Abstract
Recent studies have shown that histone proteins can act as antimicrobial peptides in host defense against extracellular bacteria, fungi, and Leishmania promastigotes. In this study, we used human recombinant histone proteins to further study their leishmaniacidal effects and the underlying mechanisms. We found that the histones H2A and H2B (but not H10) could directly and efficiently kill promastigotes of Leishmania amazonensis, L. major, L. braziliensis, and L. mexicana in a treatment dose-dependent manner. Scanning electron microscopy revealed surface disruption of histone-treated promastigotes. More importantly, the preexposure of promastigotes to histone proteins markedly decreased the infectivity of promastigotes to murine macrophages (Mφs) in vitro. However, axenic and lesion-derived amastigotes of L. amazonensis and L. mexicana were relatively resistant to histone treatment, which correlated with the low levels of intracellular H2A in treated amastigotes. To understand the mechanisms underlying these differential responses, we investigated the role of promastigote surface molecules in histone-mediated killing. Compared with the corresponding controls, transgenic L. amazonensis promastigotes expressing lower levels of surface gp63 proteins were more susceptible to histone H2A, while L. major and L. mexicana promastigotes with targeted deletion of the lipophosphoglycan 2 (lpg2) gene (but not the lpg1 gene) were more resistant to histone H2A. We discuss the influence of promastigote major surface molecules in the leishmaniacidal effect of histone proteins. This study provides new information on host innate immunity to different developmental stages of Leishmania parasites.
Leishmaniasis is a sand fly-transmitted tropical disease that threatens 350 million people in the world. It is estimated that 12 million people are infected, and the mortality rate is approximately 60,000 per year (33). Based on clinical symptoms, the disease can be classified as visceral, cutaneous, and/or mucocutaneous leishmaniasis. Visceral leishmaniasis is mainly caused by Leishmania donovani and L. infantum, while the cutaneous and mucosal leishmaniasis forms are caused by several Leishmania species, including L. major in Old World countries and L. amazonensis and L. braziliensis in New World countries (11, 23). Leishmania parasites are present as extracellular promastigotes in the gut of sand flies and enter into mammals when flies take blood meals. Promastigotes can infect different cell types, including neutrophils, monocytes, macrophages (Mφs), and dendritic cells. At the initial infection stage, some promastigotes are killed by the host's innate defense mechanisms, such as complement, while others successfully establish infection in target cells and transform into amastigotes, which replicate within the phagolysosomes of the cell. The number of leishmaniasis cases has been increasing recently, presumably due to the lack of adequate methods for vector control and efficient vaccines and the increased resistance to antileishmanial drugs (7). In addition, the number of patients with coinfection of Leishmania and HIV is increasing in subtropical and tropical regions (30). Therefore, there is a great need for the development of new strategies to control leishmaniasis and to better understand the host responses to different species and stages of Leishmania.
Leishmania's virulence and its initial infection in mammalian hosts are closely linked to promastigote surface molecules, one of which is lipophosphoglycan (LPG), a glycophosphatidylinositol (GPI)-anchored glycolipid expressed mostly on the surface of promastigotes but not on amastigotes. LPG is important for promastigotes to interact with the sand fly midgut and to establish infection in mammals (46, 49). Another important molecule involved in promastigote uptake by host Mφs and which protects promastigotes from phagolysosome degradation is gp63, a zinc protease expressed mainly on the surface of promastigotes (45). However, variations in LPG and gp63 do exist among different Leishmania species and contribute to the diverse outcome of host-parasite interactions (34).
Recently, Peters et al. have elegantly described the early events of infection triggered by the sand fly-transmitted L. major, confirming that neutrophils were the most abundant cells that migrated into the wound sites (38). Within the site of infection initiated by microbes, neutrophils are known to phagocytize the microbes and kill them by different mechanisms (8, 32), thereby contributing to pathogen clearance in the extracellular space (1). In the case of Leishmania infection, neutrophils appear to play complex and paradoxical roles: their detrimental roles are evidenced by findings that neutrophils promote promastigote infection via spreading parasites into Mφs (1), whereas reports of their protective roles stem from observations that depletion of neutrophils results in increased parasite burdens after infection with L. major or L. donovani in susceptible mice (6, 26). More recently, it was shown that neutrophils can secrete an extracellular fibril network called a neutrophil extracellular trap (NET), which can kill extracellular bacteria, fungi, and L. amazonensis promastigotes (3, 12, 52). This NET-related parasite killing is partially attributed to the histone proteins because of the reduced promastigote killing following blockage with an anti-histone H2A antibody and the enhanced promastigote killing following treatment with histone H2A purified from calf thymus (12). Histones are traditionally known as major components of the nucleosome, playing an important role in gene transcription in eukaryotic cells. They are classified as core histones (H2A, H2B, H3, and H4) and linker histones (H1 and H5) and share similar structural features and localization; however, their molecular masses vary from 14 kDa (H2A and H2B) to 21 kDa (H1) (20). Some histone proteins have also been shown to serve as antimicrobial proteins to certain bacteria and fungi (19); however, it remains unclear whether histone proteins have general or selective effects on different Leishmania species and developmental stages; if so, the mechanism(s) underlying these effects should be examined.
In this study, we used recombinant human histone H2A and H2B to examine their effects on parasite growth in vitro and on parasite infectivity to Mφs. We found that human histone H2A and H2B (but not H10) efficiently and directly killed several species of Leishmania promastigotes and that histone-treated promastigotes had markedly decreased parasite infectivity to murine Mφs. However, L. amazonensis and L. mexicana amastigotes, the disease-forming stage of the parasite, were highly resistant to histone treatment. The mechanisms underlying these differential responses to histone proteins were examined by using promastigotes with altered expression levels for gp63 and LPG. Our findings open a new area of investigation and provide insight into differential responses of Leishmania and developmental stages to host innate responses.
MATERIALS AND METHODS
Mice.
Female BALB/c mice (Harlan Sprague-Dawley, Indianapolis, IN) were used in the study. Mice were maintained under specific-pathogen-free conditions and used at 6 to 8 weeks of age. All of the protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston, TX).
Parasite species and cultivation.
The infectivity of L. amazonensis (RAT/BA/74/LV78), L. braziliensis (MHOM/BR/79/LTB111), and L. major (MRHO/SU/59/P/LV39) was maintained by regular passage through BALB/c mice. Promastigotes were cultured at 23°C in Schneider's Drosophila medium (Invitrogen, Grand Island, NY), pH 7.0, supplemented with 20% fetal bovine serum (FBS; HyClone, Logan, UT), 2 mM l-glutamine, and 50 μg/ml gentamicin. L. mexicana promastigotes, which were originally isolated from a patient (53), were cultured at 26°C in M199 medium. Unless specified, the stationary-stage promastigotes were used in all of the experiments. In some cases, metacyclic promastigotes were purified and used. Axenic amastigotes of L. amazonensis and L. mexicana were cultured at 33°C in complete Grace's insect cell culture medium (Invitrogen, Grand Island, NY), supplemented with 20% FBS, pH 5.2.
