Abstract
Leptospirosis can activate inflammatory responses through Toll-like receptors (TLRs) and may cause renal tubulointerstitial fibrosis characterized by the accumulation of extracellular matrix (ECM). We have previously demonstrated that Leptospira santorosai serovar Shermani detergent extract stimulates ECM accumulation in vitro. The aim of this study was to examine the mechanistic basis of these previous observations and, in particular, to examine the potential involvement of TLRs. The addition of serovar Shermani detergent extract led to an increase in fibronectin gene expression and production. Inhibition of TLR2 but not TLR4 expression abrogated serovar Shermani detergent extract-mediated increases in fibronectin production. This response was also blocked by the knockdown of the gene expression of the TLR2 downstream transducers myeloid differentiation factor 88 (MyD88) and tumor necrosis factor receptor-associated factor 6 (TRAF6). Serovar Shermani detergent extract also activated nuclear factor-κB, and its inhibition by curcumin-attenuated serovar Shermani detergent extract induced increases in fibronectin production. These effects were also mimicked by the specific TLR2 agonist, Pam(3)CsK(4), a response that was also abrogated by the knockdown of MyD88 and TRAF6. Similarly, the administration of live leptospires to cells also induced fibronectin production that was blocked by inhibition of TLR2 and MyD88 expression. In conclusion, serovar Shermani detergent extract can induce fibronectin production through the TLR2-associated cascade, providing evidence of an association between TLRs and leptospirosis-mediated ECM deposition.
Leptospirosis caused by leptospiral infection can result in multiple-organ failure, including acute kidney injury (7). Although tubulointerstitial nephritis is the most common histological finding in patients with leptospirosis, tubulointerstitial fibrosis eventually develops if chronic leptospiral infection is left untreated. While leptospirosis is well established as a cause of tubulointerstitial fibrosis in dogs and rats, leading to chronic renal failure (28, 30), it is also apparent that chronic leptospiral infection may result in renal disease in humans (21). Persistent infection may cause interstitial nephritis and fibrosis in human biopsy samples (21), and more recent studies have demonstrated that this may lead to end-stage renal failure (2). Leptospira santorosai serovar Shermani (referred to hereafter as serovar Shermani) is the main pathogenic leptospira causing renal failure in Taiwan (4). We have recently demonstrated that stimulation of proximal tubular cells with serovar Shermani detergent extract stimulated the accumulation of extracellular matrix (ECM) proteins such as type I and type IV collagens, supporting the notion that leptospirosis can cause tubulointerstitial fibrosis (32).
Toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns are essential for the innate immune response and play a fundamental role in antimicrobial responses against viruses and bacteria (1). For example, TLR2 and TLR4 have been implicated in cellular responses to lipopolysaccharide (LPS), and bacterial lipoproteins, the major constituents of the Gram-negative bacterial outer membrane (40). Binding of specific ligands to TLR2 leads to recruitment of an intracellular adaptor protein, myeloid differentiation factor 88 (MyD88), which subsequently activates tumor necrosis factor receptor-associated factor 6 (TRAF6) by phosphorylating interleukin-1 receptor-associated kinase 4 (IRAK-4) and IRAK-1 (16). Finally, the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase pathways are activated, thus leading to induced inflammatory gene expression. It has been shown that TLR4 enhances hepatic fibrogenesis (27). However, little is known about whether TLRs are implicated in the development of renal fibrosis.
We previously demonstrated that serovar Shermani detergent extract induces an increase in inflammatory cytokines and chemokines through the TLR2-mediated but not the TLR4-mediated pathway (36). It also stimulates ECM accumulation through transforming growth factor β (TGF-β)/Smad3-dependent signaling pathway (32). Werts et al. also demonstrate that L. interrogans outer membrane LPS and lipoproteins activate macrophages through TLR2 in human cells (34). Nevertheless, it remains unclear whether TLRs play a role in serovar Shermani detergent extract-induced alteration of ECM molecules. We show here that serovar Shermani detergent extract increases fibronectin production via the TLR2-mediated pathway, providing the first evidence that the TLR-associated signaling pathways play an important role in ECM accumulation and implying a possible association between innate immunity and renal fibrosis.
MATERIALS AND METHODS
Materials.
Curcumin was purchased from Sigma-Aldrich (St. Louis, MO) and 6-amino-4-(4-phenoxyphenylethylamino)quinazoline (QNZ) was obtained from Calbiochem (La Jolla, CA). Pam(3)CsK(4) was obtained from InvivoGen (San Diego, CA). Anti-fibronectin antibody was purchased from Abcam (Cambridge, United Kingdom).
Cell culture.
Immortalized human renal proximal tubular cells, HK-2, were cultured in Dulbecco modified Eagle medium/Ham F-12 (Life Technologies, Paisley, United Kingdom) supplemented with 5% fetal calf serum (Biological Industries, Ltd., Cumbernauld, United Kingdom), 2 mM glutamine (Life Technologies, Ltd., Paisley, United Kingdom), 20 mM HEPES buffer (Gibco-BRL, Paisley, United Kingdom), 0.4 μg of hydrocortisone/ml, 5 μg of insulin/ml, 5 μg of transferrin/ml, and 5 ng of sodium selenite/ml (Sigma Chemical Company, Ltd., Poole, United Kingdom).
To minimize the effect of serum-promoted cell proliferation and serum-mediated alteration of cell function and to avoid interference of serum-contained fibronectin in fibronectin quantification, all experiments were performed under serum-free conditions.
Live serovar Shermani was purchased from the American Type Culture Collection (ATCC; Manassas, VA). Leptospires were cultured in medium containing 10% Leptospira enrichment Elinghausen-McCullough-Johnson-Harris (EMJH) medium and 90% Leptospira medium base EMJM medium (Difco, Sparks, MD) at 28°C for 5 to 7 days (14, 38). Prior to addition to the cells, the number of leptospires was determined by direct counting in a counting chamber under dark-field microscopy (18, 25).
