi mucins, as judged by the strong reactions with Chagasic anti-α-Gal. The presence of α-Gal residues in P. brasiliensis vesicles was unequivocally shown after treatment of both whole and fractionated vesicles with α-galactosidase. On the other hand, inhibition by α-galactosidase of vesicle reactivity with MOA lectin was around 75%, compared with approximately 50% for Chagasic anti-α-Gal and about 35% for PCM anti-α-Gal, while almost complete inhibition was achieved when lipid-free vesicle fractions were tested with anti-α-Gal. These differences may be attributed to different degrees of accessibility of the enzyme to the cleavable residues under our ELISA conditions. It is also noteworthy that Ch anti-α-Gal antibodies can recognize a broader variety of linkages than does the MOA lectin. The latter is highly specific for terminal Galα1,3Gal disaccharides of blood type B, with enhanced affinity achieved after addition of β1,4GlcNAc or α1,2-l-Fuc; however, it does not bind to Galα1,2Gal and Galα1,6Gal disaccharides (41).
In T. cruzi, α-Gal epitopes are part of long, mostly branched O-linked glycans of mucins (5). In P. brasiliensis, a broad smear characterized immunoblotting reactions of anti-α-Gal with both vesicle preparations and cell extracts, suggesting antibody binding to highly glycosylated components (not shown). We found evidence for the localization of α-Gal epitopes in glycoproteins, where they are at least partially distributed in O-linked glycan chains and possibly also in N-linked chains in Pb3. Localization in glycolipids seems to occur, but those results should be better investigated. Eukaryotic O-glycans are usually attached to the β-hydroxyl group of serines or threonines. Unlike those of mammals and parasites, fungal O-glycans, such as those from C. albicans, S. cerevisiae, and Pichia pastoris, tend to be short (2 to 5 residues) and generally linear, and the main component is mannose (reviewed in reference 19). Nevertheless, terminal or side α-1,2- and α-1,3-galactopyranose (Galp) residues linked to mannose have been found in Aspergillus and Schizosaccharomyces pombe, where an α-1,2-galactosyltransferase uses UDP-galactose as a sugar donor (19). Examples of fungal galactose residues linked to other galactoses can be found in polysaccharides (1, 18).
In P. brasiliensis, we have found two annotated galactosyltransferase sequences in the Pb3 and Pb18 genomes released by the Broad Institute (http://www.broadinstitute.org/annotation/genome/paracoccidioides_brasiliensis). In this fungus, a high-molecular-mass extracellular galactomannan bears terminal β-Galp residues linked to mannose (30). Galactomannans extracted from the mycelium cell wall have α-Galf1,6α-Manp1,2 side chains, which in the yeast phase contain mostly β-Galf instead of the rare α-Galf (2). Terminal β-galactofuranosyl residues are commonly found in galactomannans of pathogenic fungi and are responsible for cross-reactivity in serum diagnosis, as exemplified in P. brasiliensis by the main diagnostic glycoprotein gp43 (7, 31). It is also noteworthy that β-Galf is an essential part of the carbohydrate epitope Galfβ1,6(Manpα1,3)Manpβ1,2-inositol contained in P. brasiliensis glycosphingolipids (35).
In C. neoformans, vesicles containing GXM are produced during macrophage infection, suggesting a role in the pathogenesis of the microorganism (33). In addition, recent in vitro data showed that vesicles can be incorporated by macrophages and stimulate expression of tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), interleukin 10 (IL-10), and nitric oxide, and hence, they increase the phagocyte microbicidal capacity (26). When α-Gal-rich vesicles secreted by T. cruzi infective trypomastigote forms were administered in vivo to BALB/c mice, increased tissue parasitism and a severe inflammatory reaction by stimulation of IL-4 and IL-10 synthesis were observed (37). In P. brasiliensis, we analyzed extracellular vesicles from both Pb18 and Pb3, which evoke experimental PCM in B10.A mice that have distinct outcomes (13). We observed that the immunoblotting antigenic profiles of Pb3 and Pb18 vesicle preparations had a few differentially reactive components (Fig. 3). Preliminary data from our group suggested that vesicle preparations from these two isolates are able to stimulate cytokine expression of macrophages in vitro and that these responses can vary according to the isolate (M. C. Vallejo, L. Ganiko, I. C. Almeida, and R. Puccia, unpublished data); however, the participation of P. brasiliensis α-Gal epitopes in the host's cellular immune response is so far speculative.