Wild-type (WT) L. major LV39 clone 5 (Rho/SU/59/P) and the homozygous gene-targeted deletion and add-back promastigotes (lpg1−, lpg1−/+LPG1, lpg2−, and lpg2−/+LPG2) generated from the parental Lv39c5 clone (48, 50) were grown in M199 medium (Invitrogen) with 10% FBS, 40 mM HEPES, 0.1 mM adenine, 1 μg/ml biotin, 5 μg/ml hemin, 2 μg/ml biopterin, and 100 U/ml penicillin-streptomycin at 26°C. For the lpg1− mutants, the culture medium contained 15 μg/ml hygromycin B (Cellgro, Herndon, VA) and 10 μg/ml puromycin (Invivogen, San Diego, CA). For lpg1−/+LPG1 parasites, the culture medium contained 15 μg/ml hygromycin B, 10 μg/ml puromycin, and 50 μg/ml Geneticin (G418; Mediatech, Inc., Manassas, VA). For lpg2− mutants, the culture medium contained 15 μg/ml hygromycin B. For lpg2−/+LPG2 parasites, the culture medium contained hygromycin B and G418 (both at 15 μg/ml). The expression levels of LPG in these parasites were confirmed via Western blotting assays (data not shown). The lpg2− L. mexicana parasites were similarly cultured.
L. amazonensis (MPRO/BR/72/M1845, LV78) promastigotes were transfected with the P6.5 vector alone (control) or with the gp63 gene either in the correct (P6.5/1.9, sense mutant) or in the reverse (P6.5/1.9R, antisense mutant) orientation (5). Transfected promastigotes were cultured in Schneider's Drosophila medium, pH 7.0, supplemented with 20% FBS, 2 mM l-glutamine, and 50 μg/ml gentamicin, and under the selective pressure of tunicamycin (10 μg/ml; Sigma), the gp63 levels were upregulated in P6.5/1.9 parasites but downregulated in P6.5/1.9R parasites (data not shown).
Parasite proliferation after histone treatment.
Recombinant human histone H2A (GenBank accession number AY131974), H2B (GenBank accession number AY131979), and H10 (GenBank accession number X03473) were purchased from New England BioLabs (Ipswich, MA). Bulk, stationary-phase promastigotes of L. amazonensis (2.5 × 106 in 50 μl of sterile PBS) were treated with histone H2A or H2B (20 to 100 μg/ml) for 3, 30, 60, and 90 min. Complete Schneider's medium was added to stop the reaction. Parasite survival was estimated by cultivation of parasites in 96-well plates in the complete medium at 23°C. After 24 h of incubation, [3H]thymidine (GE Healthcare, United Kingdom) was added for an additional 18 h of incubation. Thymidine incorporation was measured by a TopCount NXT microplate scintillation and luminescence counter (Packard Bioscience Company, Shelton, CT).
Direct killing of parasites.
Parasites (2.5 × 106 in 50 μl PBS) were treated with histone H2A or H2B (20, 100, and 200 μg/ml) for 30 min at 23°C (for promastigotes) or at 33°C (for amastigotes). A Live/Dead viability/cytotoxicity kit (Invitrogen, Eugene, OR) was used to measure the percentages of live versus dead parasites by staining with 10 μM calcein AM and 5 μM ethidium homodimer-1 (EthD-1). Stained parasites were either directly counted under an Olympus fluorescence microscope (Leeds Instruments, United Kingdom) equipped with filters for fluorescein isothiocyanate (FITC) (live parasites) or Texas Red (dead parasites) or subjected to analysis by using a C6 flow cytometer (Accuri Cytometers, Inc., Ann Arbor, MI). The flow cytometric data were analyzed with CFlow software (Accuri Cytometers, Inc.). The heat-inactivated parasites (56°C for 30 min) were all stained red, validating the accurate detection of death parasites using this assay.
Mφ cultivation and infection.
Bone marrow-derived macrophages (BM-Mφs) were generated by the methods in our previous report (40). Briefly, marrow cells (2 × 105/ml) were cultured in Iscove's modified Dulbecco's medium (Invitrogen) supplemented with 10% FBS, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 50 μg/ml gentamicin, 100 U/ml penicillin, and 20 ng/ml recombinant murine macrophage colony-stimulating factor (M-CSF) (PeproTech, Rocky Hill, NJ) for 10 days. BM-Mφs were washed twice and cultured on 24-well tissue culture plates (4 × 105 cells/well). Promastigotes, axenic amastigotes, and lesion-derived amastigotes were treated with different concentrations (20 to 100 μg/ml) of histone H2A or H2B for 30 min. Cells were infected with promastigotes or axenic amastigotes at 5:1 parasite/cell ratios and with lesion-derived amastigotes at 1:2 parasite/cell ratios. Infected Mφs were incubated at 33°C for 72 h and washed. To quantify intercellular parasites, infected Mφs were treated with 200 μl 0.01% SDS for 10 to 15 min at 37°C, and then 800 μl culture medium was added to stop cell lysis. The number of amastigotes per well was determined with a hemocytometer.
To quantify parasite loads, we also extracted genomic DNA from Mφs by using a Qiagen minikit (Qiagen, Germantown, MD), and 50 ng of DNA was used for real-time PCR analysis in the Molecular Genomics Core Laboratory at the University of Texas Medical Branch (UTMB), as described in our previous report (47). Each sample was run in duplicate, and the results were normalized by the amount of total DNA extracted. The numbers of parasites per sample were then calculated based on a standard curve, which was generated for each PCR by using a DNA mixture that contained 50 ng of uninfected Mφ DNA and increasing amounts of L. amazonensis amastigote DNA. In general, 1 pg of amastigote DNA was equivalent to 12.4 parasites, while 104 pg of amastigote DNA was equivalent to 1.2 × 105 parasites.
Scanning electron microscopy (SEM).
Histone-treated parasites were adhered to coverslips precoated with 0.01% poly-l-lysine (Electron Microscopy Sciences, Hatfield, PA) via centrifugation at 2,000 rpm for 5 min. Samples were fixed at 4°C overnight in a mixture of 2.5% formaldehyde and 0.1% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.2), containing 0.03% trinitrophenol and 0.03% CaCl2. Samples were washed with 0.1 M cacodylate buffer, postfixed in 1% OsO4 in 0.1 M cacodylate buffer (pH 7.2), dehydrated in ethanol, and processed through hexamethyldisilazane. After being air dried, coverslips were mounted onto the specimen stubs and sputter coated with iridium in an Emitech K575x sputter coater (Ashford, Kent, United Kingdom) at 20 mA for 20 s. Samples were examined in a Hitachi S4700 field emission scanning electron microscope (Hitachi High Technologies America, Electron Microscope Division, Pleasanton, CA) at 2 kV and instrumental magnifications from ×2,000 to ×50,000.
Histone binding on parasites.
