Abstract
Genital tract infections caused by Neisseria gonorrhoeae and Chlamydia trachomatis serovars D to K occur at high incidence in many areas of the world. Despite high rates of coinfection with these pathogens, investigations of host-parasite interactions have focused on each pathogen individually. We describe here a coinfection model in which female BALB/c mice were first infected with the mouse Chlamydia species C. muridarum and then inoculated with N. gonorrhoeae following treatment with water-soluble 17β-estradiol to promote long-term gonococcal infection. Viable gonococci and chlamydiae were recovered for an average of 8 to 10 days, and diplococci and chlamydial inclusions were observed in lower genital tract tissue by immunohistochemical staining. Estradiol treatment reduced proinflammatory cytokine and chemokine levels in chlamydia-infected mice; however, coinfected mice had a higher percentage of vaginal neutrophils compared to mice infected with either pathogen alone. We detected no difference in pathogen-specific antibody levels due to coinfection. Interestingly, significantly more gonococci were recovered from coinfected mice compared to mice infected with N. gonorrhoeae alone. We found no evidence that C. muridarum increases gonococcal adherence to, or invasion of, immortalized murine epithelial cells. However, increased vaginal concentrations of inflammatory mediators macrophage inflammatory protein 2 and tumor necrosis factor alpha were detected in C. muridarum-infected mice prior to inoculation with N. gonorrhoeae concurrently with the downregulation of cathelicidin-related antimicrobial peptide and secretory leukocyte peptidase inhibitor genes. We conclude that female mice can be successfully infected with both C. muridarum and N. gonorrhoeae and that chlamydia-induced alterations in host innate responses may enhance gonococcal infection.
Chlamydia and gonorrhea are the two most common notifiable infectious diseases in the United States, with over 1 million cases of chlamydia and 350,000 cases of gonorrhea reported to the Centers for Disease Control in 2008 (9). Actual rates of infection are much higher due to high rates of asymptomatic infection (50). As many as 50 to 70% of individuals with gonorrhea also have a chlamydial infection (20, 50, 55), and empirical treatment for chlamydia upon detection of N. gonorrhoeae is recommended (10, 27). Neisseria gonorrhoeae and Chlamydia trachomatis are both Gram-negative, human-specific pathogens. In symptomatic infections, both organisms elicit a proinflammatory response characterized by the influx of polymorphonuclear leukocytes (PMNs). Clinically, gonorrhea is typically more pyogenic. Postinfection complications can occur with either pathogen and complications are generally more common and more severe in women. Infections that ascend to the upper genital tract in women lead to pelvic inflammatory disease (PID), the complications of which include chronic pelvic pain, ectopic pregnancy, and infertility (28, 78).
The incidence (50, 55), transmission (45, 46, 48), and associated symptoms and complications (67, 68) of gonococcal and chlamydial coinfection have been examined in epidemiologic studies. However, differences in the pathogenesis and host response to coinfection have not been investigated in an infection model. N. gonorrhoeae is primarily extracellular but can invade and replicate within epithelial cells (18, 23). While the inflammatory response to N. gonorrhoeae can be robust in symptomatic infections, gonococcal infection induces only a transient antibody response (76). Recent evidence suggests that interleukin-17 (IL-17) responses are induced during gonococcal infection and that both IL-17 (25) and Toll-like receptor 4 (TLR4) are protective (59). In contrast, C. trachomatis is an obligate, intracellular parasite that undergoes a complex life cycle within the host cell involving the infectious, metabolically inactive elementary body (EB) and the metabolically active, replicative reticulate body (RB) (1). The primary immune response to C. trachomatis is through the Th1 pathway (16).
Despite these differences in lifestyles and host response, how one organism may alter the pathogenesis, disease severity, susceptibility, and host response to the other pathogen is not known. A small-animal model of gonococcal and chlamydial coinfection is needed to facilitate the investigation of aspects of pathogenesis that are unique to coinfection and the development of improved products. Well-established female mouse models of gonococcal or chlamydial infection currently exist. The development of a coinfection model, however, is challenged by differences in the susceptibility of mice to each pathogen with respect to the stage of the reproductive cycle. The mouse model of gonococcal genital tract infection capitalizes on the transient susceptibility of female mice to N. gonorrhoeae that occurs during the proestrus stage of the estrous cycle (15, 35). In this model, mice are treated with 17β-estradiol and antibiotics to promote long-term colonization with N. gonorrhoeae (32). In the most recent modification of this model, water-soluble estradiol is used, and serum estradiol concentrations return to physiological levels by day 3 postinfection and mice are colonized for 10 to 12 days (76). Gonococci are detected within murine vaginal and cervical tissue (76) and ascend to the upper genital tract in ca. 18 to 20% of mice (32). Infected mice have an inflammatory response that is characterized by PMN influx and the induction of tumor necrosis factor alpha (TNF-α), IL-6, macrophage inflammatory protein 2 (MIP-2), and KC (32, 58, 75). Similarly to human infection, mice elicit an unremarkable humoral response and are susceptible to repeat infection with the same strain (76). Female mice are most susceptible to Chlamydia species when in the progesterone-dominant phase of the reproductive cycle and protocols for infection with Chlamydia muridarum (formerly C. trachomatis MoPn) (3) or human serovars of C. trachomatis that use or do not use progesterone treatment have been developed (3, 6, 62). The course of infection with the mouse pneumonitis agent, C. muridarum, appears more similar to human genital chlamydial disease than infection of mice with human serovars of C. trachomatis. C. muridarum ascends from the lower to the upper genital tract of female mice (3) and causes an inflammatory response that is characterized by infiltration of both acute and chronic inflammatory cells, and postinfection sequelae, such as tubal occlusion, hydrosalpinx, and infertility (16, 19).
