Abstract
Francisella tularensis, the causative agent of tularemia, is one of the most infectious bacterial pathogens known and is classified as a category A select agent and a facultative intracellular bacterium. Why F. tularensis subsp. tularensis causes a more severe form of tularemia than F. tularensis subsp. holarctica does is not known. In this study, we have identified prominent phenotypic differences between the subspecies, since we found that F. tularensis subsp. tularensis strains contained less iron than F. tularensis subsp. holarctica strains. Moreover, strain SCHU S4 of F. tularensis subsp. tularensis was less susceptible than FSC200 and the live vaccine strain (LVS) of F. tularensis subsp. holarctica to H2O2-induced killing. The activity of the H2O2-degrading enzyme catalase was similar between the strains, whereas the iron content affected their susceptibility to H2O2, since iron starvation rendered F. tularensis subsp. holarctica strains more resistant to H2O2. Complementing LVS with fupA, which encodes an important virulence factor that regulates iron uptake, reduced its iron content and increased the resistance to H2O2-mediated killing. By real-time PCR, it was demonstrated that FSC200 and LVS expressed higher levels of gene transcripts related to iron uptake and storage than SCHU S4 did, and this likely explained their high iron content. Together, the results suggest that F. tularensis subsp. tularensis strains have restricted iron uptake and storage, which is beneficial for their resistance to H2O2-induced killing. This may be an important factor for the higher virulence of this subspecies of F. tularensis, as reactive oxygen species, such as H2O2, are important bactericidal components during tularemia.
Tularemia is an infectious disease caused by the Gram-negative bacterium Francisella tularensis (27). This facultative intracellular bacterium is one of the most infectious bacterial pathogens known and is classified as a category A select agent. In mice, as few as 10 CFU of F. tularensis subsp. tularensis causes a lethal infection, and this dose is also sufficient to establish tularemia in humans. F. tularensis subsp. tularensis causes a more severe form of tularemia than F. tularensis subsp. holarctica does. Although they exhibit differences in virulence, the two human pathogenic subspecies are highly similar in gene content; only 9 genes are unique to the highly virulent subspecies F. tularensis subsp. tularensis, and 20 genes are inactivated in all subspecies but F. tularensis subsp. tularensis (23). Recently, five of the unique genes were individually deleted in SCHU S4, the prototypic strain of F. tularensis subsp. tularensis, and the resulting mutants were found to retain full virulence in mice (10). Thus, the results did not indicate that the genes unique to F. tularensis subsp. tularensis contributed to its high virulence. Instead, the results imply that the relatively low virulence of F. tularensis subsp. holarctica strains may be a combined effect of multiple missing genes or an effect of differential regulation and expression of genes present in both subspecies.
The attenuated live vaccine strain (LVS) of F. tularensis is a spontaneous mutant of F. tularensis subsp. holarctica that has acquired a number of genomic deletions (22). FTT0918 (fupA) is a major virulence factor in both F. tularensis subsp. tularensis and F. tularensis subsp. holarctica (24, 30). In LVS, a 252-residue carboxy-terminal sequence of this gene and a 230-residue amino-terminal sequence of the downstream gene FTT0919 (fupB) are deleted. The remaining residues of fupA and fupB encode a fusion protein in LVS. A recent study demonstrated that the deletion in fupA makes a crucial contribution to the attenuation of LVS (24). The function of fupA in SCHU S4, and presumably in all strains with an intact gene, is related to regulation of siderophore-dependent and -independent iron uptake (13). Since LVS carries a deletion in fupA, it is likely that the iron uptake and regulation are distinct from those in other F. tularensis strains.
Mammalian hosts respond to infection by inducing hypoferremia; i.e., the concentration of iron in extracellular fluids and plasma drastically decreases. This has also been demonstrated to occur during tularemia in humans (17). Since iron is essential for the growth of F. tularensis, the bacterium has needed to develop means to sequester iron even under such iron-limiting conditions, but how this is performed is unknown. In vitro, in iron-deficient medium, iron uptake depends on the siderophore rhizoferrin (4, 28). Its synthesis, export, and uptake are dependent on genes in the Francisella siderophore locus (fslA to fslE). The iron uptake and storage have to be carefully regulated to avoid the possibility that intracellular iron together with H2O2 generates highly toxic hydroxyl radicals and anions through the iron-driven Fenton reaction (32). In F. tularensis, as in most bacteria, the ferric iron uptake regulator A (FurA) is the main regulator of genes related to iron uptake (8, 19). When sufficient iron is available, FurA chelates Fe2+ and binds to a Fur box near the transcription start of the gene and thereby suppresses the expression. However, under iron deficiency, the absence of Fe2+ leads to release of Fur from the box, and thereby the gene can be transcribed. The iron uptake is further regulated by the oxidative status of the bacterial cell through the oxidative stress transcriptional regulator oxyR (8). In Escherichia coli, oxyR is activated by elevated H2O2 concentrations and induces the expression of fur (33). Fur then suppresses the uptake of iron as described above. Thus, oxyR ensures that low levels are maintained under oxidative stress to reduce the iron-driven Fenton reaction.
