Abstract
Cholera toxin (CT) is a potent adjuvant for mucosal vaccination; however, its mechanism of action has not been clarified completely. It is well established that peripheral monocytes differentiate into dendritic cells (DCs) both in vitro and in vivo and that monocytes are the in vivo precursors of mucosal CD103− proinflammatory DCs. In this study, we asked whether CT had any effects on the differentiation of monocytes into DCs. We found that CT-treated monocytes, in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4), failed to differentiate into classical DCs (CD14low CD1ahigh) and acquired a macrophage-like phenotype (CD14high CD1alow). Cells differentiated in the presence of CT expressed high levels of major histocompatibility complex class I (MHC-I) and MHC-II and CD80 and CD86 costimulatory molecules and produced larger amounts of IL-1β, IL-6, and IL-10 but smaller amounts of tumor necrosis factor alpha (TNF-α) and IL-12 than did monocytes differentiated into DCs in the absence of CT. The enzymatic activity of CT was found to be important for the skewing of monocytes toward a macrophage-like phenotype (Ma-DCs) with enhanced antigen-presenting functions. Indeed, treatment of monocytes with scalar doses of forskolin (FSK), an activator of adenylate cyclase, induced them to differentiate in a dose-dependent manner into a population with phenotype and functions similar to those found after CT treatment. Monocytes differentiated in the presence of CT induced the differentiation of naïve T lymphocytes toward a Th2 phenotype. Interestingly, we found that CT interferes with the differentiation of monocytes into DCs in vivo and promotes the induction of activated antigen-presenting cells (APCs) following systemic immunization.
Adjuvant design has been mainly empirical, and the mechanism of action of the most efficacious molecules has remained obscure. It is important to investigate the mechanisms of action of already known adjuvants to facilitate the design of new effective molecules with high potency and decreased side effects. The bacterial enterotoxin cholera toxin (CT) from Vibrio cholerae is extraordinarily effective as a mucosal and systemic adjuvant, yet its capacity to amplify the immune response has not been completely clarified (12, 24, 25). It is a holotoxin, composed of an enzymatically active A subunit, noncovalently linked to a pentameric B subunit, which binds to the ganglioside GM1 on host cell membranes. Once internalized, the A subunit ADP-ribosylates the α subunit of the GTP-binding regulatory protein Gs, thereby inducing permanent adenylate cyclase activation, resulting in an increase of intracellular cyclic AMP (cAMP) (55). The mechanism of the adjuvanticity of CT may be a complex phenomenon resulting from the interaction of the toxin with different cell types present in the architecture of the mucosa. Dissecting the effect of CT on different cells types could help in understanding the contribution of each to its adjuvant activity.
Given the crucial role of dendritic cells (DCs) and other professional antigen-presenting cells (APCs) (monocytes/macrophages and B cells) in the induction of adaptive immunity (26), the potentiation of APC function can be a major aspect of adjuvant action. Cholera toxin upregulates expression of the B7-2 (CD86) costimulatory molecule and stimulates antigen presentation through enhancement of major histocompatibility complex class II (MHC-II) expression and interleukin 1β (IL-1β) production (7, 11, 37, 54). CT also interacts with lymphocytes and promotes B cell isotype-switch differentiation toward IgG1 and IgA in mice (2, 54) and enhances the antigen-presenting function of human B cells (38). We and others showed that CT, by inducing maturation of human DCs, polarizes a mixed Th1/Th2 T cell response, with a strong bias toward Th2 cells (15, 16).
The capacity of CT to interact with DCs or monocytes as innate immune cells or as precursors of DCs may represent a crucial step for its adjuvant mechanism. DCs represent heterogeneous populations that comprise distinct subtypes that vary in hematopoietic origin, life cycle, and function (4, 52). It is well established that peripheral monocytes differentiate into DCs both in vitro and in vivo (19, 20, 43-45, 48) and that monocytes are the in vivo precursors of mucosal CD103− proinflammatory DCs (5, 27, 58). More important, the role of monocytes as precursors of DCs in mediating the in vivo adjuvant effects has been described (29, 60). Therefore, in this study, we asked whether CT affected the differentiation of monocytes into DCs. By using human DCs generated from monocytes (48), we found that CT interferes with the differentiation of monocytes into DCs, giving rise to a distinct population (Ma-DCs), which displays an activated macrophage-like phenotype, induces a strong allogeneic and antigen-specific response, and promotes the polarization of naive CD4+ T lymphocytes toward a Th2 profile. Interestingly, we found that CT interferes with the differentiation of monocytes into DCs in vivo and promotes the induction of activated antigen-presenting cells following systemic immunization.
MATERIALS AND METHODS
Media and reagents.
RPMI 1640 supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco, NY), and 10% fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT) was used as complete medium in all cell cultures. Cholera toxin and cholera toxin B subunit (CT-B) were purchased from Calbiochem-Novabiochem Co. (San Diego, CA), and forskolin (FSK), ionomycin (Ion), phorbol 12-myristate 13-acetate (PMA), brefeldin A (BFA), lipopolysaccharide (LPS), and ovalbumin (OVA) were purchased from Sigma Chemicals Co., St. Louis, MO. GM-CSF and IL-4 were purchased from Immunological Science (Rome, Italy), and murine GM-CSF (mGM-CSF) was purchased from Peprotech, (Rocky Hill, NJ). Carboxyfluorescein succiniminidyl ester (CFSE) was purchased from Molecular Probes (Eugene, OR).
Animals.
Female BALB/c mice and DO11.10 TCR transgenic mice aged 6 to 8 weeks were obtained from Harlan Nossan (Correzzana, Italy) and Charles River (Calco, Italy), respectively. All mice were maintained in our animal facilities for the duration of the experiments, and all procedures were in accordance with the institutional guidelines and have been approved by the institutional committee.
Monocyte purification and cell cultures.
Human monocytes were purified from healthy donors' peripheral blood mononuclear cells (PBMC) by positive selection using anti-CD14-conjugated magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The recovered cells were 95% to 99% CD14+ as determined by flow cytometry using the FITC conjugate anti-human CD14 monoclonal antibody (MAb) (BD Biosciences, San Diego, CA). Monocytes were cultured at 1 × 106/ml in RPMI-FBS supplemented with GM-CSF (50 ng/ml) and IL-4 (35 ng/ml), in the presence of 0.03 μg/ml CT, 0.03 μg/ml CT-B, or escalating doses of FSK (10, 20, and 40 μM or 10 μM for 3 days). All stimuli were kept in culture up to 6 days, and then the cells were washed and stained with FITC or phycoerythrin (PE) conjugate anti-human CD14, CD1a, HLA-DR, HLA-I, CD80, CD86, CD209 (DC-Sign), CD64, CD32, CD89, CCR7, and CD83 (BD Biosciences, San Diego, CA) to verify their differentiation, activation, and maturation status.
Murine CD11b+ monocytes were obtained from single-cell suspensions of BALB/c femur bone marrow by positive selection using anti-mouse CD11b-conjugated magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The recovered cells were 95% to 99% CD11b+ as determinated by flow cytometry using the FITC conjugate anti-mouse CD11b MAb (BD Biosciences, San Diego, CA), and they were cultured (1 × 106/ml) in RPMI-FBS supplemented with mGM-CSF (30 ng/ml), 2-mercaptoethanol (50 μM) in the presence of 0.3 μg/ml CT. At day 2, mGM-CSF (15 ng/ml) was added again to cell cultures. After 6 days, cells were washed and stained with FITC or PE conjugate anti-mouse CD11c, MHC-II, and CD86 (BD Biosciences, San Diego, CA). Cells were acquired on a FACSCalibur instrument running CellQuest software (BD Biosciences, San Diego, CA).
