Abstract
Clostridium sordellii is an important pathogen of humans and animals, causing a range of diseases, including myonecrosis, sepsis, and shock. Although relatively rare in humans, the incidence of disease is increasing, and it is associated with high mortality rates, approaching 70%. Currently, very little is known about the pathogenesis of C. sordellii infections or disease. Previous work suggested that the lethal large clostridial glucosylating toxin TcsL is the major virulence factor, but a lack of genetic tools has hindered our ability to conclusively assign a role for TcsL or, indeed, any of the other putative virulence factors produced by this organism. In this study, we have developed methods for the introduction of plasmids into C. sordellii using RP4-mediated conjugation from Escherichia coli and have successfully used these techniques to insertionally inactivate the tcsL gene in the reference strain ATCC 9714, using targetron technology. Virulence testing revealed that the production of TcsL is essential for the development of lethal infections by C. sordellii ATCC 9714 and also contributes significantly to edema seen during uterine infection. This study represents the first definitive identification of a virulence factor in C. sordellii and opens the way for in-depth studies of this important human pathogen at the molecular level.
Clostridium sordellii is a Gram-positive, spore-forming anaerobe that causes disease in both humans and animals. In animals, C. sordellii primarily causes infections in cattle, sheep, lambs, and foals (15, 33, 34). C. sordellii also causes numerous human diseases, including myonecrosis, uterine infections, and sepsis (7, 20, 47, 55). Recently, C. sordellii has been associated with severe and life-threatening disease in both postpartum and postabortive women, particularly in those undergoing medically induced abortions with mifepristone/misoprostol (20). C. sordellii also has been associated with an increased incidence of disease in intravenous heroin users (19). Although C. sordellii infections are relatively rare in humans, the reported frequency is rising, and these infections exhibit a high mortality rate (approximately 70%) and often result in a severe toxic shock syndrome that is characterized by the sudden onset of weakness, refractory hypotension, and marked leukocytosis (1, 4).
Little is known about the pathogenesis of C. sordellii infections, although it has been suggested that the heterogeneous nature of these diseases may be related to the number and type of virulence factors expressed by the infecting strain (54). Isolates of C. sordellii are capable of producing numerous putative virulence factors, including sordellilysin (SDL), a cholesterol-dependent cytolysin (CDC), phospholipase C (PLC), neuraminidase (Neu), and two members of the large clostridial glucosylating family of toxins, known as lethal toxin (TcsL) and hemorrhagic toxin (TcsH). The production of these putative virulence factors is highly variable between strains, and the individual role that each factor plays in the clinical presentation of disease is not known (55).
TcsH (300 kDa) and TcsL (270 kDa) are both large toxins that appear to be very similar in terms of biological activity, as well as antigenicity to toxin A and toxin B, respectively, from Clostridium difficile. Collectively, these toxins are monoglucosyltransferases that act by glucosylating and disrupting the functional properties of small Rho GTPases in the host cell cytosol. These enzymes control the cell cycle, apoptosis, transcription, and the structural consequences of actin polymerization, such as cell morphology, migration, and polarity (21, 27, 29). Injection of purified TcsL into mice reproduces key features of the toxic shock syndrome observed in C. sordellii-infected humans, with lung vascular endothelial cells being particularly susceptible to the toxin (22).
Of the large clostridial glucosylating toxins, TcsL and toxin B from C. difficile have the highest similarity, being 76% identical (29). Identification of a toxin (TcdB-1470) that appears to be a hybrid between the standard C. difficile toxin B from a toxin A+ B+ strain and C. sordellii TcsL (11) suggests that genetic exchange is likely to have occurred between these clostridia.
TcsL is highly cytotoxic, having a minimum lethal dose (MLD) in a mouse lethality assay of just 5 ng, making it one of the most cytotoxic proteins characterized to date (39). TcsH, while not as cytotoxic as TcsL, is a potent enterotoxin and has an MLD in mice of 75 ng (39). In contrast, toxins A and B from C. difficile have MLDs in mice of 50 to 90 ng and 50 ng, respectively (39). For this reason, TcsL and, possibly, TcsH are thought to be the major virulence determinants of C. sordellii.
The lack of molecular tools to genetically manipulate strains of C. sordellii has made it difficult to determine the relative contribution of each virulence factor to disease. In this study, we report the development of methods that facilitate the transfer of autonomously replicating plasmids into C. sordellii using RP4-mediated conjugation from Escherichia coli. We report the first successful genetic manipulation of C. sordellii; specifically, the insertional inactivation of the tcsL gene of C. sordellii strain ATCC 9714 using targetron technology. The analysis of a number of independently derived tcsL mutants in a mouse model of infection has shown definitively that TcsL is an essential virulence factor in C. sordellii ATCC 9714.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. All bacteriological culture media were obtained from Oxoid. For mating procedures and extraction of plasmid and genomic DNA, C. sordellii strains were cultured in BHIS broth (51) supplemented with 0.1% l-cysteine HCl and 0.5% glucose or BHIS agar with the additional supplement of 0.09% FeSO4. d-Cycloserine (250 μg/ml), thiamphenicol (10 μg/ml), or erythromycin (10 μg/ml) (all from Sigma-Aldrich) was used for selection of recombinant strains, as required. C. sordellii cultures were grown in an atmosphere of 10% H2, 10% CO2, and 80% N2 at 37°C in a Coy anaerobic chamber. E. coli strains were derivatives of either Top10 (Invitrogen) or HB101(pVS520) (45) and were cultured aerobically at 37°C in 2× YT agar or broth (40). Recombinant E. coli cultures were supplemented with chloramphenicol (25 μg/ml) or tetracycline (10 μg/ml) as required.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdr17(rK− mK+) deoR thi-1 supE44 gyrA96 relA1 | Life Technologies |
| One-Shot TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 λ− | Invitrogen |
| HB101 | thi-1 hsdS20(rB− mB−) supE44 recAB ara-14 leuB5 proA2 lacY1 galK rpsL20 (Smr) xyl-5 mtl-1 | 8 |
| C. sordellii strains | ||
| ATCC 9714 | Type strain; TcsL+ TcsH− Neu+ SDL− PLC+ | 23 |
| DA108 | Clinical isolate; TcsL negative | 24,30 |
| DLL5001 | ATCC 9714 tcsL Ω targetron (mutant 1); erythromycin resistant | This study |
| DLL5002 | ATCC 9714 tcsL Ω targetron (mutant 2); erythromycin resistant | This study |
| DLL5003 | ATCC 9714 tcsL Ω targetron (mutant 3); erythromycin resistant | This study |
| Plasmids | ||
| pMTL9361Cm | E. coli-C. difficile mobilizable shuttle vector; carries pCD6 replication region, oriT, and Tmr | 10 |
| pVS520 | Tra+ Mob+ RP4 derivative; Tcr | 45 |
| pJIR3566 | Clostridial mobilizable targetron vector; carries pCB102 replication region, the lacZα gene, oriT, and Cmr | 13 |
| pDLL2 | Group II intron of pJIR3566 retargeted to the 2993/2994s site of the tcsL gene | This study |
Isolation and manipulation of DNA.
