Abstract
The genome of Borrelia burgdorferi, the causative agent of Lyme disease, is comprised of a large linear chromosome and numerous smaller linear and circular plasmids. B. burgdorferi exhibits substantial genomic variation, and previous studies revealed genotype-specific variation at the right chromosomal telomere. A correlation has also been established between genotype and invasiveness. The correlation between chromosome length and genotype and between genotype and invasiveness suggested that a gene(s) at the right chromosome telomere may be required for virulence. Of particular interest was bb0844, an RpoS-regulated gene at the right telomere, the expression of which is induced when the spirochete undergoes adaptation to the mammalian host. The structure of the right chromosomal telomere was examined in 53 B. burgdorferi clinical isolates of various genotypes. Four distinct patterns were observed for bb0844: (i) chromosomal localization, (ii) plasmid localization, (iii) presence on both chromosome and plasmid, and (iv) complete absence. These patterns correlated with the B. burgdorferi genotype. On the basis of available sequence data, we propose a mechanism for the genomic rearrangements that accounts for the variability in bb0844 genomic localization. To further explore the role of BB0844 in the spirochete life cycle, a bb0844 deletion mutant was constructed by allelic exchange, and the viability of wild-type and bb0844 deletion mutants was examined in an experimental mouse-tick infection model. The bb0844 mutant was fully infectious in C3H/HeJ mice by either needle inoculation or tick transmission with B. burgdorferi-infected Ixodes scapularis larvae. Naïve larval ticks acquired both wild-type and mutant spirochetes with equal efficiency from B. burgdorferi-infected mice. The results demonstrate that BB0844 is not required for spirochete viability, pathogenicity, or maintenance in the tick vector or the mammalian host. At present, a defined role for BB0844 in B. burgdorferi cannot be ascertained.
Borrelia burgdorferi, the Lyme disease pathogen, has a complex life cycle in nature which involves an arthropod vector and diverse mammalian hosts (6, 46). The type strain, B31, has a complex genome comprised of a linear chromosome and at least 21 linear and circular plasmids (12, 19). The linear replicons have covalently closed hairpin telomeric ends (11, 24). The chromosome of B. burgdorferi strain B31 is 910,725 bp long and includes 853 putative open reading frames (ORFs) (19). A majority of the chromosomal sequence and gene order is conserved among B. burgdorferi strains; however, the chromosomes vary in length. These differences were shown to be the result of variability in the right telomeric region (10, 11) and have been confirmed by sequencing of additional B. burgdorferi genomes (40). It has been proposed that linear plasmid fragments were added to the right chromosomal telomere by means of nonhomologous recombination, after which portions of the transposed plasmid sequences underwent mutational decay (12, 13, 25).
Numerous approaches have been employed for typing of B. burgdorferi isolates (56). For example, North American B. burgdorferi isolates can be classified into three rRNA gene spacer types (RST) based on the rRNA gene spacer sequence (32) and 17 types based on the ospC sequence (41). Importantly, a correlation between genotype and pathogenicity has been observed. Isolates with an RST1 genotype were more likely than RST3 isolates to produce disseminated infection in humans or mice (55, 58, 59). Similarly, OspC type A, B, I, H, and K isolates were most likely to disseminate (41, 58). Comparative genome hybridization analysis of 16 B. burgdorferi isolates revealed that ORFs bb0843.1 to bb0853.1 were absent from the chromosomes of most of the RST3 isolates analyzed (11, 49). Overall, the length of the right chromosomal telomere correlated with the RST; RST1 and RST2 isolates contained longer right chromosomal telomeric regions than most RST3 strains, whose chromosomes end with ORF bb0843 (11, 25, 49). The right telomere region of strain B31MI contains only two intact ORFs, bb0844 and bb0852 (19). bb0844 encodes a hypothetical protein of 323 amino acid residues with a predicted molecular mass of 37.5 kDa. The encoded sequence contains a signal peptidase II recognition signal and is predicted to be a lipoprotein. It should be noted that in the recent reannotation of the B. burgdorferi B31MI genome sequence on the Institute for Genomic Research (TIGR) comprehensive microbial resource (http://cmr.jcvi.org/cgi-bin/CMR/CmrHomePage.cgi), bb0844 has been renamed bb0860; the gene name remains as bb0844 in GenBank. In this report, bb0844 is used throughout.
Regulation of gene expression is a key strategy that B. burgdorferi employs in response to diverse environmental signals encountered in arthropod and mammalian hosts. Central to the process of differential gene expression is the alternative sigma factor RpoS, which governs differential expression of various B. burgdorferi genes during its enzootic life cycle (8, 27, 60). RpoS appears to serve a “gatekeeper” function; its induction during nymphal feeding (i.e., the RpoS-on state) results in repression of tick phase genes and the expression of genes whose products are required for transmission to the mammal or establishment and maintenance of mammalian infection. Expression of genes repressed by RpoS during mammalian infection resumes when spirochetes are acquired by naïve larvae (i.e., the RpoS-off state) (9, 36). bb0844 was reported to be an RpoS-regulated gene with an expression pattern identical to that of ospC and dbpA, two well-characterized B. burgdorferi virulence factors (3, 9, 21, 43, 48, 51, 57). bb0844 is induced under mammalian-host-like conditions (5, 9, 52). A proteome-wide array analysis revealed that BB0844 elicits an antibody response in humans and laboratory-infected mice, indicating that BB0844 is expressed during mammalian infection (2).
Given the evidence for expression of bb0844 by spirochetes infecting mammalian hosts and the variable presence of this gene among clinical isolates, which appears to correlate with virulence, we hypothesized that bb0844 might contribute to B. burgdorferi infectivity. To explore this possibility, we analyzed the right chromosomal telomeric regions of 53 B. burgdorferi clinical isolates and studied the effect of bb0844 deletion on the ability of B. burgdorferi to infect mice and to survive throughout an experimental mouse-tick-mouse cycle.
MATERIALS AND METHODS
Bacterial strains and growth.
The B. burgdorferi isolates employed in this study are listed in Table 1. Most clinical isolates were obtained from patients with early Lyme disease presenting with erythema migrans at the Lyme Disease Practice of Westchester Medical Center. A number of these isolates have been described previously (49, 55). Wild-type (c162) and rpoS mutant (c174) strain 297 isolates (7) harboring constitutively expressed PflaB-green fluorescent protein (GFP) reporters stably inserted into their endogenous cp26 plasmids (16) were used to compare expression of bb0844 within Ixodes scapularis nymphs. All isolates were cultivated in modified Barbour-Stoenner-Kelly (BSK-S) medium (54).
TABLE 1.