All organisms that live in an oxygen-rich environment have to cope with various degrees of oxidative stress, since reactive oxygen species (ROS) are by-products of the endogenous metabolism. In addition, ROS, e.g., H2O2, constitute one of the main defenses of the mammalian host against invading pathogens (18). In vitro, there is a very good correlation between resistance to H2O2 and the virulence of F. tularensis strains. SCHU S4 is more resistant than the F. tularensis subsp. holarctica strain FSC200, and LVS is the least resistant (14). The mechanism behind the high resistance of F. tularensis subsp. tularensis is not clear. In fact, there appears to be a relatively higher contribution of catalase to the virulence of LVS than to that of SCHU S4 (14). This implies that the F. tularensis subsp. tularensis subspecies of the pathogen has evolved complementary, catalase-independent mechanisms to withstand the antimicrobial effects exerted by H2O2. As described above, it is likely that the iron uptake in LVS is different from that in F. tularensis subsp. tularensis, and since iron potentiates the toxicity of H2O2 through the Fenton reaction, we asked if iron regulation is a factor that contributes to H2O2-mediated killing. Specifically, we investigated the capability of F. tularensis strains to store iron and how the different iron contents, catalase activities, and gene regulation of the bacteria influenced their resistance to H2O2-mediated killing.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are listed in Table 1. F. tularensis LVS was originally obtained from the American Type Culture Collection (ATCC 29684). The F. tularensis subsp. tularensis strains and the F. tularensis subsp. holarctica strains were obtained from the Francisella Strain Collection (FSC) of the Swedish Defense Research Agency, Umeå, Sweden. All F. tularensis subsp. tularensis strains except for FSC043 and all F. tularensis subsp. holarctica strains except for LVS carry an intact fupA gene. To complement the LVS strain, a construct containing the FTT0918 gene was sequenced and cloned into pDMK2 (SalI-NotI). The resulting clone was introduced into E. coli S17-1_pir and conjugated into LVS as described previously (7). The resulting mutant strain, denoted FSC697, was verified by PCR and sequencing (Eurofins MWG Operon). The cis complementation ensured chromosomal integration of the functional gene and expression regulated by its native promoter.
TABLE 1.
Strains used in this study
| Straina | Origin or genetic feature (reference) |
|---|---|
| F. tularensis subsp. tularensis | |
| SCHU S4 | Human, USA, 1941 |
| FSC013 | Germany, 1960 |
| FSC033 | Squirrel, USA |
| FSC042 | Hare, Canada |
| FSC043 | SCHU S4 spontaneous mutant (30) |
| FSC046 | Human, USA, 1940 |
| FSC053 | Human ulcer |
| FSC054 | Rabbit, USA, 1953 |
| FSC198 | Slovakia, 1988 |
| FSC199 | Slovakia, 1988 |
| FSC604 | USA, 1959 |
| F. tularensis subsp. holarctica | |
| FSC200 | Human ulcer |
| LVS | ATCC 29684 |
| FSC697 | LVS expressing FTT0918 in cis (this study) |
| FSC076 | Hare, Sweden, 1981 |
| FSC089 | Human, Norway, 1989 |
| FSC157 | Human, Sweden, 1994 |
| FSC226 | Human ulcer, Ljusdal, Sweden, 1998 |
| FSC244 | Human, Sweden, 1995 |
| FSC663 | Human, Sweden |
| FSC812 | Human, Sweden |
| FSC831 | Human, Sweden |
All Francisella strains were from the FSC, FOI Swedish Defense Research Agency, Umeå, Sweden, and the strain collection number is indicated.
All bacteriological work was carried out in a biosafety level 3 facility certified by the Swedish Work Environment Authority and also certified to be select agent compliant by the U.S. Centers for Disease Control and Prevention (CDC).
Preparation of growth media.
Chamberlain's defined medium (CDM) (2) iron depleted by chelation (C-CDM) was produced as described previously (3, 13). Briefly, 1% (wt/vol) Chelex-100 (Bio-Rad, Hercules, CA) was added to CDM without FeSO4, and the mixture was kept in rotation for 24 h at 4°C. The Chelex-100 was removed by filtering the medium through a 0.2-μm-pore-size Millipore filter (Biosciences, Stockholm, Sweden), and the chelating step was repeated once. The medium was thereafter supplemented with essential cations (MgSO4, 0.55 mM; ZnCl2, 1.4 μM; CuSO4, 0.2 μM; MnCl2, 1.0 μM; CaCl2, 5 μM). Sterility was ensured by a second filtration.
Deferoxamine (DFO) is an iron chelator and was used to prepare agar plates devoid of free iron in the medium (DFO plates). These plates were composed of 1 part of 4% agar GC medium base (BD Diagnostic Systems, Sparks, MD), 1 part of C-CDM, and 25 μg/ml DFO (Sigma-Aldrich, St. Louis, MO).