Plasmid DNA from E. coli strains grown overnight in 5 ml of broth, with appropriate antibiotic selection, was isolated using QIAprep spin miniprep columns (Qiagen) according to the manufacturer's instructions. C. sordellii genomic DNA was prepared as previously described (44). DNA was quantified using a NanoDrop spectrophotometer (NanoDrop Technologies) and stored at −20°C. Standard methods for the digestion, modification, ligation, and analysis of plasmid and genomic DNA and PCR products were used (48). Nucleotide sequence analysis was carried out using a Prism BigDye terminator cycle sequencing kit (Applied Biosystems) according to the manufacturer's instructions. Sequence detection was performed by Micromon at Monash University, and sequences were analyzed using contigExpress (Invitrogen).
Transfer of plasmid DNA into C. sordellii by conjugation.
The DNA transfer procedure was carried out essentially as described previously for E. coli-to-C. difficile conjugations (10). Bacterial pellets collected from 1.5-ml aliquots of an appropriate overnight E. coli donor culture were resuspended in 200-μl aliquots of C. sordellii ATCC 9714 which had been collected from 20-ml overnight cultures incubated at 37°C in an anaerobic chamber. This mating mixture, together with the appropriate mating controls, was then spread onto a thick BHIS agar plate and incubated for 6 h or overnight at 37°C under anaerobic conditions. Bacterial growth was harvested by flooding the agar plate three times with 1 ml of sterile phosphate-buffered saline (PBS). Aliquots (100 μl) of the combined cell suspension were then spread onto BHIS agar plates supplemented with d-cycloserine to prevent growth of the E. coli donor and thiamphenicol to select for C. sordellii transconjugants carrying the appropriate plasmids. The plates were incubated under anaerobic conditions for 24 to 72 h.
Identification of targetron insertion sites.
We designed and implemented Perl software to predict potential intron insertion sites. This software, designated “Intron Finder,” determines and ranks candidate intron insertion sites within a defined input DNA sequence. A position-specific scoring matrix (PSSM) was derived from a training set of 56 intron insertion sites having a predicted E-value of 0.2 or less (introns with an E-value of 0.5 or less are classed as efficient using the Sigma-Aldrich system). RSAT matrix scan (53) was then used with the PSSM to identify high-scoring target sites within the input DNA sequence. The Intron Finder software then generates the sequences of the three oligonucleotide primers (EBS1, 60 bp; EBS2, 49 bp; and IBS1, 53 bp) required for the specific retargeting of group II introns for each predicted target site. Intron Finder is available at http://vbc.med.monash.edu.au/∼torsten/intron_site_finder/.
Construction of the tcsL inactivation plasmid and conjugative donor strain.
To insertionally inactivate the tcsL gene of C. sordellii, Intron Finder was used to predict potential intron insertion sites and to design the oligonucleotide primers used for specific retargeting of the group II intron for the tcsL gene (primers DLP1, DLP2, and DLP3). Intron retargeting then was performed using splicing by overlapping extension PCR (SOE-PCR). The retargeting SOE-PCR was performed as described in the targetron user's guide supplied by Sigma-Aldrich with the following modifications: primers DLP1 (5′-AAAAAAGCTTATAATTATCCTTAATTGTCGAAGGAGTGCGCCCAGATAGGGTG-3′), DLP2 (5′-CAGATTGTACAAATGTGGTGATAACAGATAAGTCGAAGGAAGTAACTTACCTTTCTTTGT-3′), DLP3 (5′-TGAACGCAAGTTTCTAATTTCGGTTACAATCCGATAGAGGAAAGTGTCT-3′), and DLP4 (5′-CGAAATTAGAAACTTGCGTTCAGTAAAC-3′) were used to make the primer master mix, a 1-μl aliquot of which was then added to the final PCR. The final PCR was carried out with Phusion DNA polymerase (NEB) and failsafe reaction buffer E (Epicenter).
The 350-bp product generated was purified using the PCR purification kit according to the manufacturer's instructions (Qiagen) and then digested using restriction enzymes HindIII and BsrGI. The digested fragment was then purified by extraction from a 0.8% agarose gel using a QIAquick Gel extraction kit according to the manufacturer's instructions (Qiagen). The purified fragment was then subcloned into the clostridial targetron plasmid pJIR3566 (13), which had previously been digested with HindIII and BsrGI. This cloning step replaces the lacZα region of pJIR3566 with the retargeted intron fragment, thus facilitating blue/white selection. The resulting plasmid, pDLL2, was subsequently introduced into E. coli HB101(pVS520) by electroporation to facilitate conjugative transfer of the plasmid into C. sordellii.
Isolation of C. sordellii mutants.
C. sordellii transconjugants carrying pDLL2 were grown overnight in 20 ml of BHIS broth supplemented with thiamphenicol. Aliquots (100 μl) were spread onto BHIS agar supplemented with erythromycin, which was then incubated for 24 to 48 h. All erythromycin-resistant isolates were then cross-patched onto BHIS agar supplemented with thiamphenicol to ensure loss of pDLL2. Isolates resistant to erythromycin and sensitive to thiamphenicol were then analyzed further.
Southern hybridization analysis.