B. burgdorferi isolates used in this study
| RST type | Isolate(s)a | OspC type | Biological sourced |
|---|---|---|---|
| RST1 | B55, B88, B253, B360, B404, B470, B479, B489, B515, B160 | A | Human EM skin biopsy specimen |
| BL206, BL564 | Lyme disease patient blood | ||
| B242, B250, B310, B350, B134, MR623, MR660 | B | Human EM skin biopsy specimen | |
| BL214, BL325 | Lyme disease patient blood | ||
| RST2 | 297b | K | Lyme disease patient CSF |
| B379 | Human EM skin biopsy specimen | ||
| RST3 | BL515, BL538 | C | Lyme disease patient blood |
| JD1c | I. scapularis | ||
| B283, B361, B408, B460, B500, B331 | I | Human EM skin biopsy specimen | |
| BL333, BL280, BL624, BL638 | Lyme disease patient blood | ||
| B240, B453, B477 | D | Human EM skin biopsy specimen | |
| B143, B399, B485 | U | Human EM skin biopsy specimen | |
| B268, B369, B385, MR616 | G | Human EM skin biopsy specimen | |
| B348, B418, B146 | E | Human EM skin biopsy specimen | |
| B236 | J | Human EM skin biopsy specimen | |
| BL285 | Lyme disease patient blood | ||
| B356 | M | Human EM skin biopsy specimen | |
| BL522 | Lyme disease patient blood |
PCR amplification and DNA sequence analysis.
The presence of the right chromosomal telomeric ORFs was determined by PCR using primers listed in Table 2. B. burgdorferi DNA was isolated from 1 × 109 cells using a Puregene DNA purification kit (Gentra Systems Inc., Minneapolis, MN). PCR conditions were optimized for each primer set, and the products were analyzed by electrophoresis in 1.5% agarose gels. For sequencing, PCR products were purified using a QIAQuick PCR purification kit (Qiagen, Valencia, CA). DNA sequencing was performed by GeneWiz Inc., South Plainfield, NJ. Sequence alignments were performed using ClustalW (http://www.ebi.ac.uk/clustalw/).
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence (5′→3′)a | Coordinates in B31 chromosomeb | Purpose |
|---|---|---|---|
| BB0843F | TTATGGTATCAACTTTGGGATAACAG | 901760-901785 | Right-telomere ORF detection |
| BB0843R | ACAATAGAAAAGAAATAACCCAAAGC | 903285-903260 | |
| BB0843-4F | TCAGTTGTTATTGCCATGCAG | 903064-903084 | |
| BB0843-4R | TTAAAATCAAAGTCTTCTGTTAAAGG | 903902-903877 | |
| BB0844F | TTGCAATCTGTAATGCCTTTT | 903553-903573 | |
| BB0844R | GCAACAAGATGGTTTTGTTTCT | 905174-905153 | |
| BB0852F | AAAAGGATATGAAAATTATTCGCTAAA | 908198-908224 | |
| BB0852R | ATAGGGGCCTTTTACTTTTCTTGA | 909782-909759 | |
| BB0844outF | TTGTTTGATAGGATTAGAACAGAGG | 903496-903520 | Mutant construction or confirmation |
| BB0844outR | TGAATCGTTTTAAAAGGGGGTA | 905304-905283 | |
| BB0844EcoRIL | GGGAATTCCACATAGTAATTTAGCAATACAGGC | 904140-904116 | |
| BB0844EcoRIR | GGGAATTCCATCAACTAATTTAGCAAATTCAAGACTCCC | 904537-904567 | |
| flgBpF | TAATACCCGAGCTTCAAGGAAG | 304150-304129 | |
| aadAR | GACGTCATTATTTGCCGACTACC | NA | |
| 156a lp28-1F | GAGGCAGAGGATGCTTTAAACCAAT | NA | bb0844 localization in plasmid |
| 156a lp28-1R | AACTCTTAAGGCGTTGTAATTCGTAATTT | NA | |
| WI91-23 lp28-1R | CCTAATTTCCCGATGCTCTTCAAAAC | NA | |
| BB0844seqR | ACAAGCAGAGGTGCATGTTG | 904448-904429 | |
| FlaB-F | CTTTTCTCTGGTGAGGGAGCTC | 148259-148238 | Real-time RT-PCR |
| FlaB-R | GCTCCTTCCTGTTGAACACCC | 148288-148308 | |
| BB0844-574F | TTGCAGATGCTCTCTCGCTAAG | 904237-904258 | |
| BB0844-674R | GGCCGATTTTTACGTTTGTTATTT | 904158-904181 | |
| FL-Probe | FAM-CTTGAACCGGTGCAGCCTGAGCA-BHQ1 | 148264-148286 |
Underlining indicates an incorporated restriction site. FAM, 6-carboxyfluorescein.
NA, not applicable.
Analysis of bb0844 expression in ticks by real-time reverse transcription (RT)-PCR.
Total RNA was isolated from infected ticks (∼150 fed larvae or flat nymphs; ∼75 engorged nymphs) using TRIzol (Invitrogen) according to the manufacturer's instructions. Contaminating genomic DNA was removed using Turbo DNAfree (Ambion, Inc. Austin, TX); the absence of contaminating DNA was confirmed by testing each treated RNA in Taq polymerase amplification reaction mixtures containing primers specific for flaB as previously described (8). DNase-treated RNAs (∼4 μg per sample) were converted to cDNA using SuperScript First-Strand Synthesis for RT-PCR (Invitrogen). A control reaction lacking reverse transcriptase was carried out for each sample using total RNA to ensure that no DNA contamination was present. The cDNA thus generated was used as a template for quantitative PCR (qPCR) using primers and probes specific for flaB and bb0844 (Table 2). Reaction mixtures containing cDNA generated without reverse transcriptase or containing primers alone were included as controls. For quantification, flaB and bb0844 amplicons were cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA), and recombinant plasmid DNAs were prepared to generate standard curves. Transcript copy numbers were calculated using the iCycler postrun analysis software based on internal standard curves and then normalized against copies of flaB present in the same cDNA. The statistical significance of observed differences in expression was evaluated by an unpaired t test with two-tailed P values and a 95% confidence interval using GraphPad (La Jolla, CA) Prism software (v. 5.0).
Construction of a bb0844 deletion mutant.