Promastigotes and axenic amastigotes of L. amazonensis were treated with the previously indicated concentrations of H2A for 30 min. Treated parasites were washed and stained on ice with a mouse anti-human histone H2A monoclonal antibody (MAb) (Cell Signaling, Danvers, MA) and then with FITC-conjugated rat anti-mouse IgG1 (eBioscience, San Diego, CA). For intracellular staining, histone-treated parasites were fixed/permeabilized with a Cytofix/Cytoperm kit (BD Biosciences, Franklin Lakes, NJ) prior to antibody staining. Parasites were read on a C6 flow cytometer and analyzed with CFlow software. In each staining experiment, some samples were side-by-side evaluated for the live/dead parasites, as described above. The unstained parasite and parasites stained with the secondary antibody alone gave no or negligible levels of binding (data not shown). Similar studies were conducted with wild-type L. major promastigotes and lpg1− and lpg2− mutants.
Statistical analysis.
Differences between two groups were determined by using Student's t test. A one-way analysis of variance (ANOVA) was used for multiple group comparisons. Graphs were prepared by using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). The difference between two groups was considered significant when the P value was ≤0.05.
RESULTS
Histone H2A and H2B can suppress Leishmania promastigote proliferation.
To test the effect of histone proteins on parasites, we treated stationary-phase L. amazonensis promastigotes with 20 or 100 μg/ml of histone H2A or H2B for 3 to 90 min and assessed the ability of surviving parasites to replicate following addition of fresh medium. As shown in Fig. 1, a high concentration of H2A (100 μg/ml) resulted in a nearly 50% reduction in parasite replication, as judged by [3H]thymidine incorporation. Likewise, parasite replication of H2B-treated promastigotes decreased by 30 to 40% compared to their controls. At a low concentration (20 μg/ml), H2A and H2B had no major effects on parasite replication, except for the H2B treatment for 90 min, which showed a small (27%), but significant, reduction in parasite replication. Therefore, the suppression of promastigote proliferation was dependent on the histone concentrations, but such effects appeared to be independent of treatment time.
FIG. 1.
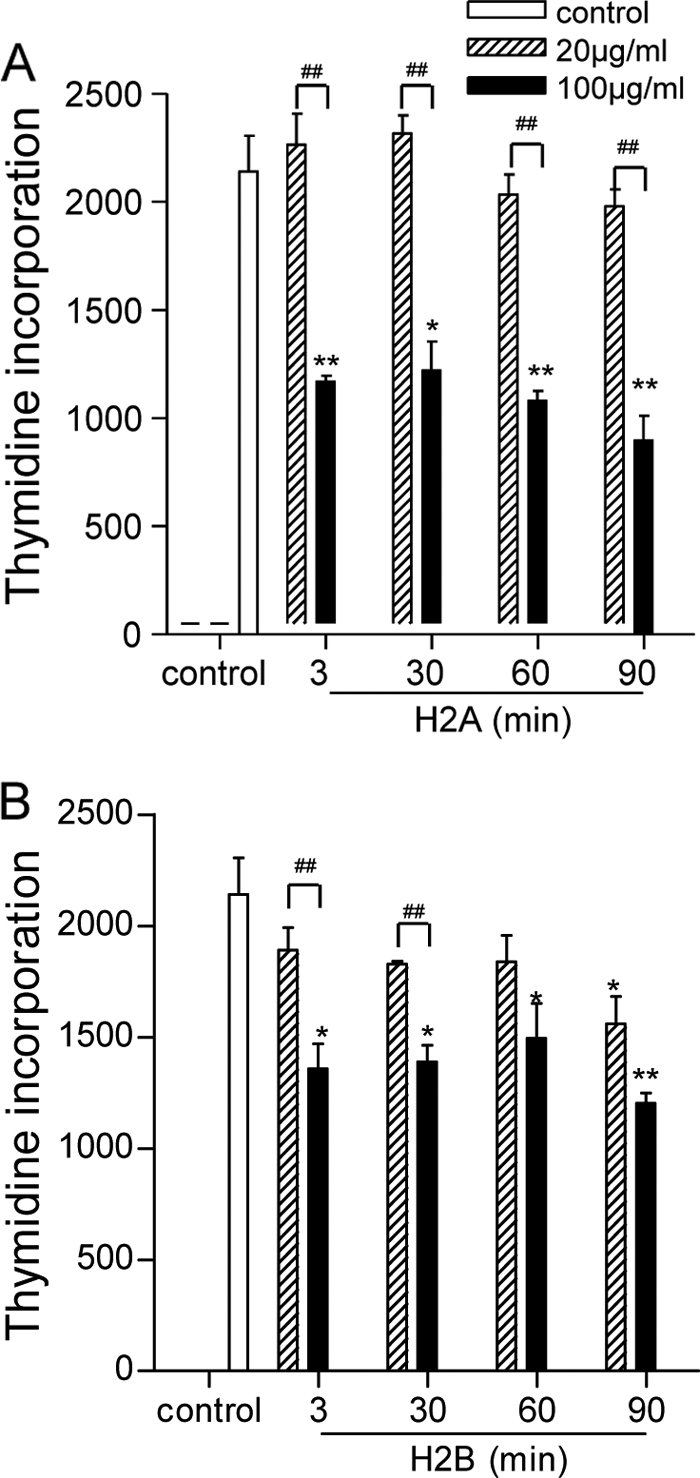
Suppressive effects of histone H2A and H2B on parasite proliferation. L. amazonensis promastigotes (2.5 × 106 in 50 μl PBS) were treated with 20 or 100 μg/ml of histone H2A (A) or H2B (B) at 23°C for indicated time periods, after which complete Schneider's medium was added. After 24 h of incubation, [3H]thymidine was added for an additional 18 h of incubation. Incorporated [3H]thymidine was measured and presented as counts per minute (cpm). Data are pooled from two independent repeats and shown as the means ± the standard errors. * (P < 0.05) and ** (P < 0.01) indicate statistically significant differences between the control and treated groups. # (P < 0.05) and ## (P < 0.01) indicate the statistical differences between the marked groups.
Histone H2A and H2B can directly kill Leishmania promastigotes but not amastigotes.
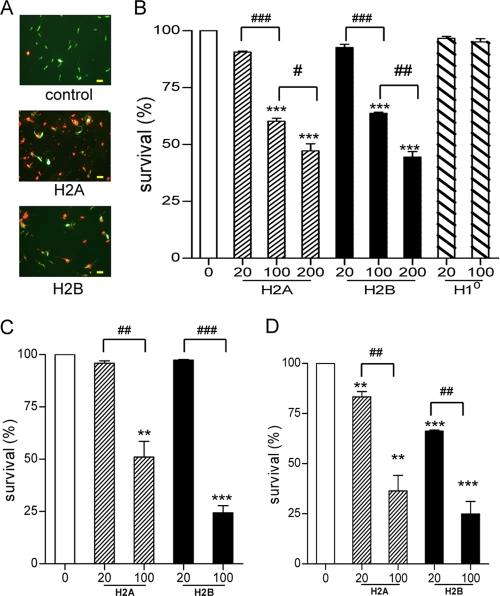
The reduced thymidine incorporation seen above could be due to parasite death or replication arrest. To examine whether histones can kill promastigotes directly, we treated promastigotes of L. amazonensis, L. braziliensis, and L. major with histones (20 to 200 μg/ml), and then used fluorescent reagents to stain live (green) versus dead (red) parasites (Fig. 2 A). It was evident that histone-treated promastigotes had significantly lower survival rates than did those of the untreated groups. At the concentration of 100 μg/ml, for example, the survival rates of histone H2A-treated promastigotes decreased by 40% in L. amazonensis (Fig. 2B), 49% in L. major (Fig. 2C), and 63% in L. braziliensis (Fig. 2D). Likewise, the survival rates of histone H2B-treated promastigotes decreased by 37% in L. amazonensis and by 75% in both L. major and L. braziliensis. Compared to the other two species, L. braziliensis promastigotes seemed more sensitive to histone treatment, judged by the significant reduction in survival rates even at 20 μg/ml of H2A or H2B (Fig. 2D). To confirm the specific effects of H2A and H2B, we also performed similar studies with histone H10, a component of chromatin linker proteins. In contrast to a previous report citing an antimicrobial effect of H10 (15), we did not observe any major changes in H10-treated L. amazonensis (Fig. 2B).
