Abstract
Interleukin 17 (IL-17) contributes to development of Th1 immunity and neutrophil influx during Chlamydia muridarum pulmonary infection, but its role during C. muridarum genital tract infection has not been described. We detected similar numbers of Chlamydia-specific Th17 and Th1 cells in iliac nodes of wild-type mice early during genital C. muridarum infection, while Th1 cells predominated later. il17ra−/− mice exhibited a reduced chlamydia-specific Th1 response in draining iliac nodes and decreased local IFN-γ production. Neutrophil influx into the genital tract was also decreased. However, il17ra−/− mice resolved infection normally, and no difference in pathology was observed compared to the wild type. Macrophage influx and tumor necrosis factor alpha (TNF-α) production were increased in il17ra−/− mice, providing a compensatory mechanism to effectively control chlamydial genital tract infection despite a reduced Th1 response. In ifnγ−/− mice, a marked increase in cellular infiltrates and chronic pathology was associated with an increased Th17 response. Although neutralization of IL-17 in ifnγ−/− mice decreased neutrophil influx, macrophage infiltration remained intact and the bacterial burden was not increased. Collectively, these results indicate that IL-17 contributes to the generation of Th1 immunity and neutrophil recruitment but is not required for macrophage influx or normal resolution of C. muridarum genital infection. These data highlight the redundant immune mechanisms operative at this mucosal site and the importance of examining site-specific responses to mucosal pathogens.
Expansion of the Th1/Th2 paradigm to include the Th17 subset has provided important insight into the complexities of the mucosal immune response. Initially, interleukin 17 (IL-17) was identified as a key factor in the development of autoimmune inflammation and pathology (11, 23, 30); however, subsequent data have demonstrated both protective and pathological roles for IL-17, depending upon the pathogen and target tissue (2, 3, 12, 13, 22, 24, 40, 44, 46, 47). In addition, Th17 cells link innate and adaptive immunity (20) and play a role in augmenting memory responses (21, 29), providing an attractive target for promoting vaccine-induced immunity.
Sexually transmitted Chlamydia infections are a significant public health concern due to their prevalence and adverse effects on female reproduction. Chlamydia muridarum is a natural pathogen of mice and causes disease in both the respiratory tract and the genital tract, allowing the determination of site-specific mucosal immune responses to the pathogen.
Resolution of chlamydial infection and protection from pathology are associated with a strong Th1 response and gamma interferon (IFN-γ) production (5, 6, 26, 27, 31). Recent work has demonstrated that IL-17 is critical for host defense against C. muridarum pulmonary infection, as bacterial clearance and development of Th1 immunity were compromised in the absence of IL-17 (3). In this mouse model, IL-17 was necessary for dendritic cell (DC) production of IL-12p70 and downstream development of a protective Th1 response (3). However, elevated levels of IL-17 have been associated with increased disease susceptibility during C. muridarum respiratory tract infection via recruitment of neutrophils (16, 47). Enhanced neutrophil influx and neutrophil release of matrix metalloproteases has been directly linked to tissue pathology in mouse models of C. muridarum genital tract infection (34, 37). In addition, human studies have suggested that increased neutrophil activation has been associated with increased genital tract disease in females infected with C. trachomatis (43). Due to the pleiotropic effects of IL-17 on Th1 immunity and neutrophil induction and the significance of these responses in host defense against chlamydiae, it is important to directly examine the role of IL-17 in protection versus pathogenesis in the genital tract model of chlamydial infection.
We determined the numbers of chlamydial antigen-specific Th17 and Th1 cells in the draining iliac nodes of wild-type C57BL/6 mice after genital infection with C. muridarum. Although similar numbers of Th17 and Th1 cells were observed on day 7 of infection, by day 20, Th1 cells predominated. We then infected IL-17 receptor-deficient (il17ra−/−) mice on the C57BL/6 background. Decreased IFN-γ production was observed in both NK cells in the cervical tissues and chlamydia-specific CD4+ T cells in the iliac nodes of il17ra−/− mice. In addition, neutrophil influx was decreased in the il17ra−/− mice. Despite diminished Th1 and neutrophil responses, infection resolved normally and pathology was not increased. Enhanced macrophage influx and macrophage production of tumor necrosis factor alpha (TNF-α) provided a compensatory host defense mechanism in the il17ra−/− mice.
To further determine whether IL-17 plays a protective role during C. muridarum genital infection, we evaluated the role of IL-17 in the absence of a protective IFN-γ response, removing any inhibition of IL-17 that might be imposed by IFN-γ. ifnγ −/− mice clear the majority of chlamydiae from their genital tracts, indicating a role for alternative mechanisms of bacterial control. We observed a significantly increased Th17 response and markedly elevated neutrophil infiltrates in ifnγ−/− mice. Although neutralization of IL-17 in ifnγ−/− mice resulted in a significant decrease in neutrophil numbers in the oviducts, control of infection was not further compromised and pathology was not improved. Alternate immune mechanisms continue to control infection, demonstrating the marked redundancy of host defense mechanisms operative at this mucosal site. Macrophage influx was sustained during IL-17 depletion, indicating a potential role for these cells as important mediators of host defense in the genital tract.
MATERIALS AND METHODS
Animals.
Mice homozygous for the ifnγtm1Ts targeted mutation (ifnγ−/−) and wild-type C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). IL-17 receptor-deficient (il17ra−/−) mice on the C57BL/6 background have been previously described (44). All mice were acclimated between 6 and 8 weeks of age and infected between 8 and 12 weeks of age. Mice were infected in groups of five except where otherwise indicated; the groups were age matched for all experiments. The mice were given food and water ad libitum in an environmentally controlled room with a cycle of 12 h of light and 12 h of darkness. All animal experiments were approved by the University Institutional Animal Care and Use Committee.
Reagents and bacteria.
C. muridarum (Nigg 1942), was cultured in mycoplasma-free McCoy or HeLa 229 cells, and chlamydial elementary bodies (EBs) were harvested from infected cells as previously described (33). Where specified, gradient-purified C. muridarum Nigg EBs inactivated under UV light (UV-EBs) were used as an antigen at a concentration of 5 μg/ml (35). Immunostimulatory peptides GNEVFVSPAAHIIDRPG (RplF51-59), SPIYVDPAAAGGQPPA (PmpG1303-311), and AFHLFASPAANYIHTG (PmpE/F2351-359) were synthesized and purified by Synthetic Biomolecules (San Diego, CA). These chlamydial peptides correspond to major histocompatibility complex (MHC) class II epitopes that were discovered by Karunakaran et al. using immunoproteomics (18). Pooled peptides were solubilized in dimethyl sulfoxide (DMSO) (4 mg/ml) and used at a concentration of 2 μg/ml each in medium as stimulatory antigens for enzyme-linked immunospot (ELISPOT) assays.
Murine infection and monitoring.
Seven days prior to infection, mice were subcutaneously injected with 2.5 mg of progesterone (Depo-Provera; Upjohn, Kalamazoo, MI) to synchronize all mice in a state of anestrus and to facilitate successful intravaginal infection (39). The mice were infected with 3 × 105 inclusion-forming units (IFU) of C. muridarum Nigg intravaginally. The mice were monitored for cervicovaginal shedding as described previously (19), and numbers of IFU were calculated as previously described (8).
Cytokine and chemokine analysis of genital tract secretions and iliac node supernatants.
Genital tract secretions were collected from mice on multiple days throughout the course of infection as previously described (9, 10). At specified intervals prior to and during infection, an aseptic surgical sponge (2 by 5 mm) (DeRoyal, Powell, TN) was inserted into the vagina of an anesthetized animal and retrieved 30 min later. The sponges were stored at −70°C until cytokine assays were performed. Each sponge was placed in a Spin-X microcentrifuge tube (Fisher Scientific, Pittsburgh, PA) containing a 0.2-mm cellulose acetate filter and incubated in 300 ml of sterile phosphate-buffered saline (PBS) plus 0.5% bovine serum albumin (BSA) and 0.05% Tween 20 for 1 h on ice and then centrifuged for 5 min. The Spin-X filters were first preblocked with 0.5 ml of sterile PBS plus 2% BSA and 0.05% Tween 20 for 30 min at 25°C, centrifuged, and washed twice with 0.05 ml of sterile PBS. Samples were kept on ice and promptly loaded into an enzyme-linked immunosorbent assay (ELISA) plate prepared for a specific cytokine assay. Genital tract sponge eluates were assayed for cytokines and chemokines using Quantikine ELISA kits (R&D Systems, Minneapolis, MN) for IL-17, IL-6, TNF-α, KC (CXCL1), and MIP-2 (CXCL2). IL-17, IFN-γ, and IL-12p70 levels in iliac node mononuclear cell supernatants were similarly analyzed. Transforming growth factor beta (TGF-β) was quantified using a luciferase bioassay as previously described (1). Cytokines and chemokines in oviduct homogenates were quantified using the Multiplex Cytometric Bead Array (Millipore, Billerica, MA).