Preparation of serovar Shermani detergent extract.
The extraction method to obtain L. santorosai serovar Shermani detergent extract has been described previously (37). Briefly, serovar Shermani (ATCC 43286) was grown in 10% EMJH leptospiral enrichment medium (Difco). Culture medium (500 ml) containing 108 leptospires/ml enumerated by using dark field-microscopy was collected (18). The serovar Shermani detergent extract was extracted with 1% Triton X-114, 10 mM Tris (pH 8), and 1 mM EDTA. After centrifugation, the hydrophobic fraction was collected, and 2% Triton X-114 was used for further phase partitioning. After centrifugation for 10 min at 2,000 × g, the aqueous phases were removed, and the hydrophobic detergent fraction was precipitated with acetone. The precipitant was then dissolved in water, resulting in a serovar Shermani detergent extract yield of 250 μg.
Gene silencing by siRNA.
To knock down MyD88 and TRAF6 expression, RNA interference was used to inhibit functions of MyD88 and TRAF6 mRNAs. On-Target plus SMARTpool short interfering RNA (siRNA) of MyD88 and TRAF6 was purchased from Dharmacon, Inc. (Lafayette, CO).
Briefly, 0.5 to 1.5 μg of siRNA against MyD88 or TRAF6 or control siRNA was diluted in serum-free medium to give a final volume of 100 μl. Subsequently, RNAiFectI transfection reagent was mixed with diluted siRNA at a ratio from 6:1 to 3:1. After incubation for 15 min at room temperature, the mixture was added to the culture medium. After transfection for 24 h, cells were incubated in the presence or absence of Leptospira santarosai serovar Shermani OMP under serum-free condition for 24 or 48 h. Cells were then lysed and subjected to real-time PCR or Western blot analysis.
Real-time PCR.
The real-time PCR method has been previously described (31). Briefly, total RNA was isolated from the cells and reverse transcribed to DNA. Real-time PCR was performed according to the manufacturer's instructions using an ABI Prism 7700 with SYBR green I as a double-stranded DNA-specific dye (PE-Applied Biosystems, Cheshire, Great Britain). The primers used to assay fibronectin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript levels (fibronectin, 5-GTGTGACCCTCATGAGGCAAC-3 and 5-CTGGCCTCCAAAGCATGTG-3; GAPDH, 5-TTCCAGGAGCGAGATCCCT-3 and 3-CACCCATGACGAACATGGG-5) were constructed to be compatible with a single reverse transcription-PCR thermal profile (95°C for 10 min, 40 cycles at 95°C for 30 s, and 60°C for 1 min) (13). The accumulation of the PCR product was monitored in real time (PE-Applied Biosystems). Experimental results are presented as the transcript levels of the analyzed genes relative to GAPDH transcript level.
Western blot analysis.
Total cellular protein extraction was performed as described previously (31). Equal amount of proteins were mixed with an equal volume of reducing sodium dodecyl sulfate (SDS) sample buffer and boiled for 5 min at 95°C. Protein samples were resolved using SDS-10% polyacrylamide gel electrophoresis (PAGE) and then electroblotted on nitrocellulose membranes (Millipore, Billerica, MA). After electroblotting, nonspecific binding was blocked with a 5% nonfat milk solution. The membrane was then incubated with primary antibodies overnight at 4°C, followed by incubation with a horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The proteins were visualized by using enhanced chemiluminescence (Amersham Biosciences, Amersham, United Kingdom). The same blot was reprobed with anti-tubulin antibody (NeoMarkers, Fremont, CA) to examine tubulin expression as a control.
Electrophoretic mobility shift assay (EMSA).
Nuclear proteins were prepared according to the method of Satriano and Schlondorff (24). Briefly, cells were harvested with trypsin and collected by centrifugation. Cell pellets were washed with ice-cold phosphate-buffered saline (PBS) and suspended with buffer A containing 10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 3.5 mM dithiothreitol (DTT), and protease inhibitor (Complete; Boehringer Mannheim). Cell suspensions were incubated on ice for 10 min and centrifuged for 5 min at 650 × g. The pellets were resuspended in the same buffer A containing 0.5% Nonidet P-40 on ice for 15 min and mixed by pipetting vigorously for lysis. The nuclear fractions were pelleted for 5 min at 6,000 × g and resuspended in buffer B containing 5 mM HEPES (pH 7.9), 26% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, and 0.4 M NaCl. After incubation on ice for 30 min and centrifugation for 10 min at 12,000 × g, the supernatants containing nuclear protein were subsequently used.
Whole-cell nuclear extracts were subjected to assays for NF-κB-binding activity using NF-κB consensus oligonucleotide (59-AGTTGAGGGGACTTTAGGC-39; Promega, Madison, WI) radiolabeled with [γ-32P]dATP by T4 polynucleotide kinase 3 (Amersham Pharmacia Biotech, Uppsala, Sweden). A total of 10 mg of nuclear protein was incubated with 70 to 80 kcpm of 32P-labeled NF-κB oligonucleotide in a binding mixture (50 mM HEPES [pH 7.9], 20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, 0.5 mg of poly(dI-dC) [Pharmacia Biotech], H2O) to a final volume of 15 ml. After incubation at room temperature for 20 min, the protein-DNA complexes were resolved on native 4% polyacrylamide gel in a Tris-borate-EDTA buffer system and run at 200 V for 1.5 h in a 4°C cold room. Gels were transferred to Whatman paper, dried, and exposed to Kodak XR5 film (Rochester, NY) in film holders for 4 to 16 h at 280°C.
Immunohistochemistry.