McLeod agar plates (MC plates) were composed of 1% (wt/vol) hemoglobin (Oxoid Ltd., Hampshire, England), 3.6% (wt/vol) GC agar base (BD Diagnostic Systems), and 1% (vol/vol) IsoVitaleX (BD Diagnostic Systems).
Ferrozine assay.
A ferrozine-based method was used to measure the total amount of iron in the bacterial samples (21). Ferrozine forms a complex with Fe2+ that absorbs strongly at 562 nm. Bacteria cultivated overnight on MC plates or DFO plates were resuspended to an A600 of 1.0 in phosphate-buffered saline (PBS). One milliliter of the bacterial suspension was centrifuged at 13,000 rpm for 5 min, and the resulting bacterial pellet was lysed with 100 μl of 50 mM NaOH. The solution was mixed thoroughly to ensure complete lysis of the bacteria. One hundred microliters of 10 mM HCl was added to the lysate. To release protein-bound iron, the samples were treated with 100 μl of a freshly prepared solution of 0.7 M HCl and 2.25% (wt/vol) KMnO4 in H2O and incubated for 2 h at 60°C. All chemicals used were from Sigma-Aldrich. Thereafter, the samples were mixed with 100 μl of the iron detection reagent, composed of 6.5 mM ferrozine, 6.5 mM neocuproine, 2.5 M ammonium acetate, and 1.0 M ascorbic acid dissolved in water. The samples were incubated for 30 min, and insoluble particles were removed by centrifugation. Two hundred microliters of the supernatant was transferred to a 96-well plate, and the A562 was determined in a microplate reader (Paradigm; Beckman Coulter, Bromma, Sweden). The iron content of the sample was calculated by comparing its absorbance to that of a range of samples with FeCl3 concentrations in the range of 0 to 40 μM that had been prepared identically to the test samples. The correlation coefficients of the standard curves varied from 0.998 to 0.999. The detection limit of the assay was 1 μM Fe. The intrasample variations (i.e., variations among samples from the same culture) were less than 0.3 nmol/3 × 109 bacteria.
H2O2 susceptibility assay.
Bacteria cultivated overnight on MC plates were resuspended and diluted in PBS to an estimated 3 × 106 CFU/ml. The bacterial suspension of each F. tularensis strain was divided into test tubes with H2O2 added or control tubes that were untreated. The tubes were incubated for 4 h at room temperature without shaking. Thereafter, the samples were serially diluted and spread on MC plates that were incubated at 37°C in 5% CO2 for 3 days before enumeration of the CFU. The data are expressed as the percent survival of a strain exposed to H2O2 relative to the viability of that strain in the control tube not exposed to H2O2.
Catalase assay.
Bacteria cultivated overnight on MC plates or DFO plates were resuspended to an A600 of 1.9 in PBS. Bacteria from 1 ml of the suspension were collected by centrifugation at 13,000 rpm for 5 min. Protein extracts were prepared from the bacterial pellet using 300 μl of B-PER bacterial protein extraction buffer (Thermo Scientific, Rockford, IL). No DNase or lysozyme was supplied to the lysis buffer. The bacteria were mixed thoroughly in the buffer and left for 10 min on the bench to allow complete disruption of the bacteria. Thereafter, the lysate was centrifuged for 3 min to remove any cell debris. The protein concentration in the extracts was determined using the Bradford reagent, according to the manufacturer's instruction (Fermentas, St. Leon-Rot, Germany).
The catalase assay was performed in a 96-well plate by mixing 110 μl of PBS with 10 μl of the protein extract. Then, 80 μl of H2O2 (100 mM) was supplied to start the reaction. The decomposition of H2O2 was measured by monitoring the decrease in absorbance at 240 nm using a microplate reader (Paradigm; Beckman Coulter). The initial linear portion of the curve was used to calculate the change in the A240. A molar extinction coefficient (ɛ) of H2O2 at 240 nm of 43.6 M−1cm−1 was used to calculate the concentration of H2O2 using the Beer-Lambert law, A = ɛcl, where A is absorbance, c is the molar concentration, and l is the path length. One unit of catalase was defined as the amount that decomposes 1 μmol of H2O2 per minute at 25°C per mg protein. In total four (LVS and FSC697) or eight (SCHU S4 and FSC200) protein extracts were analyzed per strain and growth condition.
Analysis of gene expression.
Bacteria cultivated overnight on MC plates were resuspended to an optical density at 600 nm of 1.0 in PBS, and 1 ml of the bacterial suspension was centrifuged at 13,000 rpm for 5 min. RNA was extracted from the bacterial pellet using Trizol, according to the manufacturer's protocol (Invitrogen). cDNA was synthesized from this RNA, and quantitative real-time PCR (RT-PCR) was used to analyze the cDNA samples. In order to remove contaminating DNA, the RNA samples were treated with DNase (DNA-free kit; Ambion, Inc., Austin, TX) in accordance with the protocol supplied by the manufacturer. The RNA was quantified by use of a Nanodrop device (Thermo Fisher Scientific, Wilmington, DE). cDNA was synthesized from 1 μg of the extracted RNA using RNiscript reagent (Bio-Rad), according to the protocol provided by the manufacturer. A negative control was prepared by excluding the enzyme from the cDNA synthesis. In order to degrade any remaining RNA, the cDNA was treated with 2.0 μl of 2.5 M NaOH at 42°C for 10 min, after which the pH was adjusted by the addition of 5 μl of 1 M HCl. The samples were thereafter diluted and stored at −20°C.