Quantification of antigen-specific IL-17- and IFN-γ-producing CD4+ T cells by ELISPOT.
C. muridarum antigen-specific IFN-γ- and IL-17-producing CD4+ T cells from infected iliac nodes were quantified using a previously described ELISPOT assay (22). Iliac nodes were harvested from nine C57BL/6 mice on days 7 and 20 postinfection. Cells from the nodes of 3 mice were pooled and processed into single-cell suspensions. CD4+ T cells were enriched by magnetic activated cell sorting (MACS) (Miltenyi Biotec, Auburn, CA) and were routinely >90% pure. Cell culture plates (Multiscreen-HA; Millipore, Billerica, MA) were coated overnight at 4°C with monoclonal purified anti-mouse IFN-γ (clone R4-6A2; eBioscience, San Diego, CA) or monoclonal purified anti-mouse IL-17 (clone 50101.111; R&D Systems, Minneapolis, MN) in PBS. The plates were then incubated with blocking solution (Dulbecco's modified Eagle's medium [DMEM] containing 100 U/ml penicillin, 100 U/ml streptomycin, and 10% fetal bovine serum [FBS]) (all additives were from Sigma-Aldrich, St. Louis, MO). Iliac node cells were plated at an initial concentration of 1 × 105 cells/well in antibody-coated plates before being serially diluted to a concentration of ∼3,125 cells/well. Irradiated splenocytes from uninfected mice were used as antigen-presenting cells at a concentration of 1 × 106 cells/well. The cells were cultured in the presence of IL-2 alone (10-U/ml final concentration), C. muridarum UV-EBs (5 μg/well) plus IL-2, or pooled C. muridarum peptides (1 μg/ml/peptide) (see above) plus IL-2. The spots were visualized using streptavidin-alkaline phosphatase (DakoCytomation, Ft. Collins, CO) and 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (Sigma-Aldrich, St. Louis, MO) as a substrate. The spots were quantified using a CTL-ImmunoSpot S5 UV Analyzer and a CTL ImmunoSpot Professional Software version 5.0 reader. Data are expressed as mean numbers of spot-forming cells per million iliac node cells ± standard deviations (SD) calculated from triplicate determinations.
In vivo neutralization of IL-17 in ifnγ−/−mice.
Five ifnγ−/− mice were treated intraperitoneally with rat anti-mouse IL-17 monoclonal antibody (MAb) (clone 50104; 100 μg in 100 μl PBS; R&D Systems, Minneapolis, MN) every other day from day 1 to day 19 postinfection. A control group of five ifnγ−/− mice were treated similarly with rat IgG2a (clone 2A3; BioXCell, West Lebanon, NH). Both groups were infected with 3 × 105 IFU of C. muridarum Nigg as indicated above. The mice were sacrificed on day 21 of infection. One oviduct was harvested and analyzed for bacterial burden, cytokines, and cellular influx (see “Processing of cervical and oviduct homogenates for flow cytometry” below). The second oviduct was fixed in formalin and used for histopathological assessment. None of the mice exhibited signs of serum sickness over the course of antibody administration.
Assessment of lymphocyte proliferation and in vitro chlamydial antigen-specific cytokine responses.
Iliac nodes of il17ra−/− mice and C57BL/6 mice infected intravaginally with C. muridarum were harvested on days 7, 21, and 35. The nodes of infected ifnγ−/− and C57BL/6 mice were harvested on days 7, 21, and 28. The nodes were processed into a single-cell suspension and placed in culture with medium alone, concanavalinA (ConA) (5 μg/well), or UV-EBs (5 μg/well) with or without anti-CD4 (1 μg/well; clone RM4-5; BD Biosciences, San Diego, CA), which sterically blocks antigen-specific T cell proliferation due to CD4-T cell receptor (TCR) coclustering. T cell proliferation was measured by incorporation of tritiated thymidine (1 μCi/well) after 96 h of culture and was expressed as counts per minute, as measured with a scintillation counter. Supernatants were collected for quantification of cytokines and chemokines as described above.
Processing of cervical and oviduct homogenates for flow cytometry.
For analysis of the cervical immune response, three C57BL/6 and three il17ra−/− mice were sacrificed on day 4, 8, 9, or 14 postinfection. Cervical tissues were harvested in 90 μl of medium (RPMI plus 1% FBS). The tissues were minced, and the volume was expanded to 500 μl with medium. For analysis of cervical cytokines, 100 μl was removed and stored at −80°C. Collagenase I (1 mg/ml; Sigma-Aldrich, St. Louis, MO) was added to bring the volume to 1 ml, and the suspension was incubated at 37°C for 20 min with shaking to disperse the cells. After incubation, 4 μl of 0.1 M EDTA and 500 μl of medium were added, and the homogenate was repeatedly passed through a 70-μm filter to yield a single-cell suspension.
Single-cell suspensions of at least 5 × 105 cells were stimulated with phorbol myristate acetate (PMA) (50-ng/ml final concentration; Sigma-Aldrich, St. Louis, MO) and ionomycin (500-ng/ml final concentration; Sigma-Aldrich) for 4 h at 37°C in the presence of GolgiPlug (1:1,000 final dilution; BD Biosciences, San Diego, CA) prior to analysis for cytokine production via flow cytometry. After incubation, the cells were washed and prepared as described below.
For surface staining, single-cell suspensions of stimulated or unstimulated cells were resuspended in cold fluorescence-activated cell sorter (FACS) buffer (PBS, pH 7.2, 0.5% BSA, and 2 mM EDTA) at 4 × 107 cells per/ml. To block nonspecific antibody binding, 25 μl (1 × 106 cells) of cell suspension and 25 μl of Fc Block (CD16/CD32; BD Pharmingen, San Diego, CA) were combined in a 96-well V-bottom plate and incubated on ice for 20 min. The following antibodies were utilized for cell surface staining in our experiments: CD45 peridinin chlorophyll protein (PerCP)-Cy5.5 (clone 30-F11); Ly6G/C fluorescein isothiocyanate (FITC), phycoerythrin (PE), and eFluor 450 (clone RB6-8C5); F4/80 allophycocyanin (APC) and PerCP (clone BM8); Ly6G PE-Cy7 (clone 1A8); NK1.1 PE-Cy7 (clone PK136); CD3 Alexa Fluor 700 (clone 17A2); CD4 eFluor 450 (clone RM4-5); andCD8α FITC (clone 53-6.7), all from eBioscience, San Diego, CA. Antibodies for CD11b FITC (clone M1/70) and CD11c PE and PE-Cy7 (clone HL3) were obtained from BD Pharmingen, San Diego, CA. Staining antibodies were added in 50 μl of FACS buffer for a final dilution of 1.25 μl antibody per 100-μl total volume. After being incubated for 20 min on ice, the stained cells were washed, resuspended in Live/Dead Fixable Stain (Invitrogen, Carlsbad, CA), and incubated for 20 min on ice in the dark. After being washed, the cells were fixed in 2% paraformaldehyde.
If cells were stimulated for intracellular cytokine staining, suspensions were treated as described above for surface and Live/Dead staining. They were then fixed and permeabilized according to the manufacturer's instructions (Cytofix/Cytoperm Kit; BD Biosciences, San Diego, CA). Intracellular cytokine staining was conducted as described above for surface staining. The stained cells were washed and incubated in 2% paraformaldehyde until analysis was done. The following antibodies were utilized for intracellular cytokine staining: IFN-γ APC (clone XMG1.2), IL-17A PE (clone TC11-18H10), and TNF-α FITC (clone MP6-XT22), all from BD Pharmingen, San Diego, CA.
For analysis of the oviducts, five C57BL/6 and five ifnγ−/− mice were sacrificed on day 7, 14, or 21 following intravaginal infection. The oviducts were homogenized as for cervical tissues but did not require collagenase treatment to yield single-cell suspensions. Aliquots were removed as described above for cervical samples, except that the total volume of oviduct sample plus medium was brought to 1 ml prior to removal of 100 μl for titration of the bacterial burden or cytokine levels. Staining was conducted as described above. Flow cytometric data were analyzed with FACSDiva or FlowJo Software.
Quantitative PCR analysis to determine the C. muridarum burden in the oviducts.
