Abstract
Optimal hepatic resistance to Leishmania donovani in mice requires the coordinated effort of a variety of leukocyte populations that together induce activation of local macrophages to a leishmanicidal state. Although nitric oxide and reactive oxygen intermediates are potent leishmanicidal effector molecules operating in the acquired phase of immunity, there have long been suggestions that other mechanisms of leishmanicidal activity exist. We recently discovered that Irf-7 regulates a novel innate leishmanicidal response in resident splenic macrophages that line the marginal zone. Here, we tested whether this mechanism also operates in Kupffer cells, the resident macrophage population of the liver and the major target for hepatic infection by L. donovani. Comparing the Kupffer cell responses in situ in B6 and B6.Irf-7−/− mice, we found no evidence that Irf-7 affected amastigote uptake or early survival. However, we did find that Irf-7-deficient mice had impaired acquired resistance to hepatic L. donovani infection. This phenotype was attributable to a reduction in the capacity of hepatic CD4+ T cells, NK cells, and NKT cells to produce gamma interferon (IFN-γ) and also to defective induction of NOS2 in infected Kupffer cells. Our data therefore add interferon regulatory factor 7 (IRF-7) to the growing list of interferon regulatory factors that have effects on downstream events in the acquired cellular immune response to nonviral pathogens.
The leishmaniases remain a global health problem, and an understanding of the mechanisms that underpin host resistance and susceptibility to these parasites is likely to have an important bearing on the development of new drugs and vaccines for human use. Leishmania parasites reside within the phagosomal compartment of their host cell, and this cellular location dictates the options available for parasite elimination. A variety of mechanisms have been shown to operate to contain and/or kill intracellular amastigotes within phagosomes. Early genetic studies with mice identified a natural resistance gene (Scl11a1) operating in macrophages to limit the growth rate of L. donovani and L. chagasi (6, 7, 19, 40). In contrast to the leishmanistatic effects of Slc11a1, the activation of the respiratory burst can be leishmanicidal (25), and mice defective in gp91(phox) have increased early susceptibility to hepatic infection with L. donovani (26), in spite of the fact that Kupffer cells (KCs) have a limited capacity for secretion of reactive oxygen intermediates (ROIs) (20). Further studies demonstrated the more dominant effect of NOS2-regulated nitric oxide production for the resolution of L. donovani infection in mice (26). Although there is an overwhelming body of data supporting the role of NO and ROIs as mediators of leishmanicidal activity, other pathways may remain to be uncovered. For example, in doubly phox/NOS2-deficient mice, an alternative mechanism of control serves to limit relapse following chemotherapy (27). For some intracellular pathogens, including L. major, a significant role for small GTPases has been identified (38).
We recently demonstrated that Irf-7 had a profound effect on the leishmanicidal activities of splenic marginal zone macrophages (MZM) and marginal metallophilic macrophages (MMM) in situ and of a stromal macrophage cell line (14M1.4) in vitro (12, 30). Unlike many other populations of macrophages studied in vitro and in vivo, these macrophages expressed an innate gamma interferon (IFN-γ)-independent level of leishmanicidal activity that was capable of reducing the intracellular parasite burden by ∼60% within 24 h of infection (30). An examination of genes differentially expressed between 14M1.4 macrophages and a Leishmania-permissive macrophage population (RAW264.7) identified a strong type I interferon-associated transcriptional signature that accompanied infection of 14M1.4 macrophages, including upregulation of Irf-7. Gene knockdown experiments using RNA interference (RNAi) and infection of Irf-7 −/− mice confirmed a functional role for this transcription factor in the early host response to L. donovani, that could not be bypassed by exogenous IFN-α and that was independent of NOS2 (30). The long-term consequences of Irf-7 deficiency were not established at that time, however.
Although interferon regulatory factor 7 (IRF-7) has not been studied in the context of acquired immunity to any Leishmania species to date, a number of other IRFs have been studied from this perspective. Irf-1 is involved in the downregulation of interleukin-12 (IL-12) in dendritic cells (DC) observed following infection with L. amazonensis (41). L. major induces IRF1 and IRF8 in human DC, and their protein products bound to the IL-12p35 promoter (15). L. donovani promastigotes stimulate IRF-1 expression in an lipophosphoglycan (LPG)-dependent manner in murine J774.1 cells (2). However, in a family study in a region of Brazil where visceral leishmaniasis (VL) is endemic, no genetic association was found between delayed-type hypersensitivity (DTH) positivity, a marker of resistance to L. chagasi, and single-nucleotide polymorphisms (SNPs) in Irf-1 (16). Irf-4 has been shown to play a T cell-intrinsic role in Th2 development in L. major (39). Intriguingly, Irf-8 has been shown to be a regulator of Slc11a1, acting to coordinate gene expression through a mechanism involving c-myc and Miz-1 (1). Irf-8 also plays a role in Th1 determination by acting as a regulator of IL-12 production, and Irf-8−/− mice were highly susceptible to L. major infection (11).
In this report, we show that B6.Irf-7−/− mice have a reduced capacity to mount an effective hepatic response to L. donovani infection. In contrast to splenic macrophages, Kupffer cells (KCs) did not express innate Irf-7-dependent leishmanicidal activity toward amastigotes. Nevertheless, the peak parasite burden in B6.Irf-7−/− mice was increased by ∼4-fold compared to that in wild-type B6 mice. The reduced hepatic resistance of B6.Irf-7−/− mice was shown to be associated with reduced IFN-γ production within the hepatic CD4+ T cell, NK cell, and NKT cell compartments and with a commensurate rise in the frequency of CD4+ T cells making IL-10. This altered cytokine environment in the liver was reflected by severely compromised NO production by infected KCs at the core of the hepatic granuloma. Our data add IRF-7 to the list of interferon regulatory factors that influence host resistance to Leishmania species.