Purified genomic DNA was digested with HaeIII and EcoRI, separated by gel electrophoresis on a 0.8% agarose gel, and transferred to a nylon membrane (Hybond N; Amersham) using standard procedures (44). The blots were hybridized with either an ermB-specific PCR product (14), a catP-specific PCR product (36), or an internal tcsL PCR product amplified from C. sordellii ATCC 9714 genomic DNA using primers DLP5 (5′-GGTAAATGGATAAATAAAGAAG-3′; binds at tcsL nucleotide positions 2274 to 2295) and DLP6 (5′-GATATATGAGTAGCATATTCAGAG-3′; binds at tcsL nucleotide positions 2753 to 2776). The probes were labeled by random PCR incorporation of digoxigenin (DIG)-labeled nucleotides according to the manufacturer's instructions (Roche). Hybridization was then detected using a chemiluminescence detection system according to the manufacturer's instructions (Roche).
Detection of TcsL by Western blotting.
TcsL was partially purified by ammonium sulfate precipitation from cell-free culture supernatants of C. sordellii following growth for 72 h. Protein was precipitated from 100 ml of broth culture using a concentration of 70% ammonium sulfate. The precipitate was collected, dissolved in 2 ml of distilled water, and dialyzed against 10 mM Tris-HCl buffer, pH 7.0, for 24 h with three changes of buffer. Proteins were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE [8%]) (32) and transferred by electrophoresis (48) to nitrocellulose membranes (Whatman). The membranes were incubated for 1 h in 1× TBS-Tween (5 mM Tris-HCI, 15 mM NaCI, pH 7.4, 0.05% Tween 20) containing 5% dried milk powder (Bonlac Foods, Melbourne, Australia) and then incubated for 2 h with immunopurified antibodies specific for C. sordellii TcsL (obtained from M. Popoff). TcsL-bound antibodies were then detected with peroxidase-conjugated anti-rabbit sheep antibodies (Chemicon) using a Western lightning chemiluminescence reagent kit (Perkin Elmer) according to the manufacturer's instructions.
Vero cell cytotoxicity assays.
To prepare the supernatants used in the toxin assays, C. sordellii was grown in 90 ml of TY broth (37), with a starting optical density at 600 nm (OD600) nm of 0.02, for 48 h, and the cells pelleted by centrifugation. The supernatants were filter sterilized and stored on ice prior to use. Cells were cultured in minimum essential medium (MEM alpha medium; GIBCO, Invitrogen) containing 10% heat-inactivated fetal calf serum (HI FCS), 100 units/ml penicillin, and 100 μg/ml streptomycin in culture flasks at 37°C in 5% CO2. The cells were grown to a confluent monolayer and subcultured by incubation in 1 to 2 ml of 0.1% trypsin in 1 mM EDTA. The cells were counted and resuspended in fresh medium at a concentration of 1 × 105 cells/ml. A 100-μl aliquot of the cell suspension was seeded into each well of 96-well plates. The plates were incubated for 20 to 24 h, and the culture medium removed. Serial 2-fold dilutions of the C. sordellii culture supernatants were made in MEM alpha medium supplemented with 1% HI FCS, and 100 μl added to each well. Negative controls were treated with 100 μl of MEM alpha medium supplemented with 1% HI FCS. The plates were incubated at 37°C in 5% CO2 and morphological changes were observed by microscopy after 24 h. The endpoint was scored as the last dilution at which cytopathic effect (CPE) was observed. The assays were performed in triplicate on independent culture supernatants. An Olympus 1X71 inverted microscope was used to visualize the cells at ×10 and ×20 magnifications.
Vero cell viability assay.
Viability assays were performed using the same tissue culture plates that were used for the Vero cell cytotoxicity assays described above. Following the scoring of CPE, a 20-μl aliquot of Thiazolyl blue tetrazolium bromide (MTT; Sigma) at a concentration of 0.5% (wt/vol) in PBS was added to each well and the plate shaken gently. The plate was then incubated for 4 h at 37°C in 5% CO2 in the dark. Then, the culture medium containing MTT was removed from each well and 200 μl of dimethyl sulfoxide (Sigma) was added. Absorbance was then measured using a Tecan infinite M200 plate reader with a test wavelength of 570 nm and a reference wavelength of 630 nm. The viability assay results were then converted to percent cell death using the following formula: % death = 1 − (OD630 nm of test well/OD630 nm of negative control) × 100.
Virulence experiments.
Virulence experiments in mice were performed as previously described (5, 24). Wild-type female 129/SvJ mice or BALB/c mice (8 to 10 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were treated according to National Institutes of Health guidelines for the use of experimental animals with the approval of the University of Michigan Committee for the Use and Care of Animals. C. sordellii was grown overnight in RCM broth (24), and the cells pelleted and then resuspended in PBS to a concentration of approximately 4 × 108 CFU/ml. Groups of 5 mice were then injected intraperitoneally (i.p.) with 100 μl PBS containing approximately 2 × 108 CFU of either wild-type C. sordellii or the isogenic tcsL mutants (DLL5001 and DLL5003). Infection was allowed to proceed for 7 days, and survival was recorded daily. In separate experiments, female 129/SvJ mice were infected i.p. with 2 × 108 CFU of C. sordellii strain DLL5003. Seven days after infection, spleens were harvested and homogenized in 1 ml of sterile PBS and the numbers of CFU determined by serial 10-fold dilutions onto RCM agar under anaerobic conditions. PCR analysis of splenic C. sordellii isolates was performed with primers DLP5 and DLP6 to confirm the presence of the correct strain in the infected animals. The wild-type and mutant strains yield a 503-bp and a 2,262-bp PCR product, respectively, with these primers.
Histology.
BALB/c mice were infected by intrauterine (i.u.) inoculation into the right uterine horn with 1 × 105 CFU of either ATCC 9714 or isogenic tcsL mutant DLL5003 according to our previously published protocol (24). Uterine tissues were harvested and immersed in formalin for 48 h postinoculation and processed by standard histological methods, and 5-μm hematoxylin-and-eosin-stained sections were generated (McClinchey Histology Labs, Inc., Stockbridge, MI). Histological changes were assessed by a board-certified veterinary pathologist (I.L.B.).
RESULTS
RP4-mediated conjugation facilitates the efficient transfer of plasmid DNA from E. coli to C. sordellii.