Aliquots (5 μl) were removed from 100 μl of frozen oviduct homogenates or genital tract swab eluate and diluted into 195 μl of Epicentre DNA Extraction Solution before being processed according to the manufacturer's instructions. Real-time PCRs were carried out using iQ SYBR green supermix (Bio-Rad, Hercules, CA) in a Bio-Rad iCycler using primers directed against chlamydial 16S rRNA (sense, 5′ CGTTAATACCCGCTGGATTTGAG 3′; antisense, 5′ GCCCCGATCTTTGACAACTAAC 3′) in a two-step reaction: 95°C for 10 s and 55°C for 30 s for a total of 40 cycles. Melt curve analysis showed that the accumulation of SYBR green-bound DNA was gene specific and not due to primer dimers. The samples were assayed in duplicate. The chlamydial load in the oviducts of each mouse, expressed as genome equivalents, was extrapolated from a standard curve generated using a 16S rRNA-amplified PCR product of known concentration and adjusted for the presence of two copies of the gene in C. muridarum.
Quantification of infectious C. muridarum in oviduct homogenates.
In the experiment using ifnγ−/− mice and C57BL/6 mice that were not treated with antibody, a 100-μl aliquot of the homogenized oviducts was serially diluted in 1× PBS for titration of bacteria using a plaque assay (28). On day 7 and day 14, the limit of detection of the plaque assay was ≤99 organisms, and on day 21, the limit of detection was ≤19 organisms. Samples with a negative titer were assigned a value of zero for statistical analysis.
Microscopic histopathological assessment.
Mice were sacrificed on specified days following intravaginal infection; the genital tracts were removed en bloc, fixed in 10% buffered formalin, and embedded in paraffin. Longitudinal 4-μm sections were cut and stained with hematoxylin and eosin. Each anatomic site (ectocervix, endocervix, uterine horn, and oviduct) was assessed independently for the presence of neutrophilic inflammation, lymphocytic/monocytic inflammation, plasma cells, and fibrosis and assessed using a four-tiered semiquantitative scoring system to evaluate the extents of inflammation and fibrosis, as previously described (8, 10).
Statistics.
Statistical comparisons between the murine strains for level of infection and cytokine production over the course of infection were made by a two-factor (days and murine strain) analysis of variance with a post hoc Tukey test as a multiple-comparison procedure. The Wilcoxon rank sum test was used to compare the durations of infection in the respective strains over time. A Mann-Whitney U test was used to determine significant differences in the pathological data between groups. Differences in lymphocyte proliferation, in vitro cytokine production, ELISPOT cytokine data, and oviduct homogenate cytokine and flow cytometric data were analyzed by Student's t test or by analysis of variance (ANOVA), as appropriate. SigmaStat software (SPSS) was utilized for all statistical analysis. P values of <0.05 were considered significant.
RESULTS
C. muridarum antigen-specific CD4+ Th1 and Th17 cells are present in similar numbers in the iliac nodes of C57BL/6 mice on day 7 postinfection, but Th1 cells predominate by day 20.
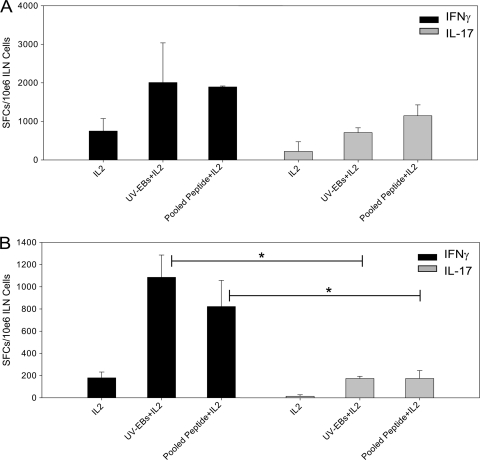
To compare the Th1 and Th17 responses during genital tract infection in wild-type mice, ELISPOT assays were performed using purified CD4+ T cells harvested from iliac nodes on day 7 and day 20 postinfection (Fig. 1). The primary objective of this experiment was to show that Th17 cells are detectable in the draining lymph nodes following C. muridarum genital tract infection in wild-type mice and to examine the kinetics of this response in relation to the development of the Th1 response. Similar numbers of chlamydia-specific Th1 and Th17 cells were detected on day 7 (Fig. 1A). In contrast, by day 20, Th1 cells were significantly increased with respect to Th17 cells (Fig. 1B), indicating that as infection resolves in wild-type mice, the Th1 response predominates. The reduction in T cell numbers detected at day 20 compared to day 7 is likely due to reduced bacterial burden and reduced antigen stimulation related to in vivo clearance of infection. It is possible that a percentage of cells produced both IFN-γ and IL-17 (45). Experiments were then conducted to examine the role of the IL-17/Th17 response during genital tract chlamydial infection.
FIG. 1.
C. muridarum antigen-specific Th1 and Th17 cells are detected in similar numbers in the iliac nodes of C57BL/6 mice on day 7 postinfection, but Th1 cells predominate on day 20. The numbers of antigen-specific IFN-γ- and IL-17-producing CD4+ T cells in iliac nodes (ILN) of C57BL/6 mice were quantified by ELISPOT on day 7 (A) and day 20 (B) postinfection. Significantly increased numbers of IFN-γ-producing CD4+ T cells and IL-17-producing CD4+ T cells were noted on both day 7 (A) and day 20 (B) postinfection after stimulation with UV-EBs or C. muridarum pooled peptide compared to stimulation with IL-2 alone (P < 0.05 by one-way ANOVA). On day 7 postinfection, the numbers of UV-EB- and pooled-peptide-specific Th1 (IFN-γ-producing) and Th17 (IL-17-producing) cells were not significantly different. By day 20, the numbers of UV-EB- and pooled-peptide-specific Th1 (IFN-γ-producing) cells were significantly greater than those of Th17 (IL-17-producing) cells (*, P < 0.05 by one-way ANOVA). The data are representative of two individual experiments in which the cells from three groups of three mice each were pooled and analyzed. The error bars indicate SD. SFCs, spot-forming cells.
The Th1 response is reduced in the iliac nodes of il17ra−/− mice.
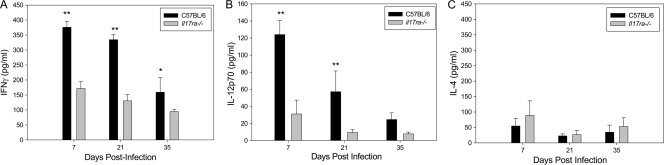
Significantly lower production of IFN-γ by chlamydia-specific CD4+ T cells from the iliac nodes of il17ra−/− mice was detected on days 7, 21, and 35 postinfection than in C57BL/6 mice (Fig. 2 A). Although we noted a contraction of the T cell response in the wild-type mice at day 20 in the previous experiment (Fig. 1B), it is not possible to directly compare the ELISPOT data, which measures the antigen-specific cytokine-producing cells, with the protein levels detected by ELISA in this experiment due to differences in methodology and experimental read-out. Since IL-12p70 is essential for induction of Th1 immunity, we assayed IL-12p70 in supernatants of chlamydia-stimulated iliac node cells harvested from infected mice. The levels were significantly reduced in il17ra−/− mice on days 7 and 21 postinfection compared to wild-type mice (Fig. 2B). Despite reduced IFN-γ and IL-12p70, IL-4 was not increased, indicating that a shift toward a Th2-like response did not occur (Fig. 2C).
FIG. 2.
il17ra−/− mice display reduced IFN-γ and IL-12p70 production in the iliac nodes. Iliac node mononuclear cells from individual il17ra−/− mice or C57BL/6 mice intravaginally infected with C. muridarum were stimulated in vitro with UV-inactivated EBs for 96 h. The supernatants were analyzed for IFN-γ (A), IL-12p70 (B), and IL-4 (C). Levels of IFN-γ and IL-12p70 were significantly reduced in il17ra−/− mice. Addition of a blocking anti-CD4 antibody resulted in >95% inhibition of the IFN-γ response, and the levels were undetectable in cells incubated with medium alone (data not shown). The data points represent the means and SD of values from 5 mice in a single independent experiment. *, P < 0.050; **, P < 0.001 (Student's t test).
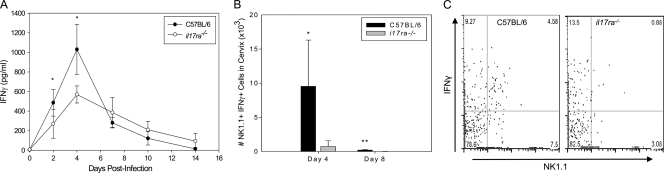
IL-17RA deficiency results in decreased cervical NK cell IFN-γ production.