RT-PCR was performed in an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA) using a SYBR green I PCR kit (Applied Biosystems), as recommended by the manufacturer. Each reaction mixture contained 12.5 μl of the SYBR green mix, 250 nM forward and reverse primers, and 5 μl of cDNA, and the total volume was adjusted to 25 μl with water. Forward and reverse primers were obtained from Invitrogen, and their sequences are listed in Table S1 in the supplemental material. The reactions were performed in a MicroAmp 96-well plate (Applied Biosystems) capped with MicroAmp optical adhesive seal. The reaction mixtures were incubated at 50°C for 2 min and 10 min at 95°C, followed by 45 cycles of 15 s at 95°C and 1 min at 60°C. The final cycle consisted of incubation at 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s. The threshold cycle (CT) values of selected genes were normalized to the CT value of the housekeeping gene polA (28). The relative gene expressions were calculated as described by Gavrilin et al. (6). Normalized CT values were used for statistical evaluation of the data.
Statistical analysis.
For statistical evaluation, one-tailed Student's t test in the statistical software program SPSS was used.
RESULTS
Bacterial iron content.
SCHU S4, FSC200, LVS, and LVS complemented with fupA, FSC697, were cultivated on MC plates, and iron contents were determined by use of the ferrozine assay. FSC200 contained, on average, 6-fold more iron than SCHU S4 (P < 0.001) (Fig. 1) but less iron than LVS (P < 0.001). FSC697, LVS expressing fupA in cis, contained less iron than both LVS and FSC200 (P < 0.001) but more iron than SCHU S4 (P < 0.001) (Fig. 1). All strains grown on agar plates with DFO had an iron content lower than the detection limit of the assay, 0.2 nmol. Henceforth, strains grown on MC plates and DFO plates are denoted iron replete and iron deplete, respectively.
FIG. 1.
Iron content of F. tularensis strains cultivated on MC plates. The bars represent the averages of at least 12 independent observations. The error bars show the standard errors of the means.
Resistance to H2O2-mediated killing.
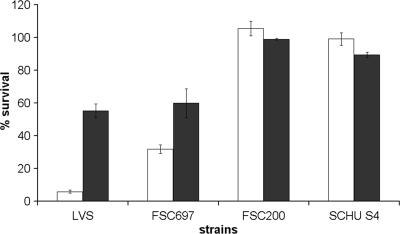
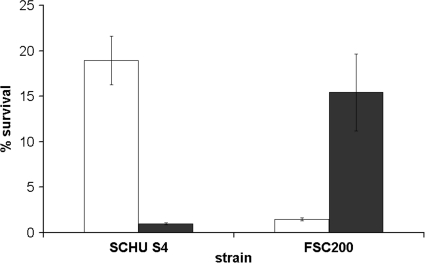
Iron in combination with H2O2 generates highly reactive hydroxyl radicals and anions through the Fenton reaction. In view of this, we determined if the iron content of the bacteria affected their susceptibility to H2O2. Iron-replete and iron-deplete SCHU S4, FSC200, LVS, and FSC697 were exposed to 1 mM H2O2 for 4.0 h. Iron-replete FSC697 survived significantly better (P < 0.001) than LVS (Fig. 2). In contrast, iron-deplete LVS and FSC697 survived the H2O2 challenge equally well and significantly better (P < 0.001) than they did in the iron-replete state. The viability of SCHU S4 and FSC200 was not affected by 1 mM H2O2 (Fig. 2), so they were therefore exposed to 6 mM H2O2 (Fig. 3). Iron-replete SCHU S4 survived the 6 mM H2O2 challenge significantly better than did FSC200 (P < 0.02) (Fig. 3). Similar to LVS and FSC697, FSC200 became more resistant to H2O2 when it was depleted of iron (P < 0.02). Notably, iron-deplete FSC200 survived the H2O2 challenge as well as iron-replete SCHU S4 did. In contrast to the other strains tested, the H2O2-mediated killing of SCHU S4 increased dramatically when it was depleted of iron relative to the level of killing in the iron-replete state (Fig. 3). LVS and FSC697 were rapidly eradicated when they were exposed to 6 mM H2O2 (data not shown).
FIG. 2.
Susceptibility to H2O2 of F. tularensis strains cultivated on MC plates (iron replete, white bars) or plates containing the iron chelator DFO (iron deplete, black bars). These bacteria were resuspended in PBS to a concentration of 3 × 106 CFU/ml and exposed to 1 mM H2O2 for 4 h at room temperature. The bars represent the averages of at least three independent experiments with three observations in each one. The error bars show the standard errors of the means.
