Abstract
Many animal studies investigating adaptive immune effectors important for protection against Pseudomonas aeruginosa have implicated opsonic antibody to the antigenically variable lipopolysaccharide (LPS) O antigens as a primary effector. However, active and passive vaccination of humans against these antigens has not shown clinical efficacy. We hypothesized that optimal immunity would require inducing multiple immune effectors targeting multiple bacterial antigens. Therefore, we evaluated a multivalent live-attenuated mucosal vaccination strategy in a murine model of acute P. aeruginosa pneumonia to assess the contributions to protective efficacy of various bacterial antigens and host immune effectors. Vaccines combining 3 or 4 attenuated strains having different LPS serogroups were associated with the highest protective efficacy compared to vaccines with fewer components. Levels of opsonophagocytic antibodies, which were directed not only to the LPS O antigens but also to the LPS core and surface proteins, correlated with protective immunity. The multivalent live-attenuated vaccines overcame prior problems involving immunologic interference in the development of O-antigen-specific antibody responses when closely related O antigens were combined in multivalent vaccines. Antibodies to the LPS core were associated with in vitro killing and in vivo protection against strains with O antigens not expressed by the vaccine strains, whereas antibodies to the LPS core and surface proteins augmented the contribution of O-antigen-specific antibodies elicited by vaccine strains containing a homologous O antigen. Local CD4 T cells in the lung also contributed to vaccine-based protection when opsonophagocytic antibodies to the challenge strain were absent. Thus, multivalent live-attenuated vaccines elicit multifactorial protective immunity to P. aeruginosa lung infections.
Pseudomonas aeruginosa lung infections cause substantial morbidity and mortality in humans, manifesting as acute life-threatening infection, often with bacteremia, in hospitalized and/or immunocompromised patients or as chronic localized lung infection in patients with cystic fibrosis (CF). In hospital-acquired lung infections, which are most commonly ventilator-associated pneumonias, P. aeruginosa is the leading Gram-negative causative bacterial agent (31). In these infections, the crude mortality rate associated with the bacterium is higher than that due to other bacterial etiologies (30). Despite the widespread and significant impact of P. aeruginosa infections, along with the increasing rates of antibiotic treatment failure due to drug resistance (33), vaccines and immunotherapeutic agents for the disease are still in the early stages of preclinical and clinical development (7).
In animal studies, lipopolysaccharide (LPS) O antigens of P. aeruginosa induce potent serogroup-specific protection (i.e., protection against P. aeruginosa strains within the same LPS O-antigen serogroup) (23, 24). However, even within a serogroup there are structural, and hence antigenic, variants, referred to as subgroup or subtype antigens, giving rise to 20 to 30 different O antigens encountered in the clinical setting (23). This necessitates a multivalent O-antigen vaccine strategy for comprehensive coverage. This approach has been problematic in that animals vaccinated with a multivalent LPS O-antigen vaccine composed of antigens from serologically distinct strains within the same overall serogroup (i.e., subtype-heterologous strains) showed interference in the immunogenicity of the individual components (11). Moreover, an octavalent O-antigen-based immunoprophylaxis trial (passive immunization) failed in a phase 3 clinical evaluation to reduce the incidence and severity of P. aeruginosa infection (5), which may indicate a limitation of protective immunity in humans if such immunity is directed solely to the LPS O antigens.
Other vaccine candidates for P. aeruginosa infections include outer membrane proteins (OMPs) (7), flagella (4, 6), flagellin-OMP fusion proteins (36, 37), alginate (25, 34), and the PcrV component of the type III secretion system (9). Some of these antigens are more conserved among different strains than the LPS O antigens, although in a clinical trial of a vaccine for prevention of infection in CF using the two most common flagellar antigens (types a and b) there was evidence for infection in vaccinated individuals by P. aeruginosa strains expressing a flagellar antigen serologically distinct from the two vaccine antigens (6). Additionally, the actual genetic, protein, and thus serologic variability in PcrV among diverse P. aeruginosa isolates has not been studied. More importantly, the opsonophagocytic and/or protective activities of antibodies elicited by OMPs, flagella, and PcrV are not as high as those achieved by LPS O antigens. Finally, some of the conserved antigens are not required for full virulence in acute pneumonia or systemic dissemination, which raises a concern that vaccines targeting one of these components may select for the emergence of vaccine-resistant strains.
For the induction of full-fledged protection against various P. aeruginosa clinical isolates, vaccine-based immunity should ideally be induced against multiple bacterial antigenic components, with diverse immunologic effectors generated by the host. However, we have an incomplete understanding of the range of effectors of acquired immunity that contribute to protection against P. aeruginosa infections in humans and no assurance that a limited array of effectors is sufficient to protect against the range of clinically relevant strains and sites of infection that are encountered by humans. Animal studies have identified virtually all aspects of humoral and cellular effectors as mediators of adaptive immune protection against P. aeruginosa infection (17, 21, 22, 26). These findings suggest that vaccine-induced immune effectors may need to encompass multiple cellular and humoral activities in order to cover the numerous manifestations of P. aeruginosa infections in different tissues.
We previously reported that P. aeruginosa live-attenuated vaccine strains confer protection against acute fatal pneumonia in mice caused by serogroup-homologous strains (29) with some limited protection against serogroup-heterologous highly virulent ExoU-positive cytotoxic strains (26). The high virulence of these strains allows the use of lower bacterial challenge doses during survival experiments, thereby allowing the actions of less potent immune effectors to be observed. In the present study, we used a mucosal vaccination route to deliver monovalent to quadrivalent combinations of live attenuated P. aeruginosa strains and found that a multifactorial immunity, involving antibodies as well as T cells, is simultaneously induced by this immunization strategy and targets diverse bacterial antigens. This multifactorial immunity was associated with broad in vivo protection against acute fatal lung infection caused by a variety of P. aeruginosa strains, including both ExoU-negative and -positive strains.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in these experiments, along with their relevant characteristics and sources, are listed in Table 1. The aroA mutants of the different P. aeruginosa strains were constructed as previously described (27). These mutants are unable to synthesize aromatic amino acids and cannot acquire them to any significant degree from the host and hence survive at detectable levels for only 3 to 4 days following high-dose infection (27). Escherichia coli HB101 was used as a negative control in the vaccine studies. We focused on the O2/O5, O6, O10, and O11 serogroups, since these are common serogroups of non-CF clinical respiratory P. aeruginosa isolates (8).
TABLE 1.
Bacterial strains used in this study
| P. aeruginosa strain | Description | Source and/or reference |
|---|---|---|
| PAO1 | Wild-type strain, LPS smooth, serogroup O2/O5 | M. Vasil |
| PAO1ΔaroA | aroA deletion mutant of PAO1 | 27 |
| IT3 | Wild-type strain, LPS smooth, serogroup O2/O5 | 11 |
| IT3ΔaroA | aroA deletion mutant of IT3 | This study |
| Habs16 | Wild-type strain, LPS smooth, serogroup O2/O5 | 11 |
| Habs16ΔaroA | aroA deletion mutant of Habs16 | This study |
| 170003 | Wild-type strain, LPS smooth, serogroup O2/O5 | 11 |
| 170006 | Wild-type strain, LPS smooth, serogroup O2/O5 | 11 |
| 170007 | Wild-type strain, LPS smooth, serogroup O2/O5 | 11 |
| IT7 | Wild-type strain, LPS smooth, serogroup O2/O5 | 11 |
| PAO6 | Wild-type strain, LPS smooth, serogroup O6 | 1 |
| 6294 | Clinical isolate (corneal infection), LPS smooth, noncytotoxic, serogroup O6 | BPEIa, |
| PA14 | Wild-type strain, LPS smooth, cytotoxic, serogroup O10 | F. Ausubel |
| PA14ΔaroA | aroA deletion mutant of PA14 | 26 |
| PA14 galU | galU transposon MAR2 × T7b insertion mutant, LPS rough with incomplete outer core | F. Ausubel 20 |
| PA14 wbpM | wbpM transposon MAR2 × T7b insertion mutant, LPS rough with complete core | F. Ausubel 20 |
| PA103 | Wild-type strain, LPS smooth, cytotoxic, serogroup O11 | J. Goldberg |
| PA103 galU | galU mutant of PA103 in which the galU gene is disrupted by an aacC1 cassette; Gmr | 28 |
| B312 | Clinical isolate (blood), LPS smooth, cytotoxic, serogroup O11 | R. Ramphal |
BPEI, Bascom-Palmer Eye Institute, Miami, FL.
