Abstract
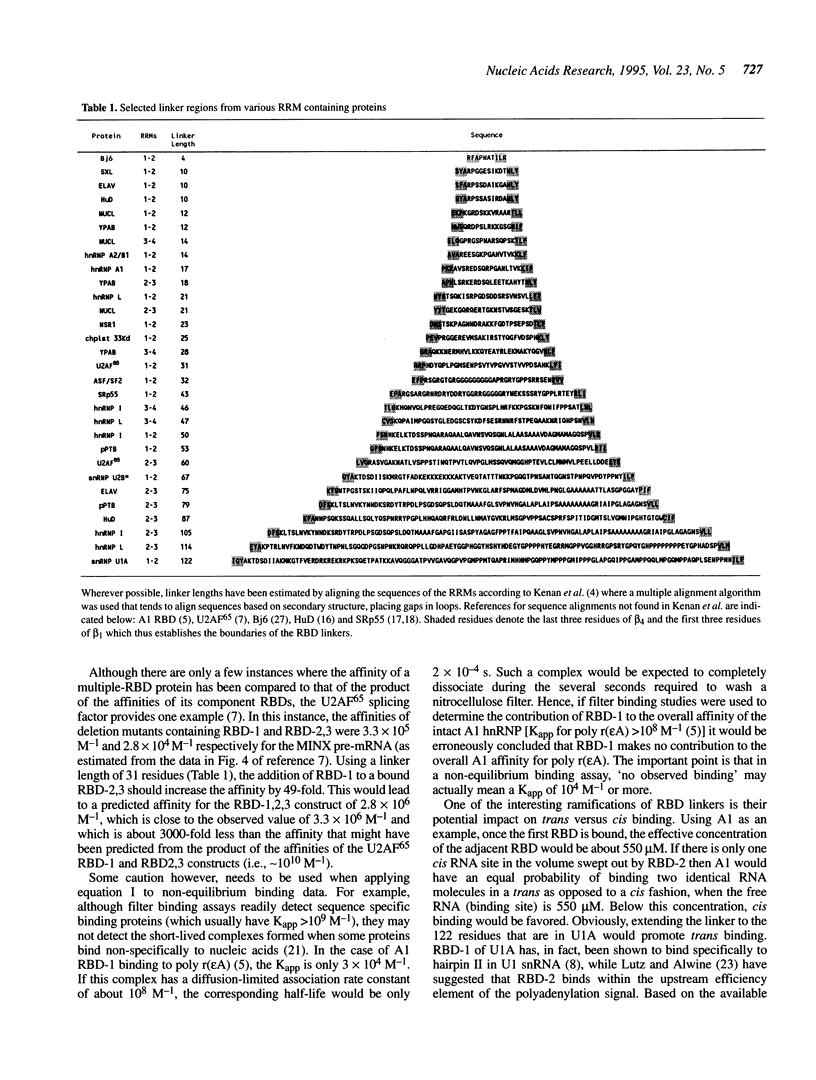
One of the most common motifs for binding RNA in eukaryotes is the RNA binding domain (RBD) or RNA Recognition Motif (RRM). One of the more intriguing aspects of these proteins is their modular nature. Proteins have been found containing from one to four RRMs. In most instances, these domains have some basal level of non-sequence specific RNA binding affinity. In addition, many also have a higher affinity for a specific structure or sequence of RNA. In the cases of heterogenous nuclear ribonucleoprotein A1 (hnRNP A1), yeast poly-A binding protein and splicing factor U2AF65, the individual free energy of binding of the RBDs for RNA are not strictly additive. By invoking a model in which the amino acids connecting adjoining RBDs are considered to be flexible linkers with an interresidue spacing of about 3.5 A, it is possible to predict the apparent association constants for at least some multi-RBD proteins to single-stranded RNA. We have surveyed the literature and found that individual RBDs are separated by 'linker' sequences of highly variable length. These linkers provide a critical determinant of binding affinity and may modulate cis versus trans binding. A clearer understanding of multi-RBD binding is essential to critically evaluating the role of these proteins in RNA splicing, packaging and transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Nakagawa T., Swanson M. S., Woodruff T. K., Dreyfuss G. mRNA polyadenylate-binding protein: gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol Cell Biol. 1986 Aug;6(8):2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrute S. B., Abdul-Manan Z., Pandey V., Williams K. R., Modak M. J. Purification and nucleic acid binding properties of a fragment of type C1/C2 heterogeneous nuclear ribonucleoprotein from thymic nuclear extracts. Biochemistry. 1994 Jul 12;33(27):8282–8291. doi: 10.1021/bi00193a015. [DOI] [PubMed] [Google Scholar]
- Bentley R. C., Keene J. D. Recognition of U1 and U2 small nuclear RNAs can be altered by a 5-amino-acid segment in the U2 small nuclear ribonucleoprotein particle (snRNP) B" protein and through interactions with U2 snRNP-A' protein. Mol Cell Biol. 1991 Apr;11(4):1829–1839. doi: 10.1128/mcb.11.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. G., Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994 Jul 29;265(5172):615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Casas-Finet J. R., Karpel R. L., Maki A. H., Kumar A., Wilson S. H. Physical studies of tyrosine and tryptophan residues in mammalian A1 heterogeneous nuclear ribonucleoprotein. Support for a segmented structure. J Mol Biol. 1991 Sep 20;221(2):693–709. doi: 10.1016/0022-2836(91)80081-5. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Metzger H. The influence of polyvalency on the binding properties of antibodies. Immunochemistry. 1972 Mar;9(3):341–357. doi: 10.1016/0019-2791(72)90097-3. [DOI] [PubMed] [Google Scholar]
- Cáceres J. F., Krainer A. R. Functional analysis of pre-mRNA splicing factor SF2/ASF structural domains. EMBO J. 1993 Dec;12(12):4715–4726. doi: 10.1002/j.1460-2075.1993.tb06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D. S., Lodi P. J., Shamoo Y., Williams K. R., Clore G. M., Gronenborn A. M. Determination of the secondary structure and folding topology of an RNA binding domain of mammalian hnRNP A1 protein using three-dimensional heteronuclear magnetic resonance spectroscopy. Biochemistry. 1994 Mar 15;33(10):2852–2858. doi: 10.1021/bi00176a015. [DOI] [PubMed] [Google Scholar]