MATERIALS AND METHODS
Animals and parasites.
C57BL/6 mice (Charles River Laboratories, Margate, United Kingdom) were used at 6 to 10 weeks of age and housed under specific-pathogen-free conditions. B6.Irf-7−/− mice (backcrossed to B6 for >8 generations [14]) were obtained from the RIKEN Bioresource Center (Ibaraki, Japan) with kind permission of T. Taniguchi, University of Tokyo, Japan. L. donovani (LV9) and tdTomato-expressing L. donovani (4) were maintained by passage in RAG-2−/− mice. Mice were infected by injecting 3 × 107 amastigotes intravenously (i.v.) via the lateral tail vein. Mice were killed by cervical dislocation. Parasite burdens were determined from Giemsa-stained impression smears and expressed as Leishman-Donovan units (LDU) (number of parasites per 1,000 host cell nuclei × organ weight). Granuloma maturation was assessed from hematoxylin-eosin (H&E)-stained tissue sections as described elsewhere (34). All animal experiments were approved by the University of York Animal Procedures and Ethics Committee and performed under United Kingdom Home Office license.
Confocal microscopy.
Confocal microscopy was performed on 8- to 20-μm frozen sections. For tissue containing tdTomato-expressing parasites, tissue was fixed in 4% paraformaldehyde (PFA) for 2 h before incubation in 30% sucrose overnight and embedding in optimal-cutting-temperature (OCT) medium (Sakura). Sections were stained with Alexa 488-conjugated F4/80 (eBioscience, United Kingdom). Parasite burdens were evaluated with three-dimensional (3D) reconstructed images generated from z-stacked 0.8- to 1-μm optical slices. 3D rendering of serial sections was generated using Volocity software (Improvision) (4). For all other sections, tissue was snap-frozen in OCT and sections fixed in ice-cold acetone. F4/80, CD11c, and CD11b antibodies were conjugated to Alexa 488 or Alexa 647 (eBioscience) and purified rabbit anti-mouse inducible nitric oxide synthase (iNOS) (Abcam, United Kingdom) detected with donkey anti-rabbit Alexa 647. Sections were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), mounted in Pro-Long Gold antifade (Invitrogen), and visualized using a Carl Zeiss inverted LSM META 510 confocal microscope. Quantification of NOS2 staining was performed on randomly selected fields for each mouse (≥10 mice for each time point). The degree of staining was detected using Adobe Photoshop CS3, and the fractions of positively stained pixels relative to the number of pixels for field of view and within granulomas (depicted by F4/80) were counted and expressed as percentage of area positive per field of view or per granuloma.
Flow cytometry and cell sorting.
Hepatic nonparenchymal cells (including myeloid and lymphoid cells) were prepared from the livers of naïve or infected wild-type and Irf-7−/− mice without collagenase digestion as previously described (4). Isolated cells were labeled with fluorochrome-conjugated antibodies to NK1.1 (eBioscience, United Kingdom), CD3 (BioLegend), and CD19, B220, CD4, CD8, DX5, CD11c, GR-1, F4/80, and CD11b (eBioscience, United Kingdom). Intracellular cytokine staining was performed on phorbol myristate acetate (PMA)-ionomycin-restimulated liver cell suspensions incubated with brefeldin A at 37°C for 4 h. Cells were surface stained with phycoerythrin (PE)-Cy7-conjugated anti-CD3, peridinin chlorophyll protein (PerCP)-conjugated anti-CD4, allophycocyanin (APC)-Cy7-conjugated anti-CD8, Alexa Fluor 647-conjugated F4/80, PE-conjugated anti-CD11b (BD Pharmingen), CD11c-PE-Cy7, and NK1.1-PE (e-Bioscience). LIVE/DEAD fixable aqua dead-cell stain (Invitrogen) was added in some experiments, and cells were then fixed with 2% paraformaldehyde, permeabilized with 0.5% saponin, and stained with Pacific blue-conjugated anti-IFN-γ, PE-conjugated anti-tumor necrosis factor alpha (anti-TNF-α), and APC-conjugated anti-IL-10 (eBioscience). B cell frequency was assessed by staining spleen cell suspensions with PerCP-conjugated anti-CD45R (BD Pharmingen). Cells were analyzed using a CyAn flow cytometer and Summit software (Beckman Coulter).
Real-time RT-PCR.
RNA was isolated from livers by using an RNeasy kit according to the manufacturer's instructions (Qiagen). RNA was then reverse transcribed into cDNA using the first-strand cDNA synthesis kit according to the manufacturer's instructions (Invitrogen). Oligonucleotides (5′ to 3′) used for the specific amplification were for IL-10 and hypoxanthine phosphoribosyltransferase (HPRT) (1a), IFN-γ (forward, CCTCCTGCGGCCTAGCTC; reverse, GTAACAGCCAGAAACAGCCATG), and NOS2 (forward, CCCTTCCGAAGTTTCTGGCAGCAGCAGC; reverse, GGCTGTCAGAGCCTCGTGGCTTTGG). Real-time quantitative reverse transcription-PCR (RT-PCR) was performed with the SYBR green PCR kit in an ABI Prism 7000 sequence detection system (Applied Biosystems) according to the manufacturer's instructions. Expression of target genes was normalized to HPRT and expressed as relative expression using the change in cycle threshold (ΔΔCT) analysis method (relative expression of the gene in the naïve compared to the infected counterpart).