Fungal components carrying α-Gal epitopes recognized by both anti-α-Gal and MOA lectin could be observed, not only in P. brasiliensis vesicles, but also along the cell wall and in intracellular vacuoles, where labeling seemed to target vesicle-like structures distributed inside and near the vacuolar membrane. Vacuoles are important fungal components where diverse membrane-trafficking pathways can converge. They contain molecules coming from the secretory pathway on their way to be secreted, as well as molecules that have been endocytosed or derived from autophagy that are meant to be disposed of or degraded (40). The biogenesis of fungal extracellular vesicles has recently been addressed using S. cerevisiae mutants with defects in conventional secretion pathways and multivesicular body trafficking (27, 28). Vesicular export has not been affected in any of the mutants analyzed; however, the relative peptide abundances varied in a considerable number of vesicular proteins. In mutants with affected Golgi-dependent secretion mechanisms, a decrease in the rate of vesicle release was observed. These results suggest that vesicular transport involves conventional and unconventional pathways. Components bearing α-Gal epitopes should use the conventional secretory pathway in order to be glycosylated, but they seem to be stored in P. brasiliensis vacuoles that resemble multivesicular bodies. This supposition is consistent with the existence of different points of convergence between Golgi-derived and endosome-related secretory pathways (39).
The finding of extracellular vesicles in P. brasiliensis opens new areas to explore in terms of the host-parasite relationship. Antigenic components are exported by this route, and also molecules that can interact with the host's cellular immune system. The role played by P. brasiliensis vesicles in vivo and the differences in contents that could account for distinct disease outcomes depending on the isolate are subjects to be addressed. The present work describes the presence of highly immunogenic and complex α-galactosyl epitopes in components that are transported by vesicles to the extracellular milieu, stored in intracellular vacuoles, and abundantly seen on the cell wall, where they are likely to be transiently trapped on their way outward. The importance of these carbohydrate epitopes in the interface with the host remains to be explained.
ACKNOWLEDGMENTS
We thank Luiz R. Travassos, Marcio L. Rodrigues, and Leonardo Nimrichter for discussions and suggestions. We are grateful to the Biomolecule Analysis Core Facility at the BBRC/Biology/UTEP.
This work was funded by FAPESP, CNPq, and NIH (grants 5G12RR008124-16A1 and 5G12RR008124-16A1S1).
Footnotes
Published ahead of print on 7 January 2011.
REFERENCES
- 1. Ahrazem O., Prieto A., Leal J., Jimenez-Barbero J., Bernabe M. 2002. Fungal cell wall galactomannan isolated from Apodus deciduus. Carbohydr. Res. 337:1503–1506 [DOI] [PubMed] [Google Scholar]
- 2. Ahrazem O., et al. 2003. Structural differences between the alkali-extracted water-soluble cell wall polysaccharides from mycelial and yeast phases of the pathogenic dimorphic fungus Paracoccidioides brasiliensis. Glycobiology 13:743–747 [DOI] [PubMed] [Google Scholar]
- 3. Albuquerque P. C., et al. 2008. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 10:1695–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almeida I. C., Ferguson M. A., Schenkman S., Travassos L. R. 1994. GPI-anchored glycoconjugates from Trypanosoma cruzi trypomastigotes are recognized by lytic anti-alpha-galactosyl antibodies isolated from patients with chronic Chagas' disease. Braz. J. Med. Biol. Res. 27:443–447 [PubMed] [Google Scholar]
- 5. Almeida I. C., Ferguson M. A., Schenkman S., Travassos L. R. 1994. Lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J. 304:793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almeida I. C., Milani S. R., Gorin P. A., Travassos L. R. 1991. Complement-mediated lysis of Trypanosoma cruzi trypomastigotes by human anti-alpha-galactosyl antibodies. J. Immunol. 146:2394–2400 [PubMed] [Google Scholar]
- 7. Almeida I. C., et al. 1996. Structure of the N-linked oligosaccharide of the main diagnostic antigen of the pathogenic fungus Paracoccidioides brasiliensis. Glycobiology 6:507–515 [DOI] [PubMed] [Google Scholar]
- 8. Ansorge W. 1985. Fast and sensitive detection of protein and DNA bands by treatment with potassium permanganate. J. Biochem. Biophys. Methods 11:13–20 [DOI] [PubMed] [Google Scholar]
- 9. Batista W. L., et al. 2006. The PbMDJ1 gene belongs to a conserved MDJ1/LON locus in thermodimorphic pathogenic fungi and encodes a heat shock protein that localizes to both the mitochondria and cell wall of Paracoccidioides brasiliensis. Eukaryot. Cell 5:379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradford M. M. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 11. Buissa-Filho R., et al. 2008. The monoclonal antibody against the major diagnostic antigen of Paracoccidioides brasiliensis mediates immune protection in infected BALB/c mice challenged intratracheally with the fungus. Infect. Immun. 76:3321–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calich V. L., Vaz C. A., Burger E. 1998. Immunity to Paracoccidioides brasiliensis infection. Res. Immunol. 149:407–417 [DOI] [PubMed] [Google Scholar]
- 13. Carvalho K. C., et al. 2005. Virulence of Paracoccidioides brasiliensis and gp43 expression in isolates bearing known PbGP43 genotype. Microbes Infect. 7:55–65 [DOI] [PubMed] [Google Scholar]