Significantly lower levels of IFN-γ were detected in genital secretions of infected il17ra−/− mice on days 2 and 4 postinfection (Fig. 3 A) and in cervical homogenates on day 4 and day 8 (data not shown). Flow cytometric analysis of cervical tissues revealed decreased numbers of IFN-γ-producing NK1.1+ cells (Fig. 3B and C) on day 4 and day 8 in il17ra−/− mice compared to the wild type. Cytometric analysis of NK1.1 and CD3 markers revealed that both NK and NK-T cell populations were significantly decreased in the il17ra−/− mice, but NK cells (NK1.1+ CD3−) were the predominant producers of IFN-γ (>80% of NK1.1+ cells) in the cervix (data not shown).
FIG. 3.
il17ra−/− mice display reduced IFN-γ production and fewer IFN-γ-producing NK cells in the lower genital tract. (A) IFN-γ levels were significantly lower in genital secretions of il17ra−/− mice on days 2 and 4, but the levels were similar to those of the wild type (black circles) from days 6 to 14. The data points represent the means ± SD of 5 mice per group from a single individual experiment. *, P < 0.050 by two-way RM ANOVA with multiple-comparison procedure. (B and C) Cell-specific IFN-γ production in infected il17ra−/− and C57BL/6 mice was measured via flow cytometric analysis of intracellular cytokine staining and revealed significantly decreased numbers of IFN-γ-producing NK cells on day 4 and day 8 in the cervixes of il17ra−/− mice. (B) The bars represent the mean numbers and SD of live NK1.1+ IFN-γ+ cells in the cervical tissues from 3 mice from a single individual experiment. (C) Flow cytometric plot gated on live cells. *, P < 0.050; **, P < 0.010 (Student's t test).
The decreased Th1-IFN-γ response observed in the absence of IL-17 signaling parallels findings in the pulmonary model of C. muridarum infection during anti-IL-17 administration (3). The marked reduction in NK cell IFN-γ is similar to results obtained during infection of il17ra−/− mice with the intracellular pathogen Francisella tularensis (24). In both of these pulmonary-infection models, increased IL-4 production was noted. We did not detect increased levels of IL-4 during chlamydial genital tract infection, indicating that alternative cell types might be activated in the genital tract to prevent this shift.
Neutrophil recruitment is decreased in the lower genital tract of il17ra−/− mice during C. muridarum infection.
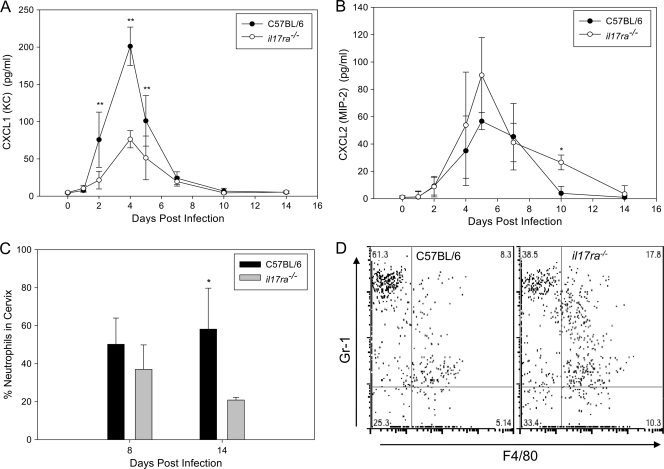
In addition to promoting Th1 immunity, IL-17 has been shown to contribute to neutrophil recruitment in chlamydial pulmonary-infection models (16, 47). Therefore, we measured neutrophil chemokines in vaginal secretions of infected C57BL/6 and il17ra−/− mice. Lower levels of the neutrophil chemoattractants CXCL1 (KC) (Fig. 4 A) and granulocyte colony-stimulating factor (G-CSF) (data not shown) were observed in the secretions of il17ra−/− mice. However, levels of CXCL2 (MIP-2) were not compromised in the il17ra−/− mice (Fig. 4B). The percentages and absolute numbers (data not shown) of neutrophils in the cervix were similar on day 4 but were decreased in il17ra−/− mice on days 8 and 14 of infection (Fig. 4C and D). We suspect that the decrease in CXCL1 expression detected early on in the genital secretions of the il17ra−/− mice is maintained in the tissue throughout infection, driving a lower influx of neutrophils over time.
FIG. 4.
il17ra−/− mice exhibit reduced chemokine levels and fewer neutrophils in the lower genital tract during early C. muridarum infection. il17ra−/− mice and C57BL/6 mice were intravaginally infected with C. muridarum. (A and B) Measurement of neutrophil-promoting chemokines in vaginal secretions revealed significantly decreased CXCL1 (KC) (P < 0.001; two-way RM ANOVA) (A), but secretion of CXCL2 (MIP-2) remained intact (P < 0.050; two-way RM ANOVA) (B) (*, P < 0.01; **, P < 0.001 for individual days). The data points represent the means ± SD of five mice analyzed in a single individual experiment. (C and D) The influx of neutrophils into the cervixes of i17ra−/− mice was decreased on day 8 and day 14 (*, P < 0.02; Student's t test). (C) The bars represent the percentages of CD45+ cells that were neutrophils (CD45+ Ly6G/Chigh F4/80−) plus SD for 3 mice from a single individual experiment. (D) Flow plot gated on live CD45+ cells. Neutrophils were Ly6/C (Gr-1) high F4/80−. Macrophages were Ly6G/C (Gr-1)+ F4/80+.
il17ra−/− mice exhibit a normal course of infection and no increase in chronic pathology compared to the wild type.
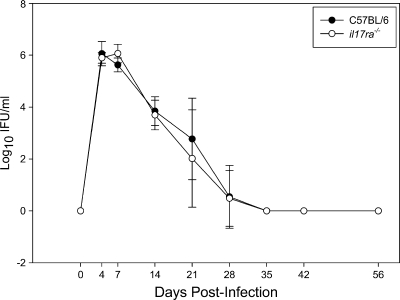
Despite dampened Th1 and neutrophil responses in the absence of IL-17 signaling, no delay in bacterial clearance from the lower genital tract was observed in il17ra−/− mice (Fig. 5). The degrees of chronic oviduct dilatation and uterine horn distension observed after resolution of infection were similar between wild-type and il17ra−/− mice (Fig. 6 A and B) on days 35 and 56 postinfection. In addition, no differences in histopathology between wild-type and il17ra−/− mice were detected in the oviducts (Fig. 6C and D), uterine horns (Fig. 6E and F), or cervixes (data not shown) on days 35 and 56 postinfection.
FIG. 5.
C57BL/6 and il17ra−/− mice exhibit no difference in the course of C. muridarum infection. Groups of five C57BL/6 and five il17ra−/− mice were intravaginally infected with C. muridarum, and the course of infection was monitored via endocervical swabs. Titration of live bacteria revealed that the bacterial burdens in the lower genital tract were similar between strains. The data points represent the means ± SD of IFU values from both culture-positive and -negative mice at each time point of a single experiment that is representative of 2 independent experiments.
FIG. 6.
il17ra−/− mice do not exhibit enhanced genital tract pathology during C. muridarum genital infection. Genital tract tissues were removed en bloc from C. muridarum-infected mice at 35 and 56 days postinfection (n = 5 mice per strain per time point). Tissues were scored for oviduct dilatation (A) and uterine horn distension (B). Scores for individual mice on day 35 (D35) and scores for day 56 (D56) are indicated. The median scores are indicated by dark lines. No differences in median pathology scores were noted between groups on either day examined (Mann-Whitney U test). Tissues were also examined grossly and histologically. Oviducts from C57BL/6 (C) and il17ra−/− (D) mice displayed similar inflammatory scores and oviduct dilatation. Uterine horns from C57BL/6 (E) and il17ra−/− (F) mice exhibited no significant differences in inflammation or fibrosis. The tissues were representative samples of the oviducts (C and D; magnification, ×40) and uterine horns (E and F; magnification, ×40) from C57BL/6 mice and il17ra−/− mice on day 56 postinfection.
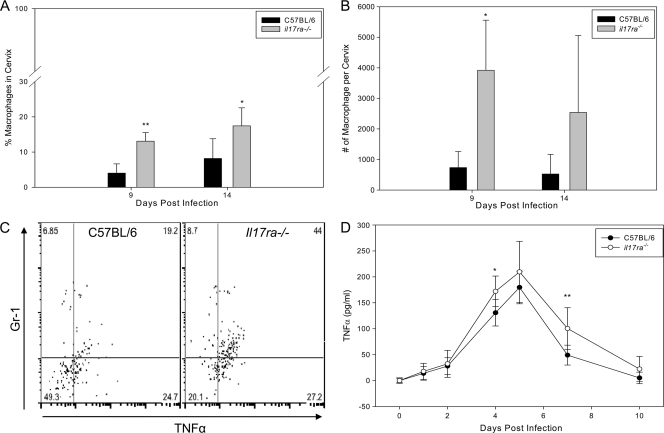
Macrophage influx and cytokine production are increased in il17ra−/− mice.