FIG. 3.
Susceptibility to H2O2 of F. tularensis strains cultivated on MC plates (iron replete, white bars) or plates with the iron chelator DFO (iron deplete, black bars). These bacteria were resuspended in PBS to a concentration of 3 × 106 CFU/ml and exposed to 6 mM H2O2 for 4 h at room temperature. The bars represent the averages of two independent experiments with three observations in each one. The error bars show the standard errors of the means.
In summary, strains of F. tularensis subsp. holarctica origin (FSC200, LVS, and FSC697) contained significantly more iron than SCHU S4 and were more susceptible to H2O2-mediated killing. These strains became more resistant to H2O2-mediated killing when their iron content was lowered. Notably, FSC697 contained less iron than LVS and was more resistant to H2O2-mediated killing. Thus, a low intracellular iron concentration appeared to increase the resistance of F. tularensis subsp. holarctica strains to H2O2-mediated killing.
Iron contents of F. tularensis subsp. tularensis and F. tularensis subsp. holarctica strains.
To establish if the relatively low iron content of SCHU S4 and high iron content of FSC200 are typical of other strains belonging to the respective subspecies, the iron contents of 11 F. tularensis subsp. tularensis strains and 9 F. tularensis subsp. holarctica strains were measured by the ferrozine assay. All F. tularensis subsp. tularensis strains tested contained less than 8 nmol iron, and all F. tularensis subsp. holarctica strains tested contained more than 18 nmol iron (Fig. 4). Thus, in general, F. tularensis subsp. tularensis strains contained less iron than F. tularensis subsp. holarctica strains after cultivation on iron-rich medium. Noteworthy, this was true also for FSC043, a spontaneous SCHU S4 mutant that contains the same fusion of fupA and fupB as LVS (26, 30).
FIG. 4.
Iron content of F. tularensis strains cultivated on MC plates. The bars represent the averages of three independent observations. The line through each box shows the median, with quartiles being at either end of each box. The vertical lines indicate maximum and minimum values.
Expression of genes related to iron uptake and storage.
We hypothesized that the difference in iron content of strains of the two subspecies may be due to differential expression of genes related to iron uptake and storage. The relative mRNA copy numbers of ferrous uptake proteins A and B (feoA and feoB), ferric uptake protein A (fupA), ferric uptake regulator A (furA), the iron storage ferritin (ftn), and genes of the fsl operon (fslA to fslG) in the strains were measured by real-time PCR. FSC200, LVS, and FSC697 expressed significantly higher levels of fslA than did SCHU S4 (Table 2). In addition, fslD was upregulated in FSC200 relative to the level of expression in SCHU S4. fslE and fslF were significantly downregulated in LVS and FSC697 relative to the levels of expression in SCHU S4, and fslF was also significantly downregulated in FSC200. The ftn copy number was significantly elevated in all strains of F. tularensis subsp. holarctica relative to the level of expression in SCHU S4. All other genes tested were expressed at similar levels in the F. tularensis strains.
TABLE 2.
Real-time PCR analysis of the expression of genes related to iron uptake or storage
| Gene | RCNa |
|||
|---|---|---|---|---|
| SCHU S4 | FSC200 | LVS | FSC697 | |
| fslA | 381 ± 87b | 1,100 ± 147b | 987 ± 205c | 1,214 ± 184b |
| fslB | 193 ± 49 | 291 ± 64 | 94 ± 17 | 161 ± 40 |
| fslC | 121 ± 39 | 171 ± 8 | 75 ± 17 | 138 ± 38 |
| fslD | 37 ± 6 | 78 ± 19c | 27 ± 5 | 53 ± 8 |
| fslE | 57 ± 20 | 28 ± 8 | 12 ± 5c | 18 ± 14c |
| fslF | 153 ± 17 | 47 ± 17c | 29 ± 11b | 16 ± 5d |
| feoA | 26 ± 6 | 27 ± 2 | 36 ± 6 | 45 ± 7 |
| feoB | 17 ± 10 | 18 ± 8 | 8 ± 3 | 11 ± 5 |
| fur | 243 ± 59 | 95 ± 19 | 167 ± 42 | 123 ± 36 |
| fupA | 351 ± 129 | 149 ± 73 | 341 ± 94 | 72 ± 3 |
| ftn | 53 ± 7 | 123 ± 11b | 139 ± 29c | 225 ± 48b |
Copy number of respective gene relative to the housekeeping gene FTT0111. The average of the relative copy numbers obtained from three to five cDNA samples prepared on different days and standard error of the mean are shown.
P < 0.01 relative to SCHU S4.
P < 0.05 relative to SCHU S4.
P < 0.001 relative to SCHU S4.
Genes related to defense against oxidative stress, oxyR, katG, gpx, ahpC1, sodB, sodC, trxA1, trxB, gshA, gshB, grxA, grxB, msrA1, and msrA2, were also analyzed by real-time PCR, but there were no significant differences in expression levels between the F. tularensis strains (data not shown).