Naïve CD4+ T cell purification.
PBMC were isolated from healthy donors by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density centrifugation. CD4+ T lymphocytes were purified by negative selection using immunomagnetic cell sorting (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, PBMC were labeled using a cocktail of the hapten-conjugated MAbs anti-CD8, -CD11b, -CD14, -CD16, -CD19, -CD36, -CD56 -CD123, -TCRγ/δ, and -glycophorin A in combination with MACS microbeads coupled to an anti-hapten monoclonal antibody. The magnetically labeled cells were depleted by retaining them on a column using a MidiMACS cell separator. CD4+ T cells were further purified in CD4+ CD45RO+ and CD4+ CD45RA+ T cells by positive selection using anti-CD45RA microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany).
Cytokine production.
Supernatants from monocytes differentiated in the presence or in the absence of CT or CT-B for 6 days and further cultured for 48 h with or without LPS (250 ng/ml) were collected and stored at −80°C. The levels of IL-1β, IL-6, TNF-α, IL-10, and IL-12 were detected by Quantikine immunoassay kits (Endogene, Cambridge, MA) and were measured as absorbance (450 nm) on an enzyme-linked immunosorbent assay (ELISA) reader.
Proliferation and polarization assays.
The ability of monocytes differentiated in the presence or in the absence of CT (0.03 μg/ml) to activate T cells was evaluated by the use of the mixed lymphocyte reaction or antigen-specific T cell response. At day 6, cells were washed, starved for 8 h, and cocultured with allogeneic PBMC (1 × 105) at different PBMC/DC ratios (4:1, 2:1, and 1:1) for 5 days in round-bottom, 96-well plates. Proliferation was evaluated by [3H]thymidine incorporation. Plates were incubated for 48 h at 37°C with 5% CO2, and [3H]thymidine (Amersham, Aylesbury, United Kingdom) was added (1 μCi/well). After 18 h, cells were harvested, and the incorporated radioactivity was measured by Microβ counting. To evaluate the ability of monocytes differentiated in the presence or in the absence of CT (0.03 μg/ml) or FSK (10 μM) to stimulate an antigen-specific T cell response, cells were isolated from donors immune to the tetanus toxoid (TT) antigen. DCs were cocultured with autologous CFSE-labeled peripheral blood lymphocytes (PBLs) (1 × 105 at a ratio of 1:1) in the presence of TT (2 μg/ml). Briefly, autologous PBLs (107/ml) were washed in phosphate-buffered saline (PBS) 1% fetal calf serum (FCS), incubated with 0.5 μM CFSE for 10 min at 37°C, and then washed three times with complete medium. After 5 days, antigen-specific proliferation was evaluated by CFSE dilution, and cells were double stained with anti-CD4-PE and anti-CD8-Cy5.5 MAbs and acquired and analyzed on a FACSCalibur instrument running CellQuest software.
The ability of monocytes differentiated in the presence or in the absence of CT (0.03 μg/ml) to stimulate and polarize naïve T cells was evaluated. At day 6, cells were washed, starved for 8 h, and cocultured with purified naïve CD4+ CD45RA+ T cells (ratio of 1:5) in 48-well plates, at 37°C for 11 days. IL-2 (20 UI/ml) was added to the culture at days 5, 7, and 9 of the cocultures, and after 11 days, cells were harvested, washed, and analyzed for cytokine production by intracellular staining. In brief, cells were stimulated with 50 ng/ml PMA and 1 μg/ml ionomycin; after 1 h, 10 μg/ml of brefeldin (Sigma Chemicals Co., St. Louis, MO) was added, and they were incubated for a further 5 h at 37°C. Cells were washed twice in PBS, 1% bovine serum albumin (BSA), and 0.1% sodium azide and stained with anti-CD4 MAb for 15 min at 4°C, and then they were fixed with lysing solution (BD Biosciences, San Diego, CA), permeabilized with permeabilizing solution (BD Biosciences, San Diego, CA), and stained with PE or FITC conjugate anti-human IL-4 and gamma interferon (IFN-γ) (BD Biosciences, San Diego, CA). Samples were acquired and analyzed on a FACSCalibur instrument running CellQuest software.
Adoptive transfer experiments.
Purified CD11b+ monocytes were labeled with CFSE (0.5 μM) at room temperature (RT) in the dark for 8 min, washed three times with RPMI-10% FBS and intraperitoneally (i.p.) injected (8 × 106) into BALB/c recipient mice. In all experiments, OVA-specific T cells (2 × 106) obtained from the spleen and lymph nodes of DO11.10 TCR transgenic mice were intravenously injected. Adoptively transferred mice were immunized i.p. with OVA (5 μg/dose) or OVA plus CT (1 μg/dose). After 3 days, the mediastinal lymph nodes were collected, stained with anti-CD11b, -CD11c, -MHC-I (H2Kd), -MHC-II (I-A/I-E), -CD86, -CD4 (BD Biosciences, San Diego, CA), and -DO11.10 TCR (eBioscience, San Diego, CA) and acquired on a FACSCanto instrument running Diva software (BD Biosciences, San Diego, CA).
Statistical analysis.
Microsoft Excel (Microsoft Corp., Redmond, WA) was used for statistical analysis. Data were expressed as the mean ± standard deviation (SD), and statistical significance was determined by Student's t test; a P value of <0.05 was considered statistically significant.
RESULTS
Cholera toxin interferes with the differentiation of monocytes into DCs, giving rise to a macrophage-like population (Ma-DCs).
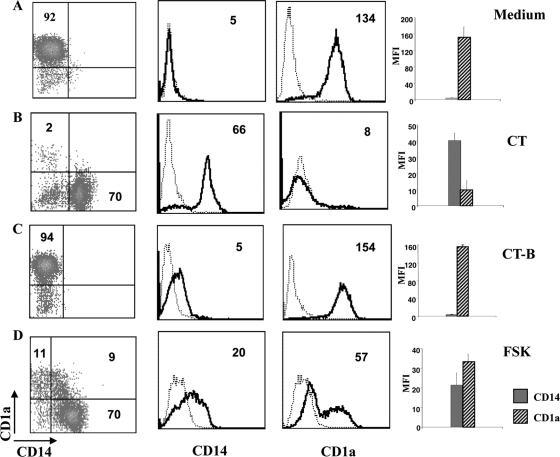
To investigate the effects of CT on the differentiation of monocytes into DCs, human monocytes were cultured with GM-CSF and IL-4 for 6 days in the presence or absence of CT. In the absence of CT, monocytes differentiated into immature DCs characterized by the expression of CD1a and the loss of CD14 molecules. In contrast, cells derived from CT-treated monocytes did not express CD1a and retained the expression of CD14, suggesting that they acquired a monocyte/macrophage-like phenotype (Ma-DCs) (Fig. 1 A and B). To investigate whether the GM1-binding B subunit (CT-B) or the enzymatic activity of the A subunit of CT was important for the differentiation toward this monocyte/macrophage-like population, monocytes were induced to differentiate into DCs in the presence of CT-B or forskolin (FSK), an activator of adenylate cyclases. Monocytes treated with CT-B showed a phenotype similar to that of untreated cells, whereas FSK-stimulated monocytes differentiate into a macrophage-like population similar to that derived from CT-treated monocytes (Fig. 1C and D). This suggests that the enzymatic activity of CT and its capacity to increase intracellular cAMP levels is important for the skewing of monocytes toward a macrophage-like phenotype.