- Hoffman D. W., Query C. C., Golden B. L., White S. W., Keene J. D. RNA-binding domain of the A protein component of the U1 small nuclear ribonucleoprotein analyzed by NMR spectroscopy is structurally similar to ribosomal proteins. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2495–2499. doi: 10.1073/pnas.88.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen T. H., Oubridge C., Teo C. H., Pritchard C., Nagai K. Identification of molecular contacts between the U1 A small nuclear ribonucleoprotein and U1 RNA. EMBO J. 1991 Nov;10(11):3447–3456. doi: 10.1002/j.1460-2075.1991.tb04909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Lutz-Freyermuth C., Query C. C., Keene J. D. Quantitative determination that one of two potential RNA-binding domains of the A protein component of the U1 small nuclear ribonucleoprotein complex binds with high affinity to stem-loop II of U1 RNA. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6393–6397. doi: 10.1073/pnas.87.16.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C. S., Alwine J. C. Direct interaction of the U1 snRNP-A protein with the upstream efficiency element of the SV40 late polyadenylation signal. Genes Dev. 1994 Mar 1;8(5):576–586. doi: 10.1101/gad.8.5.576. [DOI] [PubMed] [Google Scholar]
- Mayeda A., Zahler A. M., Krainer A. R., Roth M. B. Two members of a conserved family of nuclear phosphoproteins are involved in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1301–1304. doi: 10.1073/pnas.89.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Jessen T. H., Li J., Evans P. R. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990 Dec 6;348(6301):515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- Roth M. B., Zahler A. M., Stolk J. A. A conserved family of nuclear phosphoproteins localized to sites of polymerase II transcription. J Cell Biol. 1991 Nov;115(3):587–596. doi: 10.1083/jcb.115.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W., Kornberg R. D. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol Cell Biol. 1987 Sep;7(9):3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Boelens W., Dathan N. A., van Venrooij W. J., Mattaj I. W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B'' and their cognate RNAs. Nature. 1990 Jun 7;345(6275):502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- Shamoo Y., Abdul-Manan N., Patten A. M., Crawford J. K., Pellegrini M. C., Williams K. R. Both RNA-binding domains in heterogenous nuclear ribonucleoprotein A1 contribute toward single-stranded-RNA binding. Biochemistry. 1994 Jul 12;33(27):8272–8281. doi: 10.1021/bi00193a014. [DOI] [PubMed] [Google Scholar]
- Szabo A., Dalmau J., Manley G., Rosenfeld M., Wong E., Henson J., Posner J. B., Furneaux H. M. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell. 1991 Oct 18;67(2):325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- Wittekind M., Görlach M., Friedrichs M., Dreyfuss G., Mueller L. 1H, 13C, and 15N NMR assignments and global folding pattern of the RNA-binding domain of the human hnRNP C proteins. Biochemistry. 1992 Jul 14;31(27):6254–6265. doi: 10.1021/bi00142a013. [DOI] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- Zuo P., Manley J. L. Functional domains of the human splicing factor ASF/SF2. EMBO J. 1993 Dec;12(12):4727–4737. doi: 10.1002/j.1460-2075.1993.tb06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Besser H., Schnabel P., Wieland C., Fritz E., Stanewsky R., Saumweber H. The puff-specific Drosophila protein Bj6, encoded by the gene no-on transient A, shows homology to RNA-binding proteins. Chromosoma. 1990 Dec;100(1):37–47. doi: 10.1007/BF00337601. [DOI] [PubMed] [Google Scholar]