Previous studies in our laboratory resulted in the construction of clostridial shuttle plasmids that carry the origin of transfer (oriT) region of the broad-host-range conjugative plasmid RP4 (37). These plasmids have successfully been mobilized into previously genetically intractable strains of Clostridium perfringens (37), C. difficile (38), and Clostridium septicum (31) via conjugation from an appropriate E. coli donor strain. We have exploited this same RP4-based conjugation system in this study to successfully introduce plasmid DNA into the C. sordellii type strain, ATCC 9714. Given the relatively close phylogenetic relationship of C. sordellii to C. difficile, attempts were made to transfer the shuttle plasmid pMTL9361Cm (10), which is based on the native C. difficile plasmid pCD6 (41), into C. sordellii. Plasmid pMTL9361Cm was introduced into the E. coli donor strain HB101(pVS520) (45), after which plate matings were performed with C. sordellii strain ATCC 9714. Using this method, pMTL9361Cm was successfully transferred to C. sordellii at a relatively high frequency of between 2.7 × 10−4 and 6 × 10−3 transconjugants per donor cell.
Construction of C. sordellii tcsL mutants.
To generate C. sordellii tcsL mutants, we utilized targetron technology, which has previously been used successfully to mutagenize numerous genes in the clostridia (12, 13, 25, 50). The ATCC 9714 reference strain was used in this study as it was the only strain tested that was amenable to genetic manipulation under these conditions. C. sordellii ATCC 9714 was originally isolated from an infected patient suffering edema (23) and, despite its limited repertoire of putative virulence factors, is highly lethal in animal models of infection, reproducing typical features of human toxic shock syndrome (24, 30).
Using Intron Finder, we identified a number of putative intron insertion sites within the tcsL gene of C. sordellii. The group II intron encoded by the clostridial targetron plasmid pJIR3566 (13) was retargeted to integrate within the top-ranked site, with intron insertion predicted to occur at tcsL nucleotide position 2993 on the DNA sense strand. The tcsL-targeted plasmid, pDLL2, was introduced into C. sordellii by RP4-mediated conjugation, after which a number of thiamphenicol-resistant plasmid-containing transconjugants were isolated. Thiamphenicol-resistant transconjugants from three independent matings were subcultured onto erythromycin-containing medium, resulting in the isolation of several putative independently derived erythromycin-resistant tcsL mutants.
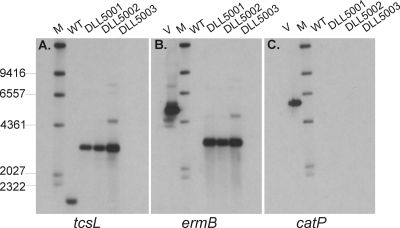
To confirm the successful inactivation of the tcsL gene, PCR and Southern blotting were performed. PCR analysis of a number of erythromycin-resistant isolates, using primers flanking the predicted insertion site of the intron within tcsL, resulted in a product of 2.3 kb, which corresponded to the expected size of the insertionally inactivated gene. The wild-type ATCC 9714 yielded a product of 500 bp, as expected (data not shown). Chromosomal DNA from ATCC 9714 and three independently derived tcsL mutants (DLL5001 to DLL5003) was digested with EcoRI and HaeIII and subjected to Southern blot analysis using DIG-labeled tcsL-, ermB-, and catP-specific probes (Fig. 1). As expected, the tcsL-specific probe bound to DNA extracted from all strains, with a band of 3.1 kb observed from the mutant strains, compared to the wild-type strain where a band of 1.8 kb was seen (Fig. 1A). This size difference corresponded to the expected size of the inserted targetron element within tcsL. In each of the mutants, the ermB probe hybridized to a 3.1-kb band, as seen with the tcsL-specific probe, but it did not bind to DNA extracted from ATCC 9714, as expected. The catP probe hybridized to the positive control, pDLL2, but did not hybridize with DNA extracted from any of the strains, confirming that pDLL2 was no longer present in the mutant strains. Finally, the 5′ and 3′ genomic tcsL-targetron junctions were PCR amplified and sequenced, confirming that the modified targetron had inserted into the predicted nucleotide position (data not shown). Taken together, these data confirmed that the tcsL gene had been insertionally inactivated in each of the independently derived mutants.
FIG. 1.
Southern hybridization analysis of tcsL mutants. Genomic DNA was purified and digested with HaeIII and EcoRI prior to electrophoresis on 0.8% agarose gels. The Southern blots were probed with probes specific for (A) tcsL, (B) ermB, and (C) catP. WT, wild-type ATCC 9714; DLL5001 to DLL5003, tcsL mutants; V, vector control pDLL2; M, DIG-labeled λ-HindIII molecular size markers (kb).
Inactivation of tcsL abolishes the production of lethal toxin.
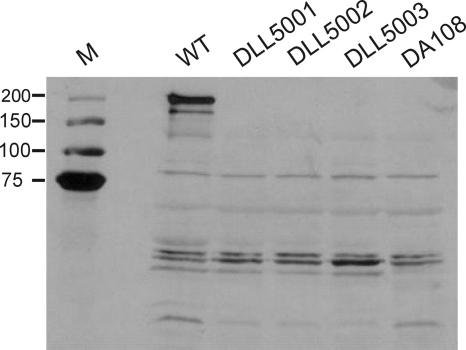
To determine the effect of tcsL inactivation on the production of TcsL, Western blots were performed using a TcsL-specific antibody. As expected, an immunoreactive protein of >200 kDa was produced by the wild-type strain ATCC 9714. This band was absent in the supernatants of both the negative-control strain DA108, which does not encode a tcsL gene (24), and each of the independently derived tcsL mutants, indicating that these strains no longer produce any protein that reacts with the TcsL-specific antiserum (Fig. 2).
FIG. 2.
Comparative analysis of TcsL production. The wild type and isogenic mutant derivatives were examined by Western immunoblotting using TcsL-specific antibodies and precipitated supernatant proteins from the strains indicated. WT, wild-type ATCC 9714; DA108, tcsL-nonexpressing negative-control strain; DLL5001 to DLL5003, tcsL mutants; M, molecular mass size markers (kDa).