With the permission of the Ethics Committee of our hospital, residual renal biopsy specimens in our tissue bank obtained from five leptospirosis patients between January 2000 and December 2003 were analyzed for the deposition of fibronectin and type I collagen. Normal tissues away from the edge of renal cell carcinoma (RCC) obtained from five RCC patients undergoing nephrectomy were used as negative controls. The average time from the onset of leptospiral infection to the day of renal biopsy was 37 ± 22 days.
Kidney specimens were fixed with 4% paraformaldehyde and embedded in paraffin. After deparaffinization, sections were treated with 10 mM sodium citrate and heated in a microwave for 10 min to expose antigenicities. Sections were then immersed in 3% H2O2 in methanol and blocked with 5% bovine albumin-PBS for 20 min. After incubation with rabbit anti-fibronectin or goat anti-type I collagen for 1 h, the sections were treated with relevant biotin-conjugated antibodies, and the fibronectin or type I collagen immunostaining was then displayed by using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
To quantify fibronectin immunostaining, the integrated image intensity in eight consecutively selected nonoverlapping and area-fixed fields of renal cortex and medulla at ×400 magnification in each kidney specimen was calculated by using MetaMorph imaging software (Molecular Devices Co., Sunnyvale, CA) (33).
Statistical analysis.
All data are presented as means ± the standard error of the mean. Statistical analysis was performed by using analysis of variance with Tukey's post hoc test. A P value of <0.05 was considered statistically significant.
RESULTS
L. santorosai serovar Shermani detergent extract induced an increase in fibronectin gene expression and protein production.
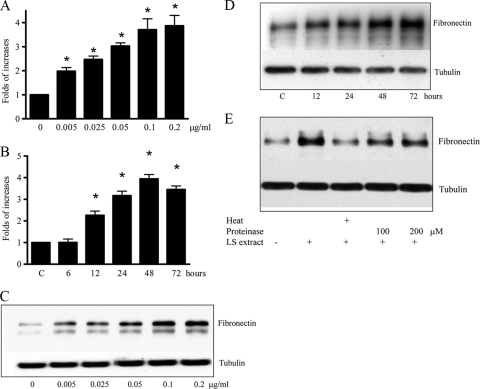
To assess whether the pathogenic leptospira, serovar Shermani increased fibronectin, we first assessed fibronectin production after the addition of serovar Shermani detergent extract to renal proximal tubular cells (HK-2 cells). After the addition of 0.005 to 0.2 μg of serovar Shermani detergent extract/ml for 48 h, both real-time PCR and Western blot analysis demonstrated a dose-dependent increase in fibronectin gene expression and protein, respectively (Fig. 1A and C). Maximal increase in fibronectin gene expression and protein production was seen after the addition of 0.1 μg of serovar Shermani detergent extract/ml. Similarly, addition of serovar Shermani detergent extract led to a time-dependent increase of fibronectin gene expression (Fig. 1B and D). Fibronectin gene expression and protein production reached maximal response after a 48-h stimulation.
FIG. 1.
Serovar Shermani detergent extract-induced increase in fibronectin production. (A and C) HK-2 cells were serum deprived for 24 h and then grown in the presence or absence of different concentrations of serovar Shermani detergent extract (0.005 to 0.2 μg/ml) under serum-free condition for 2 days. (B and D) HK-2 cells were treated with 0.1 μg of serovar Shermani detergent extract/ml for different periods as indicated. Cells were incubated in serum-free medium for 72 h as a control (lane C). (E) Serovar Shermani detergent extract (0.1 μg/ml) was inactivated by boiling (100°C) for 30 min or pretreatment with proteinase K (100 to 200 μM) at 37°C for 30 min and added to cells for further 2 days. Untreated detergent extract was used as the control. Fibronectin gene expression was then quantified by real-time PCR (A and B). The results of real-time PCR are expressed as the relative fold increase in fibronectin gene expression over that of the untreated group and presented as mean ± the standard error (SE) of triplicate measurements from four independent experiments (*, P < 0.05 versus the untreated group). Cell lysate was subjected to Western blot analysis for measurement of fibronectin production (C, D, and E). The results for one representative experiment of at least three individual replicate experiments are shown.
Lipoproteins are the most abundant components in the leptospiral total protein profile (5, 10, 42). To assess whether the protein components are important for stimulation of fibronectin production, serovar Shermani detergent extract was inactivated by boiling for 30 min or treatment with proteinase K (100 to 200 μM) at 37°C for 30 min. The result of Western blot analysis demonstrated that the heat- or proteinase-pretreated serovar Shermani detergent extract-stimulated fibronectin induction was reduced compared to untreated leptospiral detergent extract (Fig. 1E). This result suggests that the protein components in serovar Shermani detergent extract may be required to promote fibronectin production.
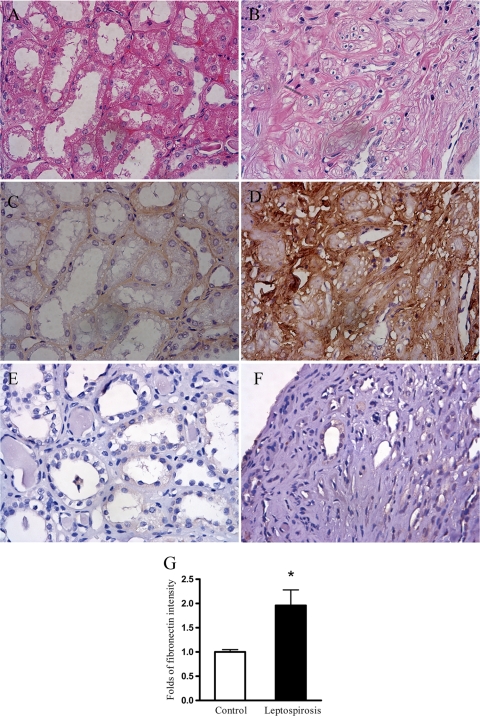
To determine whether fibronectin deposition is increased in patients with leptospirosis, fibronectin immunostaining in kidney specimens obtained from five patients with leptospiral infection was assessed by immunohistochemistry. Renal histology using hematoxylin and eosin (H&E) stain in leptospirosis patients demonstrated prominent tubulointerstitial fibrosis (Fig. 2B). Immunohistochemistry results showed that the fibronectin immunostaining intensity was markedly increased in the kidneys of patients with leptospiral infection (Fig. 2D) compared to that in normal kidney tissues (Fig. 2C). Similarly, type I collagen immunostaining intensity was also increased in leptospirosis patients (data not shown). Immunostaining intensity quantification results confirmed a significant increase in fibronectin deposition in the kidneys of patients with leptospirosis (Fig. 2G).
