Abstract
Severe sepsis is associated with early release of inflammatory mediators that contribute to the morbidity and mortality observed during the first stages of this syndrome. Although sepsis is a deadly, acute disease, high mortality rates have been observed in patients displaying evidence of sepsis-induced immune deactivation. Although the contribution of experimental models to the knowledge of pathophysiological and therapeutic aspects of human sepsis is undeniable, most of the current studies using animal models have focused on the acute, proinflammatory phase. We developed a murine model that reproduces the early acute phases but also the long-term consequences of human sepsis. We induced polymicrobial acute peritonitis (AP) by establishing a surgical connection between the cecum and the peritoneum, allowing the exit of intestinal bacteria. Using this model, we observed an acute phase with high mortality, leukopenia, increased interleukin-6 levels, bacteremia, and neutrophil activation. A peak of leukocytosis on day 9 or 10 revealed the persistence of the infection within the lung and liver, with inflammatory hepatic damage being shown by histological examination. Long-term (20 days) derangements in both innate and adaptive immune responses were found, as demonstrated by impaired systemic tumor necrosis factor alpha production in response to an inflammatory stimulus; a decreased primary humoral immune response and T cell proliferation, associated with an increased number of myeloid suppressor cells (Gr-1+ CD11b+) in the spleen; and a low clearance capacity. This model provides a good approach to attempt novel therapeutic interventions directed to augmenting host immunity during late sepsis.
Local inflammatory mechanisms triggered by an infection are usually enough to eradicate the pathogen. However, if the infection is not contained, the pathogen, its toxins, and diverse mediators of the host are released to the circulation, producing a systemic inflammatory response syndrome that can cause severe sepsis or septic shock (8). Sepsis is usually treated in intensive care units (ICUs), and about 30% of the patients with severe sepsis die; this percentage rises to 50 to 70% if septic shock, the main cause of mortality in the ICU, develops (46).
As death by septic shock has been associated with an early excessive inflammatory response, most of the treatment strategies have been designed to block the inflammatory mediators involved in this phenomenon. This theory is based on animal models where the administration of large amounts of bacteria or lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria, generates a cytokine storm and where the blockade of these molecules increases the survival of animals (11). Nevertheless, clinical trials designed to neutralize inflammatory mediators have failed (45). The sepsis syndrome is not restricted to the activation of the inflammatory response, as compensatory anti-inflammatory mechanisms are also triggered, usually leading to immunosuppression. Patients in this state have a poor prognosis and show a greater susceptibility to acquisition of opportunistic life-threatening infections (7). In fact, the majority of deaths occur in patients with sepsis who are immunosuppressed (1, 34).
Most of the studies conducted so far investigate therapeutic alternatives during the early phases of sepsis, mainly in the proinflammatory one. However, in many patients the treatments begin when they are journeying from a proinflammatory to an anti-inflammatory stage and when the damage in different organs is already evident. This is why it is necessary to develop a model that reproduces not only the acute period of the disease but also the delayed phase of immunosuppression.
The aim of the present work was to develop a murine model of polymicrobial acute peritonitis (AP) that could allow the study of the different stages observed in septic patients. For this purpose, we established a surgical connection between the cecum and the peritoneum (by placing a sterile 18-gauge silicon tube across the cecum) that allows the exit of intestinal bacteria to the peritoneal cavity. We analyzed different immunological parameters early (24 h) and late (9 and 20 days) after the induction of AP. This model could contribute to the study of the pathophysiology of sepsis and the investigation of possible therapeutic alternatives.
MATERIALS AND METHODS
Mice.
BALB/c mice were bred in the animal facility of the Department of Experimental Medicine, Academia Nacional de Medicina, Buenos Aires, Argentina. Male mice aged 16 to 20 weeks and weighing 20 to 25 g were used throughout the experiments. They were maintained under a 12-h light and 12-h dark cycle at 22 ± 2°C and provided standard diet and water ad libitum. The experiments performed in the present study were conducted according to principles set forth in the Guide for the Care and Use of Laboratory Animals (30).
Surgical procedure for AP induction.
AP was induced surgically under sterile conditions. With the mice under complete anesthesia and after disinfection of the abdomen, the abdominal wall was opened through a 1-cm midline incision. After exposure of the cecum, a silicon sterile tube of 18 gauge was introduced through the cecum wall into the lumen of the cecum and was then fixed with two stitches. To ensure proper intraluminal positioning of the tube, stool from the cecum was gently milked until a small drop of stool appeared. Fluid resuscitation of the animals was performed by flushing 0.5 ml of sterile warm saline solution into the peritoneal cavity before closure of the abdominal walls (two layers, muscle, and skin). For control purposes, sham operations were performed by stitching the tube to the cecum without penetrating it. After surgery, the animals were immediately placed on a thermal pad (37°C) until their complete recovery.
Blood sample collection and cell count determination.
Blood samples were obtained by puncture of the retro-orbital plexus at different times after surgery. A differential count of leukocytes in whole blood was performed with a Neubauer chamber using Turk's solution (0.1% gentian violet in 2% glacial acetic acid).
Peritoneal lavage.
The animals were killed and the peritoneal content was collected by peritoneal lavage at different times after surgery. The skin of the abdomen was cut open in the midline after thorough disinfection and without injuring the muscle. Sterile saline solution (3 ml) was injected into and aspirated out of the peritoneal cavity twice, using a sterile syringe and needle, to rinse out the peritoneal content from the peritoneal cavity. Cells were washed and counted as described above for whole blood, and the peritoneal liquid was used for bacterial culture as described below.
Cell tissue collection.
All tissues were collected under sterile conditions. While the mice were under anesthesia, the hepatic artery was cut and 2 ml of saline solution was gently perfused through the retro-orbital plexus to remove blood circulating within the lungs. Then, the lungs were removed from the thorax and blotted with gauze to remove blood, minced, and pressed gently through a stainless steel mesh to obtain a single-cell suspension. Finally, the mice were killed, their tissues were dissected, and single-cell suspensions of the spleen, liver, and kidney were prepared by homogenization through a sterile stainless steel mesh. When required, cells were counted with a Neubauer chamber using Turk's solution.
Bacterial cultures.