FIG. 2.
Direct killing of Leishmania promastigotes, but not amastigotes, by histone proteins. Promastigotes of L. amazonensis (A and B), L. major (C), and L. braziliensis (D) were treated with 20 to 200 μg/of of histone H2A, H2B, or H10 for 30 min and then stained with calcein AM and ethidium homodimer for evaluating live (green) versus dead (red) parasites, respectively. Shown are representative results from three independent repeats, and data are presented as mean survival rates (%) ± standard errors. ** (P < 0.01) and *** (P < 0.001) indicate statistically significant differences between the control and treated groups. # (P < 0.05), ## (P < 0.01), and ### (P < 0.001) indicate the statistical differences between the marked groups.
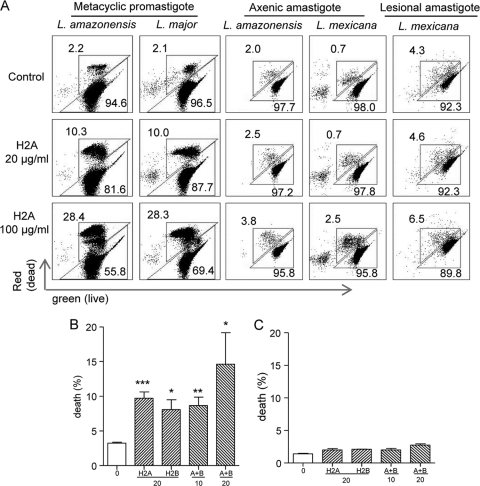
To validate and extend these findings, we purified metacyclic promastigotes of L. amazonensis and L. major, treated them with histone H2A, and measured parasite death rates by using flow cytometry, a relatively sensitive method that allows accurate quantification of the percentages of live versus dead parasites. Consistently, we detected approximately 10% and 27% of dead metacyclic promastigotes following treatment with 20 and 100 μg/ml H2A, respectively (Fig. 3 A). Since Leishmania amastigotes are the disease-forming stage of the parasite and have a long-term interaction with the host, we examined axenic and lesion-derived amastigotes of L. amazonensis and L. mexicana. Interestingly, we consistently detected a relatively low level of increase in the percentages of dead parasites even when axenic amastigotes were treated with 100 μg/ml of H2A, as judged by flow cytometry (Fig. 3) and fluorescent microscopy (data not shown). Although lesion-derived amastigotes reached a death rate of 6.5% following treatment with 100 μg/ml of H2A (Fig. 3A), the net increase was marginal (1.5%) compared to that of the untreated controls (4.3%). To test whether different histone proteins have any synergic effects, we used H2A plus H2B (each at 10 or 20 μg/ml) to treat promastigotes or axenic amastigotes but found no synergic effects between H2A and H2B on promastigotes or amastigotes (Fig. 3B and C). We also tested whether low-pH conditions (pH 5) can influence the histone killing. While the low-pH condition by itself increased the percentages of dead promastigotes (but not amastigotes), this low pH did not change the differential sensitivity of these parasites to histones (data not shown). Together, these results clearly indicate that the leishmaniacidal effects are selective for H2A and H2B (but not H10) proteins and are generally efficient for promastigotes. In contrast, axenic and lesion-derived amastigotes of L. amazonensis and L. mexicana are relatively resistant to histone treatment.
FIG. 3.
Analysis of the death rates of histone-treated parasites by flow cytometry. (A) Metacyclic promastigotes of L. major and L. amazonensis were purified by using Ficoll gradient centrifugation and treated with an indicated concentration of histone H2A. Axenic amastigotes of L. amazonensis and L. mexicana, as well as lesion-derived amastigotes of L. mexicana, were treated similarly. Parasites were stained with calcein AM and ethidium homodimer and analyzed on a C6 flow cytometer. The numbers represent the percentages of dead (top left numbers) and live (bottom right numbers) parasites. (B and C) Promastigotes (B) and axenic amastigotes (C) of L. amazonensis were treated with histone H2A or H2B alone or in combination at indicated concentrations (μg/ml) and analyzed as described for panel A. Shown are representative results from two independent repeats, and data are presented as mean death rates (%) ± standard errors. * (P < 0.05), ** (P < 0.01), and *** (P < 0.001) indicate statistically significant differences between the control and treated groups.
Preexposure to histone proteins markedly decreases promastigote infectivity in macrophages.
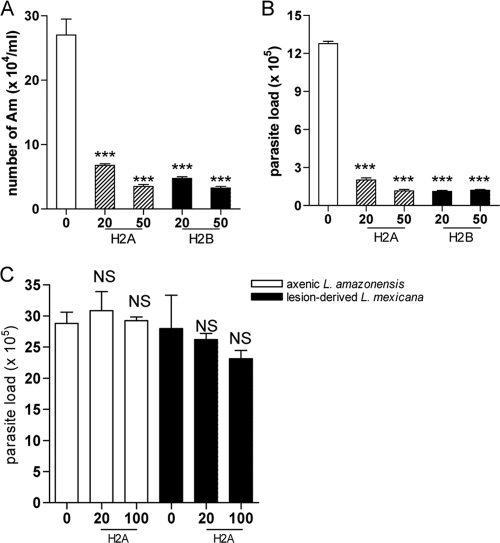
Leishmania parasites need to establish an infection and replicate within target cells in order to trigger pathological changes. To further examine the leishmaniacidal effects of histones, we pretreated promastigotes and axenic or lesion-derived amastigotes of L. amazonensis with histone proteins for 30 min and used these parasites to infect BM-Mφs. Parasite loads were evaluated by two different methods. First, we used 0.01% SDS to lyse Mφs at 72 h of infection and counted parasite numbers per well under a microscope. It was evident that pretreatment with 20 or 50 μg/ml of H2A or H2B resulted in a significant reduction in parasite numbers (P < 0.001) (Fig. 4 A). Second, we extracted the genomic DNA from cells at 72 h of infection to examine parasite loads by real-time PCR analysis and confirmed the significant reduction in parasite loads when promastigotes were pretreated with H2A or H2B (Fig. 4B). Again, we found that histone pretreatment had no major effects on Mφ infection by axenic or lesion-derived amastigotes (Fig. 4C), which was consistent with our results in Fig. 3A and C. Therefore, preexposure to histone proteins can markedly decrease the infectivity of promastigotes, but not amastigotes, in Mφs.
FIG. 4.