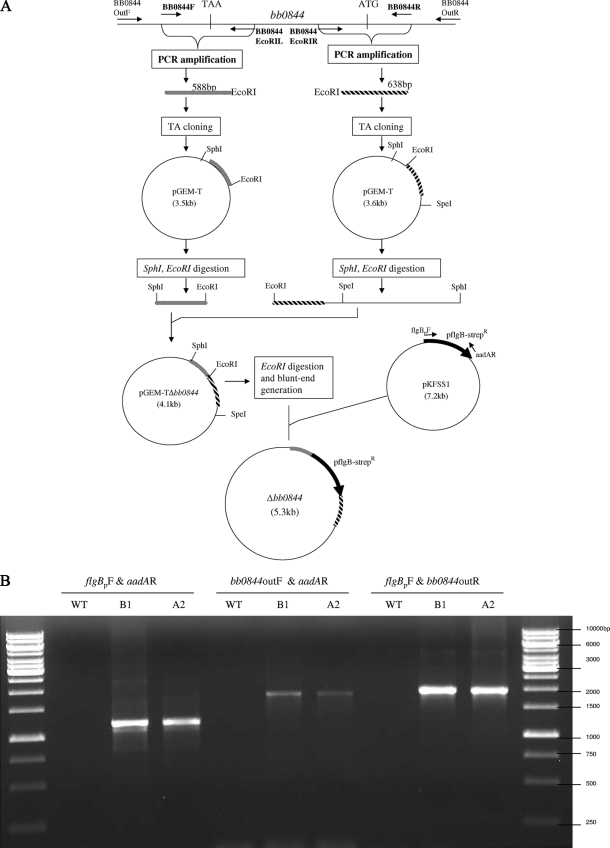
The strategy for construction of a bb0844 mutant is presented schematically in Fig. 1. This resulted in a 397-bp deletion in the central portion of bb0844 (positions 363 to 760 in in the bb0844 coding region) that was replaced with a 1,222-bp sequence containing a streptomycin/spectinomycin resistance cassette driven by the B. burgdorferi flgB promoter. Specifically, a 638-bp fragment encompassing the region immediately upstream (including 363 bp of bb0844 coding sequence) and a 588-bp fragment encompassing the region immediately downstream (including 212 bp of bb0844 coding sequence) of bb0844 were PCR amplified using primer pairs BB0844R/BB0844EcoRIR and BB0844F/BB0844EcoRIL, respectively. Each of the PCR products was ligated separately into pGEM-T (Promega, Madison, WI) and transformed into Escherichia coli DH5α, and transformants were selected by growth on LB agar containing 100 μg/ml ampicillin. Recombinant pGEM-T plasmids were digested with EcoRI and SphI (New England BioLabs, Ipswich, MA) or EcoRI and SpeI in order to identify clones carrying inserts in the desired orientations. These plasmids were digested with EcoRI and SphI, and the digestion products were ligated and transformed into E. coli DH5α as described above. The presence of both portions of bb0844 in the resultant plasmids was confirmed by digestion with SphI and SpeI, which yielded the expected 1.25-kb insert; this plasmid was designated pGEM-TΔbb0844. A 1,222-bp fragment containing a streptomycin/spectinomycin resistance gene from Shigella flexneri under the B. burgdorferi flgB promoter (flgBp-aadA) was amplified from plasmid pKFSS1 (18) using primers flgBpF and aadAR and converted to blunt ends with T4 DNA polymerase. To introduce a selective marker between the upstream and the downstream fragments, pGEMTΔbb0844 was digested with EcoRI, converted to blunt ends with T4 DNA polymerase, and ligated with the flgBp-aadA blunt-ended PCR product. The ligation mixture was transformed into E. coli DH5α, colonies were selected on LB agar plates containing 100 μg/ml of spectinomycin, and plasmids from selected colonies were analyzed by SpeI and SphI digestion. The ampicillin resistance gene was inactivated in the resulting plasmids by digestion with Psp1406I (New England BioLabs), which introduces a 300-bp deletion into bla, and ampicillin-sensitive, spectinomycin-resistant clones were selected after transformation into E. coli DH5α. The desired plasmids were isolated, electroporated into competent B. burgdorferi B31A3 cells, selected in BSK-S medium containing streptomycin (100 μg/ml), and analyzed by PCR to confirm that a double-crossover event had occurred at the bb0844 locus. The total plasmid content of the bb0844 mutant clones (Δbb0844) was determined as described previously (28), and those clones that retained all the plasmids present in the parental strain were selected for further study.
FIG. 1.
Construction and characterization of a bb0844 deletion mutant. (A) Strategy for partial deletion of bb0844. (B) PCR analysis of the wild type and two independent deletion mutant clones (B1 and A2). The combination of primers used for each PCR is shown at the top, and the positions of the primers are shown in panel A. The migration positions of a 1-kb DNA ladder are shown on the right.
Syringe inoculation of mice.
Four- to six-week-old pathogen-free C3H/HeJ mice (Jackson Laboratories, Bar Harbor, ME) were divided into groups of four and inoculated subcutaneously with 0.1 ml of either wild-type B31A3 or Δbb0844 mutant spirochetes (1 × 104 spirochetes/mouse) or an equal volume of fresh BSK-S medium. Ear punch samples were collected at 14 and 21 days postinoculation, washed several times in 70% ethanol or phosphate-buffered saline (PBS), and cultured in BSK-S medium supplemented with Borrelia antibiotic mixture (Sigma-Aldrich, St. Louis, MO). Twenty-one days after inoculation, skin biopsy samples from the site of inoculation were collected from each mouse and cultivated as described above. Blood samples (200 μl) were collected on day 14 postinoculation and assessed for serum IgG response to B. burgdorferi antigens using a Marblot Strip Test System (MarDx Diagnostics, Inc., Carlsbad, CA). The mice were sacrificed 35 days after inoculation, following which the skin, joints, and urinary bladders were collected aseptically for culture. All cultures were maintained at 37°C for at least 4 weeks and examined weekly for the presence of live spirochetes by dark-field microscopy.
Spirochete acquisition and transmission by ticks.
The pathogen-free I. scapularis larvae used in this study were obtained from a colony maintained at Yale University and kindly provided by Durland Fish. For spirochete acquisition, approximately 400 naïve larvae were placed on mice infected with either wild-type or Δbb0844 mutant spirochetes (two mice/isolate) and allowed to feed to repletion. Engorged (fed) larvae were collected at drop-off over a 5-day period and allowed to molt to the nymphal stage. Immersion-fed larvae used for qRT-PCR studies were infected with B. burgdorferi wild-type and ΔrpoS isolates as previously described (36).
For transmission studies, nymphs infected with either wild-type or Δbb0844 mutant spirochetes were placed on naïve C3H/HeJ mice (10 nymphs/mouse) and allowed to feed to repletion. Three weeks after infestation, the mice were sacrificed, and joints, urinary bladder, and ear punch biopsy specimens were cultivated and examined for spirochete infection as described above.
RESULTS
Structure of the right chromosomal ends of B. burgdorferi clinical isolates.