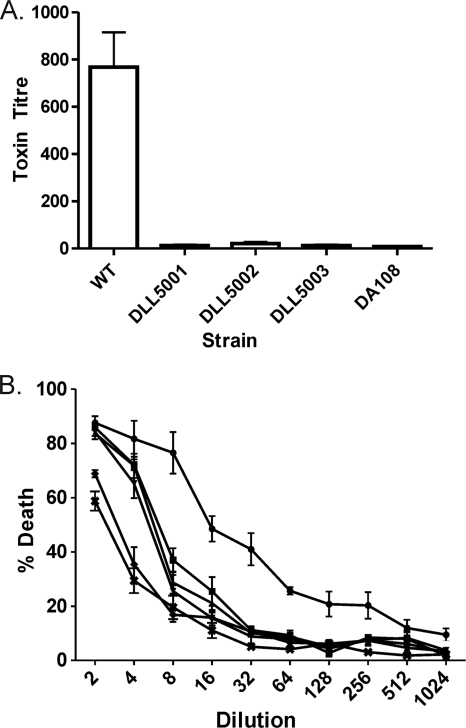
To quantify TcsL toxin production, doubling-dilution Vero cell cytotoxicity assays were performed using cell-free culture supernatants from the tcsL mutants and control strains. The wild-type strain ATCC 9714 produced high levels of TcsL (Fig. 3), as indicated by the substantial cytopathic effect (CPE) observed on Vero cells following the addition of culture supernatant from this strain (Fig. 3A). The wild-type strain was significantly more cytotoxic than all of the tcsL mutants (P < 0.0001, 95% confidence interval, t test), which produced levels of CPE that were not statistically different from those observed with the negative control DA108. As an additional measure of TcsL production, cell viability was analyzed following the incubation of Vero cells with culture supernatants collected from the wild-type C. sordellii strain ATCC 9714, each of the three independently derived tcsL mutants(DLL5001, DLL5002, and DLL5003), and strain DA108. As can be seen in Fig. 3B, supernatant from strain ATCC 9714 resulted in significantly higher levels of cell death than supernatants from the tcsL mutants or strain DA108 (P < 0.0001, 95% confidence interval, t test), indicating that these strains produce significantly less toxin than the wild-type strain. It is also apparent that the TY medium used for the growth of C. sordellii is toxic to Vero cells at the lowest dilutions (Fig. 3B); this may account for much of the cytotoxicity seen from the tcsL mutants and strain DA108. Taken together, these data further confirmed that the tcsL mutants no longer produced functional lethal toxin.
FIG. 3.
Quantitative Vero cell cytotoxicity assays. Serial doubling dilutions of C. sordellii culture supernatants were made in MEM alpha medium supplemented with 1% HI FCS and used in cytotoxicity assays. (A) The morphological changes of the Vero cells were observed and scored by microscopy after 24 h. The endpoint was taken as the last dilution at which CPE was observed. The toxin titer is the reciprocal of the endpoint dilution. WT, wild-type ATCC 9714; DA108, tcsL-nonexpressing negative-control strain; DLL5001 to DLL5003, tcsL mutants. (B) Cell viability following incubation of Vero cells with C. sordellii supernatants for 24 h was determined by using MTT reagent. The percent death was calculated by comparison to untreated wells, which were assumed to have 100% viability. •, wild-type ATCC 9714; ▪, tcsL mutant DLL5001; ▴, tcsL mutant DLL5002; ▾, tcsL mutant DLL5003; ♦, tcsL-nonexpressing negative-control strain DA108; ✖, TY medium control. All assays were performed in duplicate on three independent culture supernatants, and the mean values of these assays are shown together with the standard errors of the means.
Attempts were made to complement the tcsL mutation with the wild-type gene, but these experiments were unsuccessful as all constructs isolated contained tcsL gene deletions. The intact gene has never been cloned successfully, possibly because of its large size (approximately 7 kb) and extensive repeat regions (9, 52). Similar difficulties have been reported for the large C. difficile toxin genes tcdA and tcdB (36). In addition, there are currently no reports in the literature describing the successful complementation of any LCT gene in the clostridia. LtrA-mediated reversion of the targetron mutants (49) using plasmid pJIR3566 was also attempted. In this approach, plasmid pJIR3566 (which expresses ltrA) was introduced into each independent tcsL mutant strain, and the production of TcsL assessed using Western immunoblots and Vero cell cytotoxicity assays (data not shown). In each case, despite the presence of pJIR3566 (confirmed by catP-specific PCR), the strains that were generated produced no detectable TcsL, i.e., no excision of the targetron was detected at the phenotypic level. For this reason, two independently derived mutants were used in subsequent virulence experiments, to exclude the possibility that any observed phenotypic effects were the result of secondary mutations.
TcsL is essential for virulence in the mouse infection model.
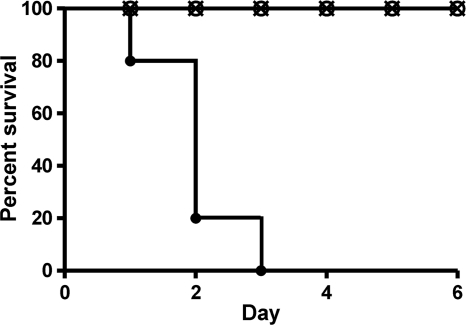
To determine the effect of the tcsL mutation on the virulence of C. sordellii strain ATCC 9714 in vivo, we utilized an intraperitoneal (i.p.) model of infection in mice (24). The i.p. injection of 2 × 108 CFU of the wild-type C. sordellii ATCC 9714 strain into female 129/SvJ mice was rapidly lethal, with 100% of mice dying within 72 h of inoculation (Fig. 4). Soon after infection, mice exhibited typical signs of illness, including huddling, bradykinesia, and fur ruffling. In stark contrast, the i.p. administration of the same number of either of two independently derived tcsL mutants (DLL5001 and DLL5003) failed to induce any visible signs of disease. These mice were still alive and healthy 7 days after inoculation (Fig. 4). In separate experiments, mice inoculated i.p. with either of the two tcsL mutant strains survived at least 3 weeks without overt signs of illness (data not shown).
FIG. 4.
Kaplan-Meier survival curve using the mouse infection model. The graph shows days from inoculation with C. sordellii to death. Two groups of 5 female 129/SvJ mice were injected intraperitoneally with each of the indicated strains, and mouse survival was monitored for 6 days. •, wild-type ATCC 9714; ○, tcsL mutant DLL5001; ×, tcsL mutant DLL5003.