We describe here the successful coinfection of female mice with N. gonorrhoeae and C. muridarum. Differences in colonization load and the host immune response occurred in coinfected mice compared to mice infected with either pathogen alone. This model should serve as a useful research tool for further study of gonococcal and chlamydial coinfection and the development of prophylactic and therapeutic agents against bacterial cervicitis and PID.
MATERIALS AND METHODS
Bacterial propagation.
N. gonorrhoeae strain FA1090 (porB1b, AHU [an auxotype for arginine, hypoxanthine, and uracil], serum resistant) was originally isolated from a female with disseminated gonococcal infection (12). Frozen stocks of piliated, OpaB-expressing FA1090 bacteria isolated from a male volunteer (33) were passaged on solid GC agar containing Kellogg's supplement I (37) and 12 μM Fe(NO3)3 and incubated at 37°C in a humidified 7% CO2 incubator. GC agar with antibiotic selection (GC-vancomycin, colistin, nystatin, trimethoprim sulfate, and streptomycin [VCNTS]) and heart infusion agar (HIA) were used to isolate N. gonorrhoeae and facultatively anaerobic commensal flora, respectively, from murine vaginal mucus as described previously (32). C. muridarum strain Nigg (70) was propagated in L929 mouse fibroblast cells (a gift from Anthony T. Maurelli, Uniformed Services University, Bethesda, MD), and C. trachomatis serovar D (a gift from Anthony T. Maurelli) was propagated in ME180 human cervical epithelial cells, similarly to the method described by O'Connell and Nicks (57). Briefly, monolayers of L929 or ME180 cells in 24-well plates were inoculated with C. muridarum or C. trachomatis, respectively, suspended in infection media (1× Dulbecco modified Eagle medium [DMEM] supplemented with 10% fetal bovine serum [FBS], 50 μg of gentamicin/ml, and 100 μg of cycloheximide/ml) at a multiplicity of infection (MOI) of 1. Plates were centrifuged at 1,600 × g for 1 h at 37°C, and the medium was replaced with fresh infection medium. Infected monolayers were incubated at 37°C in 5% CO2 for 36 h before being harvested into SPG buffer (218 mM sucrose, 3.8 mM KH2PO4, 7.2 mM K2HPO4, 4.9 mM l-glutamate [pH 7.2]), sonicated briefly, and frozen at −80°C. The titer of inclusion-forming units (IFU) in each stock was determined by immunofluorescence as described by Kelly et al. (39) except that inclusions were stained with Chlam-III antichlamydial lipopolysaccharide (LPS) antibody (Santa Cruz Biotechnology) at a 1:500 dilution and goat anti-mouse AlexaFlour-488 secondary antibody (Invitrogen) at a 1:2,000 dilution. L929 mouse fibroblast cells were maintained in DMEM with 10% FBS and grown to large quantities in suspension culture in RPMI with 5% FBS. ME180 human cervical epithelial cells were maintained in McCoy's 5A medium supplemented with 10% FBS and 2.2 g of NaHCO3/liter. Solid agar, cell culture reagents, and chemicals were purchased from Difco, Quality Biological, and Sigma, respectively, unless otherwise noted.
Coinfection protocol.