MAR2 × T7 (Gmr) is a Himar1 derivative that originates from the eukaryotic mariner transposon family (20). Gmr, gentamicin resistant.
Preparation of bacterial inocula for in vivo studies.
A sample from a frozen bacterial stock was plated directly onto tryptic soy agar (TSA) and grown overnight at 37°C, except for strain PA103 galU, which was grown on TSA containing 150 μg gentamicin/ml. For intranasal immunizations with the attenuated vaccine strains, bacteria were suspended in 0.9% NaCl, whereas for infection with wild-type P. aeruginosa strains, bacteria were suspended in phosphate-buffered saline (PBS) containing 1% heat-inactivated fetal calf serum (FCS) (HyClone). Bacterial concentrations were adjusted spectrophotometrically, and the actual doses administered to mice were confirmed by dilution and plating for enumeration of CFU on TSA after overnight incubation at 37°C.
Immunization of mice.
All animal experiments complied with the guidelines of the institutional animal care and use committee. Six- to 8-week-old female C3H/HeN mice were purchased from Harlan Laboratories. The mice were housed under virus-free conditions in microisolator-topped cages. The mice were anesthetized by injection of ketamine and xylazine (1.6 mg and 0.33 mg per mouse, respectively) and then vaccinated by placing 10 to 15 μl of the live P. aeruginosa ΔaroA vaccine strains, or live E. coli HB101 as a control, on each nostril. Escalating doses of 1 × 108, 5 × 108, and 1 × 109 CFU (doses of individual strains for monovalent, bivalent, and trivalent vaccines) were administered at weekly intervals, except for the quadrivalent vaccine preparation, which was given at 5 × 107, 2.5 × 108, and 5 × 108 CFU per strain weekly due to the viscosity of the preparation when 4 individual bacterial strains were combined. The mice were rested for 3 weeks after the final dose to allow nonspecific immune activation to subside.
Murine acute pneumonia model.
We used our previously described model of acute fatal pneumonia following intranasal inoculation (1). In this model, 67 to 100% of the bacterial inoculum is consistently and rapidly translocated to the lungs from the noses of anesthetized mice (1). The mice were followed for survival for 7 days after the bacterial challenge. Mice were sacrificed when they reached a moribund state (ruffled fur, shaking, and loss of mobility) and counted as dead for the purposes of these experiments.
In vivo CD4 depletion.
Seventy-two and 24 h prior to bacterial challenge, mice were given 500 μg intraperitoneally (i.p.) and 100 μg intranasally (i.n.) per dose of a monoclonal antibody (MAb) to CD4 (clone GK1.5; BioXCell). Intranasal administration was performed after the mice were anesthetized as described above. Normal rat IgG (Sigma-Aldrich) at the same doses was used as a control.
Opsonophagocytic assays.
Antibody-dependent opsonophagocytic killing was evaluated as described previously (29). Briefly, a 100-μl aliquot of 10% infant rabbit serum as a complement source (Accurate Chemical), polymorphonuclear leukocytes (PMNs) (2 × 107 cells/ml) from human volunteers, a P. aeruginosa target strain (5 × 106 CFU/ml), and antisera were mixed in a sterile microcentrifuge tube and incubated at 37°C for 90 min with end-over-end rotation. The opsonophagocytic activity of each antiserum was calculated as the percent decrease in bacterial levels in the experimental tube compared to bacterial levels in tubes incubated with complement, neutrophils, and media without antisera. Sera were pooled from 8 to 32 mice per immunization group. For comparing the killing activities of different sera that have sufficiently high killing activities to calculate the concentration of serum required to kill 50% of the bacteria (EC50), serial 2-fold dilutions of antisera from 1:10 to 1:640 or 1:1,280 were tested. For comparing the killing activities of different antisera that have positive but relatively low killing activities, 1:10 dilutions of antisera were used. In some of the opsonophagocytic assays, LPS was added as an inhibitor to antisera (see below); in other studies, antiserum adsorbed by different bacterial strains to remove antibodies to specific P. aeruginosa antigens was added to the assay mixture (see below). In these assays, the dilution factor that was previously determined to give 60% to 90% opsonic killing was used, as indicated in Results. By convention, we report the serum concentrations as the input dilutions rather than the final concentrations (the initial serum dilution is further diluted 4-fold in the assay tube). P. aeruginosa mutant strains PA103 galU, PA14 wbpM, and PA14 galU were grown on TSA with gentamicin (150 μg/ml) overnight at 37°C and then subcultured into tryptic soy broth (TSB) with 1% glycerol and also gentamicin (150 μg/ml). We confirmed that revertant mutations of these strains do not occur during the 90 min of the assay by plating bacteria on both TSA and TSA with gentamicin and obtaining identical counts. When the opsonophagocytic activities of different antisera against the same P. aeruginosa strain are compared, the data shown are from the same experiment. Assays were performed in duplicate for each sample, except for the experiments testing the bacterium-adsorbed antisera, which were performed in triplicate.
Antibody adsorption by P. aeruginosa strains.
A single colony of P. aeruginosa strain PA14 wbpM (serogroup O10 antigen deficient with intact outer LPS core), PA14 galU (O antigen and outer LPS core deficient), or Habs16 (serogroup O2/O5; LPS smooth) were taken from overnight cultures on TSA (containing 150 μg gentamicin/ml for strains PA14 galU and PA14 wbpM), suspended in TSB (for strain Habs16) or TSB with 150 μg gentamicin/ml (for strains PA14 galU and PA14 wbpM), and grown overnight at 37°C with shaking at 250 rpm. The concentrations of the bacteria in the overnight culture were 2 × 1010 CFU/ml for strains PA14 galU and PA14 wbpM and 3 × 1010 CFU/ml for strain Habs16. The bacterial cells were recovered from 2 ml of the overnight culture by centrifugation, washed twice with PBS, and resuspended in 500 μl of test antiserum for antibody adsorption. Before antibody adsorption, the test antiserum was diluted in RPMI (Invitrogen) with 10% FCS to a concentration previously determined to give 60 to 90% killing in the opsonic assay. For some experiments, adsorptions were performed using strain PA14 wbpM or Habs16, which was first heated at 100°C for 20 min to destroy protein antigens. Bacteria suspended in the antiserum were incubated for 30 min at 4°C with gentle mixing by tumbling, the bacterial cells were removed by centrifugation, and the serum was transferred to a tube with a second aliquot of identical adsorbing cells that were resuspended for a second adsorption step. After this procedure, the bacteria were removed by centrifugation and the serum was filter sterilized through 0.22-μm centrifuge tube filters (Costar) twice, followed by a 0.22-μm syringe filter (Millipore). Lack of viable bacteria was confirmed by plating 10-μl aliquots of this filtered antibody-adsorbed serum onto TSA.
Inhibition of opsonophagocytic killing by purified LPS.
For inhibition assays, we used LPS purified as described previously (10) from P. aeruginosa strain AK1401, a phage-selected smooth/rough (S/R) O-antigen variant of strain PAO1 (18). LPS from strain AK1401 contains a single O-antigen trisaccharide in which the terminal N-acetyl fucosamine linked to the LPS core is in a different conformation than the linkage in the O-antigen repeat unit (12). This LPS also contains some d-rhamnan neutral polysaccharide, which is not a target for opsonic antibody (10). LPS was added to a concentration of the test antisera that was previously determined to give 60% to 90% opsonic killing starting at an initial concentration of 100 μg LPS/ml, with 2-fold serial dilutions of the inhibitor added to additional tubes. The opsonophagocytic activities of these LPS-inhibited antisera were compared to those of antisera without added LPS. To ensure that adding LPS to the opsonophagocytic assay did not interfere with the biological activities of PMNs and complement, we added the AK1401 LPS to an opsonophagocytic assay that used an antiserum raised to the P. aeruginosa alginate antigen conjugated to keyhole limpet hemocyanin that mediates opsonic killing of alginate-expressing P. aeruginosa strains (34).