Notably, mice infected with the C. sordellii tcsL mutants did not appear ill. To confirm that the mice were inoculated with viable bacteria, splenic tissue was harvested and homogenized 7 days after i.p. inoculation with 2 × 108 CFU of the tcsL mutant DLL5003 and anaerobic culture was used to detect bacteria. Viable C. sordellii cells were isolated from the spleens of all inoculated mice (n = 5), demonstrating that sustained visceral organ dissemination of C. sordellii had occurred without inducing overt signs of illness. Subsequent PCR analysis (primers DLP5 and DLP6) of bacterial isolates from the spleen confirmed that the C. sordellii strains present were those used to initiate infection (data not shown). Taken together, these data provide compelling evidence that TcsL is an essential virulence factor in C. sordellii ATCC 9714.
TcsL contributes to edema during uterine infection.
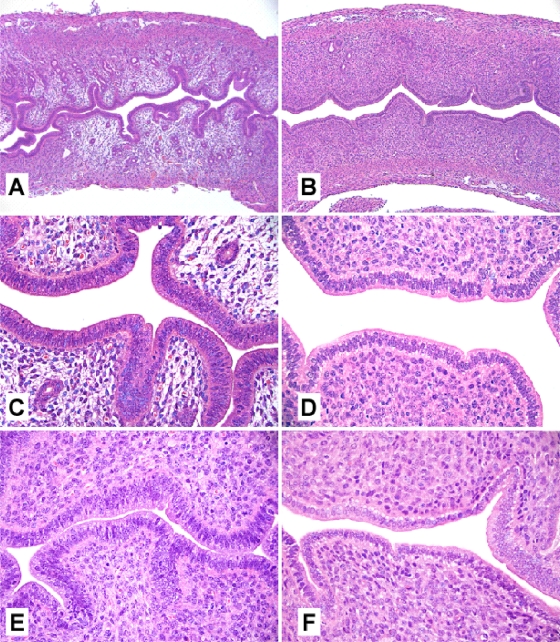
When injected as a purified protein, TcsL is known to cause edema through effects on vascular permeability (22). However, the contribution of this toxin to tissue damage during natural infection is poorly understood. To better address this issue, we utilized an established mouse model of intrauterine (i.u.) infection with C. sordellii (24), since this pathogen is an emerging cause of life-threatening reproductive tract infections in young women (26). Mice (n = 3 per group) were inoculated i.u. with 1 × 105 CFU of C. sordellii ATCC 9714 or the tcsL mutant DLL5003. Animals inoculated with ATCC 9714 became lethargic and depressed by 48 h postinfection, while animals infected with DLL5003 remained clinically normal. Histopathological analysis conducted at 48 h (Fig. 5) revealed marked edema within the lamina propria of the uterine horns inoculated with ATCC 9714 (Fig. 5A and C). The contralateral, uninfected uterine horns (Fig. 5E) appeared histologically normal, apart from mild edema near the cervix-uterus bifurcation in a minority of ATCC 9714-infected animals (data not shown). No proprial edema or other changes were observed in the infected or uninfected horns of mice infected with DLL5003 (Fig. 5B, D, and F).
FIG. 5.
Representative histological findings in mouse uterine horns 48 h after unilateral inoculation with the wild-type C. sordellii strain ATCC 9714 (A and C) or the tcsL mutant DLL5003 (B and D). Panels E and F are representative images taken from the uninfected, contralateral uterine horns of mice infected with the wild-type strain ATCC 9714 or the mutant strain DLL5003, respectively. Sections were stained with hematoxylin and eosin. Magnification: A and B, ×100; C, D, E, and F, ×400.
DISCUSSION
In this paper, we report the first construction of a mutant in C. sordellii and establish that the production of lethal toxin is essential for the virulence of C. sordellii strain ATCC 9714. This work provides new evidence that TcsL is required for the rapid lethality observed in some C. sordellii infections. Clear evidence for this conclusion comes from the observation that independently derived tcsL mutants were nonlethal in a mouse infection model, while infection with the wild-type strain, ATCC 9714, resulted in the death of all infected animals within 3 days. These studies also establish a role for TcsL in causing edema during infection, which was demonstrated in a mouse model of intrauterine sepsis.
These data confirm and extend the findings of other investigators (22, 39), which showed that purified TcsL was highly toxic when injected into mice, being some 10-fold more active than C. difficile toxin B. Furthermore, recent work from one of our laboratories (24) utilized different tcsL-expressing and -nonexpressing strains of C. sordellii (ATCC 9714 and DA108, respectively) in rodent models of endometritis to support the hypothesis that TcsL is a crucial factor involved in the pathogenesis of toxic shock syndrome during infection. The exact role of TcsL could not be conclusively determined from this study since the strains used were not isogenic and the effects of other strain differences on virulence could not be determined (24).
Our data confirm the hypothesis that, under the conditions tested, TcsL is an important virulence factor in C. sordellii and provide new information regarding the effects of TcsL on the local histopathology of infection—TcsL caused marked edema at the site of inoculation (Fig. 5). Interestingly, we did not observe abscesses, myometrial necrosis, or hemorrhage, features that have been reported, along with edema, in human cases of intrauterine infection (16, 20). The reason why these findings were absent in the mouse model is unclear but may relate to differences between this model and natural human infection.
TcsL is a member of the large clostridial glucosylating toxin family and has significant identity to toxins A and B from C. difficile, alpha-toxin from C. novyi, and TpeL from C. perfringens (2). There is growing evidence that these toxins are important virulence factors. Isogenic C. difficile strain 630 mutants that no longer produce toxin B, for example, were recently shown to be avirulent in the hamster infection model, indicating that this toxin is a major virulence factor of C. difficile (36). Similarly, C. novyi NT, which is a strain of C. novyi from which the alpha-toxin-encoding bacteriophage episome has been cured, was found to be avirulent in a mouse model, suggesting that this toxin is likely to be essential for the virulence of C. novyi (18). The role of TpeL in the virulence of C. perfringens type C strains is currently not known; however, recent studies have shown that purified TpeL toxin is lethal to mice following intravenous injection (2).