Aliquots of serial log dilutions of cell-free peritoneal fluid, organ suspensions, and blood were plated on a selective growth medium for intestinal bacteria, MacConkey agar (Britania, Argentina), or a nutritionally nonselective rich medium, Luria-Bertani (LB) agar (Britania). Plates were incubated under aerobic conditions at 37°C, and colonies were counted after overnight incubation. Bacterial counts are expressed as the number of CFU per organ or per milliliter of peritoneal lavage fluid or blood. For identification, bacteria were grown in Columbia sheep blood agar (Laboratorio Argentino, Argentina) and brain heart infusion (BHI) medium (Difco, BD Diagnostics), and tests were conducted in accordance with routine bacteriological methods (29).
Cytometric studies.
Whole blood (100 μl) or 500,000 cells were incubated for 30 min with specific antibody against fluorescein isothiocyanate-conjugated CD11b and/or phycoerythrin-conjugated Ly-6G (BD, NJ). Whole blood was subjected to hypotonic shock, and cells were washed and resuspended in paraformaldehyde (0.5%). The percentage and mean fluorescence intensity (MFI) were determined by flow cytometry on 20,000 events. Neutrophils were identified according to their high levels of expression of Ly-6G (also known as Gr-1) and the forward and side scatter (FSC/SSC) profiles.
TNF-α determination.
The amount of tumor necrosis factor alpha (TNF-α) was determined using sensitive fibroblast cell line L929 as previously described (17). Briefly, 100 μl of serial dilutions of supernatants from macrophage cultures or serum was incubated with L929 cells in the presence of 1 mg/ml of actinomycin D, to restrain cellular replication. After 18 h, the cells were washed, fixed, and dyed with a solution of 0.1% crystal violet in 20% methanol. After the cells were washed, the incorporated colorant was released with a 30% acetic acid solution. The resulting absorbance was measured at 550 nm. Results were expressed as the inverse of the dilution where 50% lysis lethal dose (LD50) was observed.
Determination of IL-10 and IL-6.
Interleukin-10 (IL-10) and IL-6 levels were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, San Diego, CA), according to the instructions provided by the manufacturer.
Hemagglutination test.
To induce a primary humoral immune response, 0.1 ml of 20% (vol/vol) washed sheep red blood cells (SRBCs) was inoculated intraperitoneally (i.p.), and 1 week later blood was collected and sera were prepared. Serial 2-fold dilutions of sera were made, and the dilutions were mixed with an equal volume of a 0.35% (vol/vol) solution of SRBCs in a round-bottom 96-well plate. The plates were left at room temperature for 24 h. The titer of antibodies was considered the inverse of the greatest dilution which inhibits hemagglutination.
T cell proliferation.
Splenocytes were obtained and resuspended in RPMI 1640 medium with 10% fetal calf serum (FCS), 0.1% 2-mercapoethanol, 1% l-glutamine, and 1% antibiotic-antimycotic. Lymphocytes (0.2 × 105) were seeded in a 96-well plate in triplicate in the presence or absence of concanavalin A (ConA; 5 μg/ml; Sigma) for 72 h at 37°C in 5% CO2. Then, the cells were incubated with 0.5 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma), and the plate was left at 37°C in 5% CO2 for an additional period of 4 h. MTT is reduced to purple formazan in living cells (28). To dissolve the insoluble purple formazan product, a solution of 20% SDS-50% dimethyl formamide was added and the mixture was left overnight at 37°C. The absorbance of the colored solution was quantified at 550 nm. The mean absorbance in the absence of ConA was subtracted from the mean absorbance in its presence for each sample.
Clearance of bacteria.
Intestinal bacteria were collected from the cecum of an untreated animal and left overnight in saline with 10% FCS at 37°C. After centrifugation, bacteria were resuspended in saline. Bacterial inocula were determined by measurement of colony counts after serial dilution, and 5 × 106 bacteria were injected i.p. Four hours later, peritoneal lavages were performed with sterile saline solution, the cells were counted, and the numbers of CFU of the remaining bacteria were determined using MacConkey agar plates. The plates were left for 24 h at 37°C, and the numbers of CFU were counted.
Statistics.
The comparisons between two groups were made using the t test. The comparisons between multiple groups were made by analysis of variance, applying the correction of Bonferroni. For mortality curves, a χ2 test was used. P values of <0.05 were considered significant.
RESULTS
Survival after AP induction.
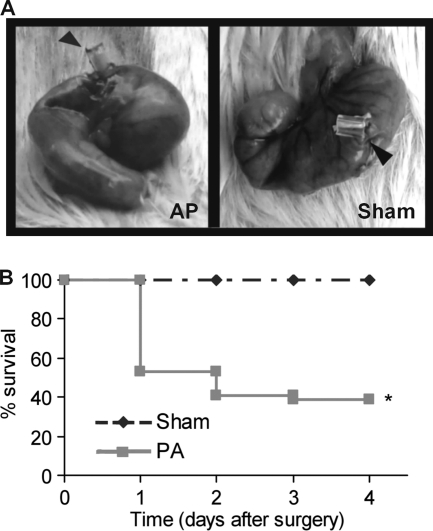
AP was induced surgically by placing a silicon sterile tube of 18 gauge through the cecum wall into the lumen and allowing the exit of intestinal bacteria to the peritoneal cavity (Fig. 1 A, left). The rate of survival after AP induction was 54% on day 1 and descended to 41% on day 2 (Fig. 1B). Beyond day 4, no changes were observed. The animals that died showed typical signs of septic shock, like hypothermia (rectal temperature below 30°C), tremor, diarrhea, lethargy, and bristled hair. The animals that survived also showed diarrhea and bristled hair on days 1 and 2, but their rectal temperature on day 1 was similar to that of the sham treatment group (referred to here as the sham group; 36.2 ± 0.4°C for sham treatment group, 35.9 ± 0.2°C for AP survivors). No mortality was observed in the sham group, where the silicon tube was fixed without penetrating the cecum wall (Fig. 1A, right). Additionally, animals in the sham group showed normal behavior after they recovered from anesthesia.
FIG. 1.
(A) Surgical induction of AP. Representative photographs of the ceca of mice with the tube (arrowheads) inserted through the cecum wall to cause the acute peritonitis (left) or fixed without penetrating the cecum wall (right). (B) Percent survival after AP induction (n = 24 per group; *, P < 0.05 versus sham group).
Systemic and local responses.