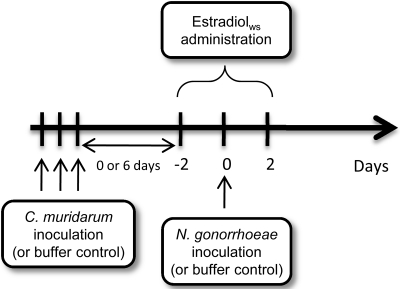
Female BALB/c mice at 4 to 6 weeks of age were purchased from the National Cancer Institute (Bethesda, MD). The infection protocol is shown in Fig. 1. Except when noted, each experiment consisted of four groups of mice: mice coinfected with N. gonorrhoeae and C. muridarum, mice infected with either pathogen alone, and mice inoculated with buffers alone as a control for inflammation (n = 10 to 11 mice per group in each of three experiments). Inoculations with C. muridarum were performed on anesthetized mice for the purpose of immobilization by intraperitoneal (i.p.) injection of a ketamine-xylazine mixture (10 and 1.5 mg, respectively, per 100 g of body weight). Mice were then vaginally inoculated with 3 × 105 IFU of C. muridarum in 20 μl of 2-SP buffer (200 mM sucrose, 12 mM K2HPO4, 8 mM KH2PO4) by pipette on three consecutive days to increase the likelihood that mice were in the diestrus stage of the reproductive cycle (62). The dose of C. muridarum was calculated based on the titer of the frozen stock determined as described above. Mice infected with N. gonorrhoeae alone and control mice were similarly anesthetized and mock inoculated with 10 μl of 2-SP buffer. On the final day of C. muridarum inoculation, vaginal smears from all mice were prepared on glass slides and stained with a Hema-3 stain (Fisher Scientific) to identify mice in the diestrus stage as described previously (15). Mice with a predominance of PMNs and nucleated epithelial cells, rather than squamous epithelial cells, were considered to be in diestrus. Mice found to be in diestrus were then treated with a subcutaneous injection of 0.5 mg of water-soluble 17β-estradiol (estradiolws; Sigma) (76) approximately 6 h after the final inoculation with C. muridarum or buffer and again 2 and 4 days later. At 4 h after the second dose of estradiolws, mice were vaginally inoculated with 106 CFU of N. gonorrhoeae in 20 μl of phosphate-buffered saline (PBS) (N. gonorrhoeae-only and coinfected groups) or mock inoculated with PBS (C. muridarum-only and uninfected control groups). The N. gonorrhoeae inoculum was prepared as described elsewhere (32), and the dose was confirmed by quantitative culture. All mice were given vancomycin hydrochloride (0.6 mg, twice daily) and streptomycin sulfate (2.4 mg, twice daily) via i.p. injection beginning 2 days before N. gonorrhoeae inoculation and maintained for 7 days to control overgrowth of commensal flora. In two experiments, C. muridarum infection was allowed to proceed for 8 to 10 days prior to treatment with estradiolws and inoculation with N. gonorrhoeae. In these experiments, n = 5 and 10, and the combined results are reported.
FIG. 1.
Time line for coinfection protocol. Mice were inoculated with C. muridarum on three consecutive days to establish chlamydial infection. After the final inoculation with C. muridarum, mice in the diestrus stage of the estrous cycle were treated with water-soluble estradiol and inoculated with a single dose of N. gonorrhoeae 2 days later. In the experiments described, mice were infected with C. muridarum either 2 to 4 days or 8 to 10 days prior to inoculation with N. gonorrhoeae.
Quantitation of colonization load and PMN influx.
The numbers of gonococci and chlamydiae recovered from each group were determined daily for 10 days in three separate experiments consisting of four groups (n = 10 to 11 mice per group in each experiment). Vaginal mucus was collected with a PBS-soaked polyester swab, and a small portion of the sample was inoculated onto an HIA plate for recovery of facultatively anaerobic commensal flora and a glass slide for enumeration of PMNs. The percentage of PMNs per 100 vaginal cells was determined by cytological differentiation of stained vaginal cells as described previously (15). The remaining sample was then suspended in 1 ml of transport buffer (2-SP buffer supplemented with 3% FBS and 0.5 mg of vancomycin/ml). Suspensions were cultured onto GC-VCNTS agar to isolate N. gonorrhoeae using the Autoplater 4000 (Spiral Biotech) and then frozen at −80°C for culture of C. muridarum. The number of CFU of N. gonorrhoeae recovered was enumerated using the Spiral Biotech Q-Counter Software. For C. muridarum, suspensions were diluted and cultured onto monolayers of L929 cells and quantified by using immunofluorescence as described above. The limits of detection were 20 CFU (N. gonorrhoeae) and 12.5 IFU (C. muridarum) per ml of vaginal swab suspension. Upper genital tracts (uterine horns, oviducts, and ovaries) were cultured for N. gonorrhoeae and C. muridarum 10 days after inoculation with N. gonorrhoeae or PBS (chlamydiae alone) in a single experiment consisting of 12 mice per group. Upper genital tracts were homogenized in 2-SP buffer. The homogenate was serially diluted in PBS and cultured for N. gonorrhoeae on GC-VCNTS. The remaining homogenate was frozen at −80°C and C. muridarum titers were determined by a plaque assay as described previously (57), except that monolayers of L929 cells were used.
Enzyme-linked immunosorbent assay (ELISA) for C. muridarum- and N. gonorrhoeae-specific antibodies.
Vaginal washes and sera were collected on days 10 and 28 postchallenge with N. gonorrhoeae (N. gonorrhoeae-infected and coinfected groups) or PBS (uninfected and C. muridarum-infected groups) in the three experiments performed to assess gonococcal and chlamydial colonization and examined for chlamydia- or gonococcus-specific antibodies. EBs from C. muridarum strain Nigg were purified by using a Renografin (Bracco Diagnostics) gradient (8), and outer membrane vesicles (OMVs) from N. gonorrhoeae strain FA1090 were prepared as described previously (7). Ninety-six-well plates were coated with 50 μl of either C. muridarum EBs (5 μg/ml) or N. gonorrhoeae OMVs (10 μg/ml) in 0.5 M NaHCO3 overnight at room temperature. All washes were performed with PBS containing 0.1% Tween 20 by using a Skan Washer (Molecular Devices). Wells were blocked with PBS containing 15% FBS for 30 min at 37°C in a humidified chamber. Sera were diluted 1:100 and 1:900, and vaginal washes were diluted 1:30 and 1:100 in the block solution and added to the wells (50 μl), followed by incubation with secondary antibody (goat anti-mouse IgG, IgM, or IgA conjugated to horseradish peroxidase, Sigma) diluted 1:10,000 in the block solution. Incubations with primary and secondary antibodies were for 1 h at 37°C in a humidified chamber. TMB-peroxidase detection solution (Bio-Rad) was added to detect bound secondary antibody. The reaction was stopped after 10 min with 0.1 N H2SO4, and the optical density at 450 nm (OD450) was read using Biotek Instruments EL80 Universal microplate reader and KC Junior software.