FIG. 2.
Increased deposition of fibronectin immunostaining in the tubulointerstitium of patients with leptospirosis. The typical renal histology of H&E staining in five leptospirosis patients is shown in panel B. Tubulointerstitial fibronectin deposition in these patients was assessed by immunostaining for fibronectin, as shown in panel D. Normal tissues cut from residual kidney specimens obtained from five nephrectomized patients with renal cell carcinoma were used as the control and stained for H&E staining (A) or fibronectin immunostaining (C). (E and F) A negative control from normal tissues (E) and diseased tissues (F) for fibronectin immunostaining was performed using only the secondary antibody with omission of the primary antibody (anti-fibronectin). (G) The integrated image intensity of fibronectin in eight consecutively selected nonoverlapping and area-fixed fields at a ×400 magnification in each kidney specimen was calculated as described in Materials and Methods. The results are presented as the relative fold immunostaining intensity over that of the control ± the SE (*, P < 0.05, leptospirosis patients versus control patients).
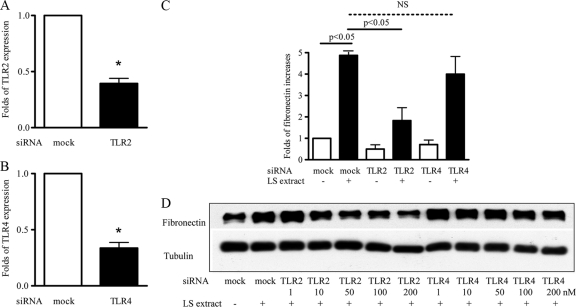
The serovar Shermani detergent extract-induced increase in fibronectin production was mediated through TLR2.
To define the role of TLR2 and TLR4 in serovar Shermani detergent extract-induced increase in fibronectin production, TLR2 and TLR4 gene expressions were manipulated by gene silencing using siRNA (12). Reduced TLR2 and TLR4 gene expression was confirmed by real-time PCR (Fig. 3A and B). Both real-time PCR and Western blot analysis showed that the suppression of TLR2 expression reduced the serovar Shermani detergent extract-induced increase in fibronectin production (Fig. 3C and D). In contrast, inhibition of TLR4 expression did not affect this reaction.
FIG. 3.
Inhibition of serovar Shermani detergent extract-induced increase in fibronectin production by TLR2 gene silencing. HK-2 cells were transfected with TLR2, TLR4, or scramble siRNA (mock) at a concentration of 100 nM for 24 h. Cells were then treated with serovar Shermani detergent extract (0.1 μg/ml) under serum-free condition for further 2 days. TLR2 (A), TLR4 (B), or fibronectin (C) mRNA expression was determined by real time-PCR. The results are expressed as the mean relative fold increase in TLR2, TLR4, or fibronectin mRNA expression compared to the mock transfection ± the SE of triplicate measurements from four independent experiments (*, P < 0.05 versus the mock transfection; NS, no significance). (D) Fibronectin production was assessed by Western blot analysis. The results of one representative experiment of at least three individual replicate experiments are shown.
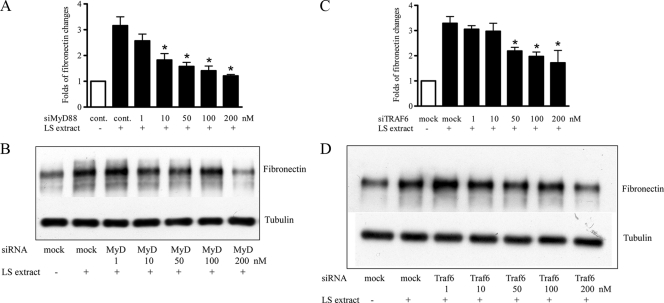
The serovar Shermani detergent extract-induced increase in fibronectin production was mediated through the TLR2-associated signaling pathway.
MyD88 and TRAF6 are important downstream mediators of the TLR2-associated signaling pathway (16). To assess whether MyD88 and TRAF6 are implicated in the serovar Shermani detergent extract-induced increase in fibronectin production, MyD88 and TRAF6 gene expressions were knocked down by siRNA. The results of real-time PCR and Western blot analysis demonstrated that inhibition of MyD88 gene expression by MyD88 siRNA reduced the serovar Shermani detergent extract-induced increase in fibronectin expression and protein production (Fig. 4A and B). In contrast, treatment with control siRNA did not affect this response. Similarly, inhibition of TRAF6 expression by gene silencing led to a reduction in the serovar Shermani detergent extract-induced increase in fibronectin expression (Fig. 4C) and protein production (Fig. 4D).
FIG. 4.
Inhibitory effect of MyD88 and TRAF6 gene silencing in serovar Shermani detergent extract-induced increase in fibronectin production. HK-2 cells were transfected with MyD88 (A and B), TRAF6 (C and D), or scramble siRNA (control) at different concentrations (1 to 200 nM) for 24 h. Cells were then stimulated with serovar Shermani detergent extract (0.1 μg/ml) under serum-free condition for further 2 days. Fibronectin gene expression was assessed by real-time PCR (A and C), and protein production was determined by Western blot analysis (B and D). The results of real-time PCR are expressed as the mean relative fold increase in fibronectin gene expression over that of the untreated group ± the SE of triplicate measurements from four independent experiments (*, P < 0.05). The results of one representative result of Western blot analysis of three individual replicate experiments are shown.