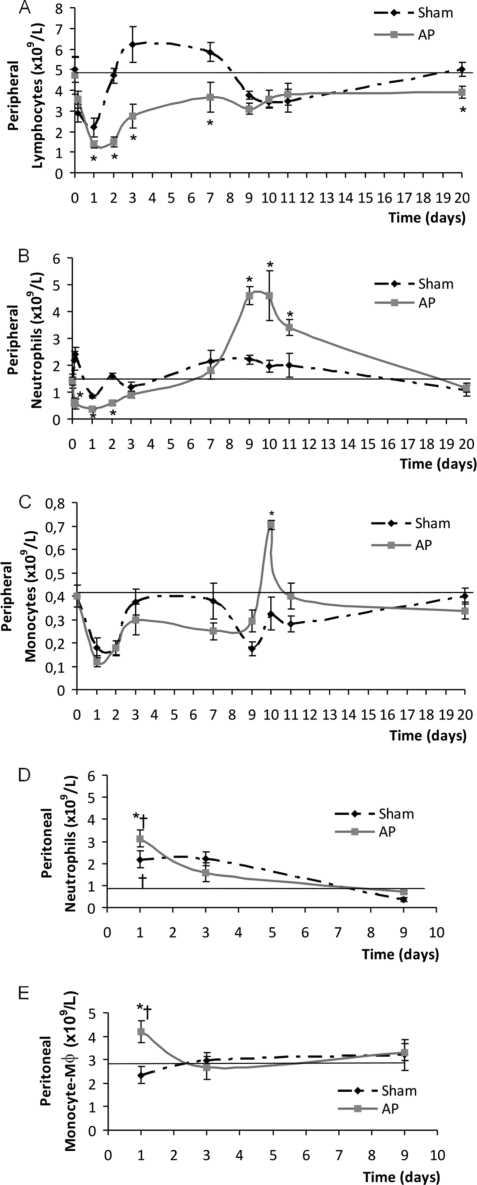
We first characterized the systemic response in mice by counting the main leukocyte subpopulations present in peripheral blood at different time points after the induction of AP. Although early (day 1) decreases in lymphocyte, neutrophil, and monocyte counts were observed in both groups, the levels of lymphocytes and neutrophils in AP mice were statistically lower than those in the sham-treated animals (Fig. 2 A and B). Neither the anesthetic solution alone nor the anesthesia plus the wound provoked by the surgical procedure caused a decrease in peripheral leukocyte counts (data not shown). The numbers of all leukocyte subpopulations returned to normal values after day 2 in the sham group and stayed relatively constant thereafter. Animals with AP showed a gradual and slow recovery in lymphocyte counts, and delayed peaks of increased neutrophils and monocytes were observed on day 9 or 10 after AP induction (Fig. 2B and C). By day 20, the lymphocyte subpopulation was slightly decreased in the AP group, and neutrophils and monocytes showed normal values.
FIG. 2.
Absolute leukocyte counts present in peripheral blood (A, B, and C) and peritoneal infiltrations (D and E) at different times after AP induction. (A) Lymphocytes; (B) neutrophils; (C) monocytes (for panels A to C, n = 6 to 30 animals for each time point, by group; *, P < 0.05 versus sham group); (D) neutrophils; (E) monocytes/macrophages (Mφ). The horizontal lines represent the values observed in healthy untreated mice (reference value) (n = 6 to 30 animals for each time, by group; *, P < 0.05 versus sham group; †, P < 0.05 versus reference value).
Leukocyte counts in the peritoneal cavity were also determined. No variations in the lymphocyte subpopulation were observed at the different time points studied (data not shown). Both experimental groups (sham and AP) showed an increase in neutrophil counts on day 1 postsurgery compared to the reference values observed in healthy untreated animals (Fig. 2D). However, the values achieved in the AP group were statistically higher than those achieved in the sham group. Regarding the monocyte/macrophage cells (Fig. 2E), only the AP group showed increased counts of this subpopulation on day 1 compared to the counts for sham-treated or untreated healthy mice. At day 9, normal values were observed for both subpopulations in the sham treatment and AP groups.
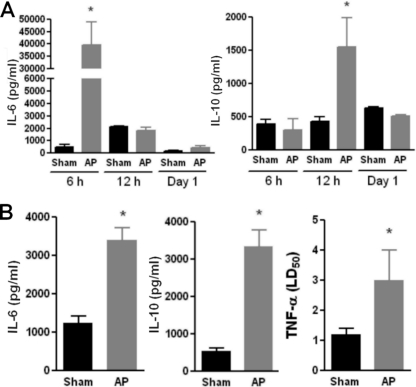
Additionally, we evaluated serum for the presence of IL-6, IL-10, and TNF-α activity at 6 and 12 h and on day 1 after surgery (Fig. 3 A). AP animals showed elevated values of serum IL-6 and IL-10 at 6 h and 12 h, respectively. No TNF-α activity was found at any time early in the course of AP, even when it was measured as early as 2 h after induction of AP (data not shown). The TNF-α results were confirmed by ELISA (data not shown). The presence of these cytokines in peritoneal lavage fluid specimens was also measured on day 1 postsurgery, and increased values of all of them were found in the AP group compared to the values for the sham group (Fig. 3B). The levels of none of these cytokines in serum or peritoneal fluid of AP mice were elevated on day 9 (data not shown).
FIG. 3.
Presence of TNF-α, IL-6, and IL-10 in serum at different time points (A) and in peritoneal fluid on day 1 (B) after AP induction. Serum and peritoneal fluid specimens were obtained from sham and AP mice at different times after surgery. TNF-α was determined using a biological test, and the results are expressed as the 50% lethal dose (LD50), as described in Materials and Methods. IL-10 and IL-6 levels were determined by ELISA (n = 12; *, P < 0.05 versus sham group).
Activation of neutrophils early during course of AP.
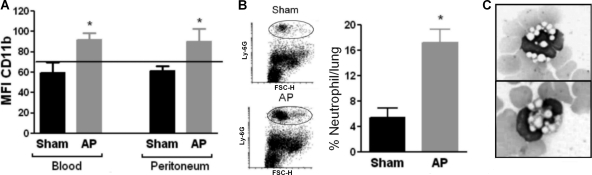
Neutrophils are central effector cells that possess bactericidal enzymes and oxygen radicals that are capable of inflicting damage to cells. Quantitative and qualitative changes in CD11b expression and adhesiveness of neutrophils are early consequences of neutrophil activation. Therefore, we evaluated the functional state of neutrophils early after AP induction (day 1) by measuring CD11b expression and in vivo adhesion to lung vessels. We found that peripheral neutrophils from the AP group showed increased CD11b expression compared to that for sham and untreated control animals (Fig. 4 A). Similarly, neutrophils that migrate to the peritoneal cavity in AP mice showed a statistically higher level of CD11b expression than that in sham or untreated control mice. Moreover, peripheral blood smears showed the presence of toxic alterations (cytoplasmic vacuoles) in neutrophils early in the course of AP (Fig. 4C). This type of alteration has been attributed to the loss of membrane integrity or to remnants of bacterial digestion. It is considered an indicator of severe infection and/or septicemia. None of the sham animals presented this type of alteration in leukocytes. Additionally, an increased percentage of neutrophils sequestered in the lung, assessed as the percentage of cells within lung vessels positive for the specific myeloid marker Ly-6G, was observed in AP animals (Fig. 4B).