Cytokine and chemokine protein analysis.
Genital tract secretions were collected for cytokine/chemokine analysis in a single experiment consisting of the following five groups: estradiol-treated mice that were infected with either single agent or both agents or left uninfected and mice infected with C. muridarum and not treated with estradiol (n = 4 to 5 mice/group). Genital tract secretions were collected with absorbent sponges (DeRoyal Earwick), and proteins were eluted as described previously (17). The levels of gamma interferon (IFN-γ), IL-1β, IL-10, MIP-2, RANTES, TNF-α, and IL-17 protein were measured by using a Millipore Mouse Cytokine/Chemokine Milliplex map kit as instructed. Reactions were read on a Luminex 100 IS instrument, and the mean fluorescence intensity was compared to standard curves to calculate pg/ml concentrations of each protein by using the Luminex 100 IS software.
Immunohistochemical tissue analysis.
Tissue was collected for histological examination during a single experiment consisting of coinfected mice, mice infected with either pathogen alone, and uninfected control mice. Whole genital tracts were harvested from five mice per group on day 2 postinoculation with N. gonorrhoeae or PBS (chlamydiae alone), and tissue was fixed in 10% buffered formalin for 24 h and then stored in 70% ethanol prior to embedding in paraffin and sectioning onto slides for immunohistochemical analysis. Sections were double immunolabeled for C. muridarum using the Chlam-III antichlamydial antibody (Santa Cruz Biotechnology) at a 1:50 dilution and mouse antiserum raised against N. gonorrhoeae strain FA1090 outer membrane vesicles at a 1:500 dilution by Histoserv, Inc. (Germantown, MD).
Tissue culture adherence assay.
ME180 human cervical epithelial cells were cultured as described above. IEC4.1 mouse intestinal epithelial and BM1.11 mouse oviduct cells (gifts from Harlan Caldwell [Rocky Mountain Laboratories, Hamilton, MT] and Raymond Johnson [Indiana University School of Medicine, Indianapolis, IN], respectively) were cultured as described elsewhere (69). Cells were seeded into 24-well plates and cultured to obtain monolayers of 80 to 90% confluence and inoculated with C. trachomatis (ME180 cells) or C. muridarum (IEC4.1 and BM11.1 cells) at an MOI of 1 or mock inoculated with SPG buffer with centrifugation in culture medium, as described above. After 20 h of incubation in normal culture medium, N. gonorrhoeae strain FA1090 was diluted in PBS to an OD600 of 0.07, followed by dilution in RPMI (Quality Biological) supplemented with 10% FBS and 0.3 μM Fe(NO3)3, and monolayers were inoculated with 500 μl of the N. gonorrhoeae suspension (final MOI = 1). After 2 h at 37°C in 7% CO2, the number of cell-associated gonococci was determined by washing monolayers four times with PBS, followed by host cell lysis with 0.5% saponin in PBS and quantitative culture. Invasion was measured by a gentamicin (Gm) protection assay. For intracellular bacteria, monolayers were washed two times with PBS after 2 h of incubation, and 500 μl of RPMI supplemented with 10% FBS, 0.3 μM Fe(NO3)3, and 50 μg of Gm/ml was added for 1.5 h. Monolayers were washed five times with PBS, and cells were lysed in 0.5% saponin in PBS, followed by quantitative culture. The results are expressed as the percentage of cell-associated bacteria relative to the inocula (percent adherence) or as the percentage of Gm-protected bacteria relative to the number of adherent bacteria under the same conditions (percent invasion). Conditions were performed in triplicate and each experiment was repeated at least three times.
Gene expression analysis by reverse transcription-PCR (RT-PCR).
Vaginal material was collected with a PBS-soaked polyester swab and suspended directly in 500 μl of RNA-Later (Ambion) from uninfected or C. muridarum-infected mice 15 min prior to inoculation with N. gonorrhoeae to determine expression levels of the cathelicidin-related antimicrobial peptide (CRAMP) gene cnlp, and the secretory leukocyte peptidase inhibitor (SLPI) gene. Samples were stored at −80°C until use. Total RNA was extracted by using the Qiagen mini-RNeasy isolation kit according to the manufacturer's instructions. RNA was converted to cDNA using the SABioscience RT2 EZ First Strand cDNA kit and then used for real-time PCR. cDNA reaction mixtures (20-μl total volume) were diluted to a final volume of 100 μl in nuclease-free water, and 5 μl of diluted cDNA template was subjected to PCR amplification using an ABI 7500 sequence detector (25-μl total reaction volume consisting of template, 12.5 μl of SYBR green master mix [ABI], and 10 μl of primer mix, which contained both forward and reverse primers at concentrations of 1 μM). Reactions were performed according to the following parameters: 10 min at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The cycle threshold (CT) value was determined by using Sequence Detector v.1.7a software (ABI). The data were analyzed with Microsoft Excel using the comparative CT method (ΔΔCT) for relative quantification of gene expression using β-actin as the normalizer, as described previously (58). Expression of the CRAMP and SLPI genes was measured by normalizing to the β-actin gene. The calculation used included the difference between the CT values of the normalizer (β-actin) and the CT values of the test genes (cnlp and SLPI) in individual samples, as follows: ΔCT(C.muridarum infected or uninfected) = CT(β-actin) − CT(test gene). ΔCT values for all uninfected mice were calculated, and the mean of these values was used as a baseline for calculating the ΔΔCT value for each C. muridarum-infected mouse. The relative difference in gene expression between each C. muridarum-infected mouse and the uninfected baseline value on day 0 (prior to inoculation with N. gonorrhoeae) was defined as the difference of normalized gene expression levels as follows: 2ΔΔCT = ΔCT(uninfected baseline) − ΔCT(infected). The experiment was performed twice with four to five mice per group. The sequences of the oligonucleotide primers are shown in Table 1.