FIG. 1.
Cholera toxin impairs differentiation of human monocytes into DCs. Dot and histogram plots show the phenotype of human CD14 monocytes isolated from healthy donors' PBMC and cultured for 6 days with GM-CSF (50 ng/ml) and IL-4 (35 ng/ml) in the presence of medium alone (A), CT (0.03 μg/ml) (B), CT-B (0.03 μg/ml) (C), and FSK (10 μM) (D). Cells were double stained using anti-CD14-FITC and anti-CD1a-PE MAbs and analyzed by flow cytometry. Dot and histogram plots are representative of one experiment out of six performed. The histogram bars show the means (+SD) of the relative mean fluorescence intensity (MFI) of CD14 and CD1a expression from six independent experiments performed. The numbers within the plots indicate the percentage of positive cells (dot plots) and the mean fluorescence intensity (histogram plots).
Ma-DCs express DC-Sign and FcR patterns typical of terminally differentiated myeloid cells.
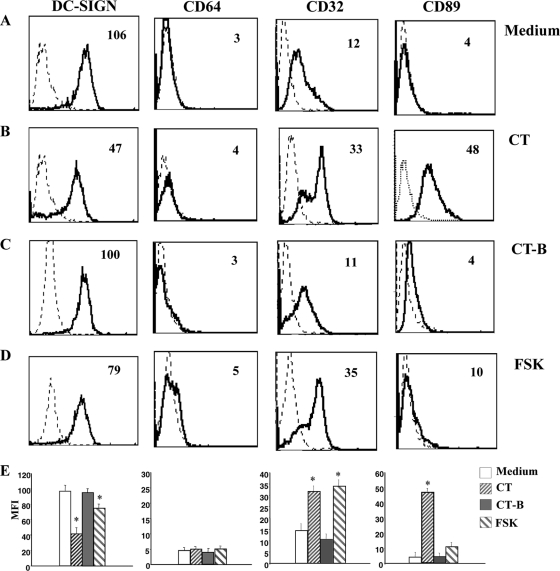
We evaluated the effect of CT on surface expression of DC-Sign (CD209), a DC-restricted C-type lectin involved in the early interaction between DCs and naïve T cells and also the DC trafficking and internalization of Ag (17, 56). DC-Sign is upregulated during the differentiation of monocytes into DCs (18), and this upregulation was not completely inhibited by treatment with CT or FSK (Fig. 2 B); however, CT- or FSK-treated monocytes expressed significantly (P = 0.0004, n = 4 for CT and P = 0.0009, n = 4 for FSK) lower levels of DC-Sign than control cells did. We also analyzed the expression of Fc-γRI (CD64), the high-affinity IgG Fc receptor (FcR), which is constitutively expressed on monocytes and down-modulated during DC differentiation. CT-treated or untreated monocytes induced to differentiate into DCs did not express CD64 molecules, showing that the down-modulation of CD64 was not affected by CT treatment (Fig. 2B).
FIG. 2.
Cholera toxin induces the differentiation of monocytes into cells with a macrophage-like phenotype (Ma-DCs). Histogram plots show the phenotype of human CD14 monocytes, isolated from healthy donors' PBMC, cultured for 6 days with GM-CSF (50 ng/ml) and IL-4 (35 ng/ml) in the presence of medium alone (A), CT (0.03 μg/ml) (B), CT-B (0.03 μg/ml) (C), and FSK (10 μM) (D). Cells were stained using anti-CD209-FITC, -CD64-PE, -CD32-FITC, and -CD89-PE MAbs and analyzed by flow cytometry. Histogram plots are representative of six experiments performed. The numbers within the plots indicate the mean fluorescence intensity. (E) The histogram bars show the means (+SD) of the relative mean fluorescence intensity (MFI) of the different markers analyzed from six independent experiments performed. Asterisks indicate that the differences between cells differentiated in the presence of CT or FSK and the control cells are significant (P < 0.05).
Terminal differentiation of myeloid cells is characterized by a switch of expression from Fc-γRI/CD64 to low-affinity Fc-γRII/CD32 and other FcRs, such as CD89, the IgA-specific FcR (8, 41). Therefore, we compared the expression of the low-affinity receptor CD32 and, because of the peculiar capacity of CT to promote mucosal IgA production, the expression of CD89 in cells differentiated from CT-treated and untreated monocytes. CD32 is expressed on monocytes and upregulated during either macrophage or, to lesser extent, DC differentiation. Treatment with CT during monocyte differentiation into DCs caused an increase of CD32 greater than that of control cells (P = 0.009, n = 6), a further indication of a macrophage-like phenotype (Fig. 2A and B). Once again treatment with FSK induced the expression of CD32 at levels comparable to that of CT-treated cells (P = 0.0003, n = 6) (Fig. 2D), suggesting that the modulation of CD32 is dependent on the adenylate cyclase activation capacity of the toxin. Further, a strong upregulation of CD89 FcR was observed in cells differentiated in the presence of CT (P = 0.005, n = 6), but not in cells treated with FSK or CT-B (Fig. 2A to D), suggesting that the expression of CD89 depends on different signaling pathways other than an increase of cAMP.
Taken together, these data suggest that the developmental program of monocytes toward DCs is affected by CT. Treated cells are CD14+ and CD1a− and morphologically different from MoDCs (forward-scatter characteristics [FSC], 250 ± 19 versus 370 ± 3; and side-scatter characteristics [SSC], 200 ± 29 versus 350 ± 58). However, unlike monocyte populations, they express DC-Sign and FcR patterns typical of terminally differentiated myeloid cells.
Monocytes differentiated in the presence of CT upregulate molecules involved in antigen presentation and show an enhanced ability to activate T cell proliferation.
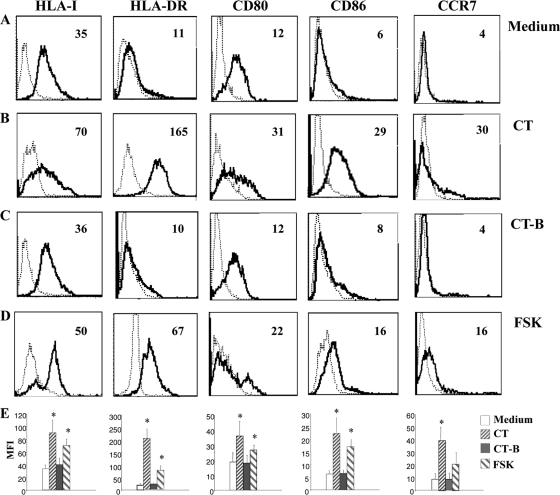
In order to better characterize the phenotype of monocytes differentiated in the presence of CT, we analyzed the expression of different markers that are upregulated during DC differentiation. We found that monocytes differentiated in the presence of CT upregulate MHC-I (P = 0.02, n = 6), MHC-II (P = 0.009, n = 6), CD80 (P = 0.003, n = 6), and CD86 (P = 0.006, n = 6) compared to control DCs (Fig. 3 A and B). Monocytes induced to differentiate into DCs in the presence of CT-B show a phenotype similar to that of untreated cells (Fig. 3C), whereas FSK induces monocytes to differentiate into an activated macrophage-like population similar to that derived from CT-treated monocytes (Fig. 3D). Although the expression levels of the differentiation markers elicited by FSK were lower than those in CT-treated cells, their expression scaled up with higher doses of FSK in a dose-dependent manner (Table 1), suggesting that the effects on DC differentiation by CT are dependent on the increase of intracellular cAMP levels. Furthermore, CCR7, the key chemokine receptor involved in the migration of mature DCs to the lymph nodes is upregulated by CT treatment (P = 0.002, n = 6) and to a lesser extent by FSK (P = 0.03, n = 6) (Fig. 3).