FIG. 4.
Functional state of neutrophils on day 1 after AP induction. (A) The MFI of the CD11b activation marker was measured in peripheral and peritoneal neutrophils (Ly-6G cells) by flow cytometry. The horizontal line represents the MFI value for untreated healthy mice (n = 9 mice per group; *, P < 0.05 versus sham group). (B) The adhesive properties of neutrophils were evaluated by measuring in vivo the percentage of cells within lung vessels that were positive for the myeloid marker Ly-6G, as described in Materials and Methods (n = 6 mice per group; *, P < 0.05 versus sham group). Representative dot plots are shown. (C) Representative photographs (May-Grünwald-Giemsa staining) showing toxic alterations on peripheral neutrophils from AP animals.
Progression of infectious process.
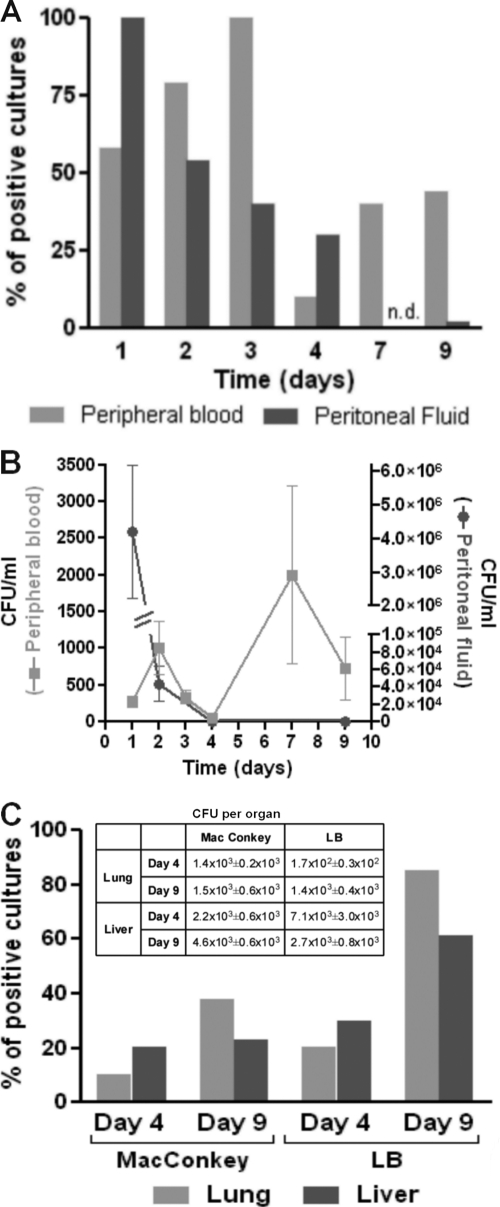
To measure systemic and local bacterial spread after AP induction, aerobic bacterial cultures of peripheral blood and peritoneal fluid, respectively, were performed at different times after surgery. As depicted in Fig. 5 A, all AP mice showed the presence of bacteria in the peritoneum on day 1, and the percentages gradually decreased, with no positive cultures detected for the mice by day 9 after surgery. Fifty percent of peripheral blood cultures were positive on day 1, reaching maximum percentages on day 3 and then decreasing by day 9. The amount of bacteria (numbers of CFU/ml) in the peritoneum gradually decreased over time, whereas in blood, two peaks were observed, one on day 2 and a second peak on day 7 (Fig. 5B). This second rebound of bacteremia preceded the leukocytosis observed on day 9 in AP mice (Fig. 2). Macroscopic examination of the peritoneal cavity at this time point revealed that the peritoneum and intestine of sham mice had a normal appearance and that the tubes in AP mice had been covered with fibrous material, and in some cases, formation of adhesions between the omentum and visceral surfaces was observed. These observations indicate that isolation of the infectious focus was complete by day 9 and that no more bacterial leakage from the intestine was occurring. In line with this observation, no bacterial growth was found in peritoneal fluids on day 20 after AP. Sham animals showed negative cultures at every time point studied.
FIG. 5.
Percentage of positive bacterial cultures and numbers of CFU in peripheral blood, peritoneal fluid, lung, and liver after AP induction. (A) Peripheral blood and peritoneal fluid were collected from AP mice and plated on MacConkey agar (n = 20; n.d., not determined); (B) numbers of CFU/ml obtained on MacConkey agar; (C) the lung and liver were collected and processed as indicated in Materials and Methods and plated on MacConkey or LB agar (n = 13). The table shows the numbers of CFU per organ.
Since bacteremia is an indicator of a persistent infectious process, different organs were tested for the presence of bacteria. On days 4 and 9 after AP induction, the lungs and livers were positive for bacterial growth (Fig. 5C). As some bacterial species may not be represented using the MacConkey selective medium, LB agar was used to detect all bacteria in the organs of AP mice. As expected, the percentages obtained with the nonselective (LB) medium were higher than those obtained with the selective one, and both organs showed similar bacterial loads. Moreover, cultures of tissues obtained from the kidneys and spleen were negative. By day 20, 38% of mice still showed bacteria in their lungs, as determined with LB medium, but none of them showed positive cultures of samples from liver tissues (data not shown). Routine bacteriological identification revealed that the bacteria found in the livers and lungs of mice belong to Enterococcus and Streptococcus spp., species typically present in mouse intestinal flora.
Analysis of delayed phase of AP model.