Previous comparative genomic studies revealed substantial genotype-dependent variation at the right chromosomal telomere beginning with bb0843 (12, 49). Of the ORFs in this region, only two, bb0844 and bb0852, appear to encode potentially functional genes (25, 49). The structure of the right chromosomal telomeric region was explored in 53 B. burgdorferi clinical isolates representing all three RST and 11 ospC genotypes. The presence of four chromosomal loci, bb0843, bb0843.1, bb0844, and bb0852, was determined by PCR using primers listed in Table 2. The results are summarized in Table 3. All strains contained bb0843, in agreement with previous reports that this is the last ORF in B. burgdorferi isolates with short chromosomes (10). All 21 RST1 and 2 RST2 isolates analyzed carried all four loci, indicative of the presence of longer chromosomes. In contrast, RST3 strains were heterogeneous with respect to the presence of the intergenic spacer between bb0843 and bb0844 (bb0843.1), bb0844, and bb0852; bb0843.1 and bb0844 were detected in 14 of 30 (47%) and 23 of 30 (77%) RST3 isolates, respectively, whereas bb0852 was present in only 3 (Table 3). Particularly noteworthy, 10 isolates contained bb0844 but not bb0843.1 or bb0852, suggesting that bb0844 may be present on a genomic element other than the chromosome in some strains.
TABLE 3.
Presence of right chromosomal loci in RST1, RST2, and RST3 isolates
| RST | OspC type | No. tested | Presence ofa: |
|||
|---|---|---|---|---|---|---|
| bb0843 | bb0843.1 | bb0844 | bb0852 | |||
| 1 | A | 12 | + | + | + | + |
| B | 9 | + | + | + | + | |
| 2 | K | 2 | + | + | + | + |
| 3 | C | 3 | + | + | + | + |
| D | 3 | + | + | + | − | |
| G | 4 | + | + | + | − | |
| U | 3 | + | + | + | − | |
| I | 10 | + | − | + | − | |
| E | 3 | + | − | − | − | |
| J | 2 | + | − | − | − | |
| M | 2 | + | − | − | − | |
+, present; −, absent.
Linkage between right chromosomal telomere structure and ospC genotype.
Previous work has shown that the RST and ospC genotypes are linked (22), and further, that disseminated infection is correlated with the genotype (41, 58, 59). It was, therefore, of interest to investigate whether a correlation exists between the structure of the right telomeric region, the presence of bb0844, and the ospC genotype. Four different patterns were observed (Table 3). As noted, RST1 (OspC types A and B), RST2 (OspC type K), and some RST3 (OspC type C) isolates scored positive for all four loci, indicating the presence of a long B31-like chromosome. Isolates harboring OspC types D, G, and U contained bb0844, but not bb0852, suggesting that their chromosomes are shortened by deletion of sequences distal to bb0844; the precise termini of these deletions were not explored further. Isolates containing OspC types E, J, and M yielded a PCR product only for bb0843, consistent with a shorter chromosome. Interestingly, all 10 OspC type I isolates tested positive for bb0843 and bb0844 but not for the chromosomal region between these loci (bb0843.1), suggesting an alternative genomic location for bb0844.
To further explore this possibility, total genomic DNA from several OspC type I isolates was separated by pulsed-field gel electrophoresis and probed for bb0844 by Southern blotting. The results indicated that bb0844 is located on a smaller replicon, most likely on a linear plasmid of 25 to 28 kbp, rather than on the chromosome (data not shown). Genomic sequences of several B. burgdorferi strains have recently been reported (40). BLAST analysis revealed a sequence with >99% identity to bb0844 on plasmid lp28-1 of strain WI91-23. Based on ospC sequence, WI91-23 is OspC type I and has a shorter chromosome of 896 to 935 kbp in length (10, 40). Primers were designed based on the WI91-23 lp28-1 sequence (accession no. CP001456). Primer WI91-23 lp28-1R is located outside the bb0844 coding region and was used in combination with primer BB0844seqR, which is within the coding region, to perform PCR amplification with genomic DNAs from B500 and B408, two previously described OspC type I isolates (55). The resulting 1.5-kb amplicons were purified and sequenced; these sequences aligned perfectly with that from WI91-23 lp28-1. Thus, OspC type I isolates have a shorter chromosome terminating at bb0843 but include a complete bb0844 on a linear plasmid, most likely lp28-1.
BLAST analysis also revealed the presence of bb0844 on lp28-1 of isolate 156a, an RST2, OspC type H strain (1). Primers 156a lp28-1F and 156a lp28-1R (Table 2) were designed based on the 156a lp28-1 sequence (accession no. CP001273) and used for PCR amplification with genomic DNA of isolates 297 and B379 (both RST2, OspC type K strains). These strains contain a complete copy of bb0844 based on PCR analysis (Table 3). A 1.2-kb region that spans the junction of bb0844 and lp28-1 was PCR amplified, and sequence analysis indicated that all three isolates contained identical sequence in this region. Unlike OspC type I isolates, strains 156a, 297, and B379 contain a complete copy of bb0844 on the chromosome, whereas the 752 nucleotides at the 5′ end of the bb0844 coding region are deleted from the copy on lp28-1. Thus, these strains contain only one complete copy of bb0844 located on the chromosome.
Strain 64b, an OspC type B isolate, also contained bb0844 sequences in two different genomic locations. Both the chromosomal copy (Bbu64b_0872; accession no. ABKA02000005) and the plasmid copy on lp28-1 (Bbu64b_F0009 of lp28-1; accession no. CP001423.1) are 99% identical to the B31 bb0844 sequence. Thus, this strain contains two complete copies of bb0844.
Sequence analysis of bb0844 in selected RST 3 isolates.
The DNA sequence of bb0844, including 223-bp upstream and 325-bp downstream flanking regions, was determined for four OspC type I isolates (B361, B460, B500, and BL333) and one OspC type C isolate (BL515) following PCR amplification using primers BB0844F and BB0844R (Table 2). The bb0844 coding region was highly conserved, containing only six nucleotide polymorphisms among the isolates that would result in mostly conservative amino acid changes in the deduced protein sequences. The upstream region of bb0844 was identical in all isolates, including the B31MI type strain, and encompasses a sequence with significant homology to the B. burgdorferi consensus RpoS promoter (see Table 4 in reference 9). Greater variability was observed in the 3′ flanking region, particularly between 94 and 150 bp downstream of the bb0844 termination codon, where several nucleotide changes were observed in RST3 isolates compared to B31MI (data not shown). The results suggest that expression of bb0844 is regulated similarly regardless of the genomic location.
TABLE 4.