TABLE 1.
Oligonucleotide sequences used in this study
Statistical analysis.
Differences in gonococcal and chlamydial colonization load between coinfected and single-pathogen-infected groups were assessed by repeated-measures ANOVA. PMN influx was compared between all groups by ANOVA using the Bonferroni correction for multiple comparisons. Unpaired t tests were performed on results from individual days. The levels of gonococcal cell association and invasion were compared by unpaired t tests, as were the cytokine and chemokine levels in genital tract secretions. P values of ≤0.05 were considered significant.
Animal use assurances.
All animal experiments were conducted in the laboratory animal facility at the Uniformed Services University, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, under a protocol approved by the university's Institutional Animal Care and Use Committee.
RESULTS
Coinfection of female mice with N. gonorrhoeae and C. muridarum.
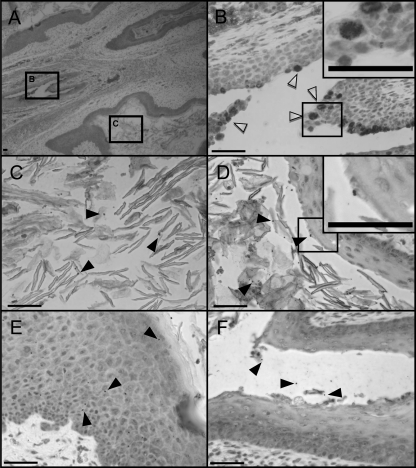
Female mice are most susceptible to infection with N. gonorrhoeae during the estrogen-dominant stage of the estrous cycle (15, 35, 41) and C. muridarum during the progesterone-dominant stage of the estrous cycle (6, 79). Progesterone treatment is not required for long-term colonization with C. muridarum provided that mice are inoculated during the diestrus stage of the estrous cycle (62). In contrast, susceptibility to long-term colonization with N. gonorrhoeae requires administration of estradiol to promote an estrus-like state. In pilot experiments, our attempts to coinfect estradiol-treated female BALB/c mice with N. gonorrhoeae and C. muridarum simultaneously were unsuccessful. However, we found that once C. muridarum infection was established, subsequent treatment with estradiol did not cause chlamydial infection to clear or alter the number of chlamydial inclusions recovered, in agreement with previous observations (38). Thus, we developed a model in which chlamydial infection was established, followed by inoculation with N. gonorrhoeae (Fig. 1). Using this protocol, we recovered viable gonococci and chlamydia from vaginal swabs for averages of 6 to 7 days and 9 to 10 days, respectively, postinoculation with N. gonorrhoeae in three separate experiments (Tables 2 and 3). N. gonorrhoeae diplococci and C. muridarum inclusions were visualized in genital tract tissues of coinfected mice by immunohistochemical staining (Fig. 2). C. muridarum inclusions were restricted to the cervix of coinfected animals. Gonococci were observed in the lumens of the vagina and the cervix, as well as deep within the vaginal tissue, 2 days after gonococcal challenge. We conclude that coinfection of female mice with N. gonorrhoeae and C. muridarum can be established.
TABLE 2.
Coinfection does not alter the duration of recovery of N. gonorrhoeae
| Expt | Mean duration (days) of bacterial recoverya |
|
|---|---|---|
| N. gonorrhoeae alone | Coinfection | |
| I | 6.4 (1-10) | 8.0 (4-10) |
| II | 6.1 (2-9) | 6.1 (4-9) |
| III | 7.6 (1-10) | 9.7 (7-10) |
| Cumulative | 6.6 (1-10) | 7.7 (4-10) |
The values represent the mean number of days of 10 consecutive days N. gonorrhoeae was recovered by vaginal swab from mice in three separate experiments, with the range (in days) represented in parentheses.
TABLE 3.
Coinfection does not alter the duration of recovery of C. muridarum
| Expt | Mean duration (days) of bacterial recoverya |
|
|---|---|---|
| C. muridarum alone | Coinfection | |
| I | 9.2 (8-10) | 9.5 (8-10) |
| II | 9.9 (9-10) | 9.8 (9-10) |
| III | 9.0 (5-10) | 8.0 (5-10) |
| Cumulative | 9.5 (5-10) | 9.2 (5-10) |
The values represent the mean number of days of 10 consecutive days C. muridarum was recovered by vaginal swab from mice in three separate experiments, with the range in days represented in parentheses.