The results presented above showed that while the local peritoneal infection has been controlled by day 9 after the induction of AP, systemic dissemination generated new infective foci in the lung and liver. Additionally, the AP group showed increased spleen weight on day 9 postsurgery (1.7 ± 0.1 g for the sham group and 3.0 ± 0.7 g for the AP group [n = 14]; P < 0.05 for AP group versus sham group) and number of splenocytes (3.2 × 108 ± 0.5 × 108 cells/spleen for the sham group and 8.6 × 108 ± 1.5 × 108 cells/spleen for the AP group [n = 14]; P < 0.05 for AP group versus sham group), evidencing a persistent systemic immune stimulation. This increase was also observed on day 20 after AP induction (2.1 × 108 ± 2.9 × 108 cells/spleen for the sham group and 9.9 × 108 ± 3.3 × 108 cells/spleen for the AP group [n = 17]; P < 0.05 for AP group versus sham group).
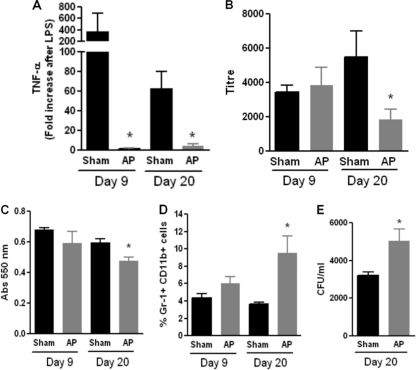
As clinical data indicate that derangements in the immune response are usually observed late in the course of sepsis (6), we evaluated different parameters of the innate and adaptive immune responses on days 9 and 20 after AP induction. The systemic response to LPS was completely impaired, as shown by the absence of TNF-α activity in the serum of AP mice 1.5 h after LPS inoculation (Fig. 6 A).
FIG. 6.
Analysis of different immunological parameters at 9 and 20 days after AP induction. (A) Systemic TNF-α production in response to an inflammatory stimulus. Sham and AP mice were injected with 5 μg of LPS, and serum was obtained 1.5 h later. TNF-α levels were determined using the L929 biological method, as described in Materials and Methods (n = 10 per group; *, P < 0.05 versus sham group). (B) Primary humoral immune response. Mice were immunized with SRBCs on day 9 or 20 postsurgery, and the levels of antibodies against SRBCs in the sera of sham and AP mice were measured 7 days later using the hemagglutination assay. Twofold serial dilutions of serum were performed, and the inverse of the maximal dilution where the antigen-antibody complex was observed is provided (n = 12 per group; *, P < 0.05 versus sham group). (C) T cell proliferation. Splenocytes were obtained from sham and AP mice, and 0.2 × 105 lymphocytes were seeded in a 96-well plate in triplicate in the presence of concanavalin A (5 μg/ml). After 72 h, cells were stained using the MTT assay, as described in Materials and Methods, and the resultant absorbance at 550 nm was measured (n = 6 per group; *, P < 0.05 versus sham group). (D) Percentage of Gr-1+ CD11b+ cells in spleens of sham and AP mice determined by flow cytometry (n = 12 per group; *, P < 0.05 versus sham group). (E) Clearance capacity was determined by inoculating bacteria in the peritoneum and measuring the remaining numbers of CFU in peritoneal lavage fluid specimens 4 h later using MacConkey agar (n = 9 per group; *, P < 0.05 versus sham group).
In order to investigate the primary humoral immune response to a T cell-dependent antigen, mice were injected with SRBCs and the hemagglutination titer in serum was measured 1 week later. As observed in Fig. 6B, although no differences between groups were found on day 9, later in the course of AP (20 days), the primary humoral immune response to SRBCs was decreased.
Additionally, T cell proliferation was decreased in AP mice compared to that in sham mice at 20 days postsurgery (Fig. 6C). A population of myeloid precursors, characterized as Gr-1+ CD11b+, has been described to suppress the immune response in different experimental and clinical pathologies (13, 26). Concomitantly with the impaired adaptive immune response, we found an increased percentage of these Gr-1+ CD11b+ cells in the spleens of AP mice at 20 days after surgery (Fig. 6D).
As the immune response is clearly compromised on day 20 after AP induction, we tested the ability of mice to eliminate a local infection at this time point. For this purpose, bacteria were inoculated in the peritoneal cavity and 4 h later the remnant numbers of CFU were determined in peritoneal lavage fluid specimens. In spite of having an increased number of migrated neutrophils (5.3 × 106 ± 0.6 × 106 for the sham group and 10.7 × 106 ± 1.9 × 106 for the AP group; P < 0.05), AP mice were less efficient in eliminating the bacteria (Fig. 6E).
Histological examination.
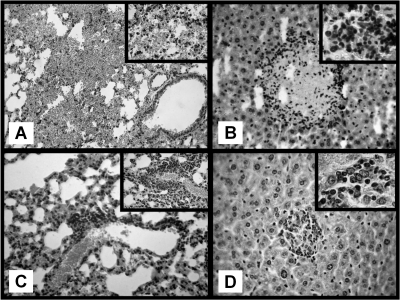
Histological examination of the lungs, liver, kidney, and spleen was performed on days 1, 4, 9, and 20 postsurgery. Congestion and intra-alveolar hemorrhage were observed in the lungs of AP mice on days 1, 4, and 9 (Fig. 7 A). By day 20, leukocyte clusters and residual hemorrhage foci were found (Fig. 7C). Additionally, AP mice showed hepatic necrosis foci with perifocal inflammatory infiltration on day 9 (Fig. 7B). Both neutrophils and lymphocytes were present within inflammatory infiltrations. By day 20, lesions within the liver were almost completely resolved, with some remaining clusters of lymphocytes within the hepatic parenchyma and hepatic degeneration and regeneration being observed (Fig. 7D). No histological alterations were observed in the spleens or kidneys of AP mice at any time point. The corresponding organs obtained from sham mice showed normal histology.
FIG. 7.
Histological examination in AP mice (hematoxylin-eosin staining). (A) Day 1 after AP, intra-alveolar lung hemorrhage. Magnification, ×250. (Inset) Destroyed alveolar septa. Magnification, ×400. (B) Nine days after AP, focus of hepatic necrosis surrounded by abundant leukocyte infiltration. Magnification, ×250. (Inset) Mixed inflammatory infiltrate with neutrophils and lymphocytes. Magnification, ×1,000. (C) Twenty days after AP, lung with focus of perivascular inflammatory infiltrate. Magnification, ×250. (Inset) A predominantly lymphocytic infiltrate. Magnification, ×400. (D) Twenty days after AP, focus of regenerated hepatocytes with chronic inflammatory infiltrate. Magnification, ×250). (Inset) Predominantly lymphocytic infiltrate. Magnification, ×1,000.