The effective control of chlamydial genital tract infection in il17ra−/− mice contrasted sharply with the outcome of respiratory tract infection in IL-17-depleted mice, where the lack of a measureable Th1 response resulted in uncontrolled infection (3). In the lung model, IL-17 depletion also resulted in enhanced neutrophil and macrophage inflammation, which was attributed to the increased bacterial burden observed in the absence of IL-17 (3). Flow cytometric analysis of cervical tissues revealed that the percentages of macrophages were increased in il17ra−/− mice on days 9 and 14 (Fig. 4D and 7 A). In addition, the absolute number of macrophages in the cervix was significantly elevated on day 9 (Fig. 7B). Remarkably, a significantly higher frequency of TNF-α+ macrophages was found in cervical tissues of il17ra−/− mice using intracellular cytokine staining (Fig. 7C). In addition, increased levels of TNF-α were observed in the secretions of il17ra−/− mice during the early days of infection (Fig. 7D). Significantly increased levels of IL-6 were detected in the secretions of the il17ra−/− mice (P = 0.048; two-way repeated-measures [RM] ANOVA; data not shown), providing corroborative evidence of enhanced macrophage activation in these mice. These findings suggest a previously undescribed role for IL-17 in diminishing monocyte/macrophage influx and activation in an infected tissue.
FIG. 7.
Macrophage influx and activation are increased in the cervixes of il17ra−/− mice. (A) Flow cytometric analysis of cervical tissues on day 9 and day 14 postinfection revealed that the percentage of CD45+ cells that were macrophages (CD45+ Gr-1+ F4/80+) was significantly higher in the cervical tissue of il17ra−/− mice (**, P < 0.005; *, P < 0.01; two-way ANOVA with multiple comparisons). (B) The absolute number of macrophages was also increased in the cervical tissue of il17ra−/− mice (*, P < 0.050 for day 9). The bars represent means and SD of 3 mice analyzed in a single independent experiment. (C) The percentage of macrophages that were TNF-α+ was significantly higher in the cervical tissues of il17ra−/− mice on day 9 (P = 0.007; Student's t test). The plot is gated on live CD45+ CD3− F4/80+ cells. (D) TNF-α levels were significantly higher in the secretions of il17ra−/− mice (P < 0.001 via two-way RM ANOVA with multiple-comparison procedure. *, P < 0.02, and **, P < 0.005 on days 4 and 7, respectively). The bars represent means and SD of 5 mice from a single independent experiment.
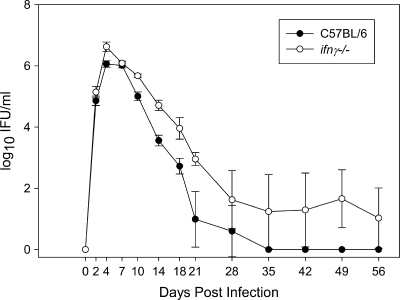
ifnγ−/− mice exhibit chronic low-level shedding of C. muridarum and increased neutrophil infiltrates throughout the genital tract in response to chlamydial infection.
Because IFN-γ has been shown to negatively regulate the Th17 response (7), we hypothesized that the severe pathology previously described in the absence of IFN-γ during chlamydial genital tract infection of ifnγ−/− mice (6, 31) may be due to enhanced induction of Th17 cells and IL-17-mediated increases in neutrophil recruitment. After intravaginal infection, similar levels of bacterial shedding were observed in wild-type and ifnγ−/− mice over the first 3 weeks, with both groups eliminating 99% of the chlamydial organisms from their genital mucosa (6, 31). Thereafter, the wild-type mice became culture negative, while ifnγ−/− mice alternated between culture-negative and culture-positive states (Fig. 8), suggesting that they remained infected at extremely low levels.
FIG. 8.
ifnγ−/− mice exhibit chronic low-level shedding of C. muridarum from the genital tract. Consistent with previously published reports (6, 31), ifnγ−/− mice and C57BL/6 mice showed similar clearance over the first 3 weeks of infection. However, wild-type C57BL/6 mice became culture negative, whereas ifnγ−/− mice continued to exhibit low-level shedding of chlamydiae, often alternating between culture-negative and -positive states. The data points represent the means ± SD of five mice and include both culture-positive and -negative mice at each time point.
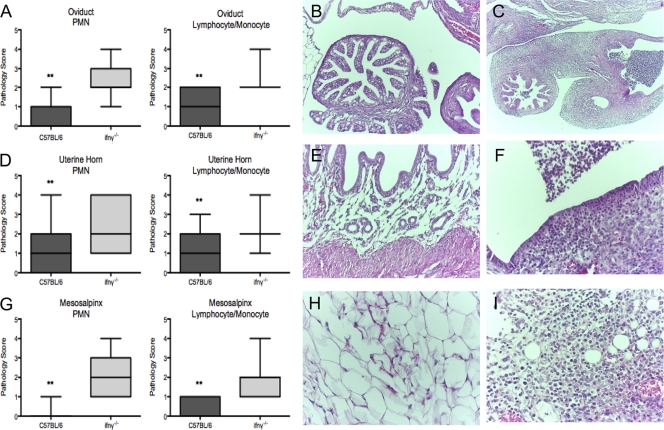
Gross examination of the peritoneal contents of ifnγ−/− mice sacrificed 35 days postinfection revealed ascites (10 of 10 mice), fibrinous peritonitis (8 of 10 mice), adhesions between the mesosalpingeal tissues and the small intestine (8 of 10 mice), and frank purulence in the uterine horns (5 of 10 mice). These gross pathological findings are similar to the findings described in humans with Fitz-Hugh-Curtis syndrome. When the genital tracts harvested 35 days postinfection were analyzed histologically, the oviducts, uterine horns, and mesosalpingeal tissues from ifnγ−/− mice demonstrated a marked increase in neutrophilic and lymphocytic/monocytic inflammation (Fig. 9 A, D, and G) compared to wild-type mice (P < 0.002 by Mann-Whitney U test).
FIG. 9.
ifnγ−/− mice exhibit enhanced genital tract pathology and an amplified neutrophil response to C. muridarum genital infection. Genital tract tissues were removed en bloc from C. muridarum-infected mice 35 days postinfection (C57BL/6, n = 21; ifnγ−/−, n = 15). Tissues were scored for neutrophilic (polymorphonuclear leukocytes [PMNs]) and lymphocytic and monocytic inflammation. Histopathologic analysis of the oviducts (A to C), uterine horns (D to F), and mesosalpingeal tissues (G to I) of C57BL/6 (B, E, and H) and ifnγ−/− (C, F, and I) mice shows significantly increased neutrophil and lymphocyte/monocyte infiltration in tissues from ifnγ−/− mice (**, P < 0.002 by Mann-Whitney U test). The boxes extend from the 25th to 75th percentiles and the bars from the 5th to 95th percentiles. Photomicrographs of oviducts (C; magnification, ×4), uterine horns (F; magnification, ×20), and mesosalpingeal tissues (I; magnification, ×20) from ifnγ−/− mice demonstrate a marked increase in neutrophilic infiltrates, edema, and loss of tissue structure compared to similar tissues from C57BL/6 mice (oviducts [B; magnification, ×4], uterine horns [E; magnification, ×20], and mesosalpinx [H; magnification, ×20]).
An increase in neutrophilic inflammation with destruction of mucosal epithelial cells, loss of luminal plicae, and a marked inflammatory exudate consisting of fibrin and neutrophils was observed in oviducts from ifnγ−/− mice (Fig. 9C) compared with oviducts from wild-type mice (Fig. 9B). Similarly, increased neutrophilic inflammation, mucosal erosions, and overlying exudate were apparent in the uterine horns of ifnγ−/− mice (Fig. 9E and F). Mesosalpingeal tissues of the ifnγ−/− mice (Fig. 9I) displayed increased inflammation, as well as fat necrosis, compared to mesosalpingeal tissues of wild-type mice (Fig. 9H).
Increased levels of Th17-related cytokines and chemokines are detected in genital tract secretions and oviducts from ifnγ−/− mice compared to wild-type mice infected with C. muridarum.
Th17 cells have been shown to promote neutrophil influx through induction of neutrophil chemokines. We examined the cytokine and chemokine milieu in the lower genital tracts and oviducts of ifnγ−/− and C57BL/6 mice following C. muridarum genital tract infection. Analysis of genital tract secretions from infected wild-type mice revealed an increase in IL-17 during the first 5 days of infection, after which IL-17 levels fell to baseline by day 10 (Fig. 10 A). The high levels of IL-17 detected in secretions of the wild-type mice on days 1 to 5 may reflect production from NK cells induced early after infection (Fig. 3B and C) (38).