Statistical analyses.
All analyses were performed using Prism software (GraphPad Software). Survival data were analyzed by Fisher's exact test and/or by Kaplan-Meier survival analysis with a log rank test incorporating Bonferroni's correction for multiple comparisons. Opsonophagocytic activities of different antisera against the same P. aeruginosa strains were compared by determining the EC50 with the 95% confidence interval (CI) calculated from a sigmoidal dose-response curve or by a t test for specific pairwise comparisons. Opsonophagocytic activities of the same antisera that were adsorbed by different P. aeruginosa strains were compared by analysis of variance (ANOVA) with the Newman-Keuls multiple-comparison test used for pairwise comparisons. ANOVA with Dunnett's multiple-comparison test was used in comparing the opsonophagocytic activities of the antisera without added LPS versus those with different concentrations of LPS added as an inhibitor.
RESULTS
Immunity induced by multivalent live attenuated P. aeruginosa vaccines within the O2/O5 serogroup.
We first tested whether immunization with live attenuated P. aeruginosa strains representing subtype variants within the O2/O5 O-antigen serogroup overcame the interference in immunogenicity and generation of opsonic-killing antibodies that we previously observed following vaccination with a multivalent preparation of purified high-molecular-weight, lipid-free LPS O antigens from strains 170007 (serogroup O2/O5; epitopes O2a, -d, and -f) and Habs16 (serogroup O2/O5; epitopes O2a, -b, and -e) (11). In vitro opsonic killing and in vivo protection using a model of acute pneumonia were compared among antisera obtained following vaccination with (i) the monovalent live attenuated P. aeruginosa strain Habs16ΔaroA or 170007 ΔaroA, (ii) a bivalent vaccine combining both of these strains, or (iii) a trivalent vaccine containing ΔaroA strains representing a broader array of O-antigen epitopes within the O2/O5 serogroup, strains Habs16(O2a, -b, and -e), IT3(O2a and -c), and PAO1(O2a and -d).
The bivalent-vaccine-containing strains Habs16ΔaroA and 170007 ΔaroA induced opsonophagocytic activities against strain 170007 (EC50, 381; 95% CI, 303 to 480) comparable to those induced by vaccination with strain 170007ΔaroA only (EC50, 397; 95% CI, 333 to 472). However, the bivalent vaccine generated better survival of mice against lung infection with strain 170007 than did the monovalent, homologous vaccine (P < 0.05, by log rank test with Bonferroni's correction) (Fig. 1 A), indicating enhanced protective efficacy not readily associated with the level of opsonic-killing antibody.
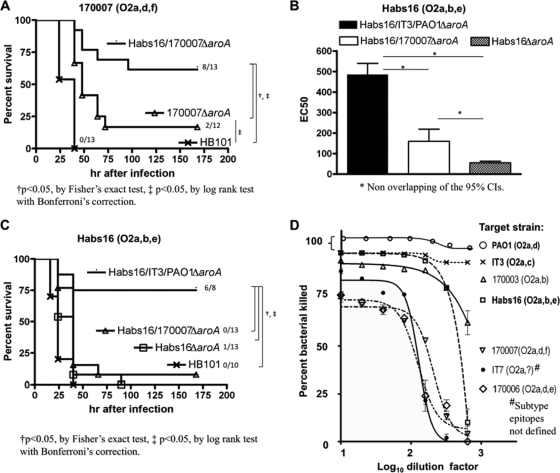
FIG. 1.
Multivalent live attenuated P. aeruginosa vaccines confer improved protective efficacy and opsonophagocytic-killing activity compared with monovalent vaccines. (A) Survival of C3H/HeN mice immunized with either monovalent P. aeruginosa strain 170007ΔaroA vaccine, bivalent P. aeruginosa strain Habs16/170007ΔaroA vaccine, or equivalent CFU of E. coli HB101 (control), followed by challenge with P. aeruginosa strain 170007 (5 × 108 CFU). (B) Levels of opsonophagocytic-killing activities against P. aeruginosa strain Habs16 in sera of mice immunized with either trivalent, bivalent, or monovalent vaccines, as indicated, that all contain a Habs16ΔaroA vaccine component. The bars represent EC50s and the error bars 95% CIs. (C) Survival of C3H/HeN mice immunized with either the trivalent Habs16/IT3/PAO1 ΔaroA vaccines, the bivalent Habs16/170007ΔaroA vaccine, the Habs16ΔaroA monovalent vaccine, or equivalent CFU of E. coli HB101 (control), followed by challenge with strain Habs16 (6 × 108 CFU). (D) Opsonophagocytic-killing activities in mouse antisera raised to the Habs16/IT3/PAO1ΔaroA trivalent vaccine against P. aeruginosa serogroup O2/O5 subgroup-heterologous strains. The points represent means and the error bars the 95% CIs. Error bars for the points without them are contained within the represented point. The ΔaroA strains included in the vaccine used to generate the antiserum are shown in boldface.
We next evaluated the opsonophagocytic-killing activities against strain Habs16 in sera of mice given either a monovalent vaccine containing only Habs16ΔaroA, a bivalent vaccine containing strains Habs16ΔaroA and 170007ΔaroA, or a trivalent vaccine containing the three ΔaroA strains Habs16ΔaroA, IT3ΔaroA, and PAO1ΔaroA. There was a progressive increase in the opsonic-killing activity with increased numbers of vaccine components (Fig. 1B) (EC50 [95% CI], 55 [48 to 63], 160 [116 to 219], and 486 [438 to 540] for the monovalent, bivalent, and trivalent vaccines, respectively). However, the mice immunized with the bivalent vaccine were not protected against lethal pneumonia caused by strain Habs16 (Fig. 1C). These findings led us to abandon the incorporation of strain 170007ΔaroA in the vaccine, as it did not seem to appreciably increase protection against the heterologous Habs16 strain, and instead, we immunized mice with a trivalent vaccine composed of ΔaroA P. aeruginosa strains Habs16, IT3, and PAO1. This trivalent vaccine elicited the highest opsonic-killing activity against strain Habs16 (Fig. 1B) and also conferred protection against challenge with strain Habs16. The 75% survival seen with the trivalent vaccine was nearly double the survival we had previously observed in mice vaccinated with the serogroup O2/O5 monovalent vaccine PAO1ΔaroA (29). Vaccination with the trivalent Habs16/IT3/PAO1ΔaroA vaccine strains induced opsonophagocytic activity against all the O2/O5 strains tested (Fig. 1D), which was associated with in vivo protection against acute lung infection caused by these strains (Table 2).
TABLE 2.
Protection from acute lethal pneumonia by the live attenuated P. aeruginosa trivalent vaccine Habs16/IT3/PAO1 ΔaroA against P. aeruginosa serogroup O2/O5 strains
| Challenge strain | Serogroup | Subtype epitopes | Inoculum (CFU) | No. surviving/no. challenged with vaccine |
|
|---|---|---|---|---|---|
| E. coli HB101 | Habs16/IT3/PAO1 ΔaroA trivalenta | ||||
| IT3 | O2/O5 | O2a, O2c | 3 × 108 | 1/10 | 9/9 |
| PAO1 | O2/O5 | O2a, O2d | 4 × 108 | 1/10 | 8/8 |
| 170003 | O2/O5 | O2a, O2b | 4 × 108 | 2/9 | 8/8 |
| 170006 | O2/O5 | O2a, O2d, O2e | 2 × 108 | 0/8 | 4/8 |
| 170007 | O2/O5 | O2a, O2d, O2f | 5 × 108 | 0/10 | 4/9 |
| IT7 | O2/O5 | O2a, ?b | 2 × 108 | 4/9 | 8/8 |
P < 0.05 by Fisher's exact test compared to E. coli HB101 control group.
?, subtype epitopes not defined.
Immunity induced against O-antigen-heterologous strains following vaccination with a multivalent live-attenuated P. aeruginosa vaccine.