FIG. 3.
Monocytes differentiated in the presence of CT upregulate molecules involved in antigen presentation and activation of T cells. Histogram plots show the phenotype of human CD14 monocytes isolated from healthy donors' PBMC, cultured for 6 days with GM-CSF (50 ng/ml) and IL-4 (35 ng/ml) in the presence of medium alone (A), CT (0.03 μg/ml) (B), CT-B (0.03 μg/ml) (C), and FSK (10 μM) (D). Cells were stained using anti-HLA-IPE, -HLA-DR-FITC, -CD80-PE, -CD86-FITC, and -CCR7-PE MAbs and analyzed by flow cytometry. Histogram plots are representative of six experiments performed. The numbers within the plots indicate the mean fluorescence intensity. (E) The histogram bars show the means (+SD) of the relative mean fluorescence intensity (MFI) of the different markers analyzed from six independent experiments performed. Asterisks indicate that the differences between cells differentiated in the presence of CT or FSK and the control cells are significant (P < 0.05).
TABLE 1.
Expression of differentiation markers on monocytes differentiated in the presence of scalar doses of FSK is dose dependent
| Treatment | Expression of differentiation markersa |
|||||
|---|---|---|---|---|---|---|
| CD14 | CD1a | HLA-I | HLA-DR | CD80 | CD86 | |
| Medium | 6.5 ± 1.3 | 102 ± 29 | 31 ± 5.2 | 14 ± 4.3 | 11.6 ± 3.5 | 7.3 ± 2.3 |
| CT, 0.03 μg/ml | 49.3 ± 14.4 | 9.3 ± 1.5 | 80 ± 10 | 170 ± 38 | 24.6 ± 6.5 | 32.6 ± 13 |
| FSK | ||||||
| 10 μM | 25.3 ± 4.6 | 57 ± 8.5 | 61 ± 8 | 72 ± 3.5 | 19 ± 5 | 22.6 ± 10.6 |
| 20 μM | 39 ± 4.2 | 31.5 ± 21 | 72 ± 7 | 140 ± 35 | 16 ± 2.8 | 32 ± 14 |
| 40 μM | 41 ± 2 | 27.5 ± 17.6 | 75 ± 3.5 | 160 ± 56 | 19 ± 5.6 | 46 ± 20.5 |
| 3 × 10 μMb | 43 ± 4.3 | 25 ± 17 | 71 ± 1 | 167 ± 53 | 18 ± 4.2 | 51 ± 29.7 |
Values are mean fluorescence intensity (MFI) ± standard deviation (three samples per group).
Forskolin was added to the cultures daily at 10 mM for 3 days.
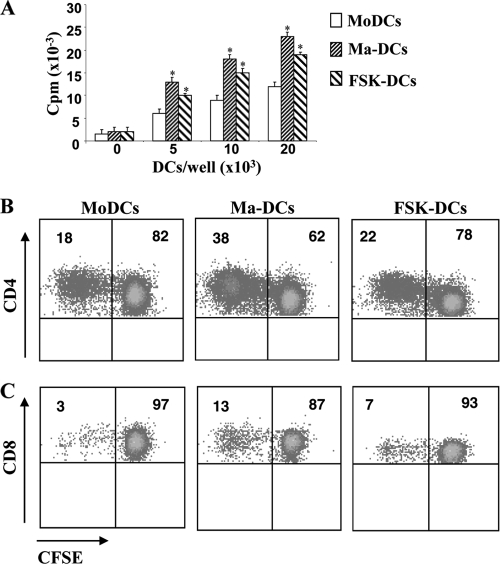
To test whether Ma-DCs could function as APC, we examined the ability to activate allogeneic or antigen-specific T cell proliferation. Monocytes differentiated in the presence of CT or FSK showed higher stimulatory ability than untreated cells did when added to allogeneic PBMC (Fig. 4 A). To evaluate the ability of monocytes differentiated in the presence or in the absence of CT or FSK to stimulate an antigen-specific T cell response, cells were isolated from donors immune to TT antigen. DCs were cocultured with autologous CFSE-labeled PBL (1 × 105 at a ratio of 1:1) in the presence of TT (2 μg/ml). After 5 days, antigen-specific proliferation was evaluated by CFSE dilution on CD4 (Fig. 4B) and on CD8 (Fig. 4C) gated populations. We found that cells differentiated in the presence of CT (Ma-DCs) show a higher capacity to stimulate TT-specific CD4 (38% of proliferating cells versus 17%) and CD8 (13% of proliferating T cells versus 3%) T lymphocytes than control cells (MoDCs) do. Cells differentiated in the presence of FSK show also a higher capacity to stimulate TT-specific CD4 (22% of proliferating cells versus 17%) and CD8 (6% of proliferating T cells versus 3%) T cells than control cells (MoDCs) do. These data are consistent with the increased expression of MHC-I and -II and costimulatory molecules CD80 and CD86 on CT- or FSK-treated cells.
FIG. 4.
Monocytes differentiated in the presence of CT show a higher T cell stimulatory capacity compared to control cells. (A) Monocytes differentiated in the presence of medium alone (MoDCs), CT (0.03 μg/ml) (Ma-DCs), or FSK (10 μM) (FSK-DCs) were cocultured with allogeneic PBMC at different ratios for 5 days. [3H]thymidine (5 μCi/ml) was added during the last 18 to 24 h, and incorporated radioactivity was measured by MicroBeta counting. (B and C) Alternatively, monocytes from donors immune to TT were cocultured with autologous CFSE-labeled PBL (1 × 105 at a ratio of 1:1) in the presence of TT (2 μg/ml). After 5 days, cells were stained with anti-CD4 and -CD8 MAbs, and the antigen-specific proliferation was evaluated by CFSE dilution on CD4+ (B) and on CD8+ (C) gated populations by cytofluorimetric analysis. The data shown are from one representative experiment of three performed. Asterisks indicate that the differences in the proliferative capacity of cells stimulated with Ma-DCs or FSK-DCs from that of control cells are significant (P < 0.05). The numbers within the plots indicate the percentage of positive cells.
Upon LPS stimulation, monocytes differentiated in the presence of CT do not upregulate CD83 and release proinflammatory and regulatory cytokines.