FIG. 10.
Genital tract secretions and oviduct homogenates from ifnγ−/− mice demonstrate increased levels of Th17-related cytokines and chemokines following C. muridarum genital tract infection. (A) C57BL/6 mice infected with C. muridarum exhibit early IL-17 responses in genital secretions that are significantly higher than those of ifnγ−/− mice on days 2 to 5 postinfection. IL-17 levels in secretions from infected ifnγ−/− mice during days 7 to 35 postinfection were significantly higher than those from wild-type mice. (B and C) IL-6 (B) and CXCL1 (KC) (C) were significantly increased in the ifnγ−/− mice from days 7 to 35 of infection. Each data point represents the mean ± SD of values from five mice in each group at each time point from a single individual experiment. P < 0.05 by two-way RM ANOVA for panels A, B, and C. (D to F) Oviducts from C. muridarum-infected C57BL/6 mice and ifnγ−/− mice were harvested on days 7, 14, and 21 postinfection. On day 7 postinfection, cytokine levels were similar in oviducts from ifnγ−/− and C57BL/6 mice. On day 14, levels of IL-17 (D), IL-6 (E), and CXCL1 (KC) (F) were significantly elevated in oviducts from ifnγ−/− mice compared to the wild type. On day 21, IL-17 (D) and CXCL1 (KC) (F) levels were significantly elevated in oviducts from ifnγ−/− mice. **, P ≤ 0.001; *, P < 0.02 by two-way ANOVA. Each bar represents the mean and SD of values from homogenates of five mice at each time point in a single individual experiment.
Although moderate increases in IL-17 were detected in ifnγ−/− mice during the first 5 days of infection, these levels were significantly lower than those of infected wild-type mice. However, by day 10, high IL-17 levels were detected in the secretions of ifnγ−/− mice, and they remained elevated through day 28 (Fig. 10A). In addition, high levels of the Th17 cytokine IL-22 were found throughout the course of infection in ifnγ−/− mice (data not shown).
Th17 responses are initiated by the combination of IL-6 and TGF-β and supported by DC IL-23 production. Th17 cells induce the production of neutrophil chemoattractants, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, and CXC chemokines, including CXCL1 (KC) and CXCL2 (MIP-2) (4, 15, 25, 41, 42). Significantly increased levels of the Th17-promoting cytokines TGF-β (data not shown) and IL-6 (Fig. 10B) were observed in the genital tract secretions of ifnγ−/− mice compared to wild-type mice. TNF-α was also significantly increased in genital tract secretions from ifnγ−/− mice compared to wild-type mice (data not shown). Thus, the cytokine milieu in the lower genital tract of ifnγ−/− mice following C. muridarum infection is conducive to induction of a Th17 response. The neutrophil chemokines CXCL1 (KC) (Fig. 10C) and GM-CSF (data not shown) (P < 0.001 by two-way RM ANOVA) were significantly increased in genital tract secretions of infected ifnγ−/− mice compared to the wild type.
Oviducts were harvested from ifnγ−/− and wild-type mice on days 7, 14, and 21, representing early, mid-, and late infection in immunologically normal mice. Similar levels of cytokines were noted in oviducts from wild-type and ifnγ−/− mice on day 7. However, on days 14 and 21, the levels of IL-17 (Fig. 10D), IL-6 (Fig. 10E), and TNF-α (data not shown) were increased in oviduct homogenates from ifnγ−/− mice, as were the chemokines CXCL1 (KC) (Fig. 10F) and CXCL2 (MIP-2) (data not shown). Thus, conditions in the upper genital tract of ifnγ−/− mice also reflect a Th17-favorable environment.
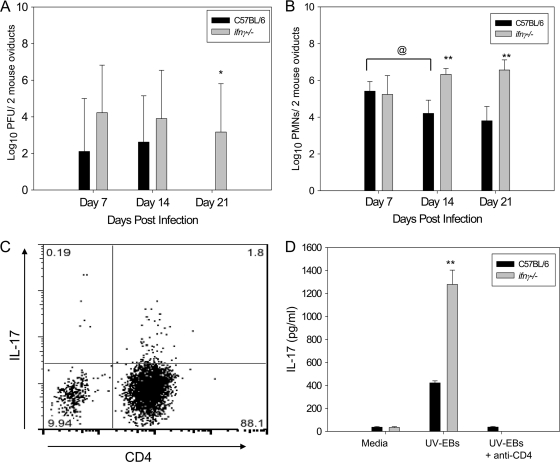
Oviducts from ifnγ−/− mice exhibit increased bacterial burden and enhanced neutrophil and Th17 infiltrates.
To determine the role of pathogen-driven inflammatory-cell recruitment to the oviduct, chlamydial burden was quantified by plaque assay and via real-time PCR for the Chlamydia 16S rRNA gene. Significantly higher levels of viable bacteria were detected in the oviducts of ifnγ−/− mice on day 21 (Fig. 11 A). Consistent with these observations, we detected significantly increased amounts of chlamydial genomic DNA in the oviducts of ifnγ−/− mice compared to wild-type mice on days 14 and 21 (P < 0.050; Student's t test) (data not shown).
FIG. 11.
Increased bacterial burden in the oviducts of ifnγ−/− mice is associated with increased infiltration of neutrophils and Th17 cells. (A) Bacterial burdens in the oviducts of 5 ifnγ−/− and 5 wild-type mice sacrificed on days 7, 14, and 21 postinfection in a single independent experiment were analyzed by plaque assay, and a significant increase in viable chlamydia in the oviduct was noted on day 21. *, P < 0.02 by two-way ANOVA. (B) Single-cell suspensions generated from two pooled oviducts of each mouse were analyzed by flow cytometry for live neutrophils (Gr-1high Cd11c− F4/80−). The data points represent the means and SD of values from 5 mice per group at each time point in a single independent experiment. **, P ≤ 0.001 by two-way ANOVA for wild-type versus ifnγ−/−; @, P < 0.02 by two-way ANOVA for day 7 versus day 14 in wild-type mice. (C) Th17 cells (CD3+ CD4+ IL-17+) were detected in the oviducts of ifnγ−/− mice on day 21 postinfection. The plot is gated on CD3+ cells. (D) Iliac node mononuclear cells from individual mice sacrificed on day 21 were stimulated in vitro with UV-inactivated EBs with and without anti-CD4 for 96 h, and the supernatants were analyzed for IL-17. The data points represent the means and SD of values from 5 mice per group at each time point in a single independent experiment. **, P < 0.001 (Student's t test).
Flow cytometric analysis revealed increased numbers of neutrophils (Fig. 11B) and CD4+ T cells (data not shown) in the oviducts of ifnγ−/− mice on days 14 and 21 compared to the wild type. IL-17-producing CD4+ T cells were detected in the oviducts of ifnγ−/− mice on day 21 (Fig. 11C) by intracellular cytokine staining, whereas, these cells were absent in the oviducts of wild-type mice on that day (data not shown).
CD4+ T cells in the iliac nodes of ifnγ−/− mice produced significantly greater amounts of IL-17 when stimulated with UV-EBs than wild-type mice on days 7 (data not shown), 21 (Fig. 11D), and 28 (data not shown). Sustained infection in the ifnγ−/− mice indicates that the enhanced Th17 and neutrophil responses were not effective compensatory bacterial control mechanisms.
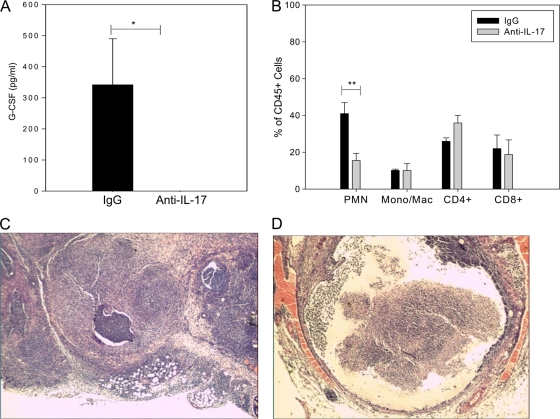
In vivo neutralization of IL-17 in ifnγ−/− mice results in a significant decrease in G-CSF and neutrophil influx but no increase in the bacterial burden.