Statistical analysis.
Data were analyzed using one-way Kruskal-Wallis analysis of variance (ANOVA) or Student's t test as appropriate. All experiments were performed independently at least twice.
RESULTS
Characterization of the immune status of B6.Irf-7−/− mice.
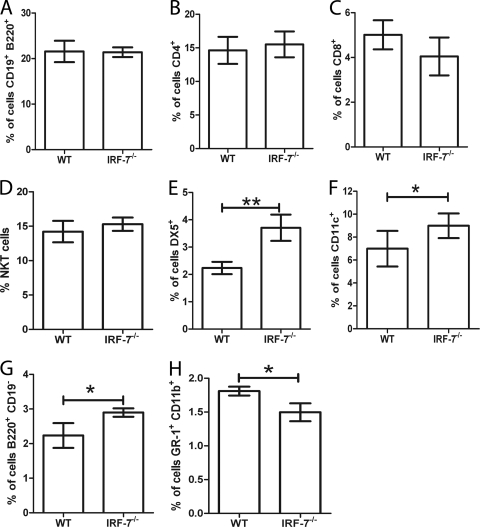
Mice defective in other interferon regulatory factor genes, including Irf-8 and Irf-4, have been shown to have a range of immune dysfunctions in the steady state, but a formal study of Irf-7-defective mice has not previously been published. We therefore first evaluated a number of cellular parameters in B6 and B6.Irf-7−/− mice, focusing particularly on cells and tissues associated with the host response during experimental VL (EVL). Comparing the livers of uninfected B6 and B6.Irf-7−/− mice, we found no differences in the number, frequency, or distribution of KCs (Fig. 1 A to E), intrahepatic B cells (Fig. 2 A), CD4+ or CD8+ T cells (Fig. 2B and C), or NKT cells (Fig. 2D). In contrast, we noted an increase in the number of NK cells (Fig. 2E) and small but significant increases in the numbers of CD11c+ F4/80− resident DC (Fig. 2F) and B220+ CD19− cells (presumptive γδ T cells) (Fig. 2G), which was somewhat offset by a reduction in Gr-1+ CD11b+ cells (presumptive granulocytes) (Fig. 2H). Similarly, in spleen no significant changes were observed in lymphoid tissue architecture (assessed by SIGNR1, CD169, ERTR-7, MAdCAM-1, CD19, and CD3) (reference 30 and data not shown). The numbers and distributions of the three CD11chi MHCIIhi splenic conventional DC (cDC) subsets were equivalent in steady-state B6 and B6.Irf-7−/− mice (B. M. J. Owens et al., unpublished data). Hence, Irf-7-deficient mice have a substantially intact immune system at steady state, compared to mice with other interferon regulatory factor deficiencies (37).
FIG. 1.
Frequency and distribution of KCs in B6.Irf-7−/− mice. (A and B) Eight-micrometer-thick liver sections from B6 (A) and B6.Irf-7−/− (B) mice were stained with anti-F4/80 (green), and nuclei were counterstained with DAPI (blue) Scale bar, 42 μm. (C and D) Representative fluorescence-activated cell sorter (FACS) plots of F4/80+ CD11b+ cells in livers of B6 (C) and B6.Irf-7−/− (D) mice. (E) Frequency of F4/80+ CD11b+ cells in livers of B6 (wild type [WT]) and B6.Irf-7−/− mice (n = 3 mice per group from one of two independent experiments; error bars indicate standard deviations [SD]).
FIG. 2.
Hepatic nonparenchymal cell populations in B6 versus B6.Irf-7−/− mice. Flow cytometric analysis of B cells (CD19+ B220+) (A), CD4 T cells (B), CD8 T cells (C), NKT cells (D), NK cells (E), dendritic cells (F), B220+ CD19− cells (G), and granulocytes (H) in livers from B6 (WT) and B6.Irf-7−/− mice is shown. Data are from two independent experiments with three to five mice per group per experiment. Data are expressed as mean absolute cell numbers ± SD. *, P ≤ 0.05; **, P ≤ 0.01.
Kupffer cells do not express IRF-7-dependent innate leishmanicidal activity.
In macrophages of the splenic marginal zone, IRF-7 regulates the extent to which intracellular amastigotes survive over the first 24 h postinfection (p.i.) (30). To determine whether the survival of L. donovani amastigotes within Kupffer cells (KCs), the principal host cell for this parasite in the liver (4), was also affected by IRF-7 deficiency, we infected wild-type B6 and B6.Irf-7−/− mice with transgenic tdTomato-expressing L. donovani. At 5 h, 24 h, and 48 h p.i., the parasite burden in individual KCs in F4/80-stained tissue sections was evaluated, using 3D reconstructed z-stacked images to obtain a full-thickness view of each individual KC. As shown in Fig. 3 A to C and Movie S1 in the supplemental material, KCs infected with L. donovani were readily detected by this approach. Unlike in spleens of B6 mice, where macrophages of the marginal zone reduce their parasite load by ∼50 to 60% over the first 24 h p.i. (30), the parasite burden in KCs of B6 mice did not decrease significantly over this time period, as determined either by the percentage of infected KCs (Fig. 3D) or by the number of parasites present within each KC (Fig. 3E). These data indicate that KCs lack the innate leishmanicidal activity of resident splenic macrophages, a finding consistent with earlier data from tritium-labeling studies that demonstrated the permissiveness of KCs for amastigote growth over the first 48 h of infection (8). Furthermore, we found no evidence to suggest that Irf-7-deficiency affected either amastigote uptake or subsequent survival within KCs during the first 24 h p.i. By 48 h p.i. there was, however, a small but significant increase in parasite burden in KCs of B6.Irf-7−/− mice (Fig. 3C and E). Hence, at the level of innate macrophage function, Irf-7 acts in a tissue-specific manner.