C. sordellii is associated with a number of different diseases in both humans and animals, which may be related to the production of numerous putative virulence factors (55). In addition to TcsL, TcsH and SDL are of particular interest and may play important roles in disease. TcsH has a significantly higher MLD than TcsL (39). As stated earlier, TcsH is thought to be similar to toxin A of C. difficile; the toxins are serologically cross-reactive. Partially purified TcsH is also able to induce a hemorrhagic response in the rabbit ileal loop in a similar manner to toxin A (39). TcsH has not been sequenced at either the nucleotide or amino acid level, and its role in disease remains to be determined. There is some evidence that TcsH might be an important virulence factor, since guinea pigs vaccinated with a TcsL-specific toxoid succumbed to infection and died following challenge with C. sordellii strain 3703, which produces both TcsL and TcsH, but survived when challenged with strain KZ1047, which only produces TcsL (3). In contrast, guinea pigs vaccinated with a combination toxoid containing components of both TcsL and TcsH were fully protected against subsequent challenge with both strains (3). A protective antigen, however, does not necessarily equate to an essential virulence factor. Several highly toxigenic TcsL-positive but TcsH-negative isolates have been characterized and shown to be rapidly lethal in an animal model, indicating that TcsH may not be essential for virulence (3, 24). As with C. difficile, there are currently no reports documenting the isolation of TcsH-positive, TcsL-negative strains of C. sordellii.
SDL forms part of the CDC family of toxins. CDCs range from being essential (listeriolysin O, streptolysin O, and anthrolysin O) (17, 35, 46) to nonessential but involved in disease pathogenesis (perfringolysin O) (6). The contribution of SDL to disease has yet to be established; however, based on the results of other studies, CDCs appear to activate the innate immune response, suggesting a possible role for these toxins early in the infection process (28, 42, 43). The presence or absence of various toxins, such as SDL, may contribute to the wide spectrum of disease observed in C. sordellii-mediated infections.
In the context of this study, it was not possible to assess the roles of SDL or TcsH in the virulence of C. sordellii, since strain ATCC 9714 does not produce either of these toxins (55). However, the observation that infection with ATCC 9714 leads to a fatal outcome in mice suggests that neither toxin is required for the virulence of this particular strain. It is not known whether TcsL is an essential virulence factor in strains of C. sordellii that do produce SDL or, perhaps more importantly, TcsH. Studies are under way in our laboratories to determine the relative roles of SDL and TcsH in the pathogenesis of C. sordellii-mediated disease.
In conclusion, we have developed methodologies which allow the transfer of plasmids into C. sordellii. Most importantly, this methodology has facilitated the construction of C. sordellii strain ATCC 9714 tcsL mutants and we have shown that TcsL is an essential virulence factor in this strain. Furthermore, the molecular tools and methodologies developed in this work will now facilitate more detailed study of C. sordellii, making it possible to use molecular approaches to explore the role of putative virulence factors in disease.
Acknowledgments
Research at Monash University was supported by grants from the Australian National Health and Medical Research Council. This work was also supported by National Institutes of Health grant HD057176 (D.M.A.).
We thank R. Poon for technical assistance, M. Popoff for the kind gift of TcsL-specific antibodies, and X. Gatsos for technical advice on the MTT cell viability assays.
Editor: A. Camilli
Footnotes
Published ahead of print on 3 January 2011.
REFERENCES
- 1.Aldape, M. J., A. E. Bryant, and D. L. Stevens. 2006. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin. Infect. Dis. 43:1436-1446. [DOI] [PubMed] [Google Scholar]
- 2.Amimoto, K., T. Noro, E. Oishi, and M. Shimizu. 2007. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology 153:1198-1206. [DOI] [PubMed] [Google Scholar]
- 3.Amimoto, K., et al. 2001. Protective effects of Clostridium sordellii LT and HT toxoids against challenge with spores in guinea pigs. J. Vet. Med. Sci. 63:879-883. [DOI] [PubMed] [Google Scholar]
- 4.Aronoff, D. M., and J. D. Ballard. 2009. Clostridium sordellii toxic shock syndrome. Lancet Infect. Dis. 9:725-726. [DOI] [PubMed] [Google Scholar]
- 5.Aronoff, D. M., et al. 2008. Misoprostol impairs female reproductive tract innate immunity against Clostridium sordellii. J. Immunol. 180:8222-8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad, M. M., D. M. Ellemor, R. L. Boyd, J. J. Emmins, and J. I. Rood. 2001. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69:7904-7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitti, A., et al. 1997. A fatal postpartum Clostridium sordellii associated toxic shock syndrome. J. Clin. Pathol. 50:259-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 9.Burger, S., et al. 2003. Expression of recombinant Clostridium difficile toxin A using the Bacillus megaterium system. Biochem. Biophys. Res. Commun. 307:584-588. [DOI] [PubMed] [Google Scholar]
- 10.Carter, G. P., et al. 2007. Binary toxin production in Clostridium difficile is regulated by CdtR, a LytTR family response regulator. J. Bacteriol. 189:7290-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaves-Olarte, E., et al. 1999. A novel cytotoxin from Clostridium difficile serogroup F is a functional hybrid between two other large clostridial cytotoxins. J. Biol. Chem. 274:11046-11052. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Y., B. A. McClane, D. J. Fisher, J. I. Rood, and P. Gupta. 2005. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl. Environ. Microbiol. 71:7542-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung, J. K., et al. 2010. The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infect. Immun. 78:3064-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiarezza, M. 2007. Analysis of the sialidases from Clostridium perfringens and Clostridium septicum. Ph.D. thesis. Monash University, Melbourne, Australia.