The serovar Shermani detergent extract-mediated increase in fibronectin production required NF-κB activation.
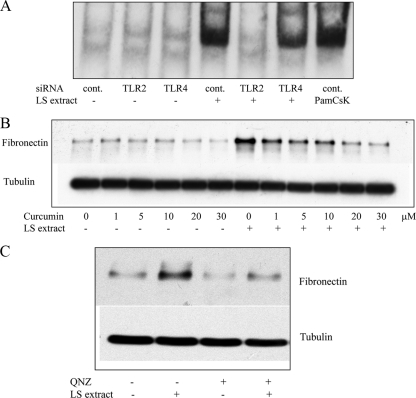
It has been shown that signal transduction of the TLR2-dependent signaling pathway propagates through transcription factors, including NF-κB, to regulate the production of cytokines and chemokines (15). The effect of serovar Shermani detergent extract on NF-κB activation was determined by EMSA to assess the amount of NF-κB binding to its consensus oligonucleotide. The results of EMSA demonstrated that addition of serovar Shermani detergent extract led to an increase in NF-κB transcription factor binding to its specific oligonucleotide (Fig. 5A). Enhancement of NF-κB binding to its oligonucleotide by serovar Shermani detergent extract was abrogated by gene silencing of TLR2 but not TLR4.
FIG. 5.
Suppressive effect of NF-κB inhibition on the serovar Shermani detergent extract-induced increase in fibronectin production. (A) HK-2 cells were treated with serovar Shermani detergent extract (0.1 μg/ml) in the presence or absence of TLR2 or TLR4 siRNA for 90 min. Mock transfection with scramble siRNA was used as a negative control (cont.). Administration of Pam(3)CsK(4) (5 μg/ml) to cells was used as an internal positive control. The NF-κB transcription factor binding to its consensus oligonucleotide was quantified by EMSA as described in Materials and Methods. (B) Cells were stimulated with serovar Shermani detergent extract (0.1 μg/ml) in the presence or absence of different concentrations of curcumin (1 to 30 μM) (B) or QNZ (10 nM) (C) for 2 days. Fibronectin production was determined by Western blot analysis. The results of one representative experiment of at least three individual replicate experiments are shown.
To further determine whether NF-κB was implicated in serovar Shermani detergent extract-induced increase in fibronectin production, an NF-κB inhibitor, curcumin, was used to block NF-κB activation prior to the administration of serovar Shermani detergent extract. The results of Western blot analysis revealed that curcumin abrogated serovar Shermani detergent extract-induced increase in fibronectin production in a dose-dependent manner, suggesting an important role for NF-κB in this reaction (Fig. 5B). Similarly, another NF-κB activation inhibitor, QNZ, also inhibited serovar Shermani detergent extract-induced increase in fibronectin production (Fig. 5C).
A specific TLR2 agonist mimicked the effect of serovar Shermani detergent extract on stimulation of fibronectin production.
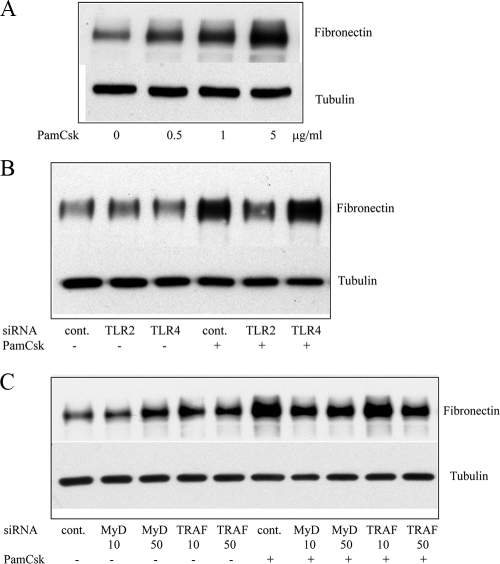
Since serovar Shermani detergent extract-induced increase in fibronectin production in HK-2 cells was mediated through TLR2, we examined whether a specific TLR2 agonist, Pam(3)CSK(4), could also stimulate increased fibronectin production. After 48-h administration of Pam(3)CsK(4) to HK-2 cells, Western blot analysis demonstrated a dose-dependent increase in fibronectin production (Fig. 6A). Specificity of the TLR2 agonist-mediated increase in fibronectin production was confirmed since inhibition of TLR2 but not TLR4 expression blocked Pam(3)CsK(4)-stimulated fibronectin production (Fig. 6B). Furthermore, inhibition of MyD88 gene expression by 10 and 50 nM MyD88 siRNA or of TRAF6 gene expression by 50 nM TRAF6 siRNA reduced the Pam(3)CsK(4)-induced increase in fibronectin production (Fig. 6C).
FIG. 6.
Inhibition of specific TLR2 agonist-stimulated increase in fibronectin production by knockdown of TLR2/MyD88/TRAF6. (A) HK-2 cells were incubated in the presence or absence of Pam(3)CsK(4) (0.5 to 5 μg/ml) under serum-free conditions for 2 days. The cell lysate was subsequently subjected to Western blot analysis to determine fibronectin production. (B and C) HK-2 cells were transfected with scramble siRNA (control), TLR2, or TLR4 at a concentration of 100 nM (B) or with MyD88 or TRAF6 siRNA at different concentrations as indicated (C) for 24 h. The cells were then incubated in the presence or absence of Pam(3)CsK(4) (5 μg/ml) for a further 2 days. Fibronectin production was assessed by Western blot analysis. The results of one representative experiment of at least three individual replicate experiments are shown.