FIG. 3.
Kupffer cells from B6 and B6.Irf-7−/− mice lack innate leishmanicidal activity. (A and B) Liver sections (10 to 20 μm) from tdTomato-expressing L. donovani-infected B6 and B6.Irf-7−/− mice were stained with F4/80 to identify infected KCs. Representative sections without (A) and with (B) F4/80 staining (green) show localization of tdTomato-expressing amastigotes (red). Scale bars, 42 μm. (C) KCs (green) infected with tdTomato-expressing amastigotes (red). Scale bar, 6 μm. Nuclei were counterstained with DAPI (blue). A z-stack projection is shown in Movie S1 in the supplemental material. (D) Numbers of infected KCs in livers of B6 mice (white bars) and B6.Irf-7−/− mice (gray bars) at 24 h and 48 h following infection. Data are shown as percent infected KCs relative to percent infected at 5 h p.i. (E) Numbers of amastigotes per KC in B6 mice (white bars) and B6.Irf-7−/− mice (gray bars) at the indicated times p.i. Data are expressed as means ± SD from two independent experiments where n = 3 to 5 mice at each time point per experiment *, P < 0.05; **, P < 0.01, ns, not significant.
Irf-7-deficient mice have diminished early control over hepatic L. donovani infection.
Having established that amastigote uptake and survival over the first 24 h of hepatic infection were similar in B6 and B6.Irf-7−/− mice, it was then possible to compare the long-term outcome of hepatic infection without the confounding issue of altered initial parasite burden. Therefore, we followed the course of hepatic infection over a 56-day period. As anticipated from previous studies, the hepatic parasite burden in B6 mice increased to day 14 p.i. and was subsequently brought under control by day 56 p.i., and in this series of experiments, B6 mice had already begun to show expression of immunity by day 28 p.i. (Fig. 4). In B6.Irf-7−/− mice the parasite burden was significantly higher at both day 14 (3-fold) (614 ± 433 LDU in B6 mice and 1,839 ± 384 LDU in B6.Irf-7−/− mice) and day 28 (4.1-fold) (545 ± 194 LDU in B6 mice and 2,240 ± 1,787 LDU in B6.Irf-7−/− mice). Nevertheless, B6.Irf-7−/− mice ultimately also cleared their hepatic parasite burden (Fig. 4). Of interest, the parasite burden was not significantly different in the spleens of these infected mice at any time point measured, in spite of the initial differences in parasite burden in the marginal zone (data not shown), again arguing for differences in the regulation of host resistance at this site (9). Thus, B6.Irf-7−/− mice have a selective defect in their expression of hepatic resistance that operates after initial amastigote-KC interactions.
FIG. 4.
Early acquired resistance to hepatic L. donovani infection is impaired in B6.Irf-7−/− mice. (A) Liver parasite burdens measured during the course of infection with L. donovani in B6 and B6.Irf-7−/− mice. Data represent the means ± SD from three independent experiments where n = 8 to 12 mice per time point. The parasite load was significantly different at days 14 and 28. (B) Number of hepatic granulomas per 50 fields of view (F.O.V.) in B6 mice (white bars) and B6.Irf-7−/− (gray bars) mice at indicated times p.i. (C) Granuloma maturation in B6 mice and B6.Irf-7−/− mice, scored as no reaction, minimal/immature reaction, mature reaction, or resolving reaction. Data are expressed as mean frequency ± SD of each maturation stage and are derived from 10 mice per time point from three independent experiments. (D and E) Representative hematoxylin- and eosin-stained liver sections showing the histopathology observed in the livers of B6 (D) and B6.Irf-7−/− (E) mice. The original magnification was ×40 (day 14) or ×100 (days 28 and d56). Arrows indicate amastigote-infected cells. **, P < 0.01; ***, P < 0.0001.
The hepatic granulomatous response is intact in Irf-7-deficient mice.
Hepatic infection with L. donovani leads to a characteristic granulomatous response, and this can be quantified either as the number of granulomas per unit area of liver tissue or by determining the maturation level of individual granulomas (34). A comparison of these parameters showed no significant differences between the granulomatous responses in B6 and B6.Irf-7−/− mice (Fig. 4B to E) in spite of the significant difference in parasite burden (Fig. 4A). Hence, the increased parasite burden in B6.Irf-7−/− mice was not a consequence of a failure to mount or a lack of maturation of the granulomatous tissue response.
IRF-7 deficiency alters the balance of IFN-γ and IL-10 production in the livers of L. donovani-infected mice.