Recovery of B. burgdorferi from mice infected with wild type, Δbb0844A2 and Δbb0844B1 strains by needle inoculation
| B. burgdorferi strain | Time postinfection | No. of positive cultures/no. tested (%) |
||||
|---|---|---|---|---|---|---|
| Ear | Skin | Joint | Bladder | Serology | ||
| B31A3 WT | Day 14 | 6/6 (100.0) | NT | NT | NT | 6/6 (100.0) |
| Day 21 | 6/6 (100.0) | 6/6 (100.0) | NT | NT | ||
| Day 35a | NTb | 4/4 (100.0) | 4/4 (100.0) | 4/4 (100.0) | ||
| Δbb0844 A2 | Day 14 | 4/4 (100.0) | NT | NT | NT | 4/4 (100.0) |
| Day 21 | 4/4 (100.0) | 4/4 (100.0) | NT | NT | ||
| Day 35a | NT | 1/2 (50.0) | 2/2 (100.0) | 1/2 (50.0) | ||
| Δbb0844 B1 | Day 14 | 4/4 (100.0) | NT | NT | NT | 4/4 (100.0) |
| Day 21 | 4/4 (100.0) | 4/4 (100.0) | NT | NT | ||
| Day 35a | NT | 2/2 (100.0) | 2/2 (100.0) | 2/2 (100.0) | ||
Only two of four animals from the mutant-infected groups were sacrificed. The remaining two mice from each group were used for larval tick feeding as described in Materials and Methods.
NT, not tested.
Differential expression of bb0844 in B. burgdorferi.
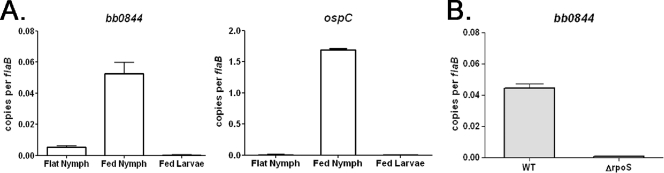
Previous microarray analyses of temperature-shifted B. burgdorferi or spirochetes cultivated under mammalian-host-like conditions indicated that, similar to ospC, expression of bb0844 is absolutely dependent on RpoS (5, 9, 52). To extend these findings to in vivo conditions, bb0844 expression was measured in B. burgdorferi-infected fed larvae and unfed and fed nymphal ticks. bb0844 transcript was detected only in fed nymphs, and expression was absolutely dependent on RpoS (Fig. 2). It should be noted that even in fed nymphs, bb0844 was poorly expressed; transcript levels were more than 30-fold lower than that for ospC, a well-characterized RpoS-dependent gene. Despite numerous attempts, bb0844 transcript was not detected in infected mouse tissue (data not shown).
FIG. 2.
(A) Real -time RT-PCR analysis of bb0844 and ospC expression in flat (unfed) nymphs, fed nymphs, and fed larvae. Ticks were infected as larvae by infestation of mice that had been syringe inoculated with B. burgdorferi strain 297. Expression of bb0844 was significantly induced upon nymphal feeding (P = 0.0005). (B) Real-time RT-PCR analysis of bb0844 in fed nymphal ticks infected as larvae by immersion with either wild-type (WT) or rpoS mutant (ΔrpoS) B. burgdorferi strain 297. Expression of bb0844 was significantly reduced in the mutant (P < 0.0001). The values represent the average flaB-normalized copy number and standard error of the mean (SEM) for each gene.
Construction and characterization of a bb0844 deletion mutant.
To determine the requirement for bb0844 during the borrelial enzootic cycle, a deletion mutant was constructed in strain B31A3 by deleting a portion of bb0844 and replacing it with a spectinomycin resistance cassette by allelic exchange (Fig. 1A). Verification of the presence of the spectinomycin resistance cassette in mutant clones and confirmation that a double-crossover event took place were accomplished by PCR using the primer sets shown in Fig. 1A. The PCR results were consistent with replacement of 397 bp of bb0844 with flgBp-aadA by a double-crossover event (Fig. 1B). The absence of any detectable bb0844 transcript in the mutant clones further confirmed functional inactivation of the gene (data not shown). There were no differences in the growth characteristics of wild-type and bb0844 mutant strains cultivated in BSK-S medium at 37°C. Furthermore, no obvious differences in spirochete morphology were observed between wild-type and bb0844 mutant strains when examined by dark-field microscopy (data not shown).
BB0844 is not required for infection of mice.
To assess the role of BB0844 in the establishment of mammalian infection, mice were needle inoculated subcutaneously with 1 × 104 spirochetes of either wild-type B31A3 or one of the two independent mutant clones (Δbb0844A2 and Δbb0844B1). Infectivity was assessed by in vitro cultivation of mouse tissues at several time points postinfection and by serology. All infected mice were culture positive for spirochetes in ear and skin tissues at 21 days postinfection (Table 4). All mice infected with wild-type and Δbb0844B1 mutant strains were culture positive for B. burgdorferi at day 35 postinoculation, and all mice seroconverted (Table 4). These findings demonstrate that bb0844 is not required for B. burgdorferi infectivity or persistence in mice when introduced by needle inoculation.
Acquisition and transmission of BB0844 deletion mutant strains by ticks.
To determine whether the absence of BB0844 influences the ability of B. burgdorferi to be acquired and transmitted by ticks, naïve larvae were allowed to feed on mice infected with either wild-type or bb0844 mutant strains (Δbb0844A2 and Δbb0844B1). Fed larvae were collected at drop-off, allowed to molt into nymphs, and used to examine the requirement for bb0844 during spirochetal transmission to a mammalian host. There was no difference between the spirochete loads in larvae fed on either wild-type- or mutant-infected mice (2,512 ± 1,265 versus 2,215 ± 815, respectively). Naïve C3H/HeJ mice were infested with 10 nymphs infected with either wild-type or bb0844 mutant spirochetes. All mice fed upon by nymphs infected with either wild-type or mutant strains were culture positive for spirochetes in ear and joint tissues (Table 5). Taken together, these results demonstrate that bb0844 is not required for spirochete acquisition, maintenance, or transmission by I. scapularis. Furthermore, bb0844 is dispensable for infection of mice by either needle inoculation or tick transmission.
TABLE 5.