Live serovar Shermani induced increased fibronectin production mainly through TLR2 and MyD88.
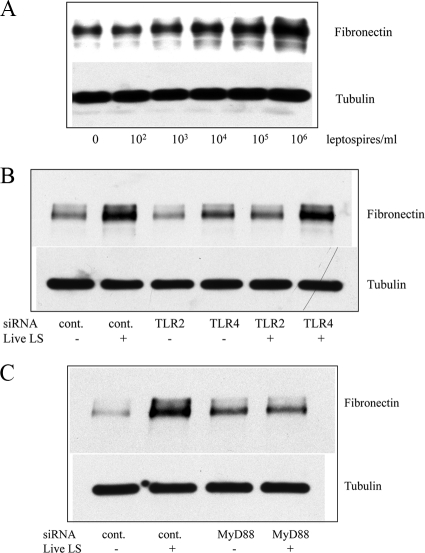
To assess whether live leptospires can also induce fibronectin production, live serovar Shermani was added to HK-2 cells. After 2-day administration of live serovar Shermani at different loads, the results of Western blot analysis showed increased fibronectin production in a dose-dependent manner (Fig. 7A). Since the major component of serovar Shermani detergent extract in triggering fibronectin production is TLR2 and MyD88 dependent (see above), we determined whether TLR2 and MyD88 are also required in live serovar Shermani-induced fibronectin production. After 24-h administration of TLR2 and MyD88 siRNA, live leptospires were added to cells for further 2 days. The results showed that knockdown of either TLR2 or MyD88 gene expression abrogated live serovar Shermani-induced fibronectin production (Fig. 7B and C). In contrast, inhibition of TLR4 gene expression by siRNA did not block this response.
FIG. 7.
Inhibition of live serovar Shermani-induced increase in fibronectin production by TLR2 or MyD88 gene silencing. (A) HK-2 cells (106) were grown in the presence or absence of live serovar Shermani (LS) from 102 to 106 leptospires under serum-free condition for 2 days. (B and C) Cells (106) were transfected with 200 nM TLR2, either TLR4 siRNA (B) or MyD88 siRNA (C), or scramble siRNA (control) for 24 h. Live serovar Shermani (105 leptospires/ml) were added to cells under serum-free condition for 2 days. At the end of the experiments, cell lysates were subjected to Western blot analysis to determine fibronectin production. The results of one representative experiment of at least three individual replicate experiments are shown.
DISCUSSION
The kidney is the main target of acute or chronic leptospiral infection (35). In humans, leptospiral infection has been shown to cause tubulointerstitial fibrosis, leading to chronic renal insufficiency and end-stage renal disease requiring hemodialysis (2, 4). Although it has been well documented that tubulointerstitial fibrosis is an important consequence of leptospiral infection in animal studies, the mechanism underlying this is poorly defined. We previously showed that human renal proximal tubular cells produce ECM proteins, including type I and type IV collagens, in response to serovar Shermani detergent extract stimulation (32). In the present study, we also demonstrated that the addition of serovar Shermani detergent extract to human renal proximal tubular cells led to an increase in another ECM protein, fibronectin.
Although many profibrotic factors such as TGF-β1 have been observed to promote tissue fibrosis, innate immunity has recently also been shown to play a crucial role in the development and progression of tissue fibrosis (27). For example, TLR4 has been implicated in hepatic fibrogenesis since TLR4 mutant mice develop less severe liver fibrosis after bile duct ligation or thioacetamide injection compared to TLR4 wild-type mice, indicating a requirement for TLR4 in the development of liver fibrosis. In cases of pulmonary fibrosis, a consequence of lung injury, Yang et al. have the demonstrated that knockout or blockade of TLR2 expression by a TLR2-neutralizing antibody attenuates the progression of pulmonary fibrosis induced by bleomycin, a TLR2 agonist (39). In the present study, a serovar Shermani detergent extract-induced increase in fibronectin was alleviated when TLR2 was knocked down by TLR2 gene silencing. In contrast, TLR4 knockdown did not alter the effect of serovar Shermani detergent extract on fibronectin production. Furthermore, a specific TLR2 agonist also induced an increase in fibronectin production, confirming an association between the activation of innate immunity and matrix deposition. We have previously demonstrated that serovar Shermani detergent extract induces increased inflammatory chemokines such as nitric oxide (iNOS) and MCP-1 through TLR2 and not TLR4 in mouse proximal tubular cells. The present study also identified a crucial role of TLR2 in pathogenesis of leptospirosis-associated matrix deposition. Although it is well documented that tubulointerstitial inflammation is closely associated with renal fibrosis (22), the molecular link remains elusive. The present study suggests that innate immunity may play a direct role in the initiation of both processes.
Both MyD88 and TRAF6 are fundamental mediators of the TLR-propagated cascade (17). MyD88 is an important adaptor molecule that interacts directly with the TLRs on the cell plasma membrane (19). In the present study, we demonstrate that the serovar Shermani detergent extract-induced increase in fibronectin production was attenuated by silencing MyD88 gene expression. It has been reported that bleomycin-induced pulmonary fibrosis is significantly reduced in MyD88-deficient mice (8). Furthermore, blockade of MyD88 by transfection of rats with dominant-negative MyD88 results in a significant reduction of cardiac hypertrophy and fibrosis in aortic banding-induced pressure overload cardiac fibrosis (9). Similarly, the present study also displayed that inhibition of TRAF6 by gene silencing reduced serovar Shermani detergent extract-induced increase in fibronectin production. Our study therefore suggests that both MyD88 and its downstream adaptor, TRAF6, lie downstream of the serovar Shermani detergent extract-induced activation of TLR and mediate subsequent matrix deposition.