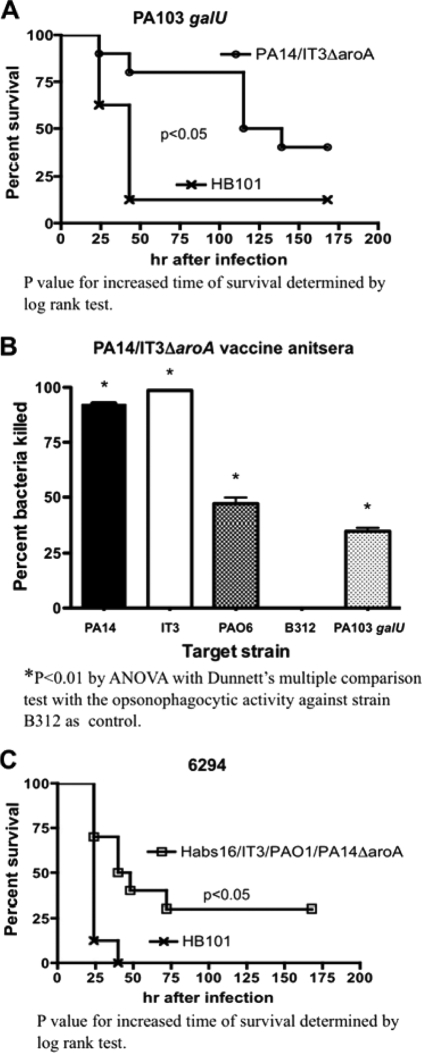
We next tested a multivalent vaccine composed of two live-attenuated O-antigen-heterologous strains, PA14 (serogroup O10) and IT3 (serogroup O2/O5), to see if this combination could induce protection against vaccine-homologous, as well as vaccine-heterologous, strains. We selected IT3 as one of the vaccine components to represent serogroup O2/O5 strains because initial experiments found that IT3 ΔaroA alone elicited protection within this serogroup broader than that elicited by strain PAO1ΔaroA (Table 3) (29). Immunization with this bivalent vaccine engendered either significant protection against (Table 3) or longer survival after (Fig. 2 A) acute lethal pneumonia due to the vaccine-homologous strains PA14 and IT3, as well as the LPS-heterologous strains PAO6 (serogroup O6), B312 (O11) (Table 3), and PA103 galU (O11 antigen deficient and serum sensitive due to galU mutation [28]) (Fig. 2A). Immunization with the PA14ΔaroA/IT3ΔaroA bivalent vaccine did not protect mice against another O6 strain, 6294, or the O11 strain PA103. Protection by the bivalent vaccine against vaccine-homologous strains PA14 and IT3, as well as against vaccine-heterologous strains PAO6 and PA103 galU, was associated with the presence of opsonophagocytic-killing activity in the sera of the immunized mice (Fig. 2B). Notably, while significant protection against strain B312 was achieved with the bivalent vaccine (Table 3), it was not associated with measurable opsonic-killing activity (Fig. 2B), further indicating nonopsonic immune effectors might be operative against some P. aeruginosa strains in this setting.
TABLE 3.
Protection from acute lethal pneumonia by live attenuated monovalent or multivalent P. aeruginosa vaccines after challenge with vaccine-homologous or -heterologous P. aeruginosa strains
| Challenge strain | Serogroup | Inoculum (CFU) | No. surviving/no. challenged with vaccine |
|||
|---|---|---|---|---|---|---|
| E. coli HB101 | PA14 ΔaroA | IT3 ΔaroA | PA14/IT3 ΔaroA bivalent | |||
| PA14 | O10 | 3 × 108 | 3/16 | 8/12a | 7/15 | 12/13a |
| IT3 | O2/O5 | 4 × 108 | 4/16 | 6/13 | 9/15 | 11/14a |
| PAO1 | O2/O5 | 7 × 108 | 0/6 | 6/6a | ||
| PAO6 | O6 | 8 × 108 | 0/8 | 4/8a | ||
| 6294 | O6 | 7 × 108 | 2/8 | 2/8 | ||
| B312 | O11 | 3 × 106 | 3/8 | 8/8a | ||
| PA103 | O11 | 4 × 106 | 0/10 | 0/10 | ||
P < 0.05 by Fisher's exact test compared to E. coli HB101 control group. Bonferroni's correction was performed in multiple comparisons.
FIG. 2.
Increased time of survival and opsonophagocytic-killing activities of the immune sera against vaccine-heterologous strains induced by multivalent live attenuated vaccines. (A) Survival of C3H/HeN mice immunized with either the PA14/IT3ΔaroA bivalent vaccine or E. coli HB101 (control), followed by challenge with the LPS-rough, vaccine-heterologous strain PA103 galU (4.0 × 107 CFU). (B) Opsonic killing in sera of mice immunized with the PA14/IT3ΔaroA bivalent vaccine against vaccine-homologous strains (PA14 and IT3) and vaccine-heterologous strains (PAO6, B312, and PA103 galU). Zero percent killing was assigned to any calculated negative-percentage kill achieved, which occurred when there was a bacterial count higher than that of the control tubes (antisera without PMNs). The bars are means and the error bars the standard errors of the mean (SEM). (C) Survival of C3H/HeN mice immunized with either the PA14/Habs16/IT3/PAO1 ΔaroA quadrivalent vaccine or E. coli HB101 (control), followed by challenge with the vaccine-heterologous strain 6294 (7.8 × 108 CFU).
We next prepared a quadrivalent vaccine containing live-attenuated strains. Habs16ΔaroA (serogroup O2/O5), IT3ΔaroA (O2/O5), PAO1ΔaroA (O2/O5), and PA14ΔaroA (O10). Immunization with the quadrivalent vaccine conferred increased time of survival (compared to E. coli-immunized controls) after challenge with strain 6294 (O6) (Fig. 2C), but not against strain PA103 (O11) (data not shown). Relatively low opsonic-killing activities of the antisera against strain 6294 were induced by the quadrivalent and bivalent vaccines (39% and 28%, respectively), which were not significantly different from each other (not shown). Opsonophagocytic-killing activity against strain PA103 was not present in immune sera from mice immunized with either the bivalent or the quadrivalent vaccine (not shown).
Opsonic-killing activity against non-LPS O-antigen components.
To determine if there was a contribution to protection by opsonic antibodies elicited to antigens other than LPS O side chains, we evaluated the opsonophagocytic-killing activities in different immune mouse sera against an LPS O-antigen-deficient strain, PA14 wbpM, which lacks the serogroup O10 O antigen but, unlike the galU mutant, has an intact LPS outer core (2, 3, 30). Sera from mice that had been immunized with monovalent live-attenuated strains (Habs16ΔaroA [serogroup O2/O5], IT3ΔaroA [O2/O5], PAO1ΔaroA [O2/O5], 170007ΔaroA [O2/O5], or PA14ΔaroA [O10]), with bivalent vaccines (IT3/PA14ΔaroA or Habs16/170007ΔaroA), or with a trivalent vaccine (Habs16/IT3/PAO1ΔaroA) were tested. All of these antisera had opsonophagocytic-killing activity against the O10 antigen-deficient strain PA14 wbpM (Fig. 3). The levels of killing of this strain in antisera raised to either Habs16ΔaroA or PAO1ΔaroA were similar and were about half the activity obtained with antisera raised to the Habs16/IT3/PAO1ΔaroA trivalent vaccine (Fig. 3A). Surprisingly, the activity in sera raised to the monovalent vaccine strain IT3ΔaroA was more than double that of opsonic-killing activity in antisera raised to the trivalent vaccine (Fig. 3A). In separate assays, the opsonic-killing activity (EC50) against strain PA14ΔwbpM in the antisera raised to strain Habs16ΔaroA was approximately 75% lower than the EC50 in antisera raised to strain 170007ΔaroA or the combination of strains Habs16/170007ΔaroA (Fig. 3B). Finally, the opsonic-killing activity against strain PA14ΔwbpM in antisera to strain IT3ΔaroA was approximately 50% lower than that achieved by immunization with the parental PA14ΔaroA strain or a combination of strains PA14/IT3ΔaroA (Fig. 3C). Overall, these various combinations of vaccine strains and target organisms indicated that the use of multivalent vaccines did not generate interference in opsonic-antibody responses, as had been observed with purified high-molecular-weight O antigens (11), and also that, in general, the multivalent vaccines elicited higher opsonic-killing activity than did the monovalent vaccines. Additionally, while clear increases in opsonic activity were achieved in some instances with the multivalent preparations, this was not always the case, indicating that superior opsonic killing against antigens other than the LPS O side chain was not necessarily just due to higher overall bacterial doses administered in the multivalent preparations.