Mφ infection with histone-treated L. amazonensis parasites. (A and B) L. amazonensis promastigotes were treated with an indicated concentration of H2A or H2B for 30 min and used to infect Mφs (at a 5:1 parasite-to-cell ratio). The number of intracellular parasites was determined at 72 h by direct counting under a hemocytometer (A) or via real-time PCR analysis (B). (C) Mφs were infected with histone-pretreated axenic amastigotes of L. amazonensis (at a 5:1 parasite-to-cell ratio) or lesion-derived amastigotes of L. mexicana (at a 1:2 parasite-to-cell ratio) for 48 h. Parasite loads were determined by real-time PCR analysis. Shown are means ± standard errors. *** (P < 0.001) indicates statistically significant differences between the control groups and histone-treated groups, and NS means no significance.
Morphological changes of histone-treated promastigotes.
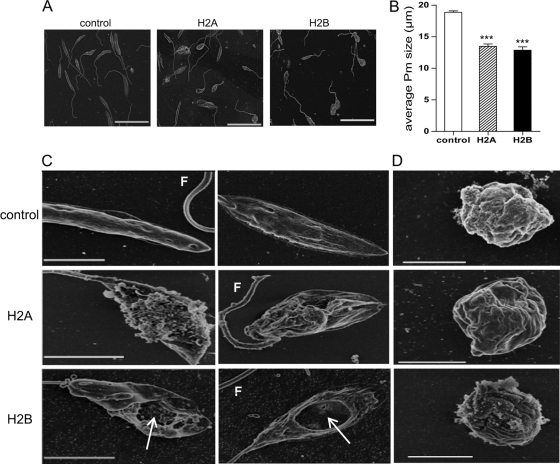
As histone proteins can efficiently kill Leishmania promastigotes, we examined the ultrastructure of histone-treated parasites by scanning electron microscopy (SEM) (Fig. 5 A) and measured the body length of L. amazonensis promastigotes at ×2,000 magnification. While the average length of control parasites was 18.6 μm, the average lengths of H2A- and H2B-treated parasites were 13.5 μm and 12.9 μm, respectively (P < 0.001) (Fig. 5B). A close-up examination revealed that while the control parasites displayed a smooth surface in the body and flagellum, the surface of H2A-treated promastigotes was uneven and disrupted (Fig. 5C). Holes of variable sizes were observed on H2B-treated promastigotes (Fig. 5C, white arrows). The similarly treated axenic amastigotes showed no notable differences from their controls (Fig. 5D), confirming the relative resistance of amastigotes to histone treatment (Fig. 3B and 4C).
FIG. 5.
Morphological and ultrastructural changes of histone-treated parasites in scanning electron microscopy (SEM). L. amazonensis promastigotes were treated with histone H2A or H2B (100 μg/ml) for 30 min, and then samples were immediately fixed for SEM analysis. (A) Representative SEM images for control and treated parasites. Bars, 20 μm. (B) Data are shown as the average lengths of promastigotes in the control and histone-treated groups. Shown are means ± standard errors. *** (P < 0.001) indicates statistically significant differences between the control and treated groups. (C and D) L. amazonensis promastigotes (C) and axenic amastigotes (D) were left untreated or treated with histone H2A or H2B (100 μg/ml) for 30 min. Samples were fixed for SEM analysis, and images were taken at ×15,000 to ×20,000 magnification. Bars, 3 μm (C) and 2 μm (D). Arrows points to variable sizes of holes on the surface of promastigotes. F, flagellum.
Binding of histone proteins to Leishmania parasites.
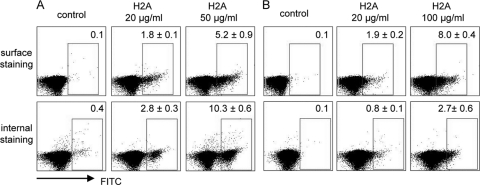
In an attempt to examine the selective binding and localization of histone proteins to parasites, we used anti-human histone H2A plus FITC-conjugated secondary antibodies to stain H2A-treated promastigotes or axenic amastigotes of L. amazonensis. We consistently detected a dose-dependent increase in the percentages of anti-H2A staining on the surface of promastigotes (Fig. 6 A) and amastigotes (Fig. 6B). When parasites were treated with H2A and then permeabilized prior to antibody staining, the percentages of internal anti-H2A staining in promastigotes were significantly higher than those in amastigotes (comparing 2.8% with 0.8%, P < 0.05). To confirm these observations, we calculated the ratios of internal to surface-bound H2A. Promastigotes treated with 20 or 50 μg/ml of H2A had an average ratio of 1.3 or 4.0, respectively, whereas amastigotes treated with 20 or 100 μg/ml of H2A had an average ratio of 0.02 or 0.4, respectively. These data imply that the surface binding and subsequent internalization of H2A are critical for promastigote killing.
FIG. 6.
Surface and internal binding of histone proteins on Leishmania parasites. L. amazonensis promastigotes (A) or axenic amastigotes (B) were treated with the indicated concentration of histone H2A for 30 min. After washing, surface-bound H2A was stained with a mouse MAb specific to human histone H2A and then with an FITC-conjugated anti-mouse IgG1 antibody. For detecting internalized H2A, parasites were permeabilized prior to and during antibody staining. Parasites were analyzed on a C6 flow cytometer. The numbers in the boxes indicate the percentages of positively stained parasites, and data are presented as means (%) ± standard errors. Shown are representative results from two independent repeats.
LPG and gp63 partially contribute to the susceptibility of promastigotes to histone proteins.