NF-κB is a crucial transcription factor in the TLR-mediated signaling pathways (41). Upon activation of TLR2 and its downstream mediators MyD88 and TRAF6, cytoplasmic NF-κB is eventually translocated into the nucleus to trigger targeted gene expression. We have previously shown that serovar Shermani detergent extract can activate NF-κB, which causes an increase in inflammatory cytokines (37). The present study demonstrated that serovar Shermani detergent extract activated NF-κB and that inhibition of NF-κB by curcumin abrogated the serovar Shermani detergent extract-induced increase in fibronectin production. An implied role of NF-κB activation in liver fibrosis has been documented (6). Chen et al. showed that inhibition of NF-κB by its inhibitor curcumin blocks LPS-induced increased connective tissue growth factor and type I collagen in cultured hepatic stellate cells, suggesting that NF-κB is a critical participant in hepatic fibrosis (3). Our data are consistent with the work on hepatic fibrosis.
Lipoproteins are the most abundant components of the leptospiral total protein profile (5, 10, 42). Over the past few decades, several leptospiral lipoproteins have been shown to be responsible for a leptospirosis-mediated immune response (26). In the present study, serovar Shermani detergent extract after inactivation by pretreatment with heat or proteinase failed to stimulate fibronectin production, suggesting that the protein components in serovar Shermani detergent extracts may play a role in promoting fibronectin production. Interestingly, Roh et al. have demonstrated that different forms of low-density lipoprotein enhance the synthesis of ECM proteins, including fibronectin (23). Whether these findings and our results share molecular mimicry requires further investigation. Nevertheless, the outer membrane extraction method used in the present study may concomitantly harvest protein components and LPS from pathogenic leptospira (11). In fact, the activity of serovar Shermani detergent extract in the stimulation of fibronectin production was partially but not completely abolished by pretreatment with proteinase (Fig. 1E). Since leptospiral LPS is also recognized by TLR2 (20), the activity of serovar Shermani detergent extract could partly be mediated by LPS contained in the extracts. It is a limitation of the present study that we cannot determine the contribution of leptospiral LPS to serovar Shermani detergent extract-induced fibronectin production.
In the present study, live serovar Shermani also induced fibronectin production that was abrogated by inhibition of TLR2 and MyD88 expression. In line with the finding that serovar Shermani detergent extract-induced fibronectin production requires TLR2 and MyD88 functionalities, live leptospire-induced fibronectin deposition also depends on TLR2 and MyD88.
It was recently shown that fibronectin proteolytic fragments can upregulate TLR2 expression in human articular chondrocytes (29). In the present study, serovar Shermani detergent extract promoted fibronectin production through TLR2. It requires further elucidation whether the increase in fibronectin production stimulated by serovar Shermani detergent extract can subsequently enhance TLR2 upregulation, thus causing a positive-feedback loop that amplifies leptospirosis-induced fibrosis.
In conclusion, we showed here that serovar Shermani detergent extract can induce fibronectin production through the TLR2-associated cascades, including its downstream adaptors, MyD88 and TRAF6, and the transcription factor NF-κB, providing evidence of an association between TLRs and leptospirosis-mediated ECM deposition and implying an association between innate immunity and renal fibrosis.
Acknowledgments
This study was supported by grants to Y.-C.T. and C.-W.Y. from the National Science Council of Taiwan.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 20 December 2010.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Atasoyu, E. M., V. Turhan, S. Unver, T. R. Evrenkaya, and S. Yildirim. 2005. A case of leptospirosis presenting with end-stage renal failure. Nephrol. Dial. Transplant. 20:2290-2292. [DOI] [PubMed] [Google Scholar]
- 3.Chen, A., and S. Zheng. 2008. Curcumin inhibits connective tissue growth factor gene expression in activated hepatic stellate cells in vitro by blocking NF-κB and ERK signaling. Br. J. Pharmacol. 153:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covic, A., D. J. Goldsmith, P. Gusbeth-Tatomir, A. Seica, and M. Covic. 2003. A retrospective 5-year study in Moldova of acute renal failure due to leptospirosis: 58 cases and a review of the literature. Nephrol. Dial Transplant. 18:1128-1134. [DOI] [PubMed] [Google Scholar]
- 5.Cullen, P. A., et al. 2005. Surfaceome of Leptospira spp. Infect. Immun. 73:4853-4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsharkawy, A. M., and D. A. Mann. 2007. Nuclear factor-κB and the hepatic inflammation-fibrosis-cancer axis. Hepatology 46:590-597. [DOI] [PubMed] [Google Scholar]
- 7.Farr, R. W. 1995. Leptospirosis. Clin. Infect. Dis. 21:1-8. [DOI] [PubMed] [Google Scholar]
- 8.Gasse, P., et al. 2007. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Invest. 117:3786-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha, T., et al. 2006. Blockade of MyD88 attenuates cardiac hypertrophy and decreases cardiac myocyte apoptosis in pressure overload-induced cardiac hypertrophy in vivo. Am. J. Physiol. Heart Circ. Physiol. 290:H985-H994. [DOI] [PubMed] [Google Scholar]