FIG. 3.
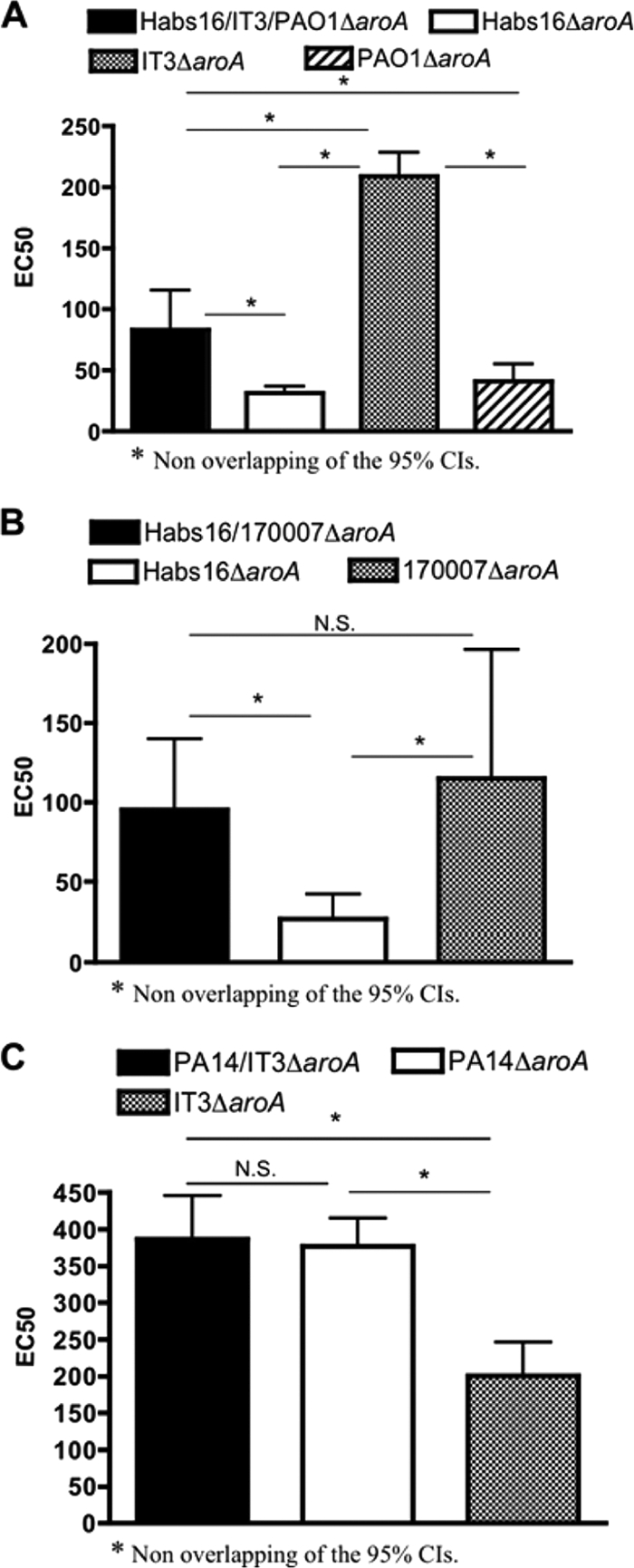
Levels of opsonophagocytic-killing activity against strain PA14 wbpM in sera of mice immunized with either a trivalent, bivalent, or monovalent vaccine constructed from serogroup O2/O5 strains (Habs16, IT3, PAO1, and 170007) or from the serogroup O10 strain PA14. (A) Opsonic-killing activities in the sera of mice induced by the Habs16/IT3/PAO1ΔaroA trivalent vaccine, the Habs16ΔaroA monovalent vaccine, the IT3ΔaroA monovalent vaccine, or the PAO1ΔaroA monovalent vaccine. (B) Opsonic killing in sera of mice immunized with the Habs16/170007ΔaroA bivalent vaccine, the Habs16ΔaroA monovalent vaccine, or the 170007ΔaroA monovalent vaccine. (C) Opsonic-killing activity elicited by the PA14/IT3ΔaroA bivalent vaccine, the PA14ΔaroA monovalent vaccine, or the IT3ΔaroA monovalent vaccine. The bars are EC50s and error bars 95% CIs. N.S., not significant.
Opsonic-killing activity against LPS core antigens.
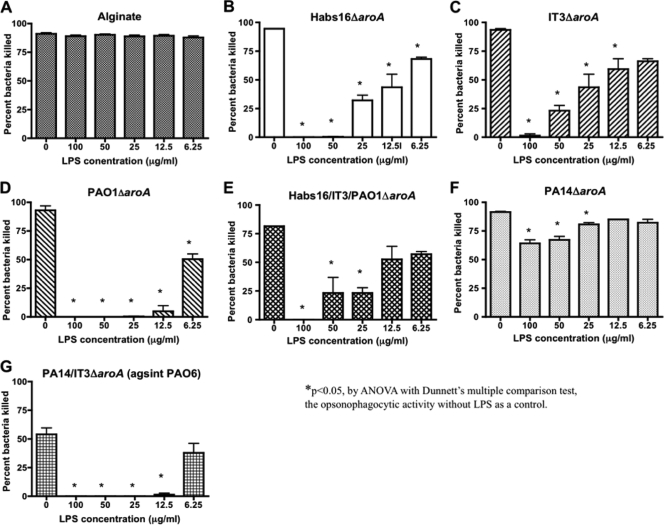
We next evaluated how much of the opsonic-killing activity was directed to LPS outer-core epitopes by using LPS antigen inhibition. We were unable to do a comparative analysis of the opsonic killing of the outer-core-proficient P. aeruginosa wbpM mutant and the outer-core-deficient P. aeruginosa galU mutant, as the latter strain was readily killed by PMNs and complement in the absence of added antibody. Among the LPS antigens of P. aeruginosa, the outer core is produced in two distinct glycoforms, one of which can bind the high-molecular-weight O antigen while the other glycoform remains unsubstituted with O antigen (15). While there is some limited structural variation in these two LPS outer cores across serogroups, there also appears to be a strong conservation of sugar substituents, suggesting that the serologic diversity in the LPS core is minimal (14, 15). To determine the proportion of opsonic antibodies directed to the LPS core epitopes in the various immune sera elicited by the different live attenuated vaccines, we measured the opsonic-killing activity against the O10 antigen-deficient strain PA14 wbpM in the presence of the LPS purified from strain AK1401 (S/R LPS with one O2/O5 antigen repeat to ensure both variant LPS outer-core glycoforms were present, and which also contained the d-rhamnan neutral polysaccharide that is not a target for opsonic antibody [10]). To ensure that this LPS did not have inhibitory effects on the PMNs or complement components of the opsonophagocytic assay, we mixed various concentrations of LPS with an immune serum raised to P. aeruginosa alginate and found no reduction in activity when 6.25 to 100 μg LPS/ml was added to the assay mixture (Fig. 4 A).
FIG. 4.
Inhibition of the opsonophagocytic-killing activities against the LPS O-antigen-deficient strain PA14 wbpM in antisera raised to different live attenuated vaccines. (A to F) Opsonic-killing activities elicited by the indicated live attenuated vaccine against strain PA14 wbpM as determined in the presence of variable concentrations of LPS purified from strain AK1401, an LPS S/R variant of serogroup O2/O5 strain PAO1. Panel A shows no effect of the LPS on killing mediated by an antibody to P. aeruginosa alginate. (G) Opsonic killing of strain PAO6 by antisera raised to the PA14/IT3ΔaroA bivalent vaccine in the presence of variable concentrations of AK1401-derived LPS. The bars are means and the error bars the SEM.