To understand the mechanism behind the increased parasite load observed within hepatic granulomas, we assessed their functionality using quantitative RT-PCR to analyze a broad range of pro- and anti-inflammatory cytokines. At the level of mRNA accumulation, we did not observe a significant difference in the IFN-γ responses of B6 mice and B6.Irf-7−/− mice, whereas IL-10 mRNA accumulation was significantly elevated at early times in B6.Irf-7−/− mice compared to B6 mice (Fig. 5 A and B). To more precisely evaluate cytokine production and identify the cellular source of IL-10 and IFN-γ, we used intracellular flow cytometry to analyze IFN-γ and IL-10 production at the protein level. At day 28 p.i., B6 mice had an increased frequency of IFN-γ-producing CD4+ T cells compared to B6.Irf-7−/− mice (Fig. 5C), whereas the reciprocal was observed for IL-10-producing CD4+ T cells (Fig. 5D). The frequency of IFN-γ+ IL-10+ CD4+ T cells was minimal in this series of experiments, though there was a trend toward an increase in the frequency of these cells in infected compared to naïve B6 mice but not in B6.Irf-7−/− mice (Fig. 5E). IFN-γ responses were also raised in infected B6 mice compared to B6.Irf-7−/− mice within both the hepatic NK cell (Fig. 5F) and NKT cell (Fig. 5G) populations. IFN-γ production by CD8+ T cells was increased in B6 mice (4.13% ± 2.3% versus 11.15% ± 2.8% in naïve versus infected mice), whereas the percentage of IFN-γ+ CD8+ T cells was reduced after infection of B6.Irf-7−/− mice, albeit from a higher resting state (9.98% ± 2.7% versus 5.58% ± 1.5% in naïve and infected mice, respectively). In contrast to these divergent results in the liver, the splenic T cell responses in the two strains were similar. Thus, the percentage of CD4+ T cells producing IFN-γ increased from 4.38% ± 0.4% to 13.7% ± 0.2% in naïve versus day 35 infected B6 mice and from 4.17% ± 0.05% to 13.98% ± 0.85% in naïve versus day 35 infected B6.Irf-7−/− mice. Similarly, the percentages of CD8+ T cells producing IFN-γ in naïve and infected B6 and B6.Irf-7−/− mice were 16.13% ± 2.26% and 17.56% ± 0.2% versus 17.83% ± 0.5% and 18.92% ± 0.3%, respectively. Therefore, B6.Irf-7−/− mice had a restricted capacity to make IFN-γ in the liver microenvironment, which was reflected by a reciprocal increase in hepatic T cell-derived IL-10.
FIG. 5.
Irf-7 deficiency alters hepatic cytokine responses. (A and B) Hepatic IFN-γ (A) and IL-10 (B) mRNA levels in B6 mice (WT) and B6.Irf-7−/− mice at the indicated times p.i. with L. donovani. Data are presented relative to mRNA accumulation in naïve controls. (C to E) At 28 days p.i., hepatic CD3+ CD4+ cells from B6 mice (WT) and B6.Irf-7−/− mice were restimulated with PMA-ionomycin and then analyzed for expression of intracellular IFN-γ (C), IL-10 (D), and both IFN-γ and IL-10 (E). (F to H) Other cellular sources of IFN-γ were identified, including CD11c+ F4/80+ cells (F), NK cells (G), and NKT cells (H). Data are presented as means ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Granulomas in B6.Irf-7−/− mice are deficient in NOS2.
NOS2 is known to be a major mediator of leishmanicidal activity in hepatic granulomas (26). As NOS2 is regulated differentially regulated by IFN-γ and IL-10, we examined the livers of B6 and B6.Irf-7−/− mice for NOS2 expression. In B6 mice, the accumulation of NOS2 mRNA in total liver increased ∼10-fold over that observed at day 14 p.i. (Fig. 6 A), commensurate with the reduction in parasite burden seen at this time (Fig. 4A). By day 56 p.i. NOS2 mRNA accumulation in B6 mice had begun to decrease. In contrast, in B6.Irf-7−/− mice, though NOS2 mRNA accumulation was also increased at day 28 compared to day 14 p.i., this response was muted compared to that in B6 mice (∼3-fold). Furthermore, the levels of NOS2 mRNA accumulation remained high at day 56 p.i. (Fig. 6A). To more specifically address the question of NOS2 levels specifically within granulomas, we used immunohistochemistry. NOS2 was readily detected in the granulomas of day 28 infected B6 mice but was far less abundant in granulomas of B6.Irf-7−/− mice (Fig. 6B and C). To quantify these results, we used computer-assisted morphometric analysis to determine the extent of NOS2 staining both in random areas of hepatic tissue (Fig. 6D) and also circumscribed within the cross-sectional area of individual granulomas (Fig. 6E). By both of these measures, the level of NOS2 staining was significantly reduced in B6.Irf-7−/− mice compared to B6 mice. Therefore, the skewed hepatic cytokine response and reduced host resistance of B6.Irf-7−/− mice compared to B6 mice correlates with defective local NOS2 production.
FIG. 6.
IRF-7 deficiency leads to diminished NOS2 in hepatic granulomas. (A) Hepatic NOS2 mRNA levels in B6 mice (WT) and B6.Irf-7−/− mice at the indicated times p.i. with L. donovani. Data are presented relative to mRNA accumulation in naïve mice. (B to E) Ten- to 20-μm-thick liver sections from B6 (B) and B6.Irf-7−/− (C) mice at 28 days p.i. were stained for F4/80 (green) and NOS2 (red). Scale bar, 42 μm. Quantification of NOS2 expression in liver tissue from B6.Irf-7−/− mice and B6 mice was compared on the bases of field of view (D) and per granuloma (E) using computer-assisted morphometric analysis *, P < 0.05; ***, P < 0.0001. Data are expressed as means ± SD.