Since oviduct epithelial cells secrete chemokines in response to chlamydial infection (17), it was possible that the enhanced neutrophil response was simply due to increased bacterial burden in the absence of IFN-γ. Therefore, we examined the contributions of IL-17 to enhanced neutrophil recruitment and to the outcome of infection in ifnγ−/− mice by administration of anti-IL-17 antibody. G-CSF was absent in the oviducts of the anti-IL-17-treated ifnγ−/− mice on day 21 postinfection (Fig. 12 A), indicating that IL-17 was effectively neutralized and that an immune response had not been mounted against the administered antibody. Flow cytometric analysis of leukocytes in the oviducts of anti-IL-17- and IgG2a-treated mice revealed a 65% reduction in the frequency of neutrophils in the anti-IL-17-treated group (Fig. 12B). Frequencies of monocytes and CD4+ and CD8+ T cells were not significantly altered by anti-IL-17 treatment (Fig. 12B). Despite the significant reduction in neutrophils observed on day 21, no increase in bacterial DNA was found in the oviducts of anti-IL-17-treated ifnγ−/− mice compared with the IgG2a-treated ifnγ−/− group (data not shown). The reduction in neutrophil influx observed in anti-IL-17-treated ifnγ−/− mice also did not alter oviduct pathology. Histologic examination revealed severe inflammation and oviduct dilatation in both groups (Fig. 12C and D). Thus, in the absence of IFN-γ, IL-17 plays a direct role in driving neutrophil influx into the oviducts but IL-17 and the downstream increase in neutrophils do not contribute to the control of infection. Sustained infection and leukocyte infiltration resulted in severe pathology in ifnγ−/− mice, even in the absence of IL-17-mediated neutrophil influx.
FIG. 12.
Administration of anti-IL-17 to ifnγ−/− mice significantly reduces G-CSF and the number of neutrophils in the oviducts but does not improve pathology. Groups of five ifnγ−/− mice infected intravaginally with C. muridarum and treated with IgG2a or anti-IL-17 were sacrificed on day 21 postinfection, and the oviducts were harvested. (A) G-CSF was completely abrogated by IL-17 neutralization (*, P < 0.05 by Student's t test). The bars represent the means and standard errors of the mean (SEM) of values from 5 mice per group in a single experiment. (B) Percentages of live neutrophils (Ly6G [1A8]+ Gr-1high), macrophages (Mac) (F4/80+), CD4+ T cells (CD3+ CD4+ CD8−), and CD8+ T cells (CD3+ CD4− CD8+) were determined by flow cytometry. A 65% reduction in the frequency of neutrophils was noted in the anti-IL-17-treated group compared with IgG2a-treated mice on day 21 (**, P < 0.01 by ANOVA). The data points represent the means and SD of values from 5 mice per group in a single independent experiment. Mono, monocytes. (C and D) Oviducts were harvested from the IgG2a-treated (C) and anti-IL-17-treated (D) groups and examined histologically. Oviduct pathology was not improved by anti-IL-17 treatment; severe inflammation and oviduct dilatation, along with destruction of mucosal epithelial cells, granuloma formation, and a marked inflammatory exudate, were noted in both groups. The photomicrographs are representative tissue samples from the oviducts of the IgG2a-treated (C; magnification, ×40) and anti-IL-17-treated (D; magnification, ×40) groups on day 21 postinfection.
DISCUSSION
Control of chlamydial infection in the genital tract requires a robust Th1 response (5, 6, 26, 27, 31). Recent investigations indicate that IL-17 potentiates Th1 immunity and consequently plays an important role in the control of intracellular pathogens (3, 24). We investigated the role of IL-17 in the control and pathological outcome of C. muridarum genital tract infection using IL-17 receptor- and IFN-γ-deficient mice. Our data demonstrate that IL-17 promotes neutrophil recruitment and augments Th1 immunity but is not required for infection control or Chlamydia-induced pathology at this site.
Following C. muridarum genital tract infection of C57BL/6 wild-type mice, we detected both Th1 and Th17 antigen-specific CD4+ T cells in the iliac nodes, the draining lymph nodes for the genital tract. We noted a contraction of the T cell response in the iliac node on day 20 compared with day 7, likely due to resolving infection and reduced antigen burden. Ongoing experiments examining the kinetics of tissue-specific, antigen-specific Th1 and Th17 responses in the genital tract tissues by multicolor flow cytometric analysis, as well as examination of alternative IL-17- and IFN-γ-producing cells (i.e., NK, NK-T, γδ, and CD4 T cells) operative in the cervical tissues and oviducts of wild-type mice will further define the role of IL-17 during chlamydial infection.
During C. muridarum pulmonary infection, IL-17 neutralization had a detrimental impact on the disease course and the development of Chlamydia-specific Th1 responses (3). This parallels findings during pulmonary infection with F. tularensis LVS, where in the absence of IL-17, Th1 immunity was compromised (24). In contrast, results obtained during pulmonary Mycobacterium tuberculosis infection of il23p19−/− mice revealed that infection is eliminated at a normal rate despite the absence of IL-17 (22). Further, resistance to infection with the intracellular pathogens Salmonella and Listeria is independent of the IL-23/Th17 cell pathway (2, 36). We determined that il17ra−/− mice did not experience increased bacterial load or augmented genital tract pathology when inoculated intravaginally with C. muridarum. We detected lower levels of IFN-γ in the genital tract secretions of il17ra−/− mice early during infection and in the iliac node supernatants through day 35. Further examination revealed that the lower levels of IFN-γ in the secretions of the il17ra−/− mice could be attributed to decreased numbers of IFN-γ-producing NK cells in the cervix, which is consistent with the documented role of IL-17 in inducing IFN-γ production by NK cells (24). It is possible that this decreased NK cell production of IFN-γ contributes to the reduction in the Th1 response we noted in the iliac nodes of il17ra−/− mice. Tseng and Rank demonstrated that depletion of NK cells during C. muridarum genital tract infection resulted in a shift toward a Th2 response and delayed resolution of infection (38). However, we did not detect increased IL-4 in the iliac nodes of infected il17ra−/− mice, indicating that such a shift did not occur. It is possible that alternative pathways are induced in il17ra−/− mice that prevent an augmented Th2 response when the NK cell IFN-γ response is decreased.
In both the C. muridarum and F. tularensis pulmonary infection models, Th1 immunity was compromised in anti-IL-17-treated or IL-17-deficient mice, a finding that was associated with significantly decreased dendritic cell production of IL-12p70 (3, 24). We detected reduced levels of both IL-12p70 and IFN-γ in the iliac nodes of il17ra−/− mice, indicating that priming of the Th1 response was compromised in the absence of IL-17. However, we did not detect the increase in bacterial load or IL-4 secretion that was observed when IL-17 was neutralized during C. muridarum pulmonary infection (3). We also observed significantly increased macrophage influx and enhanced macrophage TNF-α production in il17ra−/− mice. A role for TNF-α in contributing to control of C. muridarum genital tract infection has been described (32), suggesting that this inflammatory response may have compensated for deficiencies in the Th1 response, resulting in a normal course of infection.
C. muridarum infects both the respiratory tract and genital tract, and this provides a unique opportunity to investigate the potential for site-specific differences in the mucosal inflammatory responses to the pathogen. Although a decreased Th1 response was noted in the absence of IL-17 signaling in both the respiratory and genital tracts, we did not observe increased bacterial burden or a shift toward a Th2 response during genital tract infection of il17ra−/− mice. It is possible that these differences may indicate site specificity in the development of the adaptive response to chlamydiae. These site-specific differences could be related to distinct differences in the anatomy of the mucosal immune response in the female genital tract, including the lack of inductive mucosal sites analogous to the mucosa-associated lymphoid tissue (MALT) of the lungs or related to differences in immunologic priming by genital tract epithelial cells. Alternatively, the differences in responses may reflect variance resulting from the genetic background of the animals used in each study. C57BL/6 mice, the genetic background for both the il17ra−/− and ifnγ−/− strains used in this study, are less susceptible to pulmonary infection with C. muridarum and experience reduced bacterial load, a shortened infection course, and less severe tissue pathology than BALB/c mice (16). Thus, abrogation of the Th17 response may be more detrimental to host defense in the BALB/c strain used for the studies of pulmonary infection (9).
Because IFN-γ has been shown to negatively regulate the Th17 response (7, 14), we intravaginally inoculated ifnγ−/− mice with C. muridarum to determine the role of an unopposed Th17 response during chlamydial infection. In the absence of CD4+ Th1 IFN-γ production, we observed a significantly increased CD4+ Th17 response and increased neutrophil influx. ifnγ−/− mice displayed significantly worsened pathology and elevated bacterial burden in the lower and upper genital tracts. Administration of an anti-IL-17 antibody raised against IL-17A confirmed a direct role for IL-17A in driving neutrophil influx in ifnγ−/− mice, but IL-17 neutralization did not lead to enhanced bacterial burden, likely due to maintenance of macrophage influx. We anticipated that if neutrophils were effectors of upper-tract pathology, then administration of anti-IL-17 antibody might reduce the severity of disease. Unfortunately, the intense infiltration of nonneutrophil leukocytes observed in the absence of IFN-γ prevented adequate assessment of the role of neutrophils or IL-17 in pathology. Because the antibody utilized in these experiments targeted IL-17A, it is possible that the continued presence of IL-17F provided sufficient signaling to result in production of neutrophil-promoting chemokines and neutrophil influx, albeit at reduced levels, which could explain the absence of an effect on pathology in the antibody-treated mice. Further, continued signaling by IL-17F could also prevent enhanced macrophage influx, such as that observed in the il17ra−/− mice.