Infectivity of Δbb0844 in mice via tick bite after 3 weeks of infection
| B. burgdorferi strain | Number of mice infected/no. of mice tested (%) |
||
|---|---|---|---|
| Ear | Bladder | Joint | |
| B31A3 WT | 2/2 (100.0) | 2/2 (100.0) | 2/2 (100.0) |
| Δbb0844 A2 | 3/3 (100.0) | 2/3 (66.6) | 3/3 (100.0) |
| Δbb0844 B1 | 3/3 (100.0) | 2/3 (66.6) | 3/3 (100.0) |
DISCUSSION
With the exception of the right telomere, the linear chromosome of B. burgdorferi is highly conserved with respect to sequence and gene order (11, 49). The variation of the right telomere is the result of a 6- to 14-kbp extension of the chromosomal end in certain strains (10, 11). Among the ORFs on the chromosomal extension is bb0844, an RpoS-dependent gene (9). Given the proposed role of RpoS as a regulator of genes required for establishment of mammalian infection (9, 36), it was of interest to explore the presence of bb0844 among a collection of B. burgdorferi clinical isolates with different pathogenic properties representing most ospC genotypes. The results indicate four patterns of bb0844 genomic localization: (i) complete absence, (ii) presence only on the chromosome, (iii) presence only on a plasmid, or (iv) presence of a complete gene copy on the chromosome and either a complete copy or a 5′-truncated copy on a plasmid. The genomic location of bb0844 correlates with the B. burgdorferi genotype in a manner similar to that described previously for chromosome length variation (10, 49). OspC types A, B, C, and K have the largest chromosomes, as indicated in the current study by the presence of bb0852. B31, a typical OspC type A strain, contains an extra 8.5 kbp at the right chromosomal end; Sh-2-82 and JD1, OspC types K and C, respectively, have chromosomes which are approximately 12 kbp longer than that of B31(40). The chromosomes of Sh-2-82 and JD1 have not been sequenced, and thus, the precise nature of the right chromosomal ends in these strains beyond that of B31 is not currently known. OspC types D, G, and U contain bb0844 on the chromosome but lack bb0852, suggesting that these genotypes possess a right chromosomal end extension that is shorter than that of B31. Casjens et al. (11) analyzed the right chromosomal telomere for 31 B. burgdorferi isolates, mostly of tick or wildlife origin, by Southern blotting using probes derived from conserved and extended regions of the chromosome. They noted a pattern essentially identical to that observed in the current study. The present data extend the previous findings to human clinical isolates and provide a correlation between the genotype and the structure of the right chromosomal end.
Previous studies suggested that the B. burgdorferi genotype predicts the capacity of a strain for hematogenous dissemination. RST1 strains have a significantly greater tendency to produce disseminated infection than do RST3 isolates (29, 55, 58, 59). All 21 RST1 isolates (12 OspC type A and 9 OspC type B) analyzed in the current study had a pattern identical to that of strain B31. In contrast, RST3 isolates were heterogeneous in terms of the presence or absence of the four right chromosomal loci tested. Of particular note are strains of OspC types E, J, M, and I. None of the 17 isolates with these OspC types had bb0844 on their chromosomes, and presumably all have the shortest chromosomal right end. OspC types E, J, and M isolates completely lack the gene. Despite this, BL285 (OspC type J) and BL522 (OspC type M) were recovered from the blood of Lyme disease patients, while B348 (OspC type E) disseminates in mice (albeit with kinetics that differ from those of OspC type A strains) (15, 23, 55), indicating that bb0844 is not required for hematogenous dissemination. In contrast, OspC type I isolates lack bb0844 in the chromosome and instead carry it on a plasmid of approximately 28 kb. The recently completed sequences of OspC type I strain WI91-23 plasmids allowed a more careful analysis of the genomic localization of bb0844 in these strains. Beginning at nucleotide 394 of plasmid lp28-1, WI91-23 contains 6,654 nucleotides that are highly similar to the right chromosomal end of B31 and include ORFs bb0844 to bb0853. The coding region in WI91-23 lp28-1 designated F0011 (BBUWI91-23_F0011) and the 80 bp upstream and downstream are >99% identical to the sequence of B31 bb0844; the deduced amino acid sequence of F0011 is 98.8% identical (319/323 residues) to that of BB0844. We conclude that a complete copy of bb0844 is carried on lp28-1 in OspC type I strains and that bb0844 expression would be dependent on RpoS regardless of the genomic location of the gene.
The results also indicate that OspC type K isolates carry bb0844 on the chromosome, as they were PCR positive for all tested B31 ORFs, including bb0852. This is consistent with the results of Casjens et al. and Huang et al. (11, 25) showing that strains of this genotype have right chromosome extensions that are longer than that of B31. A complete genome sequence for an OspC type K isolate is not available; however, strain 156a, an OspC type H isolate (closely related phylogenetically to OspC type K isolates [33]), has been sequenced. Analysis revealed that this strain contains a complete copy of bb0844 on the chromosome and a partial copy on lp28-1. Interestingly, the bb0844 copy on lp28-1 is missing the 5′ 752 bp of the bb0844 coding region. Using primers based on the strain 156a sequence, we amplified identical sequences for two OspC type K strains. We conclude that OspC type K strains do not contain an intact bb0844 on lp28-1; rather, these strains carry a complete, functional copy of bb0844 only on the chromosome.
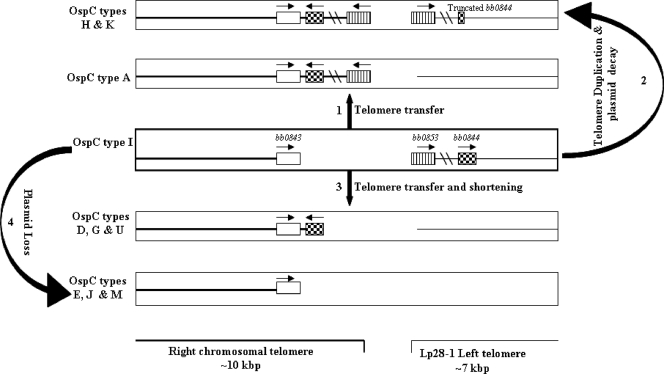
What is the mechanism underlying the variation in bb0844 content and location? Kobryn and Chaconas (30) demonstrated that hairpin telomeres could be fused by reversal of the telomere resolution reaction catalyzed by ResT. On this basis, they suggested that the most likely mechanism for variation at the chromosomal right end is extension via addition of linear plasmid sequences in a process that may be dependent on ResT, a telomere resolvase (13, 30). Telomere fusion would require base pairing between the six nucleotide sticky ends generated during the first step (substrate cleavage) (13, 30). Sequences for most of the B. burgdorferi chromosomal and plasmid telomeres have recently been reported and can be characterized into three distinct types based on the presence, sequence, and position of the so-called box 1; only telomeres with complementary sticky ends between the cleavage sites can undergo fusion by reversal of the telomere resolution (14, 53). Interestingly, the chromosome right end and the left end of lp28-1 are both type 1 telomeres with compatible sticky ends (53), and thus, fusion mediated by ResT would be permitted. Based on the data from the current study and available sequence information, we propose the following evolution of right chromosomal ends and bb0844 localization (Fig. 3). The ancestral strain is likely to have been similar to OspC type I, which has the shortest chromosome and carries bb0844 on a linear plasmid. Telomere fusion between the bb0844-containing plasmid and the right chromosomal end, followed by deletion or mutation, could result in several outcomes: (i) telomere transfer, resulting in chromosome extension and the presence of bb0844 only on the chromosome (e.g., ospC type A); (ii) telomere duplication and either maintenance of both gene copies (e.g., strain 64b) or decay of the plasmid copy of bb0844 (ospC types K and H); (iii) telomere transfer followed by shortening of the chromosomal extension, resulting in removal of regions distal to bb0844, including bb0852 (ospC types D, G, and U; alternatively, this arrangement could be derived by telomere transfer from a currently unidentified plasmid containing bb0844 but not the sequence distal to it); and finally, (iv) loss of the bb0844-containing plasmid from the OspC type I ancestral strain would yield isolates completely lacking bb0844 (OspC types E, J, and M).