Catalase activity.
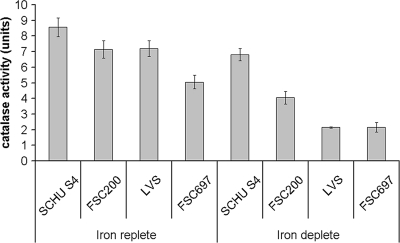
Catalase contributes to the resistance of SCHU S4 and LVS to H2O2 (14), but the role of catalase in FSC200 has not been investigated. The catalase activity of the strains was measured to determine if there were any differences that could be correlated to their susceptibility to H2O2. Moreover, as catalase is a heme-containing enzyme, we wanted to establish if the iron content of the bacteria affected their catalase activity. The activity was measured in protein extracts from iron-deplete and iron-replete strains SCHU S4, FSC200, LVS and FSC697 (Fig. 5). The catalase activities of SCHU S4, FSC200, and LVS were similar under iron-replete conditions, whereas the activity in FSC697 was lower than that in SCHU S4 (P < 0.001) and LVS (P < 0.05) under iron-replete conditions. Depletion of the iron pool reduced the catalase activity of each of the strains in relation to the activity under iron-replete conditions (P < 0.05 for SCHU S4; P < 0.001 for FSC200 and LVS; P < 0.01 for FSC697). The decrease was most pronounced in the F. tularensis subsp. holarctica strains, and the catalase activity of iron-depleted SCHU S4 thereby was significantly higher than that of the other strains (P < 0.001). Protein extracts from SCHU S4 ΔkatG and LVS ΔkatG mutants did not decompose H2O2, thereby validating the specificity of the assay (data not shown).
FIG. 5.
Catalase activity in protein extracts from F. tularensis strains cultivated on MC plates (iron replete) or on plates with DFO (iron deplete). The bars represent the averages of four to eight independent experiments. The error bars show the standard errors of the means.
DISCUSSION
The human vaccine strain LVS is highly virulent in mice and causes a tularemia-like disease. A multitude of studies with LVS in the mouse model have delineated host protective mechanisms against F. tularensis. It has been found that gamma interferon, tumor necrosis factor alpha, CD4 and CD8 T cells, neutrophils, and the inducible nitric oxide synthase all make important contributions to the host defense (5). In addition, the phagocyte oxidase (phox) is critical to restrict bacterial replication during the innate phase of the infection (15). Phox is a major source of superoxide (O2−) production (18) and the degradation product H2O2; therefore, it is not surprising that catalase, which degrades H2O2, is an important virulence factor of LVS (14). In contrast, catalase makes only a minor contribution to the virulence of SCHU S4. This implies that virulent strains of F. tularensis, unlike LVS, possess additional strategies to avoid H2O2-mediated killing. Our results identify iron regulation as a factor that determines the susceptibility of F. tularensis to H2O2-mediated killing and also demonstrate that there is a correlation between iron content and virulence. A major finding was that F. tularensis subsp. tularensis strains contained less iron than F. tularensis subsp. holarctica after growth on iron-rich medium. In line with this, the expression of ftn, encoding the iron storage protein ferritin, was expressed at higher levels in FSC200 (F. tularensis subsp. holarctica) than in SCHU S4 (F. tularensis subsp. tularensis). Despite the high iron content in FSC200, genes of the fsl operon (fslA and fslD), which are negatively regulated by FurA, were expressed at higher levels in FSC200 than in SCHU S4. A similar regulation of ftn and fslA was apparent in FSC200 and LVS. In contrast to fslA, fslE was expressed at lower levels in LVS than in SCHU S4. A reason for this may be that in LVS induction of fslE requires more stringent iron limitation than does induction of fslA (4, 11). It has previously been observed that ferritin exists in different isoforms and that some of these are unique to each F. tularensis subspecies (9, 12). Together with these reports, our results demonstrate that the fine-tuning of Fur regulation and the regulation of iron uptake and storage are fundamentally different between the two subspecies of F. tularensis.
FSC697, LVS expressing the full-length fupA gene, contained even less iron than FSC200 and was almost as virulent as FSC200 (unpublished data), in accordance with recent findings on another fupA-complemented LVS strain (24). Thus, the partial deletion of fupA in LVS is a major factor for its attenuation, and the reason is likely related to changes in iron metabolism, since it is the only function attributed to the protein. In F. tularensis subsp. tularensis, FslE is the siderophore receptor and FupA influences the expression of the fsl operon (13, 19). In contrast, the hybrid protein FupA/FupB functions as the siderophore receptor in LVS, whereas FslE has a minor role in the process (25). We have observed that FSC697, in which the hybrid gene is missing, could still utilize siderophores (unpublished data). This suggests either that FupA functions as a siderophore receptor when it is expressed in LVS or that in the presence of FupA, FslE functions as a receptor for the siderophore. The last scenario is in accordance with the finding that both FupA and FslE are required by F. tularensis subsp. tularensis to utilize siderophores (13, 19). Notably, disrupting the siderophore system in SCHU S4 had no marked effect on its virulence (13), indicating that FupA must have a siderophore-independent function that is critical for virulence.