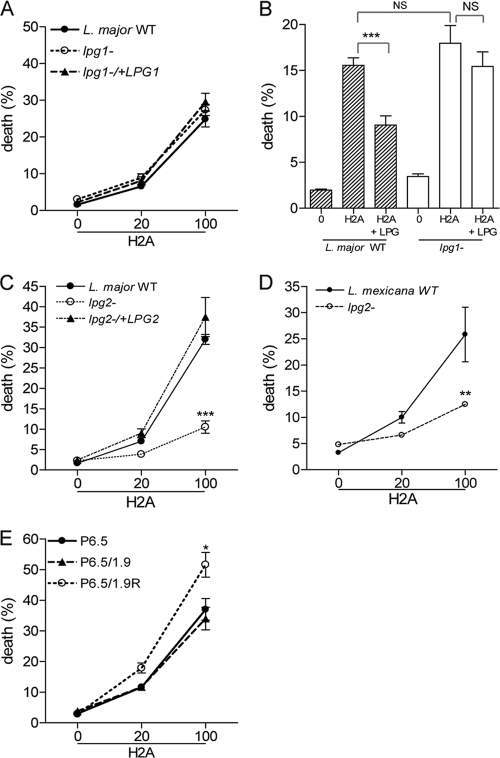
The sensitivity of Leishmania promastigotes to histone proteins prompted us to examine the possible contribution of gp63 and LPG, the most abundant surface molecules and important virulence factors for promastigotes but not at all important (or in only a limited manner) for amastigotes (28, 31). We first treated WT, lpg1−, and lpg1−/+LPG1 promastigotes of L. major with histone H2A and analyzed the stained parasites by flow cytometry. Our pooled results from several independent repeats revealed no major differences among the three parasite groups (Fig. 7 A), implying a limited role for lpg1 deficiency in the histone-mediated killing of promastigotes. To validate this observation, we incubated histone H2A (50 or 100 μg/ml) with purified L. major LPG for 30 min and then added the H2A and LPG to WT and lpg1− parasites. In WT parasites, the mortality rate was reduced significantly when H2A was preincubated with 50 μg/ml of LPG (P < 0.001) (Fig. 7B) or 100 μg/ml LPG (P < 0.05) (data not shown), leading us to postulate that the leishmaniacidal effect of H2A was hampered by the binding to purified LPG. However, the similar treatment had no major effects on lpg1− parasites, implying that other promastigote surface components were involved in binding to H2A. To examine these possibilities, we used lpg2− promastigotes of L. major and L. mexicana. As shown in Fig. 7C, histone treatment of lpg2− L. major resulted in death rates of 10%, a number about 3-fold lower than those in WT and lpg2−/+LPG2 parasites (P < 0.001). Likewise, the death rates of H2A-treated L. mexicana lpg2− parasites were about 2-fold lower than those in its WT controls (P < 0.05) (Fig. 7D). We also stained L. major WT and mutant parasites with anti-human histone H2A plus FITC-conjugated secondary antibodies, as described for Fig. 6. However, we failed to obtain a consistent trend, mostly due to the marked loss of mutant parasites during the staining procedures (data not shown). To investigate the involvement of gp63, we used different concentrations of H2A to treat L. amazonensis P6.5, P6.5/1.9R, and P6.5/1.9 promastigotes (the latter two parasites were knockdown or overexpression levels of gp63, respectively). At 100 μg/ml of H2A, the death rates of P6.5/1.9R (gp63-knockdown) parasites were significantly higher than those of P6.5 and P6.5/1.9 parasites (P < 0.05) (Fig. 7E). Together, these results implied that both LPG and gp63 partially contribute to the susceptibility of promastigotes to histone proteins.
FIG. 7.
Effects of LPG and gp63 on histone-mediated promastigote killing. (A) Wild-type (WT), lpg1−, and lpg1−/+LPG1 promastigotes of L. major were treated with the indicated concentrations (μg/ml) of histone H2A or H2B and then stained with calcein AM and ethidium homodimer, respectively. The stained parasites were then analyzed on a C6 flow cytometer. (B) Histone H2A (50 μg/ml) was preincubated with purified L. major LPG (50 μg/ml) for 30 min prior to parasite treatment and death analysis. (C) WT, lpg2−, and lpg2−/+LPG2 promastigotes of L. major were treated with indicated concentrations (μg/ml) of histone H2A and analyzed for death rates. (D) WT and lpg2− promastigotes of L. mexicana were treated with the indicated concentrations of histone H2A and analyzed for death. (E) WT (P6.5), P6.5/1.9, and P6.5/1.9R promastigotes of L. amazonensis were treated with the indicated concentrations of H2A and analyzed for death. Shown are the means ± the standard errors. * (P < 0.05), ** (P < 0.01), and *** (P < 0.001) indicate statistically significant differences between histone-treated groups. NS means no significance among these groups.
DISCUSSION
Many components in the innate immune system can contribute to protection against invading pathogens. For example, antimicrobial peptides (AMPs) are conserved components of the host defense system in plants, invertebrates, and vertebrates, and they have a wide spectrum of antimicrobial function against bacteria, fungi, viruses, and parasites (42, 43). At least two major mechanisms are known to be responsible for AMP-mediated pathogen killing. Some AMPs can bind to the surface of microbes and alter their membrane permeabilization (4), while other AMPs can directly enter the cytoplasm of microbes, whereby killing relies on an intracellular target (35). Several AMPs have antiprotozoan activities on promastigotes of L. major and L. donovani (24, 25), the procyclic and bloodstream forms of Trypanosoma brucei (13, 29), the oocysts of Cryptosporidium parvum (10), and the intraerythrocytic form of Plasmodium falciparum (9). However, our knowledge regarding the effect of AMPs on protozoan infection is still quite limited in comparison to that known for bacteria and fungi (42).
In this study, we used human histone H2A and H2B to treat Leishmania parasites. We have provided evidence that core histone proteins H2A and H2B, but not linker histone protein H10, can directly and efficiently kill several species of Leishmania promastigotes (L. amazonensis, L. major, L. braziliensis, and L. mexicana) in a dosage-dependent and time-independent manner. More importantly, we have shown that preexposure to relatively low concentrations of histone proteins can greatly reduce the infectivity of promastigotes to mouse Mφs. However, histone proteins had limited effects on axenic and lesion-derived amastigotes of L. amazonensis and L. mexicana (Fig. 3A, 4C, and 5D). These findings indicate a general, microbicidal effect of histone proteins on Leishmania promastigotes but not on amastigotes.
The differential sensitivities of promastigotes and amastigotes to histone-mediated parasite killing, as well as the marked decrease in body length and the apparent damage of treated promastigotes (Fig. 5), prompted us to examine the possible mechanism of promastigote killing. The flow cytometric studies suggested to us that the relatively high sensitivity of promastigotes to histones is due to the efficient binding and internalization of histone proteins (Fig. 6A). Given that LPG and gp63 are important virulence and survival factors for some Leishmania species (48, 51) and that their expression levels are highly regulated in parasite developmental stages (2, 39), we then focused our investigation on LPG and gp63.
Our studies with purified LPG, gene-targeted deletion, and transgenic parasites reveal that LPG and gp63 play complex roles in the histones' leishmaniacidal effects (Fig. 7) and suggest a need for additional investigation in this area. First, the enhanced susceptibility of gp63-knockdown L. amazonensis to H2A treatment implies a potential protective role for gp63 against the action of histone proteins. Since gp63 is a major surface zinc protease of promastigotes (27) that can inactivate AMPs (14, 22), it will be interesting to further examine whether our observations were due to proteolytic cleavage of histones by surface-anchored gp63. Second, our studies with LPG-pretreated histone proteins implied a possible reduction in both quantity and availability of histones for binding to parasites, which may explain the reduced susceptibility of wild-type L. major promastigotes to histone treatment. Third, the four main components of Leishmania LPG may contribute to histone binding differently. Although the lpg1− mutants lack LPG (16), they were as susceptible to H2A as were the WT promastigotes, because lpg1− mutants are still capable of synthesizing and expressing PGs attached to proteophosphoglycans (PPG) on the surface (16). In contrast, the lpg2− mutants showed an increased resistance to histone treatment, because these mutants lack all PG repeats due to the deletion of the Golgi GDP-Man transporter LPG2 but can synthesize the LPG glycan core and protein backbone of PPG. It is reported that, like other AMPs, some core histone proteins such as H2A can cross lipid bilayers and mycoplasma membranes and that the intracellular accumulation of histones is energy independent and mediated via a nonendocytic pathway (44). At this stage, the molecular basis underlying the translocation of histone proteins to promastigotes remains unclear. Given marked loss of lpg1− and lpg2− mutants in our antibody-based assay (data not shown), we suggest that other approaches such as radioactive-labeled H2A are needed to address this issue.