DISCUSSION
In this work, our main objective was to develop a useful, reproducible, and clinically relevant model of sepsis. Different animal models have been developed for more than 20 years of research in the field. The most recently developed polymicrobial models of sepsis are cecal ligation and puncture (CLP) and colon ascendant stent peritonitis (CASP). The first model induces a peritonitis triggered by two events: necrosis of the cecum (caused by ligation) and the passage of intestinal flora to the peritoneum (induced by puncture) (38). In this model, a well-tolerated abscess is formed (18, 48). On the other hand, the CASP model simulates a diffuse (not localized) peritonitis, by introducing a titanium stent in the ascending colon, allowing the intestinal leakage of bacteria into the peritoneal cavity (25). In this sense, intestinal leakage is an important factor of sepsis in patients who have undergone abdominal surgeries (3). A diffuse peritonitis instead of a septic abscess is the primary insult in this model. The CASP model may have more clinical relevance, since abscesses rarely trigger severe sepsis, whereas fecal diffuse peritonitis frequently leads to septic shock and multiorgan failure (9, 49). In our study, we introduced several modifications to the previously described CASP model and examined both early and late events triggered by the induction of AP. Silicon tubes were used instead of titanium stents, and the tube was surgically placed in the cecum instead of the ascending colon. The last modification was made, as the fixation of the tube in the ascending colon can obstruct the intestine, causing death not related to a septic phenomenon. Regardless of the introduced modifications, the mortality rate during the first 24 to 48 h in our model was similar to the one reported for the CASP model using a tube of the same diameter (∼50%) (25). In addition, the general signs and the pattern of inflammatory mediators in the animals that died strongly suggest that mortality is caused by septic shock.
Our model imitates a common cause of sepsis, which is the passage or filtration of bacteria from the intestine to the peritoneal cavity as a result of intra-abdominal lesions, such as anastomotic leakage, perforation of hollow viscus, bowel necrosis, or penetrating infectious processes (15). At day 1 postsurgery, both sham and AP mice showed decreased peripheral cell counts and increased peritoneal inflammatory cell counts, although these events were more pronounced in the AP group. Since leukocyte peripheral counts were normal only in anesthetized mice and in animals undergoing surgery without tube fixation, it is likely that the presence of the tube induces an inflammatory reaction. However, only AP mice showed a parallel and early increase in cytokine levels and neutrophil activation systemically and locally, indicating that the local infection triggered strong signals leading to a sepsis phenomenon. In this regard, IL-6 seems to be crucial, since the animals that showed the highest values of IL-6 at 6 h were dead by 24 h (data not shown). In accordance with our data, IL-6 levels predict mortality in the CLP model (36), and high IL-6 levels are also associated with poor outcomes in patients with sepsis (20, 32, 39).
Neutrophils are central in the defense against microorganisms because of their capacity to phagocytose and destroy pathogens. Although neutrophil activation is absolutely necessary to eliminate an infection, excessive, uncontrolled activation can result in tissue damage. Particularly in sepsis, neutrophil activation has been associated with organ damage and multiorgan failure (2). In our model, AP induced clear features of early (day 1) neutrophil activation both locally and systemically, as seen by the sequestration of activated neutrophils in the lungs associated with histological damage in this organ. These results are in line with those of Neumann et al., who reported that the systemic inflammation induced by CASP surgery results in a rapid (6 to 12 h) and profound increase of lung vascular permeability associated with activation and recruitment of neutrophils to the lung (31). This is an important aspect, since early activation of neutrophils, as a result of systemic inflammation, is central in the pathophysiological processes of adult respiratory distress syndrome (ARDS), a common complication that develops in the acute phase of sepsis (2).
One of the most interesting aspects of our model was the second infectious rebound. After an initial release of bacteria to the bloodstream (day 2), a second peak of bacteremia, accompanied by the presence of bacteria in the lung and liver, was observed 1 week later. At this time point, none of the mice showed bacterium-positive cultures of samples from the peritoneum or elevated acute inflammatory cytokine levels. However, the fact that the bacteria present in the lung and liver belong to mouse intestinal flora suggests that these bacteria were originally released from the induced peritonitis and probably colonized vital organs. It is important to notice that our analysis was restricted to aerobic organisms, and since much of the intestinal flora is anaerobic, this could limit the extent of our conclusions regarding bacterial clearance. However, we think that our results are valuable for several reasons. First, although sepsis and septic shock have been produced by all species of aerobic and anaerobic bacteria, aerobic Gram-negative bacilli (particularly the members of the family Enterobacteriaceae and the pseudomonads) are commonly isolated (21, 33), and aerobic Gram-positive cocci are now becoming the most common causes of sepsis and septic shock, both as the result of direct infection (with Streptococcus pneumoniae, for example) and as the result of toxin production (i.e., by Staphylococcus aureus or Streptococcus pyogenes), or both (10, 44). Second, even though anaerobic bacteria may persist longer in the peritoneum, anaerobic organisms account for only 0.5% to 9% of bacteremias, depending on geographic location, hospital patient demographics, and patient age (16). Even more, the incidence of anaerobic bacteremia is decreasing, whereas the number of positive aerobic blood cultures stayed constant in the last few years (1 to 7 positive anaerobic blood cultures versus 80 positive aerobic blood cultures per 1,000 blood cultures performed). The described trend in anaerobic bacteremia has been observed both in the decreasing number of positive anaerobic blood culture results and in the decreasing proportion of anaerobic organisms compared to the number of all organisms causing bacteremia (5, 16, 19).
Histological data revealed inflammatory hepatic and pulmonary damage in AP mice, which is consistent with the persistence of bacteria, as the liver is the major site of bacterial clearance (22, 47) and pulmonary dysfunction is the complication most frequently observed after intra-abdominal sepsis (24, 37). Although pulmonary infections in patients are usually a consequence of infections caused by nosocomial pathogens, in some cases this type of complication may arise secondary to hematologic dissemination from remote foci of infection. One interesting possibility is that early activated neutrophils (and probably tissue-activated macrophages) disarrange local immunity, rendering the liver and lung susceptible to bacterial colonization. Additional studies in order to explore this hypothesis are under investigation.