The opsonic-killing activities of antisera raised to the monovalent serogroup O2/O5 strains Habs16ΔaroA, IT3ΔaroA, and PAO1ΔaroA and antisera raised to serogroup O10 strain PA14ΔaroA vaccines, as well as the killing activity in the antisera raised to the trivalent Habs16/IT3/PAO1ΔaroA vaccine, were all inhibited by addition of the S/R LPS (Fig. 4B to E). This result identified opsonic antibodies to the LPS core that could represent a component of the opsonic-killing or protective response to P. aeruginosa strains following immunization with live attenuated vaccine strains that do not contain the homologous O antigens. Of note, addition of 100 μg LPS/ml to immune sera inhibited the opsonic activities of all the antisera tested except that of the antisera raised to the PA14ΔaroA parental strain, which maintained 70% of the killing activity compared to the serum without added LPS (Fig. 4F). This may be due to either an incomplete inhibition of the core-targeted antibodies by the heterologous AK1401-derived LPS, the presence of PA14-specific epitopes not inhibited by the AK1401-derived LPS, or the presence of opsonic antibody against other antigens in the antisera raised to the PA14ΔaroA vaccine.
The bivalent PA14/IT3ΔaroA vaccine also showed some protective efficacy against serogroup O6 strain PAO6 (Table 3), and we found that antibody to this bivalent vaccine had opsonic killing of strain PAO6 that was inhibited by the S/R serogroup O2/O5 LPS from strain AK1401 (Fig. 4G). This indicated the presence of core-specific opsonic antibody in this LPS-heterologous protective serum that was associated with the protective efficacy.
Opsonic-killing activity against bacterial surface proteins.
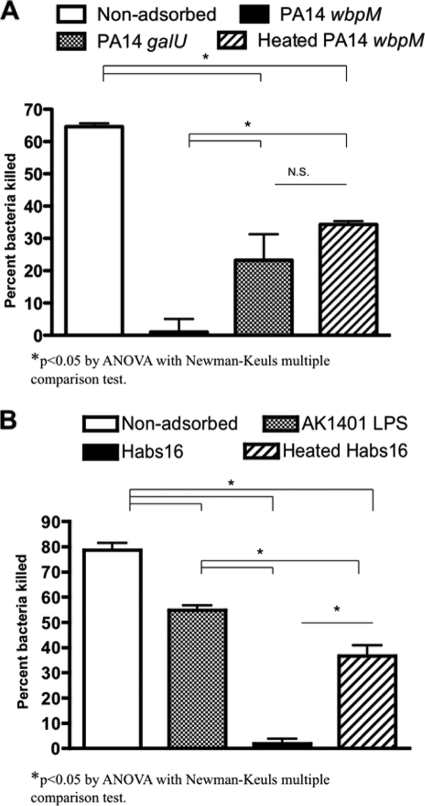
Next, we evaluated the relative contribution to opsonic killing of LPS-rough strain PA14 wbpM by antibody to heat-labile surface antigens in an antiserum raised to serogroup O10 strain PA14ΔaroA. The antiserum elicited by the PA14ΔaroA vaccine was adsorbed with strain PA14 galU (which lacks the outer LPS core and thus would primarily remove antibodies to non-LPS surface antigens) or with boiled cells of strain PA14 wbpM (which would remove antibodies to heat-stable antigens, such as LPS, and leave behind antibodies to heat-labile antigens, such as surface proteins). The controls were serum adsorbed by intact cells of the target strain, PA14 wbpM. Both PA14 galU-adsorbed antisera and antisera adsorbed by the heated PA14 wbpM strain possessed opsonophagocytic-killing activity lower than that of the unadsorbed sera, while adsorption with intact PA14 wbpM cells, as expected, removed all of the opsonic activity (Fig. 5 A) (P < 0.05 by ANOVA with a Newman-Keuls multiple-comparison test). No significant differences were observed between the killing activity of the serum raised to strain PA14ΔaroA adsorbed by either heated PA14 wbpM cells or cells of strain PA14 galU (Fig. 5A). These data indicate that immunization with strain PA14ΔaroA elicited opsonic antibody to both the LPS core and surface proteins of the vaccine-homologous strain PA14 wbpM.
FIG. 5.
Relative contribution of LPS core-targeted or bacterial surface protein-targeted immunity induced by immunization with live attenuated P. aeruginosa vaccines. (A) Opsonophagocytic-killing activities induced by strain PA14ΔaroA vaccine against the LPS O-antigen-deficient strain PA14 wbpM. The bars are means and the error bars SEM. The antisera were either untreated, adsorbed by the target strain PA14 wbpM (control), adsorbed by the outer-core-deficient mutant strain PA14 galU, or adsorbed by the heat-treated target strain PA14 wbpM. (B) Opsonophagocytic-killing activities of the Habs16/IT3/PAO1ΔaroA trivalent-vaccine antisera against one of the vaccine-homologous strains, Habs16. The antisera were either untreated, treated with the S/R LPS from strain AK1401, adsorbed by the target strain Habs16 (control), or adsorbed by heat-treated target strain Habs16. The bars are means and the error bars SEM.
Opsonic antibodies with multiple specificities associated with in vivo protection generated by the trivalent vaccine.
The range of antigenic targets for the antibodies induced by the Habs16/IT3/PAO1ΔaroA serogroup O2/O5 trivalent vaccine, which elicited the most potent in vivo protection against the vaccine-homologous P. aeruginosa strain Habs16 (Fig. 1C), was determined by measuring the opsonophagocytic activities of the trivalent-vaccine antisera adsorbed by intact or heated cells of strain Habs16, as well as opsonic killing of this antiserum in the presence or absence of AK1401-derived S/R LPS. The AK1401-derived S/R LPS inhibited the opsonic activity by 30% (Fig. 5B), suggesting the trivalent-vaccine-based immunity against the vaccine-homologous strain Habs16 is partly targeted against the bacterial LPS core. Moreover, adsorption with strain Habs16, which removed the antibodies against the serogroup O2/O5 LPS O antigen, outer core, and surface proteins, led to more than a 97% decrease in the opsonic activity of the antisera, whereas adsorption with heat-treated strain Habs16 cells, which left behind the antibodies against the heat-labile surface proteins, decreased the killing level by 50% (Fig. 5B), suggesting that the trivalent-vaccine-based immunity against the vaccine-homologous strain Habs16 is also in part associated with antibodies against the heat-labile bacterial surface proteins.
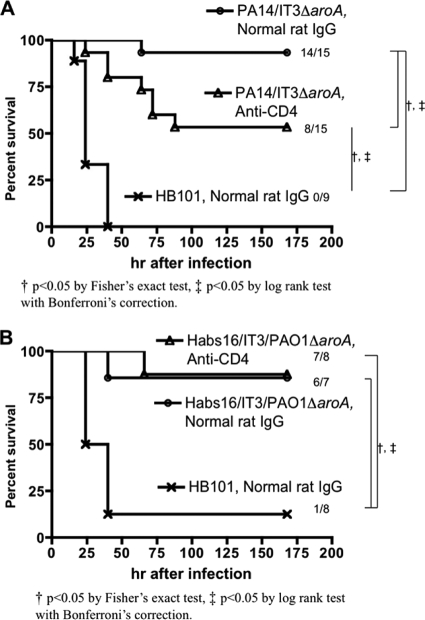
Vaccine-heterologous protection associated with CD4 T cell-mediated immunity.
An antiserum raised to the bivalent vaccine containing the PA14 (serogroup O10) and IT3 (serogroup O2/O5) ΔaroA strains did not have opsonophagocytic-killing activity against P. aeruginosa strain B312 (O11) (Fig. 2B); nonetheless, the vaccine protected mice from acute lethal pneumonia due to this strain. We hypothesized that a contribution from CD4 T cell-mediated immunity might be present in this setting. Following immunization and a 3-week rest period, we administered a MAb to CD4 both i.n. and i.p. to deplete systemic and local CD4 T cells. Preliminary experiments showed that i.p. administration of the anti-CD4 antibody depleted CD4 T cells in the spleen, but not in the lung, if measured after lung challenge (G. P. Priebe, unpublished observations). After combined i.p. and i.n. administration of the anti-CD4 MAb, flow cytometric analysis showed that more than 98% of CD4+ T cells in the lung were depleted when measured 24 and 72 h after live-bacterial challenge (data not shown). Depletion of CD4 cells in this manner just prior to challenge resulted in attenuated protection by the PA14/IT3ΔaroA vaccine (P < 0.05 by log rank test followed by Bonferroni's correction) (Fig. 6 A). However, the same CD4 depletion strategy did not change the survival of Habs16/IT3/PAO1ΔaroA-immunized mice challenged with one of the vaccine-homologous strains, PAO1 (Fig. 6B), suggesting that opsonic antibody and/or cell-mediated immunity not involving CD4 T cells was the major effector of immunity in this setting.