Our data have important implications for the prevention and treatment of human Chlamydia infections and the strategic design of vaccines and therapeutics to target chronic chlamydial disease. The presence of augmented Th17 responses and enhanced neutrophilic infiltration in the absence of IFN-γ indicate that these responses could be used as biomarkers for chronic infection and an inadequate Th1 response in humans. In models of M. tuberculosis pulmonary infection, vaccination in the presence of IL-17-inducing adjuvant promotes Th1 immunity and reduced bacterial burden (21). Recent data from Yu et al. demonstrated that a vaccine consisting of immunodominant chlamydial T cell antigens afforded protection from infection that was associated with IFN-γ/TNF-α and IFN-γ/IL-17 double-positive CD4+ T cells (45). Indeed, our data reveal that removal of IL-17 blunts Th1 priming during genital tract infection. Thus, the possibility exists that a chlamydial vaccine that induces a Th17 response may promote more effective Th1 immunity.
In summary, we have shown for the first time that the Th17 response that occurs in the genital tract due to Chlamydia infection promotes but is not essential for induction of neutrophil influx or Th1 immunity. In addition, we have shown that in the absence of the protective IFN-γ response, a heightened Th17 response induces neutrophil influx. The findings that chlamydial genital infection, in contrast to respiratory tract infection, resolves normally in the absence of IL-17 and in the presence of a diminished IFN-γ response highlight the importance of examining site-specific responses to infection. Importantly, we determined that removal of IL-17 led to decreased neutrophil influx but enhanced macrophage influx and activation. Thus, it is possible that in an immunologically intact host IL-17 not only drives neutrophil influx and Th1 immunity, but also downregulates the monocyte/macrophage response. Future studies will investigate IL-17-mediated mechanisms of monocyte/macrophage inhibition and the role of the macrophage TNF-α response in controlling chlamydial infection.
Acknowledgments
This work was supported by the Marion B. Lyon Foundation (A.M.S.); Arkansas Biosciences Institute (A.M.S.); and Public Health Service grants 1K08AI077932 (A.M.S.), AI054624 (T.D.), and AI084024 (T.D.) from the National Institutes of Health, the Bates-Wheeler Foundation and the Arkansas Children's Hospital Research Institute.
We are grateful to Alison Logar and Megan Blanchard for assistance with flow cytometry. The flow cytometric method was designed by Uma Nagarajan and James D. Sikes. We appreciate helpful discussions with Roger Rank and Uma Nagarajan.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 13 December 2010.
REFERENCES
- 1.Abe, M., et al. 1994. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216:276-284. [DOI] [PubMed] [Google Scholar]
- 2.Aujla, S. J., et al. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai, H., et al. 2009. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J. Immunol. 183:5886-5895. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli, E., et al. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235-238. [DOI] [PubMed] [Google Scholar]
- 5.Cain, T. K., and R. G. Rank. 1995. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 63:1784-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 65:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz, A., et al. 2006. Cutting edge: IFN-{gamma} regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 177:1416-1420. [DOI] [PubMed] [Google Scholar]
- 8.Darville, T., et al. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 65:3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darville, T., et al. 2001. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infect. Immun. 69:7419-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darville, T., C. W. Andrews, Jr., J. D. Sikes, P. L. Fraley, and R. Rank. 2001. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect. Immun. 69:3556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diveu, C., M. J. McGeachy, and D. J. Cua. 2008. Cytokines that regulate autoimmunity. Curr. Opin. Immunol. 20:663-668. [DOI] [PubMed] [Google Scholar]
- 12.Feinen, B., A. E. Jerse, S. L. Gaffen, and M. W. Russell. 2010. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 3:312-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Happel, K. I., et al. 2005. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 202:761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington, L. E., et al. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123-1132. [DOI] [PubMed] [Google Scholar]
- 15.Hartupee, J., C. Liu, M. Novotny, X. Li, and T. Hamilton. 2007. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 179:4135-4141. [DOI] [PubMed] [Google Scholar]
- 16.Jiang, X., C. Shen, H. Yu, K. P. Karunakaran, and R. C. Brunham. 2010. Differences in innate immune responses correlate with differences in murine susceptibility to Chlamydia muridarum pulmonary infection. Immunology 129:556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, R. M. 2004. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infect. Immun. 72:3951-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karunakaran, K. P., et al. 2008. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J. Immunol. 180:2459-2465. [DOI] [PubMed] [Google Scholar]
- 19.Kelly, K. A., E. A. Robinson, and R. G. Rank. 1996. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect. Immun. 64:4976-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khader, S. A., S. L. Gaffen, and J. K. Kolls. 2009. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khader, S. A., et al. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369-377. [DOI] [PubMed] [Google Scholar]
- 22.Khader, S. A., et al. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-{gamma} responses if IL-12p70 is available. J. Immunol. 175:788-795. [DOI] [PubMed] [Google Scholar]
- 23.Langrish, C. L., et al. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, Y., et al. 2009. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31:799-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangan, P. R., et al. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441:231-234. [DOI] [PubMed] [Google Scholar]
- 26.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connell, C. M., and K. M. Nicks. 2006. A plasmid-cured Chlamydia muridarum strain displays altered plaque morphology and reduced infectivity in cell culture. Microbiology 152:1601-1607. [DOI] [PubMed] [Google Scholar]
- 29.Parham, C., et al. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12R{beta}1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699-5708. [DOI] [PubMed] [Google Scholar]
- 30.Park, H., et al. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 32.Perry, L. L., et al. 1999. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-{gamma}-mediated inhibition. J. Immunol. 162:3541-3548. [PubMed] [Google Scholar]
- 33.Ramsey, K. H., W. J. Newhall, and R. G. Rank. 1989. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect. Immun. 57:2441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey, K. H., I. M. Sigar, J. H. Schripsema, N. Shaba, and K. P. Cohoon. 2005. Expression of matrix metalloproteinases subsequent to urogenital Chlamydia muridarum infection of mice. Infect. Immun. 73:6962-6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsey, K. H., L. S. Soderberg, and R. G. Rank. 1988. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect. Immun. 56:1320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz, S. M., et al. 2008. Protective immunity to systemic infection with attenuated Salmonella enterica serovar Enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J. Immunol. 181:7891-7901. [DOI] [PubMed] [Google Scholar]
- 37.Shah, A. A., et al. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex. Transm. Dis. 32:49-56. [DOI] [PubMed] [Google Scholar]
- 38.Tseng, C. T. K., and R. G. Rank. 1998. Role of NK cells in early host response to chlamydial genital infection. Infect. Immun. 66:5867-5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuffrey, M., and D. Taylor-Robinson. 1981. Progesterone as a key factor in the development of a mouse model for genital tract infection with Chlamydia trachomatis. FEMS Microbiol. Lett. 12:111-115. [Google Scholar]
- 40.Umemura, M., et al. 2007. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis Bacille Calmette-Guerin infection. J. Immunol. 178:3786-3796. [DOI] [PubMed] [Google Scholar]
- 41.Veldhoen, M., R. J. hocking, C. J. Atkins, R. M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179-189. [DOI] [PubMed] [Google Scholar]
- 42.Weaver, C. T., R. D. Hatton, P. R. Mangan, and L. E. Harrington. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25:821-852. [DOI] [PubMed] [Google Scholar]
- 43.Wiesenfeld, H., et al. 2002. Association between elevated neutrophil defensin levels and endometritis. J. Infect. Dis. 186:792-797. [DOI] [PubMed] [Google Scholar]
- 44.Ye, P., et al. 2001. Requirement of interleukin 17 receptor signaling for lung Cxc chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu, H., et al. 2010. Chlamydia T cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection which correlates with a high frequency of IFN-{gamma}/TNF-{alpha} and IFN-{gamma}/IL-17 double-positive CD4+ T cells. Infect. Immun. 78:2272-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, X., et al. 2009. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J. Immunol. 183:1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou, X., et al. 2009. A critical role of the IL-17/IL-17R axis in regulating host susceptibility to respiratory chlamydia infection. Infect. Immun. 77:5059-5070. [DOI] [PMC free article] [PubMed] [Google Scholar]