FIG. 3.
Proposed evolution of the right chromosomal end of B. burgdorferi and the bb0844 genomic location. The schematic diagram shows the final product following ResT-mediated reversal of telomere resolution based on the mechanisms proposed by Chaconas and coworkers (13, 14, 30). The rectangles denote the presence of the chromosome and plasmid in a single cell. The numbers refer to alternative outcomes discussed in the text.
RpoS induces expression of genes whose products are required for either transmission to the mammalian host or establishment and maintenance of mammalian infection (8). Transcription of bb0844 is induced in the mammalian-host-adapted state (5, 9, 52), and its expression is abrogated in Rrp2 and BosR mutants, two regulatory proteins required for expression of rpoS (4, 37, 38). In this study, bb0844 was found not to be expressed in fed larvae or unfed nymphs, and its transcription was induced in fed nymphs. This is the pattern expected for an RpoS-dependent gene, as rpoS is induced on nymphal feeding and is not expressed at other tick stages (9). The bb0844 transcript level is quite low in fed nymphs (0.05 copy/copy of flaB), as is the case in vitro and in dialysis membrane chambers (9). bb0844 transcripts could not be detected in infected mice (data not shown), presumably due to relatively low transcript levels and the low spirochete loads in the infected animals. Nevertheless, BB0844 induces an antibody response in both humans and mice (2), confirming not only that it is expressed in vivo, but also that the protein is highly immunogenic. Eggers et al. (17) proposed that B. burgdorferi recognizes an extended −10 region to distinguish between RpoS and σ70 promoters. The putative bb0844 promoter sequence has a 10/11 match to the B. burgdorferi consensus RpoS promoter (9). Thus, the molecular basis for the low levels of bb0844 transcript is unclear at present. It is possible that bb0844 transcript levels are subject to posttranscriptional regulation (e.g., mRNA stability).
We hypothesized that BB0844 would play a role in mammalian infection based on two considerations: comparative genomic analysis indicated the absence of bb0844 in isolates that disseminate less readily in mammals (49), and bb0844 is coordinately expressed with other genes required for infectivity (e.g., ospC) due to its RpoS dependence (9). However, absence of bb0844 had no discernible effect at any step in an experimental tick-mouse infection model. bb0844 mutant strains were fully capable of establishing infection in mice, whether by needle or tick inoculation. Mutant spirochetes were acquired by ticks and persisted through the entire tick life cycle. Therefore, bb0844 is not essential to B. burgdorferi survival in vivo, and its function is dispensable for spirochete pathogenicity and maintenance in the tick-mouse infectious cycle. This is perhaps not surprising, given that certain spirochete lineages (e.g., OspC types E, J, and M) lack bb0844 yet are maintained in the tick-mammal enzootic cycle in nature and can infect humans (23).
RpoS is a key factor that governs differential gene expression in B. burgdorferi as it transitions from growth in the tick vector to growth in the mammal. It is therefore reasonable to predict that genes that are part of the RpoS regulon are important for mammalian infection. Caimano et al. (9) reported that the RpoS regulon is comprised of 137 genes, including bb0844. The current results demonstrate that not all genes whose expression is coordinately regulated with those required for virulence are themselves necessary for that process. It should be noted that there are numerous contradictory reports about the roles of other RpoS-dependent genes (e.g., dbpA, bbk32, and bba64) in mammalian infection, although these discrepancies may depend on the experimental mode of infection (3, 20, 31, 34, 35, 42-45). Our findings underscore the necessity to demonstrate required protein function by mutagenesis experiments, rather than reliance on correlative data alone.
It is assumed that BB0844 had a required function at some point in the evolutionary history of B. burgdorferi, although the biological function of BB0844 is not known. It is possible that other proteins of unknown function can provide redundant activities and compensate for the absence of BB0844. In such a case, it might be expected that the bb0844 coding region would decay, but sequence analysis of multiple isolates revealed only a small number of nucleotide polymorphisms that would result in only conservative amino acid changes in the encoded protein. Alternatively, it is possible that BB0844 does indeed have a required function that cannot be discerned using currently available experimental models. For example, BB0844 may be required for survival in an alternative reservoir host (other than mice). At present, bb0844 can be considered an addition to the growing list of B. burgdorferi genes (e.g., ospD, luxS, and chbC) that, based upon experimental models currently available, do not appear to be required during the enzootic cycle (26, 47, 50).
Acknowledgments
We thank D. Scott Samuels for providing pKFSS1 and helpful discussions and George Chaconas for valuable insights.
This work was supported by NIH grants AI045801 (I.S.), AI029735 (J.D.R. and M.J.C.), and AI085248 (M.J.C.) and a grant from the National Research Fund for Tick-Borne Diseases.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 20 December 2010.