Another important finding in this study is the relatively high resistance of axenic and lesion-derived amastigotes of L. amazonensis and L. mexicana to the microbicidal effects of histone proteins (Fig. 3 and 4), even though appreciable percentages of amastigotes were surface positive for H2A (Fig. 6B). There are several possible explanations for the evident surface binding, but limited detection, of internal H2A in amastigotes. First, it is known that amastigotes resemble the lpg1− promastigotes in that they lack LPG1 but retain PPG (18) and that the structure of the glycans found on amastigote PPG in L. mexicana differs considerably from the simpler LPG-like linear PG polymers found on promastigote PPG (17, 21). These unique structures may allow interaction with histones but limit their translocation. Second, amastigotes reside and replicate within acidified vacuoles and are highly enriched in proteases for the digestion of host proteins. The possibility of degrading histone proteins, as suggested in our studies with gp63 transfectants (Fig. 7E), needs further investigation. Third, lesional amastigotes of L. mexicana and amastigotes residing in phagolysosomes can secrete relatively large amounts of PPG, which can bind to serum mannan-binding proteins and subsequently activate serine protease (37). Although the molecular details of surface molecules for L. amazonensis amastigotes remain largely unclear, it will be interesting to further examine how amastigotes protect themselves from damage by histone proteins. Regardless of the mechanisms, our results clearly indicate the biologically important differences between the two parasite stages in their sensitivity to histone proteins and thereby open areas for future investigation.
Histone proteins are known to be released from activated neutrophils during NET formation. Neutrophils are considered a major part of innate immunity and can utilize phagocytosis, degranulation, and NET at different time points for host defense (36). For example, phagocytosis by neutrophils plays a predominant role in killing freshly engulfed microbes, while later killing is determined mostly by degranulation and/or NET formation (36). The NET-related antimicrobial activities can be attributed to many NET components, including histones, myeloperoxidase, neutrophil elastase, bacterial permeability-increasing proteins, and defensins (36). In addition, neutrophils or purified neutrophil elastase can activate Mφs to induce a defense against L. major, a process that relies on TLR4 expression by Mφs (41). The NET-mediated killing of L. amazonensis promastigotes is attributed to histones rather than to DNA and elastase relayed from NETs (12). Recently, we have found that mice deficient in the type I interferon (IFN) receptor are less susceptible to L. amazonensis infection than are WT mice and that enhanced neutrophil recruitment, turnover, and effector functions are responsible for the enhanced resistance in the deficient mice (54). Thus, the present study extends our understanding of histone functioning in innate immunity to Leishmania promastigotes. Additional studies are needed to further examine the relevance of these interactions in Leishmania infection in vivo.
In summary, our results showed that human histone proteins are highly efficient in killing Leishmania promastigotes but not amastigotes. The finding of the differential susceptibility of promastigotes and amastigotes to histone proteins is interesting and biologically important. This study supports the view that histone proteins released from NETs contribute to host immune responses to Leishmania parasites (12) and calls for further examination of the effect of histone proteins on parasite infection in vivo and the mechanism underlying histones' antileishmanial function. Although our results revealed a minimal effect of histone proteins on Leishmania amastigotes, it will be interesting to examine whether other NET components can promote amastigote killing and how an amastigote-derived factor(s) down-modulates these antileishmanial effects.
Acknowledgments
This study was supported by NIH grants AI043003 and AI076849 to L. Soong and AI31078 to S. M. Beverley and by a China Scholarship Council grant to Y. Wang.
We thank Joao Wanderley and Julie Wen for technical assistance, Katherine Owens for assistance with lpg mutants, David Sacks for providing purified L. major LPG, Sam Turco for providing an antibody specific to L. major LPG, Michael Mandell for discussions, and Mardelle Susman for assisting in manuscript preparation.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 28 December 2010.
REFERENCES
- 1.Appelberg, R. 2007. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 15:87-92. [DOI] [PubMed] [Google Scholar]
- 2.Bahr, V., et al. 1993. Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol. Biochem. Parasitol. 58:107-121. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann, V., et al. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532-1535. [DOI] [PubMed] [Google Scholar]
- 4.Chan, D. I., E. J. Prenner, and H. J. Vogel. 2006. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim. Biophys. Acta 1758:1184-1202. [DOI] [PubMed] [Google Scholar]
- 5.Chen, D. Q., et al. 2000. Episomal expression of specific sense and antisense mRNAs in Leishmania amazonensis: modulation of gp63 level in promastigotes and their infection of macrophages in vitro. Infect. Immun. 68:80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, L., et al. 2005. The involvement of neutrophils in the resistance to Leishmania major infection in susceptible but not in resistant mice. Parasitol. Int. 54:109-118. [DOI] [PubMed] [Google Scholar]
- 7.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale, D. C., L. Boxer, and W. C. Liles. 2008. The phagocytes: neutrophils and monocytes. Blood 112:935-945. [DOI] [PubMed] [Google Scholar]
- 9.Gelhaus, C., T. Jacobs, J. Andra, and M. Leippe. 2008. The antimicrobial peptide NK-2, the core region of mammalian NK-lysin, kills intraerythrocytic Plasmodium falciparum. Antimicrob. Agents Chemother. 52:1713-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacometti, A., et al. 2003. In vitro effect on Cryptosporidium parvum of short-term exposure to cathelicidin peptides. J. Antimicrob. Chemother. 51:843-847. [DOI] [PubMed] [Google Scholar]
- 11.Gramiccia, M., and L. Gradoni. 2005. The current status of zoonotic leishmaniases and approaches to disease control. Int. J. Parasitol. 35:1169-1180. [DOI] [PubMed] [Google Scholar]
- 12.Guimaraes-Costa, A. B., et al. 2009. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U. S. A. 106:6748-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haines, L. R., et al. 2009. Killing of trypanosomatid parasites by a modified bovine host defense peptide, BMAP-18. PLoS Negl. Trop. Dis. 3:e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halle, M., et al. 2009. The Leishmania surface protease GP63 cleaves multiple intracellular proteins and actively participates in p38 mitogen-activated protein kinase inactivation. J. Biol. Chem. 284:6893-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell, S. J., D. Wilk, S. P. Yadav, and C. L. Bevins. 2003. Antimicrobial polypeptides of the human colonic epithelium. Peptides 24:1763-1770. [DOI] [PubMed] [Google Scholar]