FIG. 6.
Role of CD4 T cells at the time of infection in vaccine-based protection against vaccine-heterologous or -homologous strains. (A) Survival of C3H/HeN mice immunized with either the PA14/IT3 ΔaroA bivalent vaccine or E. coli HB101 (control), followed by challenge with the vaccine-heterologous strain B312 (4.3 × 106 to 4.5 × 106 CFU). Prior to infection, mice were given either anti-CD4 monoclonal antibody (GK1.5) or normal rat IgG. (B) Survival of C3H/HeN mice immunized with either the Habs16/IT3/PAO1ΔaroA trivalent vaccine or E. coli HB101 (control), followed by challenge with a vaccine-homologous strain, PAO1 (5 × 108 CFU). Prior to infection, the mice were given either anti-CD4 monoclonal antibody (GK1.5) or normal rat IgG.
DISCUSSION
One of the biggest challenges for developing P. aeruginosa vaccines or immunotherapies is the antigenic variation found among clinical isolates. Although P. aeruginosa components, such as LPS O antigens (23, 24), outer membrane proteins (35), flagella (4, 6), flagellin-OMP fusion proteins (36, 37), alginate (2, 3), and the PcrV component of the type III secretion system (9), have been studied as vaccine candidates, none of these, targeting a single type of bacterial antigen, has so far demonstrated broad protection against infection with multiple strains. We hypothesized that the use of live-attenuated bacteria as vaccine antigens could induce the most comprehensive immunity against multiple protective targets, as well as elicit diverse immunologic effectors and thus expand the spectrum of protection and/or the potency of vaccines for P. aeruginosa.
In the present study, we utilized a multivalent live-attenuated mucosal vaccination strategy to assess the relative contributions of various P. aeruginosa antigenic components to protective immunity in a murine model of acute pneumonia. We found that multivalent live-attenuated vaccines can induce opsonophagocytic antibodies, not only against LPS O antigens, but also against the LPS core and bacterial surface proteins. We also found that multivalent vaccines elicited a broader spectrum of in vivo protection than monovalent vaccines. With the bivalent vaccine, we also observed in vivo protection against a noncytotoxic O-antigen-heterologous strain—a degree of efficacy not previously achieved in our published studies with the monovalent PA14ΔaroA vaccine (26), which engendered heterologous protection only against cytotoxic strains (which in general require lower challenge doses in this infection model to achieve lethality). We also found that CD4 T cells are required at the time of infection for vaccine-based protection when opsonophagocytic antibodies to the challenge strain are absent. Overall, the live attenuated multivalent vaccine successfully protected mice from acute fatal pneumonia caused by a broad range of LPS-homologous and LPS-heterologous strains of P. aeruginosa. These findings indicate that multifactorial immunity targeted against multiple bacterial factors may be a means to induce full-fledged protection against diverse clinical isolates of P. aeruginosa.
In contrast to our previous findings utilizing multivalent purified LPS O-antigen vaccines (11), we did not observe diminution of the opsonophagocytic-killing activities against component strains when the live attenuated vaccines from closely related LPS O-antigen subgroup-heterologous strains were incorporated together as a multivalent vaccine. The possible mechanisms of interference in those prior studies (11) included decreased immunogenicity in the absence of conjugation of the polysaccharide O antigen to a carrier; competition among related polysaccharide antigens for a limited repertoire of membrane immunoglobulin on B cells; in vivo antigenic variation of the O antigen during infection, leading to diminished effectiveness of opsonic antibodies; and/or evolutionary selection for P. aeruginosa strains making O antigens that elicit antibody primarily to nonprotective epitopes. Theoretically, the live-attenuated vaccines described here can circumvent most of these limitations, other than the last.
We also found an association between the presence of opsonic antibodies to the LPS core and protection against some strains. There are four LPS outer-core structural variants synthesized by P. aeruginosa (13), as opposed to 20 to 30 variations in O antigens, indicating that if a properly formulated attenuated vaccine can be made that elicits the needed range of LPS core-targeted immunity, then a more expansive level of protection might be achieved. However, achieving this formulation may rely primarily on empirical determinations of antibody specificity following vaccination. For example, the opsonophagocytic activities against the LPS O10 antigen-deficient strain PA14 wbpM (i.e., LPS core- or surface-protein-targeted immunity) induced by live-attenuated vaccines constructed from strains within the heterologous O2/O5 serogroup varied significantly depending on the vaccine strains used. Similarly, the bivalent vaccine composed of the PA14ΔaroA and IT3ΔaroA strains protected mice from infection with the O11 strain B312, but not from another O11 strain, PA103. As the O11 serogroup is one of the most clinically relevant because most of the strains expressing the ExoU cytotoxin are serogroup O11 strains (8), it seems that the O antigen and/or other components from some serogroup O11 strain should be incorporated into future multivalent-vaccine candidates.
Although here we demonstrated that LPS core-targeted antibodies with opsonic-killing activity were associated with better protection against acute fatal lung infection, it is not clear whether antibodies with this specificity would also help prevent and/or eradicate chronic P. aerguinosa infection in CF patients. Based on cross-inhibition enzyme-linked immunosorbent assay (ELISA) analysis, it has been reported that antibodies induced by a P. aeruginosa LPS O-antigen-based octavalent conjugate vaccine that was tested in CF patients bound monospecifically to individual O antigens of each serotype, but during chronic lung infection with the pathogen, there was acquisition of antibodies that bound to common epitopes within the core region of LPS (19, 32, 38). As neither the LPS O-antigen-based octavalent vaccine nor the induced core-specific antibodies confer protection against either acquisition or progression of chronic infection, it appears unlikely that targeting these epitopes with a vaccine would have a high chance of success in the setting of CF.
Finally, CD4 T cell depletion performed after immunization but prior to infection diminished the vaccine-based protection during challenge with vaccine-heterologous strains when opsonophagocytic-killing activity against the strain was absent. Notably, this treatment did not affect protection during challenge with a vaccine-homologous strain when high opsonophagocytic-killing activity against the challenge strain was present in the immune sera. These findings highlight the fact that optimal adaptive immunity against the diverse P. aeruginosa strains requires both cellular and humoral effectors. Indeed, we previously showed that IL-17, a neutrophil-recruiting cytokine, plays a critical role in antibody-independent LPS-heterologous protection against P. aeruginosa lung infection after immunization with the monovalent vaccine PA14ΔaroA (26). Thus, cooperation of adaptive (CD4 T cells and antibodies) and innate (neutrophils and complement) immunity can be optimized by vaccination with live-attenuated P. aeruginosa cells.
In conclusion, our multivalent live-attenuated vaccines induced humoral effectors with multiple specificities, as well as effector CD4 T cells. This multifactorial immunity was associated with a broader range of protection than previously achieved in animal studies and more potent protection than our monovalent live-attenuated vaccine candidates. Production of these vaccine strains is straightforward, and the strains appear to have a high margin of safety in terms of attenuation of pathological consequences in both intact (29) and neutropenic (16) mice. Multivalent live-attenuated vaccines appear to induce multiple types of immune protection, increasing the likelihood that such an approach will be useful against the wide variety of infections P. aeruginosa can cause. While use of live attenuated bacterial vaccines in humans has only been validated using the Ty21A strain of Salmonella enteritidis serovar Typhi, this is an area of vaccinology that could expand with the development of the proper means to attenuate virulence while maintaining immunogenicity. Under such circumstances, an inexpensive and effective P. aeruginosa vaccine might be realized.