DISCUSSION
In the splenic marginal zone, MZM and MMM rapidly phagocytose blood-borne L. donovani amastigotes after intravenous inoculation and over the following 24 h exert innate leishmanicidal activity that reduces the parasite burden at this site by ∼60% (12, 30). We recently showed, using a combination of gene expression profiling, RNAi, and gene-targeted mice, that Irf-7 played a key role in regulating this early leishmanicidal activity, operating in a manner that could not be bypassed by exogenous type I IFNs and was independent of NO (30). However, in the current study, we have not found any evidence that a similar mechanism operated in KCs, which similarly acquire amastigotes rapidly from the circulation. Neither the level of entry nor the subsequent survival of amastigotes over 48 h was affected in a major way by Irf-7 deficiency. By using full-thickness z-stack reconstructions of individual KCs, we were able to determine the level of infection within each KC to a degree of accuracy not previously possible in situ. This analysis showed that whereas the percentage of infected KCs decreased significantly from 24 h to 48 h p.i., there was no accompanying change in the number of amastigotes per infected KC between these two time points. There are two nonexclusive explanations for this finding. First, KCs may represent a heterogeneous population, with differing capacities for leishmanicidal activity. In support of this possibility, recent studies have identified two populations of KCs that differ in their dependence on bone marrow for their renewal and in their capacity for migration following an inflammatory insult (18). To date, potential differences in antimicrobicidal activity between these two populations have not been explored. Second, KC activation for antileishmanial activity may be dependent upon extrinsic signals provided in trans by a cell(s) whose activity or number is rate limiting. In this regard, we have previously shown that NK cells, invariant NKT (iNKT) cells, and noninvariant NKT cells are all stimulated during L. donovani infection to produce IFN-γ but that only iNKT cell-produced IFN-γ is sufficient to induce KC expression of CXCL10 (35). These two options may not be mutually exclusive. For example, in order to stimulate maximal IFN-γ from NKT cells, KCs express SIRPα, yet only ∼80% of infected KCs had demonstrably upregulated SIRPα by 24 h p.i. (5). Further studies on KC heterogeneity are clearly warranted, but in any event, it is clear from our data that this late stage of KC activation (24 h to 48 h) as well as the early innate response of KCs (5 h to 24 h) are independent of functional IRF-7.
IRF-7 is most closely associated with the amplification of the type I IFN response (37). In plasmacytoid DC, IRF-7 is constitutively expressed at high levels and underlies the capacity of these cells to make a robust type I IFN (13, 14). In most other cells, including most macrophage populations studied to date, basal IRF-7 expression is low and the levels of Irf-7 mRNA accumulation and IRF-7 protein expression are tightly regulated by the immediate IFN-β response to viral infection or Toll-like receptor (TLR) ligation (37). However, low-level expression of IRF-7 is not a feature of all macrophages. While RAW264.7 macrophages had minimal IRF-7 and failed to induce an IRF-7 response following L. donovani infection, expression of IRF-7 was significantly greater at rest in the stromal macrophage cell line 14M1.4, and the response to L. donovani infection in this cell line, measured by the global gene expression profile, shows the characteristic hallmarks of a type I IFN-IRF-7-mediated response (30). Similarly, MZM and MMM in situ rapidly upregulated their expression of IRF-7 to a level detected by immunohistochemistry within 5 h of infection. In contrast, we have not to date been able to identify IRF-7 in KCs (L. Beattie, unpublished data). Though this may reflect a sensitivity issue, it is nevertheless in keeping with the lack of early IRF-7-dependent leishmanicidal activity reported here and suggests that KCs may have lower basal levels of IRF-7 than some resident splenic macrophages. Together, these findings serve to further highlight the distinct properties and functional potential of different tissue macrophage populations.
The link between Irf-7 and other IRF genes that are intimately involved in myeloid cell differentiation (37) made it important to more fully characterize the phenotype of B6.Irf-7−/− mice. We found no obvious differences in KCs (this paper) or in the subsets of conventional DC (Owens, unpublished data) in these mice, indicating that while some of the downstream effects of Irf-7 deficiency may be indirectly attributable to Irf-7-dependent regulation of other IRF genes (see below), these mice did not display the gross defects in myeloid cell differentiation observed in mice deficient in Irf-1, Irf-2, Irf-4, and Irf-8 (reviewed in reference 37). Establishing that B6 and B6.Irf-7−/− KCs did not differ in their early control of L. donovani also provided a firm platform for further characterization of the long-term response to infection without the biases potentially introduced by a differing initial parasite load. Our data indicate that the major lesion found in the hepatic immune response was at the level of NOS2 expression within the granuloma. Similarly, IRF-7-deficient mice exhibited normal granuloma assembly and maturation despite having higher parasite burdens, a profile that was also observed in iNOS-deficient mice (26). NOS2 is regulated positively by IFN-γ and negatively by IL-10, and these two cytokines were reciprocally expressed in infected B6 and B6.Irf-7−/− mice. Of note, most of the major hepatic sources of IFN-γ were affected by IRF-7 deficiency, including CD4+ T cells, NK cells, and NKT cells. Given the role of IRF-7 in the regulation of type I IFNs, a link involving this amplification loop and the regulation of IFN-γ responses might seem plausible. However, most literature, albeit from viral systems, argues against a role for type I IFNs in the regulation of murine NK cell and T cell IFN-γ production (28). This contrasts with the clear role of these cytokines in regulating IFN-γ production in human cells (31), a reflection of the species-specific difference in STAT2-dependent activation of STAT4 by type I IFNs (10, 29). Indeed, some reports have indicated that in the mouse, type I IFN acts as a negative regulator of IFN-γ in CD4+ T cells and the balance between positive and negative regulation of IFN-γ lies at the level of STAT1 (28). Hence, it is unlikely in our model that IRF-7 deficiency directly leads to a muted IFN-γ response due to a lack of available type I IFNs.