- 16.Ilg, T. 2000. Proteophosphoglycans of Leishmania. Parasitol. Today 16:489-497. [DOI] [PubMed] [Google Scholar]
- 17.Ilg, T., D. Craik, G. Currie, G. Multhaup, and A. Bacic. 1998. Stage-specific proteophosphoglycan from Leishmania mexicana amastigotes. Structural characterization of novel mono-, di-, and triphosphorylated phosphodiester-linked oligosaccharides. J. Biol. Chem. 273:13509-13523. [DOI] [PubMed] [Google Scholar]
- 18.Ilg, T., E. Handman, and Y. D. Stierhof. 1999. Proteophosphoglycans from Leishmania promastigotes and amastigotes. Biochem. Soc. Trans. 27:518-525. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki, H., and S. Iwamuro. 2008. Potential roles of histones in host defense as antimicrobial agents. Infect. Disord. Drug Targets 8:195-205. [DOI] [PubMed] [Google Scholar]
- 20.Khorasanizadeh, S. 2004. The nucleosome: from genomic organization to genomic regulation. Cell 116:259-272. [DOI] [PubMed] [Google Scholar]
- 21.Klein, C., U. Gopfert, N. Goehring, Y. D. Stierhof, and T. Ilg. 1999. Proteophosphoglycans of Leishmania mexicana. Identification, purification, structural and ultrastructural characterization of the secreted promastigote proteophosphoglycan pPPG2, a stage-specific glycoisoform of amastigote aPPG. Biochem. J. 344:775-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni, M. M., et al. 2006. The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol. Microbiol. 62:1484-1497. [DOI] [PubMed] [Google Scholar]
- 23.Lupi, O., et al. 2009. Tropical dermatology: tropical diseases caused by protozoa. J. Am. Acad. Dermatol. 60:897-925. [DOI] [PubMed] [Google Scholar]
- 24.Luque-Ortega, J. R., W. van't Hof, E. C. Veerman, J. M. Saugar, and L. Rivas. 2008. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J. 22:1817-1828. [DOI] [PubMed] [Google Scholar]
- 25.Mangoni, M. L., et al. 2005. Temporins, small antimicrobial peptides with leishmanicidal activity. J. Biol. Chem. 280:984-990. [DOI] [PubMed] [Google Scholar]
- 26.McFarlane, E., et al. 2008. Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani. Infect. Immun. 76:532-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGwire, B., and K. P. Chang. 1994. Genetic rescue of surface metalloproteinase (gp63)-deficiency in Leishmania amazonensis variants increases their infection of macrophages at the early phase. Mol. Biochem. Parasitol. 66:345-347. [DOI] [PubMed] [Google Scholar]
- 28.McGwire, B. S., W. A. O'Connell, K. P. Chang, and D. M. Engman. 2002. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis: implications for parasite virulence. J. Biol. Chem. 277:8802-8809. [DOI] [PubMed] [Google Scholar]
- 29.McGwire, B. S., C. L. Olson, B. F. Tack, and D. M. Engman. 2003. Killing of African trypanosomes by antimicrobial peptides. J. Infect. Dis. 188:146-152. [DOI] [PubMed] [Google Scholar]
- 30.Molina, R., L. Gradoni, and J. Alvar. 2003. HIV and the transmission of Leishmania. Ann. Trop. Med. Parasitol. 97(Suppl. 1):29-45. [DOI] [PubMed] [Google Scholar]
- 31.Naderer, T., J. E. Vince, and M. J. McConville. 2004. Surface determinants of Leishmania parasites and their role in infectivity in the mammalian host. Curr. Mol. Med. 4:649-665. [DOI] [PubMed] [Google Scholar]
- 32.Nathan, C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173-182. [DOI] [PubMed] [Google Scholar]
- 33.Neuber, H. 2008. Leishmaniasis. J. Dtsch. Dermatol. Ges. 6:754-765. [DOI] [PubMed] [Google Scholar]
- 34.Olivier, M., D. J. Gregory, and G. Forget. 2005. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin. Microbiol. Rev. 18:293-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otvos, L., Jr. 2005. Antibacterial peptides and proteins with multiple cellular targets. J. Pept. Sci. 11:697-706. [DOI] [PubMed] [Google Scholar]
- 36.Papayannopoulos, V., and A. Zychlinsky. 2009. NETs: a new strategy for using old weapons. Trends Immunol. 30:513-521. [DOI] [PubMed] [Google Scholar]
- 37.Peters, C., et al. 1997. Secreted proteophosphoglycan of Leishmania mexicana amastigotes activates complement by triggering the mannan binding lectin pathway. Eur. J. Immunol. 27:2666-2672. [DOI] [PubMed] [Google Scholar]
- 38.Peters, N. C., et al. 2008. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321:970-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pimenta, P. F., E. M. Saraiva, and D. L. Sacks. 1991. The comparative fine structure and surface glycoconjugate expression of three life stages of Leishmania major. Exp. Parasitol. 72:191-204. [DOI] [PubMed] [Google Scholar]
- 40.Qi, H., J. Ji, N. Wanasen, and L. Soong. 2004. Enhanced replication of Leishmania amazonensis amastigotes in gamma interferon-stimulated murine macrophages: implications for the pathogenesis of cutaneous leishmaniasis. Infect. Immun. 72:988-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro-Gomes, F. L., et al. 2007. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J. Immunol. 179:3988-3994. [DOI] [PubMed] [Google Scholar]
- 42.Rivas, L., J. R. Luque-Ortega, and D. Andreu. 2009. Amphibian antimicrobial peptides and protozoa: lessons from parasites. Biochim. Biophys. Acta 1788:1570-1581. [DOI] [PubMed] [Google Scholar]
- 43.Rivas-Santiago, B., C. J. Serrano, and J. A. Enciso-Moreno. 2009. Susceptibility to infectious diseases based on antimicrobial peptide production. Infect. Immun. 77:4690-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbluh, J., et al. 2005. Translocation of histone proteins across lipid bilayers and Mycoplasma membranes. J. Mol. Biol. 345:387-400. [DOI] [PubMed] [Google Scholar]
- 45.Russell, D. G. 1987. The macrophage-attachment glycoprotein gp63 is the predominant C3-acceptor site on Leishmania mexicana promastigotes. Eur. J. Biochem. 164:213-221. [DOI] [PubMed] [Google Scholar]
- 46.Sacks, D., and S. Kamhawi. 2001. Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu. Rev. Microbiol. 55:453-483. [DOI] [PubMed] [Google Scholar]
- 47.Sanabria, M. X., D. A. Vargas-Inchaustegui, L. Xin, and L. Soong. 2008. Role of natural killer cells in modulating dendritic cell responses to Leishmania amazonensis infection. Infect. Immun. 76:5100-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spath, G. F., et al. 2000. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. U. S. A. 97:9258-9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spath, G. F., L. A. Garraway, S. J. Turco, and S. M. Beverley. 2003. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc. Natl. Acad. Sci. U. S. A. 100:9536-9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spath, G. F., et al. 2003. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science 301:1241-1243. [DOI] [PubMed] [Google Scholar]
- 51.Thiakaki, M., B. Kolli, K. P. Chang, and K. Soteriadou. 2006. Down-regulation of gp63 level in Leishmania amazonensis promastigotes reduces their infectivity in BALB/c mice. Microbes Infect. 8:1455-1463. [DOI] [PubMed] [Google Scholar]
- 52.Urban, C. F., U. Reichard, V. Brinkmann, and A. Zychlinsky. 2006. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 8:668-676. [DOI] [PubMed] [Google Scholar]
- 53.Vinetz, J. M., and L. Soong. 2007. Leishmania mexicana infection of the eyelid in a traveler to Belize. Braz. J. Infect. Dis. 11:149-152. [DOI] [PubMed] [Google Scholar]
- 54.Xin, L., et al. 2010. Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J. Immunol. 184:7047-7056. [DOI] [PMC free article] [PubMed] [Google Scholar]