- 10.Haake, D. A., and J. Matsunaga. 2002. Characterization of the leptospiral outer membrane and description of three novel leptospiral membrane proteins. Infect. Immun. 70:4936-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haake, D. A., et al. 1991. Changes in the surface of Leptospira interrogans serovar Grippotyphosa during in vitro cultivation. Infect. Immun. 59:1131-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung, C. C., et al. 2006. Upregulation of chemokine CXCL1/KC by leptospiral membrane lipoprotein preparation in renal tubule epithelial cells. Kidney Int. 69:1814-1822. [DOI] [PubMed] [Google Scholar]
- 13.Ifon, E. T., et al. 2005. U94 alters FN1 and ANGPTL4 gene expression and inhibits tumorigenesis of prostate cancer cell line PC3. Cancer Cell Int. 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, R. C., and V. G. Harris. 1967. Differentiation of pathogenic and saprophytic leptospires. I. Growth at low temperatures. J. Bacteriol. 94:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai, T., and S. Akira. 2007. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 13:460-469. [DOI] [PubMed] [Google Scholar]
- 16.Kawai, T., and S. Akira. 2007. TLR signaling. Semin. Immunol. 19:24-32. [DOI] [PubMed] [Google Scholar]
- 17.Kawai, T., and S. Akira. 2005. Toll-like receptor downstream signaling. Arthritis Res. Ther. 7:12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 20.Nahori, M. A., et al. 2005. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J. Immunol. 175:6022-6031. [DOI] [PubMed] [Google Scholar]
- 21.Penna, D., et al. 1963. Kidney biopsy in human leptospirosis. Am. J. Trop. Med. Hyg. 12:896-901. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Iturbe, B., R. J. Johnson, and J. Herrera-Acosta. 2005. Tubulointerstitial damage and progression of renal failure. Kidney Int. 2005(Suppl.):S82-S86. [DOI] [PubMed] [Google Scholar]
- 23.Roh, D. D., V. S. Kamanna, and M. A. Kirschenbaum. 1998. Oxidative modification of low-density lipoprotein enhances mesangial cell protein synthesis and gene expression of extracellular matrix proteins. Am. J. Nephrol. 18:344-350. [DOI] [PubMed] [Google Scholar]
- 24.Satriano, J., and D. Schlondorff. 1994. Activation and attenuation of transcription factor NF-κB in mouse glomerular mesangial cells in response to tumor necrosis factor-alpha, immunoglobulin G, and adenosine 3′:5′-cyclic monophosphate. Evidence for involvement of reactive oxygen species. J. Clinical Inv. 94:1629-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreier, S., W. Triampo, G. Doungchawee, D. Triampo, and S. Chadsuthi. 2009. Leptospirosis research: fast, easy, and reliable enumeration of mobile leptospires. Biol. Res. 42:5-12. [PubMed] [Google Scholar]
- 26.Schroder, N. W., J. Eckert, G. Stubs, and R. R. Schumann. 2008. Immune responses induced by spirochetal outer membrane lipoproteins and glycolipids. Immunobiology 213:329-340. [DOI] [PubMed] [Google Scholar]
- 27.Seki, E., et al. 2007. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13:1324-1332. [DOI] [PubMed] [Google Scholar]
- 28.Sterling, C. R., and A. B. Thiermann. 1981. Urban rats as chronic carriers of leptospirosis: an ultrastructural investigation. Vet. Pathol. 18:628-637. [DOI] [PubMed] [Google Scholar]
- 29.Su, S. L., C. D. Tsai, C. H. Lee, D. M. Salter, and H. S. Lee. 2005. Expression and regulation of Toll-like receptor 2 by IL-1β and fibronectin fragments in human articular chondrocytes. Osteoarthritis Cartilage 13:879-886. [DOI] [PubMed] [Google Scholar]
- 30.Taylor, P. L., L. E. Hanson, and J. Simon. 1970. Serologic, pathologic, and immunologic features of experimentally induced leptospiral nephritis in dogs. Am. J. Vet. Res. 31:1033-1049. [PubMed] [Google Scholar]
- 31.Tian, Y. C., et al. 2007. Epidermal growth factor and transforming growth factor-β1 enhance HK-2 cell migration through a synergistic increase of matrix metalloproteinase and sustained activation of ERK signaling pathway. Exp. Cell Res. 313:2367-2377. [DOI] [PubMed] [Google Scholar]
- 32.Tian, Y. C., et al. 2006. Leptospiral outer membrane protein induces extracellular matrix accumulation through a TGF-β1/Smad-dependent pathway. J. Am. Soc. Nephrol. 17:2792-2798. [DOI] [PubMed] [Google Scholar]
- 33.Wang, L., et al. 2007. Effects of retinoic acid on the development of liver fibrosis produced by carbon tetrachloride in mice. Biochim. Biophys. Acta 1772:66-71. [DOI] [PubMed] [Google Scholar]
- 34.Werts, C., et al. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 35.Yang, C. W. 2007. Leptospirosis renal disease: understanding the initiation by Toll-like receptors. Kidney Int. 72:918-925. [DOI] [PubMed] [Google Scholar]
- 36.Yang, C. W., et al. 2006. Toll-like receptor 2 mediates early inflammation by leptospiral outer membrane proteins in proximal tubule cells. Kidney Int. 69:815-822. [DOI] [PubMed] [Google Scholar]
- 37.Yang, C. W., et al. 2000. Leptospira outer membrane protein activates NF-κB and downstream genes expressed in medullary thick ascending limb cells. J. Am. Soc. Nephrol. 11:2017-2026. [DOI] [PubMed] [Google Scholar]
- 38.Yang, C. W., et al. 2002. The Leptospira outer membrane protein LipL32 induces tubulointerstitial nephritis-mediated gene expression in mouse proximal tubule cells. J. Am. Soc. Nephrol. 13:2037-2045. [DOI] [PubMed] [Google Scholar]
- 39.Yang, H. Z., et al. 2009. Targeting TLR2 attenuates pulmonary inflammation and fibrosis by reversion of suppressive immune microenvironment. J. Immunol. 182:692-702. [DOI] [PubMed] [Google Scholar]
- 40.Yang, R. B., et al. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, G., and S. Ghosh. 2001. Toll-like receptor-mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 107:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuerner, R. L., W. Knudtson, C. A. Bolin, and G. Trueba. 1991. Characterization of outer membrane and secreted proteins of Leptospira interrogans serovar Pomona. Microb. Pathog. 10:311-322. [DOI] [PubMed] [Google Scholar]