The second peak of bacteremia was followed by a peak of leukocytosis comprising monocytes and neutrophils that are probably involved in the resolution of bacterial foci within the tissues. Interestingly, even though bacteria were almost eradicated by day 20, a late phase of defective immune responses was clearly observed, evidenced by flaws in both the innate and adaptive immunity. In this regard, we found almost absent TNF-α production in response to LPS at both 9 and 20 days after AP. This is in agreement with an LPS refractory state, known as endotoxin tolerance, as a consequence of a previous contact with Gram-negative bacterial stimuli (14). Additionally, impairments in the primary antibody immune response and T cell proliferation were also observed. Concomitant with these findings, an increased percentage of myeloid-derived suppressor cells (MDSCs), characterized by the presence of the myeloid markers Gr-1 and CD11b, was found in the spleens of AP mice. It has been demonstrated that MDSCs are able to inhibit the antigen-specific T cell response (23, 41) and that their levels increase after immunization with different antigens or after bacterial or parasitic infections in mice (4, 27). Further studies are necessary to demonstrate that in our model MDSCs are involved in the derangements observed in the adaptive immune response after AP.
Our findings showing an impaired late immunity are in line with clinical observations, where patients have typical signs of immunosuppression late in the course of sepsis (12). Defects include diminished expression of important cell surface antigens, dysregulated cytokine production, alterations in antigen-presenting ability, and accelerated apoptosis. Impaired leukocyte function has important clinical ramifications, as high mortality rates have been observed among patients displaying evidence of sepsis-induced immune deactivation, commonly associated with a diminished ability to eliminate infections (35). In this sense, in the late phase of our model we observed less of an ability to clear a local bacterial infection. Whereas in the CASP model no published work to date has determined the state of the immune response beyond 24 h, different parameters associated with immunosuppression have been reported in other models. Old work reports that the injection of moderate doses of LPS daily reduces the capacity to generate antibodies for between 1 and 7 days (42, 43), and using the CLP model, a low clearance capacity and an increased sensitivity to a second infection were observed (40, 50).
In summary, our model of sepsis presented an acute proinflammatory phase with high mortality rates, increased IL-6 levels, and neutrophil activation, but it also induced a delayed state where deranged immune responses were observed. The limitations of sepsis models are diverse, and some are difficult to overcome. Even though all models have important limitations, they have contributed significantly to the knowledge of the pathophysiological and therapeutic aspects of human sepsis. The recommendations for the treatment of severe sepsis are based on clinical tests that were designed on the basis of animal models and preclinical studies. Therefore, we consider that the effort to develop good models is extraordinarily necessary to improve the comprehension of the biology of human sepsis and design effective therapeutic strategies.
Acknowledgments
This work was supported by grants from Alberto J. Roemmers and the Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 20 December 2010.
REFERENCES
- 1.Adib-Conquy, M., and J. M. Cavaillon. 2009. Compensatory anti-inflammatory response syndrome. Thromb. Haemost. 101:36-47. [PubMed] [Google Scholar]
- 2.Aldridge, A. J. 2002. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur. J. Surg. 168:204-214. [DOI] [PubMed] [Google Scholar]
- 3.Angus, D. C., and R. S. Wax. 2001. Epidemiology of sepsis: an update. Crit. Care Med. 29:S109-S116. [DOI] [PubMed] [Google Scholar]
- 4.Atochina, O., T. Daly-Engel, D. Piskorska, E. McGuire, and D. A. Harn. 2001. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Grl(+) macrophages that suppress naive CD4(+) T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J. Immunol. 167:4293-4302. [DOI] [PubMed] [Google Scholar]
- 5.Bengualid, V., H. Singh, V. Singh, and J. Berger. 2008. An increase in the incidence of anaerobic bacteremia: true for tertiary care referral centers but not for community hospitals? Clin. Infect. Dis. 46:323-324. [DOI] [PubMed] [Google Scholar]
- 6.Benjamim, C. F., C. M. Hogaboam, and S. L. Kunkel. 2004. The chronic consequences of severe sepsis. J. Leukoc. Biol. 75:408-412. [DOI] [PubMed] [Google Scholar]
- 7.Bone, R. C. 1996. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit. Care Med. 24:1125-1128. [DOI] [PubMed] [Google Scholar]
- 8.Bone, R. C., et al. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee, American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644-1655. [DOI] [PubMed] [Google Scholar]
- 9.Bosscha, K., T. J. van Vroonhoven, and C. van der Werken. 1999. Surgical management of severe secondary peritonitis. Br. J. Surg. 86:1371-1377. [DOI] [PubMed] [Google Scholar]
- 10.Cervera, C., M. Almela, J. A. Martinez-Martinez, A. Moreno, and J. M. Miro. 2009. Risk factors and management of Gram-positive bacteraemia. Int. J. Antimicrob. Agents 34(Suppl. 4):S26-S30. [DOI] [PubMed] [Google Scholar]
- 11.Deitch, E. A. 1998. Animal models of sepsis and shock: a review and lessons learned. Shock 9:1-11. [DOI] [PubMed] [Google Scholar]
- 12.Docke, W. D., et al. 1997. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat. Med. 3:678-681. [DOI] [PubMed] [Google Scholar]
- 13.Egan, C. E., W. Sukhumavasi, A. L. Bierly, and E.Y. Denkers. 2008. Understanding the multiple functions of Gr-1(+) cell subpopulations during microbial infection. Immunol. Res. 40:35-48. [DOI] [PubMed] [Google Scholar]
- 14.Fan, H., and J. A. Cook. 2004. Molecular mechanisms of endotoxin tolerance. J. Endotoxin Res. 10:71-84. [DOI] [PubMed] [Google Scholar]
- 15.Farthmann, E. H., and U. Schoffel. 1998. Epidemiology and pathophysiology of intraabdominal infections (IAI). Infection 26:329-334. [DOI] [PubMed] [Google Scholar]