REFERENCES
- 1.Attie, O., et al. 2007. Co-evolution of the outer surface protein C gene (ospC) and intraspecific lineages of Borrelia burgdorferi sensu stricto in the northeastern United States. Infect. Genet. Evol. 7:1-12. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, A. G., et al. 2008. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect. Immun. 76:3374-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blevins, J. S., K. E. Hagman, and M. V. Norgard. 2008. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boardman, B. K., et al. 2008. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 76:3844-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorfer, W., et al. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 7.Caimano, M. J., C. H. Eggers, C. A. Gonzalez, and J. D. Radolf. 2005. Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J. Bacteriol. 187:7845-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caimano, M. J., C. H. Eggers, K. R. Hazlett, and J. D. Radolf. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caimano, M. J., et al. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casjens, S., M. DeLange, H. L. Ley III, P. Rosa, and W. M. Huang. 1995. Linear chromosomes of Lyme disease agent spirochetes: genetic diversity and conservation of gene order. J. Bacteriol. 177:2769-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casjens, S., et al. 1997. Telomeres of the linear chromosomes of Lyme disease spirochaetes: nucleotide sequence and possible exchange with linear plasmid telomeres. Mol. Microbiol. 26:581-596. [DOI] [PubMed] [Google Scholar]
- 12.Casjens, S., et al. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 13.Chaconas, G. 2005. Hairpin telomeres and genome plasticity in Borrelia: all mixed up in the end. Mol. Microbiol. 58:625-635. [DOI] [PubMed] [Google Scholar]
- 14.Chaconas, G., and K. Kobryn. 2010. Structure, function, and evolution of linear replicons in Borrelia. Annu. Rev. Microbiol. 64:185-202. [DOI] [PubMed] [Google Scholar]
- 15.Derdáková, M., et al. 2004. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl. Environ. Microbiol. 70:6783-6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunham-Ems, S. M., et al. 2009. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J. Clin. Invest. 119:3652-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggers, C. H., M. J. Caimano, and J. D. Radolf. 2006. Sigma factor selectivity in Borrelia burgdorferi: RpoS recognition of the ospE/ospF/elp promoters is dependent on the sequence of the −10 region. Mol. Microbiol. 59:1859-1875. [DOI] [PubMed] [Google Scholar]
- 18.Frank, K. L., S. F. Bundle, M. E. Kresge, C. H. Eggers, and D. S. Samuels. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser, C. M., et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore, R. D., Jr., et al. 2010. The bba64 gene of Borrelia burgdorferi, the Lyme disease agent, is critical for mammalian infection via tick bite transmission. Proc. Natl. Acad. Sci. U. S. A. 107:7515-7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm, D., et al. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanincová, K., D. Liveris, S. Sandigursky, G. P. Wormser, and I. Schwartz. 2008. Borrelia burgdorferi sensu stricto is clonal in patients with early Lyme borreliosis. Appl. Environ. Microbiol. 74:5008-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanincová, K., et al. 2008. Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl. Environ. Microbiol. 74:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinnebusch, J., and A. G. Barbour. 1991. Linear plasmids of Borrelia burgdorferi have a telomeric structure and sequence similar to those of a eukaryotic virus. J. Bacteriol. 173:7233-7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, W. M., M. Robertson, J. Aron, and S. Casjens. 2004. Telomere exchange between linear replicons of Borrelia burgdorferi. J. Bacteriol. 186:4134-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hübner, A., A. T. Revel, D. M. Nolen, K. E. Hagman, and M. V. Norgard. 2003. Expression of a luxS gene is not required for Borrelia burgdorferi infection of mice via needle inoculation. Infect. Immun. 71:2892-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hübner, A., et al. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U. S. A. 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyer, R., et al. 2003. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect. Immun. 71:3699-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones, K. L., et al. 2006. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J. Clin. Microbiol. 44:4407-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobryn, K., and G. Chaconas. 2005. Fusion of hairpin telomeres by the B. burgdorferi telomere resolvase ResT implications for shaping a genome in flux. Mol. Cell 17:783-791. [DOI] [PubMed] [Google Scholar]
- 31.Li, X., X. Liu, D. S. Beck, F. S. Kantor, and E. Fikrig. 2006. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect. Immun. 74:3305-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liveris, D., et al. 1999. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J. Clin. Microbiol. 37:565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margos, G., et al. 2008. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 105:8730-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maruskova, M., M. D. Esteve-Gassent, V. L. Sexton, and J. Seshu. 2008. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect. Immun. 76:391-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruskova, M., and J. Seshu. 2008. Deletion of BBA64, BBA65, and BBA66 loci does not alter the infectivity of Borrelia burgdorferi in the murine model of Lyme disease. Infect. Immun. 76:5274-5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulay, V. B., et al. 2009. Borrelia burgdorferi bba74 is expressed exclusively during tick feeding and is regulated by both arthropod- and mammalian host-specific signals. J. Bacteriol. 191:2783-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouyang, Z., J. S. Blevins, and M. V. Norgard. 2008. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology 154:2641-2658. [DOI] [PubMed] [Google Scholar]
- 38.Ouyang, Z., et al. 2009. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol. Microbiol. 74:1331-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piesman, J., T. N. Mather, R. J. Sinsky, and A. Spielman. 1987. Duration of tick attachment and Borrelia burgdorferi transmission. J. Clin. Microbiol. 25:557-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schutzer, S. E., et al. 8 October 2010. Whole genome sequences of thirteen isolates of Borrelia burgdorferi. J. Bacteriol. doi: 10.1128/JB.01158-10. [DOI] [PMC free article] [PubMed]
- 41.Seinost, G., et al. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 67:3518-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seshu, J., et al. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591-1601. [DOI] [PubMed] [Google Scholar]
- 43.Shi, Y., Q. Xu, K. McShan, and F. T. Liang. 2008. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect. Immun. 76:1239-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi, Y., Q. Xu, S. V. Seemanapalli, K. McShan, and F. T. Liang. 2006. The dbpBA locus of Borrelia burgdorferi is not essential for infection of mice. Infect. Immun. 74:6509-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi, Y., Q. Xu, S. V. Seemanapalli, K. McShan, and F. T. Liang. 2008. Common and unique contributions of decorin-binding proteins A and B to the overall virulence of Borrelia burgdorferi. PLoS. One 3:e3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steere, A. C., et al. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 47.Stewart, P. E., A. Bestor, J. N. Cullen, and P. A. Rosa. 2008. A tightly regulated surface protein of Borrelia burgdorferi is not essential to the mouse-tick infectious cycle. Infect. Immun. 76:1970-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart, P. E., et al. 2006. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect. Immun. 74:3547-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terekhova, D., R. Iyer, G. P. Wormser, and I. Schwartz. 2006. Comparative genome hybridization reveals substantial variation among clinical isolates of Borrelia burgdorferi sensu stricto with different pathogenic properties. J. Bacteriol. 188:6124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tilly, K., D. Grimm, D. M. Bueschel, J. G. Krum, and P. Rosa. 2004. Infectious cycle analysis of a Borrelia burgdorferi mutant defective in transport of chitobiose, a tick cuticle component. Vector Borne Zoonotic Dis. 4:159-168. [DOI] [PubMed] [Google Scholar]
- 51.Tilly, K., et al. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tourand, Y., J. Deneke, T. J. Moriarty, and G. Chaconas. 2009. Characterization and in vitro reaction properties of 19 unique hairpin telomeres from the linear plasmids of the Lyme disease spirochete. J. Biol. Chem. 284:7264-7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, G., et al. 2004. Variations in Barbour-Stoenner-Kelly culture medium modulate infectivity and pathogenicity of Borrelia burgdorferi clinical isolates. Infect. Immun. 72:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, G., et al. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, G., A. P. van Dam, I. Schwartz, and J. Dankert. 1999. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 12:633-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weening, E. H., et al. 2008. Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect. Immun. 76:5694-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wormser, G. P., et al. 2008. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J. Infect. Dis. 198:1358-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wormser, G. P., et al. 1999. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J. Infect. Dis. 180:720-725. [DOI] [PubMed] [Google Scholar]
- 60.Yang, X., et al. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]