Introduction of fupA into LVS (FSC697) reduced the iron content below the level found in F. tularensis subsp. holarctica strains. If FupA was directly responsible for the low level of iron in F. tularensis subsp. tularensis, deletion of the gene should result in increased iron content. However, the spontaneous SCHU S4 mutant FSC043, which contains the same FupA-FupB deletion as LVS (26, 30), did not demonstrate an elevated iron content. Moreover, the protein is identical in virulent strains of the two subspecies. Thus, although FupA has an important role in the iron regulation of F. tularensis strains in general, other, subspecies-specific factors play a more important role for the regulation of the total iron levels. This implies that FupA in F. tularensis subsp. tularensis operates coordinately with genes not present in F. tularensis subsp. holarctica or that these genes are differentially regulated. Together, the results illustrate the complexity of the regulatory network that controls the iron homeostasis in F. tularensis. FupA appears to be one of the important components of this network.
Our findings point to a strong correlation between the iron content of the F. tularensis subsp. holarctica strains and their susceptibility to H2O2. The first evidence was the increased resistance of LVS and FSC200 to H2O2-mediated killing after iron depletion. The second evidence was that introduction of fupA into LVS reduced the iron content and increased the resistance to H2O2-mediated killing. The catalytic activity of catalase is dependent on iron (31), so it was not surprising that the catalase activity decreased in the iron-depleted strains. However, catalase is critically required for LVS to withstand H2O2-mediated killing, so an increased resistance to H2O2 paralleled by a reduced catalase activity appears to be paradoxical (14). Rather, the results illustrate how detrimental excess iron may be for bacteria when they are exposed to H2O2. The iron-driven Fenton reaction probably decreased when the iron content was reduced, and therefore, the bacteria survived the H2O2 challenge better. This is the first study to demonstrate that the level of iron modifies the susceptibility of F. tularensis to H2O2. The important role of iron to modulate the adaptation to oxidative stress in other bacteria is well documented. For example, and similar to the results with LVS and FSC200, Staphylococcus aureus (20) and Escherichia coli (1) grown under iron-rich conditions demonstrated increased susceptibility to H2O2 compared to that of bacteria grown under iron-limiting conditions. In addition, inactivation of the fur gene (resulting in deregulation of iron metabolism) increases susceptibility to oxidative stress. This effect can be reversed by iron chelation or introducing a tonB mutation, thereby blocking iron uptake (16, 29).
The correlation between iron and the H2O2 susceptibility of the F. tularensis subsp. holarctica strains implies that the natural, low level of iron in strains of F. tularensis subsp. tularensis is an important trait required to withstand H2O2-mediated damage upon infection. Nevertheless, the resistance of SCHU S4 to H2O2 was highly dependent on iron. In contrast to LVS and FSC200, the susceptibility of SCHU S4 increased dramatically when it was in the iron-deplete versus the iron-replete state. Thus, the mechanism(s) behind the defense against H2O2-mediated damage in SCHU S4 is dependent on iron. Catalase is an important enzyme for SCHU S4 to withstand H2O2, but its activity was only slightly affected by iron depletion, so another, not yet identified factor(s) in SCHU S4 that is dependent on iron to protect the bacterium against H2O2 appears to exist.
Altogether the data presented show that the regulation of iron uptake and storage is different between F. tularensis subsp. tularensis and F. tularensis subsp. holarctica and that iron is a factor that determines the susceptibility of the bacterium to H2O2. During infection, F. tularensis has to cope with both iron deficiency and oxidative stress. Thus, the different handling of iron regulation and resistance to H2O2 by the two subspecies may be central to the differences in their virulence.
Supplementary Material
Acknowledgments
The work was funded in part by grant AI60689 from the U.S. National Institutes of Health. Grant support was also obtained from the Swedish Medical Research Council (grant K2010-56X-09485-20-3) and the Medical Faculty, Umeå University, Umeå, Sweden.
The work was performed in part at the Umeå Centre for Microbial Research (UCMR).