- 16.Fenner, L., A. F. Widmer, C. Straub, and R. Frei. 2008. Is the incidence of anaerobic bacteremia decreasing? Analysis of 114,000 blood cultures over a ten-year period. J. Clin. Microbiol. 46:2432-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez, G. C., et al. 2005. Differential expression of function-related antigens on blood monocytes in children with hemolytic uremic syndrome. J. Leukoc. Biol. 78:853-861. [DOI] [PubMed] [Google Scholar]
- 18.Fink, M. P., and S. O. Heard. 1990. Laboratory models of sepsis and septic shock. J. Surg. Res. 49:186-196. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein, E. J. 1996. Anaerobic bacteremia. Clin. Infect. Dis. 23(Suppl. 1):S97-S101. [DOI] [PubMed] [Google Scholar]
- 20.Groeneveld, A. B., A. N. Tacx, A. W. Bossink, G. J. van Mierlo, and C. E. Hack. 2003. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clin. Immunol. 106:106-115. [DOI] [PubMed] [Google Scholar]
- 21.Kale, I. T., M. A. Kuzu, H. Berkem, R. Berkem, and N. Acar. 1998. The presence of hemorrhagic shock increases the rate of bacterial translocation in blunt abdominal trauma. J. Trauma 44:171-174. [DOI] [PubMed] [Google Scholar]
- 22.Katz, S., M. A. Jimenez, W. E. Lehmkuhler, and J. L. Grosfeld. 1991. Liver bacterial clearance following hepatic artery ligation and portacaval shunt. J. Surg. Res. 51:267-270. [DOI] [PubMed] [Google Scholar]
- 23.Kusmartsev, S. A., Y. Li, and S. H. Chen. 2000. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J. Immunol. 165:779-785. [DOI] [PubMed] [Google Scholar]
- 24.Landelle, C., et al. 2008. Nosocomial infection after septic shock among intensive care unit patients. Infect. Control Hosp. Epidemiol. 29:1054-1065. [DOI] [PubMed] [Google Scholar]
- 25.Maier, S., et al. 2004. Cecal ligation and puncture versus colon ascendens stent peritonitis: two distinct animal models for polymicrobial sepsis. Shock 21:505-511. [DOI] [PubMed] [Google Scholar]
- 26.Makarenkova, V. P., V. Bansal, B. M. Matta, L. A. Perez, and J. B. Ochoa. 2006. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J. Immunol. 176:2085-2094. [DOI] [PubMed] [Google Scholar]
- 27.Mencacci, A., et al. 2002. CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. J. Immunol. 169:3180-3190. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 29.Murray, P. R., et al. (ed.). 2007. Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 30.National Institutes of Health. 1985. Guide for the care and use of laboratory animals. Office of Laboratory Animal Welfare, National Institutes of Health, Bethesda, MD.
- 31.Neumann, B., et al. 1999. Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int. Immunol. 11:217-227. [DOI] [PubMed] [Google Scholar]
- 32.Oberhoffer, M., et al. 1999. Sensitivity and specificity of various markers of inflammation for the prediction of tumor necrosis factor-alpha and interleukin-6 in patients with sepsis. Crit. Care Med. 27:1814-1818. [DOI] [PubMed] [Google Scholar]
- 33.O'Boyle, C. J., et al. 1998. Microbiology of bacterial translocation in humans. Gut 42:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osuchowski, M. F., K. Welch, J. Siddiqui, and D. G. Remick. 2006. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 177:1967-1974. [DOI] [PubMed] [Google Scholar]
- 35.Reddy, R. C., G. H. Chen, P. K. Tekchandani, and T. J. Standiford. 2001. Sepsis-induced immunosuppression: from bad to worse. Immunol. Res. 24:273-287. [DOI] [PubMed] [Google Scholar]
- 36.Remick, D. G., G. R. Bolgos, J. Siddiqui, J. Shin, and J. A. Nemzek. 2002. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17:463-467. [DOI] [PubMed] [Google Scholar]
- 37.Richardson, J. D., M. M. DeCamp, R. N. Garrison, and D. E. Fry. 1982. Pulmonary infection complicating intra-abdominal sepsis: clinical and experimental observations. Ann. Surg. 195:732-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rittirsch, D., L. M. Hoesel, and P. A. Ward. 2007. The disconnect between animal models of sepsis and human sepsis. J. Leukoc. Biol. 81:137-143. [DOI] [PubMed] [Google Scholar]
- 39.Spittler, A., et al. 2000. Relationship between interleukin-6 plasma concentration in patients with sepsis, monocyte phenotype, monocyte phagocytic properties, and cytokine production. Clin. Infect. Dis. 31:1338-1342. [DOI] [PubMed] [Google Scholar]
- 40.Sterns, T., N. Pollak, B. Echtenacher, and D. N. Mannel. 2005. Divergence of protection induced by bacterial products and sepsis-induced immune suppression. Infect. Immun. 73:4905-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talmadge, J. E. 2007. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin. Cancer Res. 13:5243-5248. [DOI] [PubMed] [Google Scholar]
- 42.Uchiyama, T., and D. M. Jacobs. 1978. Modulation of immune response by bacterial lipopolysaccharide (LPS): cellular basis of stimulatory and inhibitory effects of LPS on the in vitro IgM antibody response to a T-dependent antigen. J. Immunol. 121:2347-2351. [PubMed] [Google Scholar]
- 43.Uchiyama, T., and D. M. Jacobs. 1978. Modulation of immune response by bacterial lipopolysaccharide (LPS): multifocal effects of LPS-induced suppression of the primary antibody response to a T-dependent antigen. J. Immunol. 121:2340-2346. [PubMed] [Google Scholar]
- 44.Vincent, J. L., et al. 2006. Sepsis in European intensive care units: results of the SOAP study. Crit. Care Med. 34:344-353. [DOI] [PubMed] [Google Scholar]
- 45.Vincent, J. L., Q. Sun, and M. J. Dubois. 2002. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin. Infect. Dis. 34:1084-1093. [DOI] [PubMed] [Google Scholar]
- 46.Vincent, J. L., F. Taccone, and X. Schmit. 2007. Classification, incidence, and outcomes of sepsis and multiple organ failure. Contrib. Nephrol. 156:64-74. [DOI] [PubMed] [Google Scholar]
- 47.Wang, X., R. Andersson, J. Ding, L. Norgren, and S. Bengmark. 1993. Reticuloendothelial system function following acute liver failure induced by 90% hepatectomy in the rat. HPB Surg. 6:151-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wichterman, K. A., A. E. Baue, and I. H. Chaudry. 1980. Sepsis and septic shock—a review of laboratory models and a proposal. J. Surg. Res. 29:189-201. [DOI] [PubMed] [Google Scholar]
- 49.Wittmann, D. H., M. Sehein, and R. E. Condon. 1996. Management of secondary peritonitis. Ann. Surg. 224:10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao, H., J. Siddiqui, and D. G. Remick. 2006. Mechanisms of mortality in early and late sepsis. Infect. Immun. 74:5227-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]