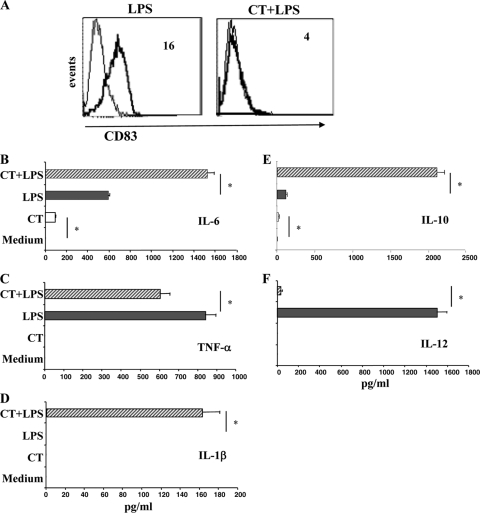
We asked how monocytes differentiated in the presence of CT could react to maturation stimuli. Therefore, we compared the ability of LPS to induce the expression of CD83, a selective DC maturation marker, in cells differentiated in the absence and in the presence of CT. Monocytes differentiated into DCs for 5 days and then stimulated with LPS for 24 h upregulated the expression of CD83 (Fig. 5 A), whereas cells differentiated in the presence of CT failed to upregulate CD83 (Fig. 5A), showing that monocytes differentiated in the presence of CT do not acquire the maturation marker of DCs.
FIG. 5.
Upon LPS stimulation, monocytes differentiated in the presence of CT (Ma-DCs) do not upregulate CD83 and produce proinflammatory and regulatory cytokines. (A) Monocytes were cultured with GM-CSF (50 ng/ml) and IL-4 (35 ng/ml) in the presence of medium alone (left) or CT (0.03 μg/ml) (right). After 6 days, cells were washed, starved for 8 h, and incubated with LPS (250 ng/ml) for a further 48 h. Cellular phenotype was analyzed by flow cytometry after staining using anti-CD83 MAbs. The data shown are from one representative experiment of six performed. The numbers within the plots indicate the mean fluorescence intensity. The accumulation of IL-6 (B), TNF-α (C), IL-1β (D), IL-10 (E), and IL-12 (F) in culture supernatants was evaluated by ELISA. The data shown represent the mean (+SD) of three independent experiments. Asterisks indicate that the differences between cells differentiated in the presence of CT and the control cells with or without LPS treatment are significant (P < 0.05).
The production of proinflammatory cytokines, such as IL-6, TNF-α, and IL-1β, by monocytes differentiated in the presence and in the absence of CT for 5 days was evaluated after a further 48 h of culture with and without LPS. In the absence of LPS, only a small amount of IL-6 was detected in the supernatants of monocytes differentiated in the presence of CT (Fig. 5B). Upon LPS stimulation, the production of IL-6 and TNF-α was induced in untreated cells, as expected (Fig. 5B and C). The presence of CT during the differentiation of monocytes into DCs increased the production of IL-6 and partially prevented the production of TNF-α by LPS-stimulated cells (Fig. 5B and C). The production of IL-1β was induced by LPS treatment only in monocytes differentiated in the presence of CT (Fig. 5D).
The accumulation of IL-10 and IL-12, which are regulatory cytokines involved in directing the immune responses, was also measured. In the absence of maturation stimuli, only a small amount of IL-10 was detected in the culture supernatants of cells differentiated in the presence of CT (Fig. 5E). The production of IL-10 induced by LPS was strongly enhanced in cultures containing monocytes differentiated in the presence of CT (Fig. 5E). On the other hand, the release of IL-12, which was induced by LPS treatment, was strongly inhibited in cells differentiated in the presence of CT (Fig. 5F). These data suggest that in the presence of maturation stimuli Ma-DCs are able to produce significantly larger amounts of proinflammatory (IL-6 and IL-1β) or regulatory (IL-10) cytokines than conventional DCs do, suggesting that they might have a different capacity in directing immune responses.
Ma-DCs induce the polarization of naive CD4+ T lymphocytes toward a Th2 profile compared to control cells.
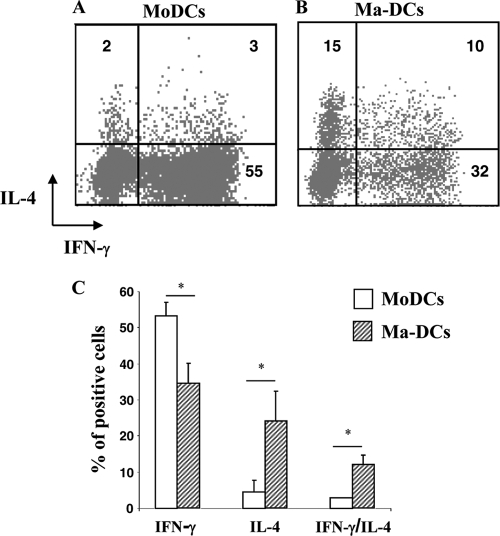
Dendritic cells have the unique capacity to stimulate naïve T lymphocytes and drive them into distinct classes of effector cells. Because of the particular pattern of cytokines produced by Ma-DCs and to evaluate whether these cells have the capacity to support naïve T cell differentiation, we performed a polarization assay. Cells differentiated in the presence or in the absence of CT were cultured with purified CD4+ CD45RA+ T cells at a ratio of 1:5. After 11 days of culture, the production of IL-4 and IFN-γ by CD4+ T lymphocytes was analyzed by intracellular staining. A lower number of naïve CD4+ T cells cultured with Ma-DCs (32% ± 2) than of cells cultured with untreated DCs (55%±3) differentiated into cells producing IFN-γ (P = 0.008, n = 6) (Fig. 6). In contrast, Ma-DCs induced a higher number of naïve CD4+ T lymphocytes to differentiate into IL-4 (15% ± 0.2%)-producing cells than untreated DC did (2% ± 1) (P = 0.02, n = 6) (Fig. 6). The number of cells producing both IFN-γ and IL-4 was higher in cocultures containing Ma-DCs (10% ± 2) than in cultures containing untreated DCs (2% ± 1) (P = 0.02, n = 6) (Fig. 6A). These data indicate that Ma-DCs are able to support T cell differentiation and induce a mixed Th1/Th2 phenotype, with skewing toward the Th2 phenotype.
FIG. 6.
Monocytes differentiated in the presence of CT (Ma-DCs) induce the polarization of naive CD4+ T lymphocytes toward a Th2 profile. Monocytes were cultured with GM-CSF (50 ng/ml) and IL-4 (35 ng/ml) in the presence of medium alone (MoDCs) or CT (0.03 μg/ml) (Ma-DCs). After 6 days, cells were washed, starved for 8 h, and cocultured with purified CD4+ CD45RA+ T cells at a ratio of 1:5. After 11 days of culture, the production of IL-4 and IFN-γ by CD4+ T lymphocytes cocultured with control cells (MoDCs) (A) or Ma-DCs (B) was analyzed by flow cytometry after intracellular staining using anti-CD4-Cy5, -IFN-γ-FITC, and -IL-4-PE MAbs. Dot plots show the percentage of CD4+ cytokine-producing cells. The data shown are from one representative experiment of six performed. The numbers within the plots indicate the percentage of positive cells. (C) The histogram bars (+SD) show the means of six independent experiments performed. Asterisks indicate that the differences between cells differentiated in the presence of CT and the control cells are significant (P < 0.05).