FIG. 2.
Immunohistochemical staining reveals the presence of N. gonorrhoeae and C. muridarum in the coinfected genital tract. (A) Genital tract tissue extracted from a coinfected mouse on day 2 after N. gonorrhoeae inoculation at ×40 magnification. Boxes B and C correspond to the regions shown in panels B and C, respectively, where they are magnified at ×400. (B) Cervical tissue at ×400 magnification showing distinct C. muridarum inclusions (open arrowheads) within nucleated epithelial cells. Panels C, D, E, and F show tissue from the same mouse at ×400 magnification with visible N. gonorrhoeae diplococci (filled arrowheads) among squamous epithelial cells in the vaginal lumen (C), squamous epithelial cells in the vaginal lumen and superficially associated with the vaginal epithelium (D), deep within the vaginal tissue (E), and in the lumen of the cervix (F). Several chlamydial inclusions and a single diplococcus are shown in the insets of higher magnification in panels B and D, respectively. All scale bars represent 50 μm. The dual staining performed by Histoserv, Inc., was done with a different color chromogen for each organism for positive identification.
We saw no difference in the rate of ascendant infection with either pathogen. Ascendant chlamydial infection was observed in 75% of C. muridarum-infected mice and this percentage was not altered by coinfection with N. gonorrhoeae (mean of 103 PFU of C. muridarum per upper genital tract in both groups; data not shown). This rate of ascension is comparable to rates seen in progesterone-treated C57/BL6 mice (56). No gonococci were recovered from the upper genital tract at day 10 after gonococcal challenge in mice with or without a preexisting chlamydial infection in these experiments.
Estradiol treatment alters the host response to C. muridarum.
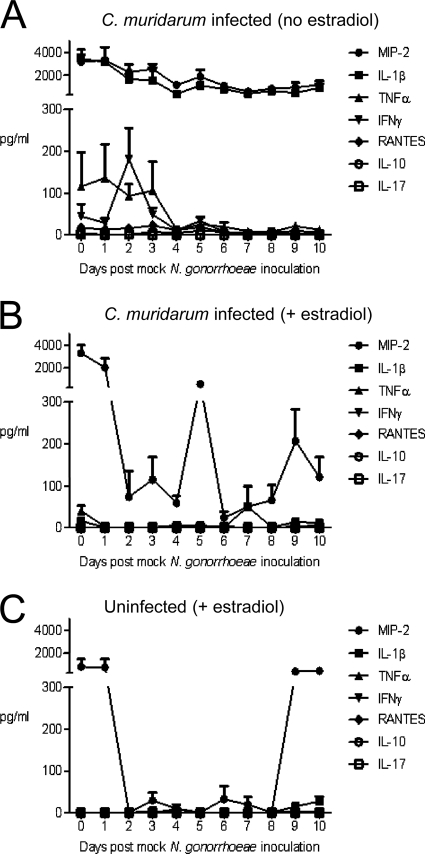
The initial inflammatory response to C. muridarum infection in progesterone-treated mice is intense and wanes over time (16). We defined the kinetics of the inflammatory response to C. muridarum in our model system by investigating the effects of estradiol on the inflammatory response to C. muridarum alone in the absence of inoculation with N. gonorrhoeae. Mice were infected with C. muridarum or mock inoculated with buffer (uninfected control) on three consecutive days. On the final day of C. muridarum inoculation, 10 diestrus-stage mice were identified and half of these mice were treated with estradiol, as per the usual protocol, and half were not (n = 5 mice per group). All diestrus-stage mice in the uninfected control group were treated with estradiol (n = 4 mice). Two days later, all mice were inoculated with PBS, rather than N. gonorrhoeae. Vaginal levels of seven different cytokines and chemokines (MIP-2, IL-1β, TNF-α, IFN-γ, RANTES, IL-10, and IL-17) that were previously implicated in gonococcal (11, 22, 25, 47, 58) or chlamydial (5, 16, 17, 63, 66, 83) infection were measured on the day of PBS challenge and over the next 10 days. Consistent with C. muridarum inducing an inflammatory response, high levels of IFN-γ, TNF-α, IL-1β, and MIP-2 were detected in mice that were not treated with estradiol on days 1 to 4 postinoculation. MIP-2 and IL-1β levels remained elevated over the next 7 days, while IFN-γ and TNF-α levels declined at a time point that corresponds to day 4 postinoculation with N. gonorrhoeae in our coinfection protocol (Fig. 3A). Administration of estradiol dampened the response to C. muridarum as evidenced by similar levels of IL-1β, TNF-α, and IFN-γ in estradiol-treated, infected (Fig. 3B) and estradiol-treated, uninfected (Fig. 3C) mice. Interestingly, estradiol treatment did not fully abrogate the MIP-2 response to C. muridarum as vaginal MIP-2 levels were elevated in estradiol-treated, infected mice compared to uninfected, estradiol-treated mice on days 2 to 8 post-PBS challenge (Fig. 3C). The high levels of MIP-2 in estradiol-treated, uninfected mice on days 0 and 1 and at late time points (days 9 and 10) when the effects of estradiol begin to wear off reflect the normal fluctuations in MIP-2 that are associated with the influx of vaginal PMNs during the metestrus and diestrus phases of the estrous cycle (77). We conclude that the requirement to administer estradiol to establish gonococcal infection reduces the inflammatory response to chlamydial infection but that significant levels of MIP-2 are still induced.