Analysis of other potential transcriptional targets for IRF-7, both experimentally in IRF-7-overexpressing cell lines (3) and by bioinformatic approaches (P. M. Kaye, unpublished data), suggests a range of potential targets through which IRF-7 deficiency may affect the IFN-γ responses independently of type I IFNs. CD80 and CD86 have been identified as potential IRF-7 target genes (3) and could regulate Th1 development through affecting levels of costimulation (21). However, in preliminary studies, we have not found any differences in activated cDC derived from L. donovani-infected B6 and B6.Irf-7−/− mice (Owens, unpublished data).
IRF-7 also binds to the Irf-1 promoter (33), a transcription factor playing a major role in cellular resistance to intracellular pathogens that arises from two independent functions. Irf-1 is an intrinsic regulator of myeloid cell IL-12 and IL-18 production (37), and consequently Irf-1 deficiency leads to loss of both CD4+ Th1 and NK cell IFN-γ responses (23, 36). In addition, Irf-1 directly binds to the macrophage NOS2 promoter, inhibiting the macrophage microbicidal response to cytokine-mediated activation (17, 24). The striking susceptibility of Irf1−/− mice to L. major infection has been suggested to result from the compound effect of both these aspects of Irf-1 function (22), and this conclusion could similarly be applied to our data with L. donovani in Irf-7−/− mice. Irf-2 has also been linked to susceptibility to L. major infection (22) and is induced by L. donovani in stromal macrophages (30), but while Irf-2 affected Th1 development through impairment of IL-12, its effect was modest compared to that of Irf-1 and Irf-2 deficiency did not directly affect macrophage NOS2 production (22). Furthermore, no direct link between IRF-7 and Irf-2 regulation has been reported.
Further studies of the response to L. donovani in mice will clearly be required to ascertain how Irf-7 and Irf-1 might cooperate to maximize hepatic resistance in this model, though we have already noted that in stromal macrophages infected with L. donovani, induction of Irf-7 occurs coordinately with Irf-1 expression (30). A dependence upon IRF-1 and not type I IFNs would also reconcile our current data with those of Rosas et al., who noted that loss of type I IFN signaling in IFN-αβR−/− mice had no impact on hepatic L. donovani infection (32). The late onset of hepatic resistance that we observe in Irf-7-deficient mice may also reflect the availability of other pathways able to activate Irf-1 in these mice, coupled to the fact that unlike Irf-1 and Irf-2 mice (37), Irf-7 mice do not show defects in other key cellular players in antileishmanial immunity.
In summary, therefore, we have shown that B6.Irf-7−/− mice have heightened susceptibility during the initial stages of hepatic infection with L. donovani and that this is linked to a defective IFN-γ response and limited induction of NOS2 in infected KCs. These data add IRF-7 to the list of interferon regulatory factors playing a role in acquired host resistance to intracellular pathogens.
Supplementary Material
Acknowledgments
This work was supported by grants from the Wellcome Trust and the Medical Research Council (to P.M.K.).
We thank the staff of the Biological Services Facility for animal husbandry and the Technology Facility for microscopy support.
We declare no conflicts of interest.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 13 December 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alter-Koltunoff, M., et al. 2008. Innate immunity to intraphagosomal pathogens is mediated by interferon regulatory factor 8 (IRF-8) that stimulates the expression of macrophage-specific Nramp1 through antagonizing repression by c-Myc. J. Biol. Chem. 283:2724-2733. [DOI] [PubMed] [Google Scholar]
- 1a.Ato, M., et al. 2002. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat. Immunol. 3:1185-1191. [DOI] [PubMed] [Google Scholar]
- 2.Balaraman, S., P. Tewary, V. K. Singh, and R. Madhubala. 2004. Leishmania donovani induces interferon regulatory factor in murine macrophages: a host defense response. Biochem. Biophys. Res. Commun. 317:639-647. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, B. J., J. Richards, M. Mancl, S. Hanash, L. Beretta, and P. M. Pitha. 2004. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J. Biol. Chem. 279:45194-45207. [DOI] [PubMed] [Google Scholar]
- 4.Beattie, L., et al. 2010. Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8 T cells. PLoS Pathog. 6:e1000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie, L., et al. 2010. Leishmania donovani-induced expression of signal regulatory protein alpha on Kupffer cells enhances hepatic invariant NKT-cell activation. Eur. J. Immunol. 40:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackwell, J. M., et al. 2001. SLC11A1 (formerly NRAMP1) and disease resistance. Cell. Microbiol. 3:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley, D. J. 1977. Regulation of Leishmania populations within the host. II. Genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin. Exp. Immunol. 30:130-140. [PMC free article] [PubMed] [Google Scholar]
- 8.Crocker, P. R., E. V. Davies, and J. M. Blackwell. 1987. Variable expression of the murine natural resistance gene Lsh in different macrophage populations infected in vitro with Leishmania donovani. Parasite Immunol. 9:705-719. [DOI] [PubMed] [Google Scholar]
- 9.Engwerda, C. R., and P. M. Kaye. 2000. Organ-specific immune responses associated with infectious disease. Immunol. Today 21:73-78. [DOI] [PubMed] [Google Scholar]