- 14. Casadevall A., Nosanchuk J. D., Williamson P., Rodrigues M. L. 2009. Vesicular transport across the fungal cell wall. Trends Microbiol. 17:158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Mattos G., de Almeida S. R., Mariano M., Lopes J. D. 2003. Characterization of gp70 and anti-gp70 monoclonal antibodies in Paracoccidioides brasiliensis pathogenesis. Infect. Immun. 71:6534–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497–509 [PubMed] [Google Scholar]
- 17. Ganiko L., et al. 2007. Paracoccin, an N-acetyl-glucosamine-binding lectin of Paracoccidioides brasiliensis, is involved in fungal growth. Microbes Infect. 9:695–703 [DOI] [PubMed] [Google Scholar]
- 18. Gómez-Miranda B., et al. 2004. Differences among the cell wall galactomannans from Aspergillus wentii and Chaetosartorya chrysella and that of Aspergillus fumigatus. Glycoconj. J. 20:239–246 [DOI] [PubMed] [Google Scholar]
- 19. Goto M. 2007. Protein O-glycosylation in fungi: diverse structures and multiple functions. Biosci. Biotechnol. Biochem. 71:1415–1427 [DOI] [PubMed] [Google Scholar]
- 20. Hanisch F. G., Jovanovic M., Peter-Katalinic J. 2001. Glycoprotein identification and localization of O-glycosylation sites by mass spectrometric analysis of deglycosylated/alkylaminylated peptide fragments. Anal. Biochem. 290:47–59 [DOI] [PubMed] [Google Scholar]
- 21. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 22. Macher B. A., Galili U. 2008. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim. Biophys. Acta 1780:75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matute D. R., et al. 2006. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol. Biol. Evol. 23:65–73 [DOI] [PubMed] [Google Scholar]
- 24. Morais F. V., Barros T. F., Fukada M. K., Cisalpino P. S., Puccia R. 2000. Polymorphism in the gene coding for the immunodominant antigen gp43 from the pathogenic fungus Paracoccidioides brasiliensis. J. Clin. Microbiol. 38:3960–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nimrichter L., et al. 2005. Structure, cellular distribution, antigenicity, and biological functions of Fonsecaea pedrosoi ceramide monohexosides. Infect. Immun. 73:7860–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oliveira D. L., et al. 2010. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 78:1601–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliveira D. L., et al. 2010. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS. One 5:e11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oliveira D. L., et al. 2010. Biogenesis of extracellular vesicles in yeast: many questions with few answers. Commun. Integr. Biol. 3:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pereira-Chioccola V. L., et al. 2000. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J. Cell Sci. 113:1299–1307 [DOI] [PubMed] [Google Scholar]
- 30. Puccia R., Schenkman S., Gorin P. A., Travassos L. R. 1986. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect. Immun. 53:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Puccia R., Travassos L. R. 1991. 43-Kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, or Jorge Lobo's disease. J. Clin. Microbiol. 29:1610–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodrigues M. L., et al. 2008. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 7:58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodrigues M. L., et al. 2007. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell 6:48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. San-Blas G., Nino-Vega G., Iturriaga T. 2002. Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med. Mycol. 40:225–242 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki E., Toledo M. S., Takahashi H. K., Straus A. H. 1997. A monoclonal antibody directed to terminal residue of beta-galactofuranose of a glycolipid antigen isolated from Paracoccidioides brasiliensis: cross-reactivity with Leishmania major and Trypanosoma cruzi. Glycobiology 7:463–468 [DOI] [PubMed] [Google Scholar]
- 36. Teixeira M. M., et al. 2009. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 52:273–283 [DOI] [PubMed] [Google Scholar]
- 37. Trocoli Torrecilhas A. C., et al. 2009. Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 11:29–39 [DOI] [PubMed] [Google Scholar]
- 38. Travassos L. R., Taborda C. P., Iwai L. K., Cunha-Neto E. C., Puccia R. 2003. The gp43 from Paracoccidioides brasiliensis: a major diagnostic antigen and vaccine canditate, p. 279–296 In Domer J. E., Kobayashi G. S. (ed.), The Mycota XII: human fungal pathogens. Springer-Verlag, Berlin, Germany [Google Scholar]
- 39. van Niel G., Porto-Carreiro I., Simoes S., Raposo G. 2006. Exosomes: a common pathway for a specialized function. J. Biochem. 140:13–21 [DOI] [PubMed] [Google Scholar]
- 40. Veses V., Richards A., Gow N. A. 2008. Vacuoles and fungal biology. Curr. Opin. Microbiol. 11:503–510 [DOI] [PubMed] [Google Scholar]
- 41. Winter H. C., Mostafapour K., Goldstein I. J. 2002. The mushroom Marasmius oreades lectin is a blood group type B agglutinin that recognizes the Galalpha 1,3Gal and Galalpha 1,3Galbeta 1,4GlcNAc porcine xenotransplantation epitopes with high affinity. J. Biol. Chem. 277:14996–15001 [DOI] [PubMed] [Google Scholar]
- 42. Yoneda A., Doering T. L. 2006. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol. Biol. Cell 17:5131–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]