FIG. 3.
The host inflammatory response to C. muridarum infection is altered by estradiol treatment. The protein concentrations (in pg/ml) of cytokines and chemokines in genital tract secretions from (A) non-estradiol-treated, C. muridarum-infected mice, (B) estradiol-treated, C. muridarum-infected mice, and (C) estradiol-treated, uninfected mice (n = 4 to 5 mice per group) were determined.
Vaginal PMN influx is increased in coinfected mice.
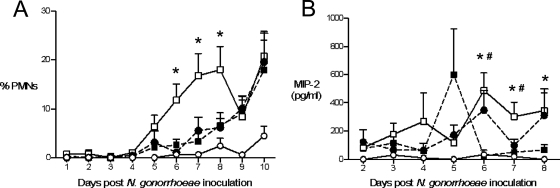
An influx of PMNs is characteristic of symptomatic infection with N. gonorrhoeae or C. trachomatis in both women and men. Similarly, a localized PMN influx is observed in gonococcal infection of BALB/c mice (32, 58, 76) and mouse models of chlamydial infection (17, 60). To determine whether the PMN response is altered during coinfection, the percentages of PMNs in vaginal smears from estradiol-treated uninfected mice, coinfected mice, and mice infected with either N. gonorrhoeae or C. muridarum alone were compared. A similar percentage of PMNs was observed on stained vaginal smears from estradiol-treated mice infected with either N. gonorrhoeae or C. muridarum alone. In both groups, vaginal PMNs were detected beginning at day 5 postchallenge with N. gonorrhoeae (gonorrhea-alone group) or PBS (chlamydia-alone group) and gradually increased, with significantly higher percentages of PMNs detected in both groups on days 9 and 10 compared to uninfected mice (Fig. 4A, solid symbols). Vaginal PMNs were also observed in coinfected mice beginning on day 5 postinoculation with N. gonorrhoeae, with significantly higher percentages of PMNs observed in coinfected mice versus uninfected mice on days 5 to 10. On days 6, 7, and 8 postinoculation with N. gonorrhoeae, a significantly higher percentage of vaginal PMNs were detected in coinfected mice compared to mice infected with either pathogen alone (Fig. 4A, open squares versus solid symbols).
FIG. 4.
A greater PMN influx occurs in coinfected mice. (A) Significantly more PMNs migrate into the lower genital tracts of mice coinfected with N. gonorrhoeae and C. muridarum (white squares, solid line) compared to mice infected with N. gonorrhoeae (black circles, dashed line) or C. muridarum (black squares, dashed line) alone, or uninfected mice (white circles, solid line) on days 6, 7, and 8 after N. gonorrhoeae inoculation (*, P < 0.05). The percent PMNs is defined as the number of PMNs counted/100 vaginal cells observed, including squamous and nucleated epithelial cells and leukocytes. The results shown are from three combined experiments (n = 30 to 32 mice per group). (B) Levels of PMN attracting chemokine MIP-2 are greatest on days 6 to 8 in mice coinfected with N. gonorrhoeae and C. muridarum (white squares, solid line) compared to mice infected with either N. gonorrhoeae (black circles, dashed line) or C. muridarum (black squares, dashed line) alone or uninfected mice (white circles, solid line) on days 6 to 8 (*, P < 0.05, coinfected versus uninfected; #, P < 0.05, coinfected versus C. muridarum alone). The results from a single experiment are shown (n = 4 to 5 mice per group). The high average level of MIP-2 detected on day 5 in C. muridarum-infected mice is due to one mouse in this group having 1,843 pg of MIP-2/ml. MIP-2 concentrations ranged from 60 to 646 pg/ml for the other four mice within this group.
MIP-2, the mouse analog of the human PMN attracting chemokine IL-8, correlates with PMN influx at the infection site in both gonococcal and chlamydial mouse infection models (17, 58). Here, levels of MIP-2 in genital tract secretions also mirrored the degree of vaginal PMN influx, with MIP-2 levels beginning to increase in coinfected mice and mice infected with either pathogen alone on day 5 and continuing to increase during the period of significantly increased PMN influx for all infected groups (days 6 to 8). MIP-2 levels, like the percentage of vaginal PMNs, were highest in the coinfected group on days 6, 7, and 8 postinoculation with N. gonorrhoeae (Fig. 4B). MIP-2 levels were elevated in C. muridarum-infected (similar to that shown in Fig. 3B) and in coinfected mice, but not N. gonorrhoeae-infected mice on days 0 and 1, and decreased to below the limit of detection by day 2. After day 8, MIP-2 levels began to increase in all groups, including uninfected mice, as the effects of estradiol treatment wore off and mice began cycling again (data not shown). Other cytokines and chemokines tested (IL-1β, TNF-α, IFNγ, RANTES, IL-10, and IL-17) were not significantly increased in coinfected mice at time points corresponding to changes in PMN influx (data not shown). In summary, these results suggest that despite the demonstrated immunosuppressive effect of the estradiol used in this model, an inflammatory response that results in a localized PMN influx is generated against mice infected with either single pathogen and that this response is intensified in mice that are coinfected with C. muridarum and N. gonorrhoeae.