Editor: S. M. Payne
Footnotes
Published ahead of print on 28 December 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Abdul-Tehrani, H., et al. 1999. Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient. J. Bacteriol. 181:1415-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamberlain, R. E. 1965. Evaluation of live tularemia vaccine prepared in a chemically defined medium. Appl. Microbiol. 13:232-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox, C. D. 1994. Deferration of laboratory media and assays for ferric and ferrous ions. Methods Enzymol. 235:315-329. [DOI] [PubMed] [Google Scholar]
- 4.Deng, K., R. J. Blick, W. Liu, and E. J. Hansen. 2006. Identification of Francisella tularensis genes affected by iron limitation. Infect. Immun. 74:4224-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2007. Innate and adaptive immunity to Francisella. Ann. N. Y. Acad. Sci. 1105:284-324. [DOI] [PubMed] [Google Scholar]
- 6.Gavrilin, M. A., et al. 2009. Pyrin critical to macrophage IL-1beta response to Francisella challenge. J. Immunol. 182:7982-7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golovliov, I., A. Sjöstedt, A. Mokrievich, and V. Pavlov. 2003. A method for allelic replacement in Francisella tularensis. FEMS Microbiol. Lett. 222:273-280. [DOI] [PubMed] [Google Scholar]
- 8.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 9.Hubalek, M., et al. 2004. Comparative proteome analysis of cellular proteins extracted from highly virulent Francisella tularensis ssp. tularensis and less virulent F. tularensis ssp. holarctica and F. tularensis ssp. mediaasiatica. Proteomics 4:3048-3060. [DOI] [PubMed] [Google Scholar]
- 10.Kadzhaev, K., et al. 2009. Identification of genes contributing to the virulence of Francisella tularensis SCHU S4 in a mouse intradermal infection model. PLoS One 4:e5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiss, K., W. Liu, J. F. Huntley, M. V. Norgard, and E. J. Hansen. 2008. Characterization of fig operon mutants of Francisella novicida U112. FEMS Microbiol. Lett. 285:270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, B. Y., M. A. Horwitz, and D. L. Clemens. 2006. Identification, recombinant expression, immunolocalization in macrophages, and T-cell responsiveness of the major extracellular proteins of Francisella tularensis. Infect. Immun. 74:4002-4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindgren, H., et al. 2009. The 58-kilodalton major virulence factor of Francisella tularensis is required for efficient utilization of iron. Infect. Immun. 77:4429-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindgren, H., et al. 2007. Resistance of Francisella tularensis strains against reactive nitrogen and oxygen species with special reference to the role of KatG. Infect. Immun. 75:1303-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindgren, H., S. Stenmark, W. Chen, A. Tärnvik, and A. Sjöstedt. 2004. Distinct roles of reactive nitrogen and oxygen species to control infection with the facultative intracellular bacterium Francisella tularensis. Infect. Immun. 72:7172-7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngok-Ngam, P., et al. 2009. Roles of Agrobacterium tumefaciens RirA in iron regulation, oxidative stress response, and virulence. J. Bacteriol. 191:2083-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pekarek, R. S., K. A. Bostian, P. J. Bartelloni, F. M. Calia, and W. R. Beisel. 1969. The effects of Francisella tularensis infection on iron metabolism in man. Am. J. Med. Sci. 258:14-25. [DOI] [PubMed] [Google Scholar]
- 18.Rada, B., and T. L. Leto. 2008. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol. 15:164-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakrishnan, G., A. Meeker, and B. Dragulev. 2008. fslE is necessary for siderophore-mediated iron acquisition in Francisella tularensis Schu S4. J. Bacteriol. 190:5353-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Repine, J. E., R. B. Fox, and E. M. Berger. 1981. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J. Biol. Chem. 256:7094-7096. [PubMed] [Google Scholar]
- 21.Riemer, J., H. H. Hoepken, H. Czerwinska, S. R. Robinson, and R. Dringen. 2004. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal. Biochem. 331:370-375. [DOI] [PubMed] [Google Scholar]
- 22.Rohmer, L., et al. 2006. Potential source of Francisella tularensis live vaccine strain attenuation determined by genome comparison. Infect. Immun. 74:6895-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohmer, L., et al. 2007. Comparison of Francisella tularensis genomes reveals evolutionary events associated with the emergence of human pathogenic strains. Genome Biol. 8:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salomonsson, E., et al. 2009. Reintroduction of two deleted virulence loci restores full virulence to the live vaccine strain of Francisella tularensis. Infect. Immun. 77:3424-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen, B., A. Meeker, and G. Ramakrishnan. 2010. The fslE homolog, FTL_0439 (fupA/B), mediates siderophore-dependent iron uptake in Francisella tularensis LVS. Infect. Immun. 78:4276-4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjödin, A., K. Svensson, M. Lindgren, M. Forsman, and P. Larsson. 2010. Whole-genome sequencing reveals distinct mutational patterns in closely related laboratory and naturally propagated Francisella tularensis strains. PLoS One 5:e11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjöstedt, A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105:1-29. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan, J. T., E. F. Jeffery, J. D. Shannon, and G. Ramakrishnan. 2006. Characterization of the siderophore of Francisella tularensis and role of fslA in siderophore production. J. Bacteriol. 188:3785-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Touati, D., M. Jacques, B. Tardat, L. Bouchard, and S. Despied. 1995. Lethal oxidative damage and mutagenesis are generated by iron in delta fur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 177:2305-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twine, S., et al. 2005. A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect. Immun. 73:8345-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlasits, J., et al. 2010. Mechanisms of catalase activity of heme peroxidases. Arch. Biochem. Biophys. 500:74-81. [DOI] [PubMed] [Google Scholar]
- 32.Winterbourn, C. C. 1995. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett. 82-83:969-974. [DOI] [PubMed] [Google Scholar]
- 33.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.