Acknowledgments
We thank Gloria Meluleni for excellent technical assistance.
This work was supported by National Institutes of Health grants K08 AI050036 and R01 HL092515 (to G.P.P.), R01 AI068112 (to J.B.G.), and R01 AI22535 (to G.B.P.).
The authors have no financial conflicts of interest.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 13 December 2010.
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the P. aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrows, L. L., D. F. Charter, and J. S. Lam. 1996. Molecular characterization of the Pseudomonas aeruginosa serotype O5 (PAO1) B-band lipopolysaccharide gene cluster. Mol. Microbiol. 22:481-495. [DOI] [PubMed] [Google Scholar]
- 3.Burrows, L. L., R. V. Urbanic, and J. S. Lam. 2000. Functional conservation of the polysaccharide biosynthetic protein WbpM and its homologues in Pseudomonas aeruginosa and other medically significant bacteria. Infect. Immun. 68:931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campodonico, V. L., et al. 2010. Evaluation of flagella and flagellin of Pseudomonas aeruginosa as vaccines. Infect. Immun. 78:746-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donta, S. T., et al. 1996. Immunoprophylaxis against Klebsiella and Pseudomonas aeruginosa infections. Federal Hyperimmune Immunoglobulin Trial Study Group. J. Infect. Dis. 174:537-543. [DOI] [PubMed] [Google Scholar]
- 6.Döring, G., C. Meisner, and M. Stern. 2007. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 104:11020-11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Döring, G., and G. B. Pier. 2008. Vaccines and immunotherapy against Pseudomonas aeruginosa. Vaccine 26:1011-1024. [DOI] [PubMed] [Google Scholar]
- 8.Faure, K., et al. 2003. O-antigen serotypes and type III secretory toxins in clinical isolates of Pseudomonas aeruginosa. J. Clin. Microbiol. 41:2158-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank, D. W., et al. 2002. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 186:64-73. [DOI] [PubMed] [Google Scholar]
- 10.Hatano, K., J. B. Goldberg, and G. B. Pier. 1995. Biologic activities of antibodies to the neutral-polysaccharide component of the Pseudomonas aeruginosa lipopolysaccharide are blocked by O side chains and mucoid exopolysaccharide (alginate). Infect. Immun. 63:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatano, K., and G. B. Pier. 1998. Complex serology and immune response of mice to variant high-molecular-weight O polysaccharides isolated from Pseudomonas aeruginosa serogroup O2 strains. Infect. Immun. 66:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irina, S., et al. 2000. Structural characterization of the outer core and the O-chain linkage region of lipopolysaccharide from Pseudomonas aeruginosa serotype O5. Eur. J. Biochem. 267:1640-1650. [DOI] [PubMed] [Google Scholar]
- 13.King, J. D., D. Kocincova, E. L. Westman, and J. S. Lam. 2009. Lipopolysaccharide biosynthesis in Pseudomonas aeruginosa. Innate Immun. 15:261-312. [DOI] [PubMed] [Google Scholar]
- 14.Knirel, Y. A. 1990. Polysaccharide antigens of Pseudomonas aeruginosa. Crit. Rev. Microbiol. 17:273-304. [DOI] [PubMed] [Google Scholar]
- 15.Knirel, Y. A., et al. 2001. Structural analysis of the lipopolysaccharide core of a rough, cystic fibrosis isolate of Pseudomonas aeruginosa. Eur. J. Biochem. 268:4708-4719. [DOI] [PubMed] [Google Scholar]
- 16.Koh, A. Y., G. P. Priebe, and G. B. Pier. 2005. Virulence of Pseudomonas aeruginosa in a murine model of gastrointestinal colonization and dissemination in neutropenia. Infect. Immun. 73:2262-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh, A. Y., G. P. Priebe, C. Ray, N. Van Rooijen, and G. B. Pier. 2009. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect. Immun. 77:5300-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kropinski, A. M., L. C. Chan, and F. H. Milazzo. 1979. The extraction and analysis of lipopolysaccharides from Pseudomonas aeruginosa strain PAO and three rough mutants. Can. J. Microbiol. 25:390-398. [DOI] [PubMed] [Google Scholar]
- 19.Lang, A. B., et al. 2004. Vaccination of cystic fibrosis patients against Pseudomonas aeruginosa reduces the proportion of patients infected and delays time to infection. Pediatr. Infect. Dis. J. 23:504-510. [DOI] [PubMed] [Google Scholar]
- 20.Liberati, N. T., et al. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. U. S. A. 103:2833-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller-Ortiz, S. L., S. M. Drouin, and R. A. Wetsel. 2004. The alternative activation pathway and complement component C3 are critical for a protective immune response against Pseudomonas aeruginosa in a murine model of pneumonia. Infect. Immun. 72:2899-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieuwenhuis, E. E., et al. 2002. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat. Med. 8:588-593. [DOI] [PubMed] [Google Scholar]
- 23.Pier, G. B. 2003. Promises and pitfalls of Pseudomonas aeruginosa lipopolysaccharide as a vaccine antigen. Carbohydr. Res. 338:2549-2556. [DOI] [PubMed] [Google Scholar]
- 24.Pier, G. B. 2007. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int. J. Med. Microbiol. 297:277-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pier, G. B., et al. 2004. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J. Immunol. 173:5671-5678. [DOI] [PubMed] [Google Scholar]
- 26.Priebe, G., et al. 2008. IL-17 is a critical component of vaccine-induced protection against lung infection by LPS-heterologous strains of Pseudomonas aeruginosa. J. Immunol. 181:4965-4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priebe, G. P., et al. 2002. Construction and characterization of a live, attenuated aroA deletion mutant of Pseudomonas aeruginosa as a candidate intranasal vaccine. Infect. Immun. 70:1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priebe, G. P., et al. 2004. The galU gene of Pseudomonas aeruginosa is required for corneal infection and efficient systemic spread following pneumonia but not for infection confined to the lung. Infect. Immun. 72:4224-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priebe, G. P., G. J. Meluleni, F. T. Coleman, J. B. Goldberg, and G. B. Pier. 2003. Protection against fatal Pseudomonas aeruginosa pneumonia in mice after nasal immunization with a live, attenuated aroA deletion mutant. Infect. Immun. 71:1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rello, J., et al. 1997. Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit. Care Med. 25:1862-1867. [DOI] [PubMed] [Google Scholar]
- 31.Safdar, N., C. Dezfulian, H. R. Collard, and S. Saint. 2005. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit. Care Med. 33:2184-2193. [DOI] [PubMed] [Google Scholar]
- 32.Schaad, U. B., et al. 1991. Safety and immunogenicity of Pseudomonas aeruginosa conjugate A vaccine in cystic fibrosis. Lancet 338:1236-1237. [DOI] [PubMed] [Google Scholar]
- 33.Tam, V. H., et al. 2010. Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:1160-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theilacker, C., et al. 2003. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect. Immun. 71:3875-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Specht, B. U., et al. 1995. Protection of immunocompromised mice against lethal infection with Pseudomonas aeruginosa by active or passive immunization with recombinant P. aeruginosa outer membrane protein F and outer membrane protein I fusion proteins. Infect. Immun. 63:1855-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weimer, E. T., S. E. Ervin, D. J. Wozniak, and S. B. Mizel. 2009. Immunization of young African green monkeys with OprF epitope 8-OprI-type A- and B-flagellin fusion proteins promotes the production of protective antibodies against nonmucoid Pseudomonas aeruginosa. Vaccine 27:6762-6769. [DOI] [PubMed] [Google Scholar]
- 37.Weimer, E. T., H. Lu, N. D. Kock, D. J. Wozniak, and S. B. Mizel. 2009. A fusion protein vaccine containing OprF epitope 8, OprI, and type A and B flagellins promotes enhanced clearance of nonmucoid Pseudomonas aeruginosa. Infect. Immun. 77:2356-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuercher, A. W., et al. 2006. Antibody responses induced by long-term vaccination with an octovalent conjugate Pseudomonas aeruginosa vaccine in children with cystic fibrosis. FEMS Immunol. Med. Microbiol. 47:302-308. [DOI] [PubMed] [Google Scholar]