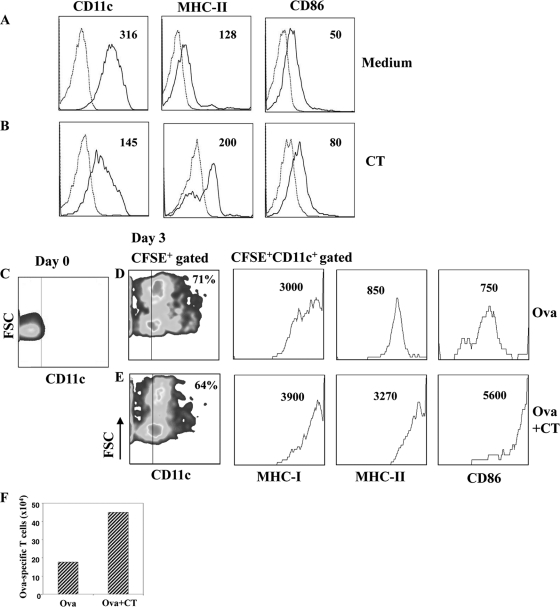
Cholera toxin interferes with the differentiation of murine monocytes into DCs and promotes the induction of activated antigen-presenting cells following systemic immunization.
To strengthen our in vitro observation of human monocytes, we investigated the effect of CT on murine cells in order to evaluate its effect in vivo. First, we evaluated the effect of CT on the differentiation of murine monocytes into DCs in vitro, and then we performed adoptive transfer experiments by using CFSE-labeled monocytes and followed their differentiation in vivo after the use of CT as a systemic adjuvant. Monocytes were purified by CD11b+ magnetic beads from the bone marrow of BALB/c mice and cultured with GM-CSF in the presence and in the absence of CT (0.3 μg/ml) for 6 days. In the absence of CT, monocytes differentiated into immature DCs characterized by the expression of CD11c, MHC-II and CD86 molecules (Fig. 7 A). In contrast, cells derived from CT-treated monocytes showed a lower expression of CD11c, but higher levels of both MHC-II and CD86 molecules than control cells did (Fig. 7B), suggesting that they acquired an activated phenotype. Next, to follow the differentiation of monocytes into DCs in vivo, CD11b+ cells were labeled with CFSE and injected intraperitoneally in mice immunized with OVA (5 μg/dose) or OVA plus CT (1 μg/dose). OVA-specific CD4+ T lymphocytes (2 × 106) isolated from DO.11.10 transgenic mice were also injected intravenously. At day 3, the phenotype of CFSE-positive (CFSE+) cells and the number of OVA-specific CD4+ T cells were analyzed in the mediastinal lymph nodes, which have been described as the draining lymph nodes after intraperitoneal immunization (29). We found that CFSE+ cells migrated to the mediastinal lymph nodes and upregulated CD11c, MHC-I, MHC-II, and CD86 costimulatory molecules. As observed during the differentiation of monocytes in vitro, in mice immunized with CT, the level of CD11c on CFSE+ cells was lower, but the expression of MHC-I, MHC-II, and CD86 on the CFSE+ CD11c+ gated population was higher than that in mice immunized in the absence of CT (Fig. 7D and E). In addition, the absolute number of OVA-specific CD4+ T cells was also higher in mice immunized with CT (Fig. 7F), showing that a good presentation of the antigen occurred. These data indicate that CT interferes with the differentiation of monocytes into DCs also in vivo and gives rise to an activated population that could play a role in the adjuvant activity of CT.
FIG. 7.
Cholera toxin interferes with the differentiation of murine monocytes into DCs in vitro and in vivo. CD11b+ monocytes purified from bone marrow of BALB/c mice were cultured with GM-CSF in the absence (A) and in the presence (B) of CT (0.3 μg/ml). At day 6, cells were stained using anti-CD11c, -MHC-II, and -CD86 MAbs and analyzed by flow cytometry. In parallel, CD11b+ cells were stained with anti-CD11c MAb at day 0 (C), labeled with CFSE and adoptively transferred in recipient mice immunized with OVA (D) or OVA plus CT (E). At day 3, cells were collected from the mediastinal lymph nodes and stained with anti-CD11c, -MHC-I, -MHC-II, and -CD86 MAbs. Dot and histogram plots show CFSE+ gated populations and are representative of one experiment out of 2 performed. The numbers within the plots indicate the percentage of positive cells (dot plots) and the mean fluorescence intensity (histogram plots). Cells from OVA-specific TCR transgenic mice (DO.11.10) were injected in the recipient mice, and their expansion is reported as an absolute number (F) after staining with anti-CD4 and anti-TCR MAbs.
DISCUSSION
Understanding the mechanisms of action of CT, one of the most effective mucosal adjuvants, could be important for the design of new effective adjuvants. The strong adjuvant activity of CT may be due to the interaction of the toxin with different cell types, including epithelial cells, B and T lymphocytes, monocytes/macrophages, and DCs, present in the mucosa (1, 16, 22, 53). Recently, it has been established that circulating monocytes differentiate into mucosal, but not splenic or lymphoid, DCs (30, 33, 57, 60) and that monocytes under the control of GM-CSF give rise to the proinflammatory CD103− CX3CR1+ mucosal DCs (46, 58). In this study, by using human DCs generated from monocytes cultured with GM-CSF and IL-4, we found that CT interferes with the differentiation of monocytes into DCs, giving rise to a population with a macrophage-like phenotype (Ma-DCs). These cells display an activated phenotype, induce a strong allogeneic and antigen-specific immune response, and have a higher capacity to induce the polarization of naive CD4+ T lymphocytes toward Th2 cells than control cells do. Interestingly, we found that CT interferes with the differentiation of monocytes into DCs in vivo and promotes the induction of activated antigen-presenting cells following systemic immunization.
Monocytes are circulating precursors of both macrophages and DCs, which can be recruited into tissues and differentiate depending on the microenvironment of the inflammatory sites (43, 47). The differentiation process is complex and regulated by cytokines (9, 10, 49, 50) and also by the interaction with pathogens, such as viruses or bacteria (35, 36, 39). Although many factors affecting DC and macrophage differentiation have been identified, the intracellular signaling pathways that regulate these processes are poorly understood. Here, by using CT, which induces permanent adenylate cyclase activation, resulting in an increase of intracellular cyclic AMP (cAMP), we found that the cAMP pathway affects differentiation of monocytes into DCs by inhibiting CD14 down-modulation and CD1a upregulation. These data are consistent with previous findings showing that the intracellular increase of cAMP impairs the differentiation of monocytes into DCs (21, 28, 40). We have previously shown that CT induces the release of cAMP in the extracellular compartment (59), and we have also observed that extracellular cAMP in turn affects monocyte differentiation (unpublished data). Consistent with the effect of the activation of the cAMP pathway during the differentiation of monocytes into DCs, CT upregulated DC-Sign, although to a lesser extent than the upregulation in control cells. Interestingly, Fc-γRI/CD64 was down-modulated, and the expression of CD32 was enhanced by CT compared to the expression in untreated cells, leading to a cell type resembling phenotypically a macrophage. Similarly, during DC and macrophage development, cAMP analogues have been found to promote a switch from CD64 to CD32 expression (8). On the other hand, a strong upregulation of the IgA receptor CD89 (Fc-αRI) was observed in cells differentiated in the presence of CT and not in cells treated with FSK or CT-B, suggesting that CD89 upregulation depends on additional signaling pathways other than an increase of cAMP or on a more sustained adenylate cyclase activation. Whether CD89 upregulation by CT plays an important role in the adjuvant mechanisms of CT remains to be specifically addressed.