- 10.Farrar, J. D., et al. 2000. Selective loss of type I interferon-induced STAT4 activation caused by a minisatellite insertion in mouse Stat2. Nat. Immunol. 1:65-69. [DOI] [PubMed] [Google Scholar]
- 11.Giese, N. A., et al. 1997. Interferon (IFN) consensus sequence-binding protein, a transcription factor of the IFN regulatory factor family, regulates immune responses in vivo through control of interleukin 12 expression. J. Exp. Med. 186:1535-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorak, P. M., C. R. Engwerda, and P. M. Kaye. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28:687-695. [DOI] [PubMed] [Google Scholar]
- 13.Honda, K., et al. 2005. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 434:1035-1040. [DOI] [PubMed] [Google Scholar]
- 14.Honda, K., et al. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 15.Jayakumar, A., M. J. Donovan, V. Tripathi, M. Ramalho-Ortigao, and M. A. McDowell. 2008. Leishmania major infection activates NF-kappaB and interferon regulatory factors 1 and 8 in human dendritic cells. Infect. Immun. 76:2138-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeronimo, S. M., et al. 2007. Genes at human chromosome 5q31.1 regulate delayed-type hypersensitivity responses associated with Leishmania chagasi infection. Genes Immun. 8:539-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamijo, R., et al. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263:1612-1615. [DOI] [PubMed] [Google Scholar]
- 18.Klein, I., et al. 2007. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood 110:4077-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq, V., M. Lebastard, Y. Belkaid, J. Louis, and G. Milon. 1996. The outcome of the parasitic process initiated by Leishmania infantum in laboratory mice: a tissue-dependent pattern controlled by the Lsh and MHC loci. J. Immunol. 157:4537-4545. [PubMed] [Google Scholar]
- 20.Lepay, D. A., C. F. Nathan, R. M. Steinman, H. W. Murray, and Z. A. Cohn. 1985. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J. Exp. Med. 161:1079-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang, L., and W. C. Sha. 2002. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr. Opin. Immunol. 14:384-390. [DOI] [PubMed] [Google Scholar]
- 22.Lohoff, M., et al. 2000. Deficiency in the transcription factor interferon regulatory factor (IRF)-2 leads to severely compromised development of natural killer and T helper type 1 cells. J. Exp. Med. 192:325-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohoff, M., et al. 1997. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity 6:681-689. [DOI] [PubMed] [Google Scholar]
- 24.Martin, E., C. Nathan, and Q. W. Xie. 1994. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J. Exp. Med. 180:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray, H. W. 1981. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J. Exp. Med. 153:1302-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, H. W., and C. F. Nathan. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray, H. W., Z. Xiang, and X. Ma. 2006. Responses to Leishmania donovani in mice deficient in both phagocyte oxidase and inducible nitric oxide synthase. Am. J. Trop. Med. Hyg. 74:1013-1015. [PubMed] [Google Scholar]
- 28.Nguyen, K. B., et al. 2000. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat. Immunol. 1:70-76. [DOI] [PubMed] [Google Scholar]
- 29.O'Shea, J. J., and R. Visconti. 2000. Type 1 IFNs and regulation of TH1 responses: enigmas both resolved and emerge. Nat. Immunol. 1:17-19. [DOI] [PubMed] [Google Scholar]
- 30.Phillips, R., et al. 2010. Innate killing of Leishmania donovani by macrophages of the splenic marginal zone requires IRF-7. PLoS Pathog. 6:e1000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogge, L., et al. 1998. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J. Immunol. 161:6567-6574. [PubMed] [Google Scholar]
- 32.Rosas, L. E., et al. 2006. STAT1 and T-bet play distinct roles in determining outcome of visceral leishmaniasis caused by Leishmania donovani. J. Immunol. 177:22-25. [DOI] [PubMed] [Google Scholar]
- 33.Sgarbanti, M., G. Marsili, A. L. Remoli, R. Orsatti, and A. Battistini. 2007. IRF-7: new role in the regulation of genes involved in adaptive immunity. Ann. N. Y. Acad. Sci. 1095:325-333. [DOI] [PubMed] [Google Scholar]
- 34.Stager, S., J. Alexander, K. C. Carter, F. Brombacher, and P. M. Kaye. 2003. Both interleukin-4 (IL-4) and IL-4 receptor alpha signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect. Immun. 71:4804-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svensson, M., et al. 2005. Invariant NKT cells are essential for the regulation of hepatic CXCL10 gene expression during Leishmania donovani infection. Infect. Immun. 73:7541-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taki, S., et al. 1997. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity 6:673-679. [DOI] [PubMed] [Google Scholar]
- 37.Tamura, T., H. Yanai, D. Savitsky, and T. Taniguchi. 2008. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26:535-584. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, G. A., C. G. Feng, and A. Sher. 2004. p47 GTPases: regulators of immunity to intracellular pathogens. Nat. Rev. Immunol. 4:100-109. [DOI] [PubMed] [Google Scholar]
- 39.Tominaga, N., et al. 2003. Development of Th1 and not Th2 immune responses in mice lacking IFN-regulatory factor-4. Int. Immunol. 15:1-10. [DOI] [PubMed] [Google Scholar]
- 40.Vidal, S., et al. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xin, L., K. Li, and L. Soong. 2008. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol. Immunol. 45:3371-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.