- 15.Clark, S. 2003. Sudden death in periparturient sheep associated with Clostridium sordellii. Vet. Rec. 153:340. [PubMed] [Google Scholar]
- 16.Cohen, A. L., et al. 2007. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstet. Gynecol. 110:1027-1033. [DOI] [PubMed] [Google Scholar]
- 17.Cossart, P., et al. 1989. Listeriolysin O is essential for the virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57:3629-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang, L. H., C. Bettegowda, D. L. Huso, K. W. Kinzler, and B. Vogelstein. 2001. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl. Acad. Sci. U. S. A. 98:15155-15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunbar, N. M., and R. C. Harruff. 2007. Necrotizing fasciitis: manifestations, microbiology and connection with black tar heroin. J. Forensic Sci. 52:920-923. [DOI] [PubMed] [Google Scholar]
- 20.Fischer, M., et al. 2005. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N. Engl. J. Med. 353:2352-2360. [DOI] [PubMed] [Google Scholar]
- 21.Geny, B., et al. 2010. Rac1 inactivation by lethal toxin from Clostridium sordellii modifies focal adhesions upstream of actin depolymerization. Cell. Microbiol. 12:217-232. [DOI] [PubMed] [Google Scholar]
- 22.Geny, B., et al. 2007. Clostridium sordellii lethal toxin kills mice by inducing a major increase in lung vascular permeability. Am. J. Pathol. 170:1003-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall, I. C., and J. P. Scott. 1927. Bacillus sordellii, a cause of malignant edema in man. J. Infect. Dis. 41:329-335. [Google Scholar]
- 24.Hao, Y., et al. 2010. Lethal toxin is a critical determinant of rapid mortality in rodent models of Clostridium sordellii endometritis. Anaerobe 16:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heap, J. T., O. J. Pennington, S. T. Cartman, G. P. Carter, and N. P. Minton. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiol. Methods 70:452-464. [DOI] [PubMed] [Google Scholar]
- 26.Ho, C. S., et al. 2009. Undiagnosed cases of fatal Clostridium-associated toxic shock in Californian women of childbearing age. Am. J. Obstet. Gynecol. 201:459 e451-e457. [DOI] [PubMed] [Google Scholar]
- 27.Huelsenbeck, S. C., I. Klose, M. Reichenbach, J. Huelsenbeck, and H. Genth. 2009. Distinct kinetics of (H/K/N)Ras glucosylation and Rac1 glucosylation catalysed by Clostridium sordellii lethal toxin. FEBS Lett. 583:3133-3139. [DOI] [PubMed] [Google Scholar]
- 28.Jones, S., and D. A. Portnoy. 1994. Intracellular growth of bacteria. Methods Enzymol. 236:463-467. [DOI] [PubMed] [Google Scholar]
- 29.Just, I., and R. Gerhard. 2004. Large clostridial cytotoxins. Rev. Physiol. Biochem. Pharmacol. 152:23-47. [DOI] [PubMed] [Google Scholar]
- 30.Kachman, M. T., M. C. Hurley, T. Thiele, G. Srinivas, and D. M. Aronoff. 2010. Comparative analysis of the extracellular proteomes of two Clostridium sordellii strains exhibiting contrasting virulence. Anaerobe 16:454-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy, C. L., et al. 2005. The alpha-toxin of Clostridium septicum is essential for virulence. Mol. Microbiol. 57:1357-1366. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Lewis, C. J., and R. Naylor. 1996. Sudden death in lambs associated with Clostridium sordellii infection. Vet. Rec. 138:262. [PubMed] [Google Scholar]
- 34.Lewis, C. J., and R. D. Naylor. 1998. Sudden death in sheep associated with Clostridium sordellii. Vet. Rec. 142:417-421. [DOI] [PubMed] [Google Scholar]
- 35.Limbago, B., V. Penumalli, B. Weinrick, and J. R. Scott. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect. Immun. 68:6384-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyras, D., et al. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lyras, D., and J. Rood. 1998. Conjugative transfer of RP4-oriT shuttle vectors from Escherichia coli to Clostridium perfringens. Plasmid 39:160-164. [DOI] [PubMed] [Google Scholar]
- 38.Mani, N., et al. 2002. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. J. Bacteriol. 184:5971-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez, R. D., and T. D. Wilkins. 1992. Comparison of Clostridium sordellii toxins HT and LT with toxins A and B of C. difficile. J. Med. Microbiol. 36:30-36. [DOI] [PubMed] [Google Scholar]
- 40.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 41.Minton, N., et al. 2004. The development of Clostridium difficile genetic systems. Anaerobe 10:75-84. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien, D. K., and S. B. Melville. 2000. The anaerobic pathogen Clostridium perfringens can escape the phagosome of macrophages under aerobic conditions. Cell. Microbiol. 2:505-519. [DOI] [PubMed] [Google Scholar]
- 43.O'Brien, D. K., and S. B. Melville. 2004. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infect. Immun. 72:5204-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Connor, J. R., et al. 2006. Construction and analysis of chromosomal Clostridium difficile mutants. Mol. Microbiol. 61:1335-1351. [DOI] [PubMed] [Google Scholar]
- 45.Palombo, E. A., K. Yusoff, V. A. Stanisich, V. Krishnapillai, and N. S. Willetts. 1989. Cloning and genetic analysis of tra cistrons of the Tra 2/Tra 3 region of plasmid RP1. Plasmid 22:59-69. [DOI] [PubMed] [Google Scholar]
- 46.Park, J. M., V. H. Ng, S. Maeda, R. F. Rest, and M. Karin. 2004. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J. Exp. Med. 200:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rorbye, C., I. S. Petersen, and L. Nilas. 2000. Postpartum Clostridium sordellii infection associated with fatal toxic shock syndrome. Acta Obstet. Gynecol. Scand. 79:1134-1135. [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 49.Sayeed, S., et al. 2008. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 67:15-30. [DOI] [PubMed] [Google Scholar]
- 50.Shao, L., et al. 2007. Targeted gene disruption by use of a group II intron (targetron) vector in Clostridium acetobutylicum. Cell Res. 17:963-965. [DOI] [PubMed] [Google Scholar]
- 51.Smith, C. J., S. M. Markowitz, and F. L. Macrina. 1981. Transferable tetracycline resistance in Clostridium difficile. Antimicrob. Agents Chemother. 19:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang-Feldman, Y. J., G. Ackermann, J. P. Henderson, J. Silva, Jr., and S. H. Cohen. 2002. One-step cloning and expression of Clostridium difficile toxin B gene (tcdB). Mol. Cell. Probes 16:179-183. [DOI] [PubMed] [Google Scholar]
- 53.Thomas-Chollier, M., et al. 2008. RSAT: regulatory sequence analysis tools. Nucleic Acids Res. 36:W119-W127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voth, D. E., and J. D. Ballard. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voth, D. E., O. V. Martinez, and J. D. Ballard. 2006. Variations in lethal toxin and cholesterol-dependent cytolysin production correspond to differences in cytotoxicity among strains of Clostridium sordellii. FEMS Microbiol. Lett. 259:295-302. [DOI] [PubMed] [Google Scholar]