The precise role of the enzyme activity of the A subunit in the adjuvant action of enterotoxins is uncertain. It has been reported that FSK, a direct activator of adenylate cyclase, had no effect on the mucosal immune response (61) and that CT or Escherichia coli heat-labile enterotoxin (LT-I) holotoxin mutants that lack ADP ribosylation retain adjuvant action in vivo (13, 42), indicating that the enzymatic activity of the enterotoxins is dispensable for adjuvant activity. However, the responses generated by the mutants of CT and LT are much weaker than those induced by the wild-type toxins, and mutants that retain partial enzymatic activity have an intermediate capacity to enhance the responses (13, 14, 42), suggesting that the capacity of increasing cAMP plays a role in the adjuvant effects. Furthermore, constructs based on the enzymatic activity of CT are strong adjuvants (3, 34). We found that the increase of intracellular cAMP levels is important for the skewing of monocytes toward professional antigen-presenting myeloid cells, and this is consistent with the cAMP-dependent adjuvant effects noted in vivo. On the other hand, it is unclear how the enzymatically inactive A subunit would support adjuvant activity, but LT and CT mutants LTK63 and CTK63 maintained the ability to bind the ADP-ribosylation factor (ARF), which is important in vesicular membrane trafficking (42). This ARF-binding activity is independent of the catalytic activity of the A subunit, although it has not yet been linked to any adjuvant mechanism. Additional studies are needed to verify the contribution of the cAMP pathway versus other intracellular pathways in CT-mediated adjuvanticity. Furthermore, when using the CT-B subunit, which lacks the enzymatic activity, we did not observe the upregulation of the activation markers. It has been reported that commercially available preparations of CT-B before the 1990s were active as adjuvants, but later it was realized that they were contaminated with traces of intact CT (22). Therefore, some attention should be given to the different sources of CT-B used. However, a more recent study by Schnitzler et al. demonstrated that in the absence of the A subunit, CT-B induces intracellular signaling associated with in vitro activation of murine B cells and peritoneal macrophages (51). In our experimental setting, when using CT-B during the differentiation of monocytes into DCs, we have not found the upregulation of activation markers, and this could be explained by having analyzed different cell types in the human rather than in the murine system. In addition, with human B cells we also did not observe the upregulation of activation markers by CT-B (38); therefore, the effects of CT-B on human versus murine cells should be further investigated.
Cells differentiated from CT-treated monocytes, despite having lower levels of specific DC markers, do acquire potent APC capability and produce proinflammatory and regulatory cytokines. The release of proinflammatory cytokines may promote the recruitment and activation of additional leukocytes, playing a central role in amplifying the immune responses. In addition, upon maturation stimuli, cells differentiated from CT-treated monocytes produced high levels of IL-10 and were not able to produce IL-12. These findings are consistent with others showing that CT through the cAMP pathway inhibits TNF-α and IL-12 production in different APCs both in vitro and in vivo (6, 23, 31, 38). The secretion of IL-10 and the inhibition of IL-12 production can account for the induction of the Th2-biased immune response induced by CT (6, 16, 32). Indeed, when cells derived from CT-treated monocytes were cultured with naïve CD4+ CD45RA+ T lymphocytes, a higher percentage of CD4+ T lymphocytes producing IL-4 and a lower percentage of IFN-γ-producing cells were induced than when untreated cells were used.
Interestingly, we found that CT interferes with the differentiation of monocytes into DCs in vivo and promotes the induction of activated antigen-presenting cells following systemic immunization. We hypothesize that in the initial step of CT interaction with the tissues, different cell types, including residents of freshly recruited monocytes, may come into contact with the toxin, and the monocytes, during their differentiation into DCs, could be induced to differentiate into a distinct population that has an activated phenotype and strong antigen-presenting capacity and promotes the polarization of naive CD4+ T lymphocytes toward Th2 cells. However, additional studies need to be performed to better clarify the role of this population in the adjuvant activity of CT following either mucosal or systemic immunization.
Acknowledgments
We thank R. Lindstedt for helpful discussions.
This work was supported by a grant from the collaboration program ISS/NIH, no. 530/0F24, and from Muvapred Exploration project no. L76/7.
We have no conflicting financial interests.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 13 December 2010.
REFERENCES
- 1.Anosova, N. G., et al. 2008. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer's patches. Mucosal Immunol. 1:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arce, S., H. F. Nawar, G. Muehlinghaus, M. W. Russell, and T. D. Connell. 2007. In vitro induction of immunoglobulin A (IgA)- and IgM-secreting plasma blasts by cholera toxin depends on T-cell help and is mediated by CD154 up-regulation and inhibition of gamma interferon synthesis. Infect. Immun. 75:1413-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley, K. C., et al. 2003. Immunogenicity of DNA vaccines that direct the coincident expression of the 120 kDa glycoprotein of human immunodeficiency virus and the catalytic domain of cholera toxin. Vaccine 21:3335-3341. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Bogunovic, M., et al. 2009. Origin of the lamina propria dendritic cell network. Immunity 31:513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, M. C., J. He, C. Y. Wu, and B. L. Kelsall. 1999. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J. Exp. Med. 189:541-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bromander, A., J. Holmgren, and N. Lycke. 1991. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J. Immunol. 146:2908-2914. [PubMed] [Google Scholar]
- 8.Cameron, A. J., M. M. Harnett, and J. M. Allen. 2001. Differential recruitment of accessory molecules by FcgammaRI during monocyte differentiation. Eur. J. Immunol. 31:2718-2725. [DOI] [PubMed] [Google Scholar]
- 9.Chomarat, P., J. Banchereau, J. Davoust, and A. K. Palucka. 2000. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 1:510-514. [DOI] [PubMed] [Google Scholar]
- 10.Chomarat, P., C. Dantin, L. Bennett, J. Banchereau, and A. K. Palucka. 2003. TNF skews monocyte differentiation from macrophages to dendritic cells. J. Immunol. 171:2262-2269. [DOI] [PubMed] [Google Scholar]
- 11.Cong, Y., C. T. Weaver, and C. O. Elson. 1997. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J. Immunol. 159:5301-5308. [PubMed] [Google Scholar]
- 12.De Magistris, M. T. 2006. Mucosal delivery of vaccine antigens and its advantages in pediatrics. Adv. Drug Deliv. Rev. 58:52-57. [DOI] [PubMed] [Google Scholar]
- 13.De Magistris, M. T., et al. 1998. Adjuvant effect of non-toxic mutants of E. coli heat-labile enterotoxin following intranasal, oral and intravaginal immunization. Dev. Biol. Stand. 92:123-126. [PubMed] [Google Scholar]
