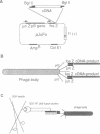
Abstract
HIV-1 reverse transcriptase is a dimeric enzyme mainly involved in the replication of the viral genome. A filamentous phage cDNA expression library from human lymphocytes was used to select cellular proteins interacting with HIV-1 reverse transcriptase Affinity selections using the bacterially expressed monomeric large subunit of reverse transcriptase (p66) yielded host beta-actin. This clone was expressed as glutathione-S-transferase fusion protein which was identified by using a specific antibody against beta-actin. Furthermore we show that also the eukaryotic beta-actin binds to either the large subunit of reverse transcriptase or to the Pol precursor polyprotein in vitro. The reverse transcriptase/beta-actin interaction might be important for the secretion of HIV-1 virions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur L. O., Bess J. W., Jr, Sowder R. C., 2nd, Benveniste R. E., Mann D. L., Chermann J. C., Henderson L. E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992 Dec 18;258(5090):1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- Blackwood E. M., Eisenman R. N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Bryant M., Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990 Jan;87(2):523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso M., Thornthwaite J., Resnick L. HIV-induced syncytium formation requires the formation of conjugates between virus-infected and uninfected T-cells in vitro. AIDS. 1991 Dec;5(12):1425–1432. doi: 10.1097/00002030-199112000-00003. [DOI] [PubMed] [Google Scholar]
- Crameri R., Suter M. Display of biologically active proteins on the surface of filamentous phages: a cDNA cloning system for selection of functional gene products linked to the genetic information responsible for their production. Gene. 1993 Dec 27;137(1):69–75. doi: 10.1016/0378-1119(93)90253-y. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Sheffield J. B., Tuszynski G. P., Warren L. Is there a role for actin in virus budding? J Cell Biol. 1977 Nov;75(2 Pt 1):593–605. doi: 10.1083/jcb.75.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T., Becherer K., Chen P. L., Yeh S. H., Yang Y., Kilburn A. E., Lee W. H., Elledge S. J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993 Apr;7(4):555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Gao Y., Thomas J. O., Chow R. L., Lee G. H., Cowan N. J. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell. 1992 Jun 12;69(6):1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- Goel R., Beard W. A., Kumar A., Casas-Finet J. R., Strub M. P., Stahl S. J., Lewis M. S., Bebenek K., Becerra S. P., Kunkel T. A. Structure/function studies of HIV-1(1) reverse transcriptase: dimerization-defective mutant L289K. Biochemistry. 1993 Dec 7;32(48):13012–13018. doi: 10.1021/bi00211a009. [DOI] [PubMed] [Google Scholar]
- Gragerov A., Zeng L., Zhao X., Burkholder W., Gottesman M. E. Specificity of DnaK-peptide binding. J Mol Biol. 1994 Jan 21;235(3):848–854. doi: 10.1006/jmbi.1994.1043. [DOI] [PubMed] [Google Scholar]
- Hafkemeyer P., Ferrari E., Brecher J., Hübscher U. The p15 carboxyl-terminal proteolysis product of the human immunodeficiency virus type 1 reverse transcriptase p66 has DNA polymerase activity. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5262–5266. doi: 10.1073/pnas.88.12.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoock T. C., Newcomb P. M., Herman I. M. Beta actin and its mRNA are localized at the plasma membrane and the regions of moving cytoplasm during the cellular response to injury. J Cell Biol. 1991 Feb;112(4):653–664. doi: 10.1083/jcb.112.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottiger M., Podust V. N., Thimmig R. L., McHenry C., Hübscher U. Strand displacement activity of the human immunodeficiency virus type 1 reverse transcriptase heterodimer and its individual subunits. J Biol Chem. 1994 Jan 14;269(2):986–991. [PubMed] [Google Scholar]
- Huse W. D., Sastry L., Iverson S. A., Kang A. S., Alting-Mees M., Burton D. R., Benkovic S. J., Lerner R. A. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989 Dec 8;246(4935):1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Jacobo-Molina A., Arnold E. HIV reverse transcriptase structure-function relationships. Biochemistry. 1991 Jul 2;30(26):6351–6356. doi: 10.1021/bi00240a001. [DOI] [PubMed] [Google Scholar]
- Karacostas V., Wolffe E. J., Nagashima K., Gonda M. A., Moss B. Overexpression of the HIV-1 gag-pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology. 1993 Apr;193(2):661–671. doi: 10.1006/viro.1993.1174. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt L. A., Wang J., Friedman J. M., Rice P. A., Steitz T. A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992 Jun 26;256(5065):1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Eriksson S. Purification from crude extract by affinity chromatography of the inhibitor of deoxyribonucleae I. Eur J Biochem. 1971 Feb;18(4):474–479. doi: 10.1111/j.1432-1033.1971.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Lutz H., Pedersen N., Higgins J., Hübscher U., Troy F. A., Theilen G. H. Humoral immune reactivity to feline leukemia virus and associated antigens in cats naturally infected with feline leukemia virus. Cancer Res. 1980 Oct;40(10):3642–3651. [PubMed] [Google Scholar]
- Mortara R. A., Koch G. L. Analysis of pseudopodial structure and assembly with viral projections. J Cell Sci Suppl. 1986;5:129–144. doi: 10.1242/jcs.1986.supplement_5.8. [DOI] [PubMed] [Google Scholar]
- Pearce-Pratt R., Malamud D., Phillips D. M. Role of the cytoskeleton in cell-to-cell transmission of human immunodeficiency virus. J Virol. 1994 May;68(5):2898–2905. doi: 10.1128/jvi.68.5.2898-2905.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stanislawsky L., Mongiat F., Moura Neto V. Presence of actin in oncornaviruses. Biochem Biophys Res Commun. 1984 Jan 30;118(2):580–586. doi: 10.1016/0006-291x(84)91342-1. [DOI] [PubMed] [Google Scholar]
- Strzelecka-Gołaszewska H., Venyaminov SYu, Zmorzynski S., Mossakowska M. Effects of various amino acid replacements on the conformational stability of G-actin. Eur J Biochem. 1985 Mar 1;147(2):331–342. doi: 10.1111/j.1432-1033.1985.tb08754.x. [DOI] [PubMed] [Google Scholar]
- Tarone G., Cirillo D., Giancotti F. G., Comoglio P. M., Marchisio P. C. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985 Jul;159(1):141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- Thimmig R. L., McHenry C. S. Human immunodeficiency virus reverse transcriptase. Expression in Escherichia coli, purification, and characterization of a functionally and structurally asymmetric dimeric polymerase. J Biol Chem. 1993 Aug 5;268(22):16528–16536. [PubMed] [Google Scholar]
- Thömmes P., Ferrari E., Jessberger R., Hübscher U. Four different DNA helicases from calf thymus. J Biol Chem. 1992 Mar 25;267(9):6063–6073. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Weber J., Grosse F. Fidelity of human immunodeficiency virus type I reverse transcriptase in copying natural DNA. Nucleic Acids Res. 1989 Feb 25;17(4):1379–1393. doi: 10.1093/nar/17.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Marzo Veronese F., Copeland T. D., DeVico A. L., Rahman R., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of highly immunogenic p66/p51 as the reverse transcriptase of HTLV-III/LAV. Science. 1986 Mar 14;231(4743):1289–1291. doi: 10.1126/science.2418504. [DOI] [PubMed] [Google Scholar]