- 14.Dickinson, B. L., and J. D. Clements. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagliardi, M. C., et al. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 16.Gagliardi, M. C., et al. 2002. Effects of the adjuvant cholera toxin on dendritic cells: stimulatory and inhibitory signals that result in the amplification of immune responses. Int. J. Med. Microbiol. 291:571-575. [DOI] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B., J. den Dunnen, and S. I. Gringhuis. 2009. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 4:879-890. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T. B., et al. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 19.Geissmann, F., et al. 2008. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol. Cell Biol. 86:398-408. [DOI] [PubMed] [Google Scholar]
- 20.Ginhoux, F., et al. 2006. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7:265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giordano, D., D. M. Magaletti, E. A. Clark, and J. A. Beavo. 2003. Cyclic nucleotides promote monocyte differentiation toward a DC-SIGN+ (CD209) intermediate cell and impair differentiation into dendritic cells. J. Immunol. 171:6421-6430. [DOI] [PubMed] [Google Scholar]
- 22.Hajishengallis, G., S. Arce, C. M. Gockel, T. D. Connell, and M. W. Russell. 2005. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J. Dent. Res. 84:1104-1116. [DOI] [PubMed] [Google Scholar]
- 23.Hajishengallis, G., H. Nawar, R. I. Tapping, M. W. Russell, and T. D. Connell. 2004. The type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect. Immun. 72:6351-6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harandi, A. M., J. Sanchez, K. Eriksson, and J. Holmgren. 2003. Recent developments in mucosal immunomodulatory adjuvants. Curr. Opin. Investig. Drugs 4:156-161. [PubMed] [Google Scholar]
- 25.Holmgren, J., et al. 2005. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol. Lett. 97:181-188. [DOI] [PubMed] [Google Scholar]
- 26.Itano, A. A., and M. K. Jenkins. 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4:733-739. [DOI] [PubMed] [Google Scholar]
- 27.Jakubzick, C., et al. 2008. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J. Immunol. 180:3019-3027. [DOI] [PubMed] [Google Scholar]
- 28.Kaliński, P., J. H. Schuitemaker, C. M. Hilkens, and M. L. Kapsenberg. 1998. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 161:2804-2809. [PubMed] [Google Scholar]
- 29.Kool, M., et al. 2008. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 205:869-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landsman, L., C. Varol, and S. Jung. 2007. Distinct differentiation potential of blood monocyte subsets in the lung. J. Immunol. 178:2000-2007. [DOI] [PubMed] [Google Scholar]
- 31.la Sala, A., et al. 2009. Cholera toxin inhibits IL-12 production and CD8alpha+ dendritic cell differentiation by cAMP-mediated inhibition of IRF8 function. J. Exp. Med. 206:1227-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavelle, E. C., et al. 2004. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J. Leukoc. Biol. 75:756-763. [DOI] [PubMed] [Google Scholar]
- 33.Liu, K., et al. 2009. In vivo analysis of dendritic cell development and homeostasis. Science 324:392-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lycke, N., and M. Bemark. 2010. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 3:556-566. [DOI] [PubMed] [Google Scholar]
- 35.Martino, A., et al. 2004. Dendritic cells derived from BCG-infected precursors induce Th2-like immune response. J. Leukoc. Biol. 76:827-834. [DOI] [PubMed] [Google Scholar]
- 36.Martino, A., et al. 2005. Non-pathogenic Mycobacterium smegmatis induces the differentiation of human monocytes directly into fully mature dendritic cells. J. Clin. Immunol. 25:365-375. [DOI] [PubMed] [Google Scholar]
- 37.Matousek, M. P., J. G. Nedrud, and C. V. Harding. 1996. Distinct effects of recombinant cholera toxin B subunit and holotoxin on different stages of class II MHC antigen processing and presentation by macrophages. J. Immunol. 156:4137-4145. [PubMed] [Google Scholar]
- 38.Negri, D. R., et al. 2009. Cholera toxin and Escherichia coli heat-labile enterotoxin, but not their nontoxic counterparts, improve the antigen-presenting cell function of human B lymphocytes. Infect. Immun. 77:1924-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niiya, H., et al. 2006. Human herpesvirus 6 impairs differentiation of monocytes to dendritic cells. Exp. Hematol. 34:642-653. [DOI] [PubMed] [Google Scholar]
- 40.Novitskiy, S. V., et al. 2008. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 112:1822-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otten, M. A., and M. van Egmond. 2004. The Fc receptor for IgA (FcalphaRI, CD89). Immunol. Lett. 92:23-31. [DOI] [PubMed] [Google Scholar]
- 42.Pizza, M., et al. 2001. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19:2534-2541. [DOI] [PubMed] [Google Scholar]
- 43.Randolph, G. J., S. Beaulieu, S. Lebecque, R. M. Steinman, and W. A. Muller. 1998. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282:480-483. [DOI] [PubMed] [Google Scholar]
- 44.Randolph, G. J., K. Inaba, D. F. Robbiani, R. M. Steinman, and W. A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11:753-761. [DOI] [PubMed] [Google Scholar]
- 45.Randolph, G. J., J. Ochando, and S. Partida-Sanchez. 2008. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 26:293-316. [DOI] [PubMed] [Google Scholar]
- 46.Rescigno, M. 2009. Before they were gut dendritic cells. Immunity 31:454-456. [DOI] [PubMed] [Google Scholar]
- 47.Sacchi, A., et al. 2007. Differentiation of monocytes into CD1a− dendritic cells correlates with disease progression in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 46:519-528. [DOI] [PubMed] [Google Scholar]
- 48.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanarico, N., et al. 2006. Human monocyte-derived dendritic cells differentiated in the presence of IL-2 produce proinflammatory cytokines and prime Th1 immune response. J. Leukoc. Biol. 80:555-562. [DOI] [PubMed] [Google Scholar]
- 50.Santini, S. M., et al. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnitzler, A. C., J. M. Burke, and L. M. Wetzler. 2007. Induction of cell signaling events by the cholera toxin B subunit in antigen-presenting cells. Infect. Immun. 75:3150-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shortman, K., and S. H. Naik. 2007. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7:19-30. [DOI] [PubMed] [Google Scholar]
- 53.Shreedhar, V. K., B. L. Kelsall, and M. R. Neutra. 2003. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T- and B-cell areas of Peyer's patches. Infect. Immun. 71:504-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons, C. P., et al. 2001. Immunomodulation using bacterial enterotoxins. Scand. J. Immunol. 53:218-226. [DOI] [PubMed] [Google Scholar]
- 55.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinman, R. M. 2000. DC-SIGN: a guide to some mysteries of dendritic cells. Cell 100:491-494. [DOI] [PubMed] [Google Scholar]
- 57.Varol, C., et al. 2007. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 204:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varol, C., et al. 2009. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31:502-512. [DOI] [PubMed] [Google Scholar]
- 59.Vendetti, S., M. Patrizio, A. Riccomi, and M. T. De Magistris. 2006. Human CD4+ T lymphocytes with increased intracellular cAMP levels exert regulatory functions by releasing extracellular cAMP. J. Leukoc. Biol. 80:880-888. [DOI] [PubMed] [Google Scholar]
- 60.Willart, M. A., et al. 2009. The lung vascular filter as a site of immune induction for T cell responses to large embolic antigen. J. Exp. Med. 206:2823-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson, A. D., A. Robinson, L. Irons, and C. R. Stokes. 1993. Adjuvant action of cholera toxin and pertussis toxin in the induction of IgA antibody response to orally administered antigen. Vaccine 11:113-118. [DOI] [PubMed] [Google Scholar]