No difference was detected in the pathogen-specific antibody response.
C. muridarum-infected mice respond robustly to infection and the antibody response peaks at approximately 4 weeks postinfection (3). In contrast, a weak antibody response occurs during N. gonorrhoeae murine infection, which is localized and transient and peaks at approximately 5 days postinfection (76). It is likely that different interactions occur between innate receptors and bacterial ligands during coinfection compared to infection with either pathogen alone. We therefore hypothesized that coinfection may alter the humoral response to one or both pathogens. As expected, we detected significant levels of serum C. muridarum-specific IgG and IgM and vaginal IgA and IgG in chlamydia-infected mice on days 10 and 28. However, we found no difference between chlamydia-infected and coinfected mice (Fig. 5 and data not shown). We were unable to detect gonococcus-specific antibodies in vaginal washes from coinfected mice or mice infected with N. gonorrhoeae alone on day 10 or 28 after gonococcal challenge; earlier time points may have shown detectable antibodies (76). We conclude that while coinfection alters the innate immune response, there is no evidence that it affects the adaptive response at the level of antibody production.
FIG. 5.
Coinfection does not alter the level of C. muridarum-specific antibodies. Levels of C. muridarum-specific serum IgG (A) and vaginal IgA (B) were measured by ELISA and the absorbance at 450 nm is shown for samples collected from mice infected with C. muridarum alone, mice coinfected with C. muridarum and N. gonorrhoeae, mice infected with N. gonorrhoeae alone, and uninfected mice in a single representative experiment. Samples were collected on day 28 postinoculation with N. gonorrhoeae. Sera samples were diluted 1:900, and vaginal washes were diluted 1:100. Symbols indicate values for individual mice; horizontal bars show the geometric mean.
Gonococcal colonization is increased in coinfected mice.
As discussed, we found no difference in the duration of infection with either pathogen in coinfected versus singly infected mice (Tables 2 and 3). We also found no difference in the number of IFU of C. muridarum recovered from the lower genital tract of mice infected with C. muridarum alone or with both C. muridarum and N. gonorrhoeae (Fig. 6A). However, a 0.5-1 log increase in the number of gonococci recovered from mice coinfected with N. gonorrhoeae and C. muridarum was observed as early as 1 day postinoculation with N. gonorrhoeae compared to mice infected with N. gonorrhoeae alone (Fig. 6B). This difference was maintained through approximately 8 days postinoculation with N. gonorrhoeae and was highly reproducible. To investigate whether an increased gonococcal colonization load would be seen in mice that were infected with C. muridarum for a longer time period before challenging with N. gonorrhoeae, we postponed estradiol treatment to 6 days after the final inoculation of C. muridarum. Mice were thus infected with C. muridarum for 8 to 10 days prior to challenge with N. gonorrhoeae, as described in Materials and Methods, which is when the initial intense inflammatory response to C. muridarum wanes and cytokine levels were the most similar between estradiol-treated and untreated C. muridarum-infected mice (Fig. 3). In two separate experiments we again observed increased gonococcal colonization in mice with a preexisting C. muridarum infection (Fig. 6C). We conclude that the effect of C. muridarum infection on gonococcal colonization is sustained during infection and can occur after the period in which the inflammatory response to C. muridarum peaks.
FIG. 6.
C. muridarum infection alters levels of N. gonorrhoeae in the lower genital tract. (A) C. muridarum colonization remained constant whether mice were coinfected with N. gonorrhoeae (white squares, solid line) or infected with C. muridarum alone (black squares, dashed line); (B) N. gonorrhoeae colonization was greater in mice coinfected with C. muridarum (white squares, solid line) than in mice infected with N. gonorrhoeae alone (black circles, dashed line) (P = 0.011 [repeated-measures ANOVA]). The results shown in panels A and B are from three combined experiments (n = 31 to 32 mice per group). (C) A similar trend in N. gonorrhoeae colonization during coinfection with C. muridarum was observed when mice with a longer pre-established C. muridarum infection were challenged with N. gonorrhoeae (n = 11 to 19 mice per group). The time point at which mice were challenged with N. gonorrhoeae in the experiment shown in panel C corresponds to day 6 in panels A and B. The results are expressed as log10 IFU or CFU per ml of vaginal swab suspension.
C. muridarum does not affect N. gonorrhoeae association with cultured murine epithelial cells.
To investigate the mechanism behind the increased gonococcal colonization load in C. muridarum coinfected mice, we used an in vitro coinfection model to determine whether gonococci are more able to associate with and invade chlamydia-infected epithelial cells. We hypothesized that chlamydial infection may alter the epithelial cell surface to allow greater gonococcal adherence or invasion, thus accounting for the increased gonococcal colonization load observed in vivo. Murine epithelial cell lines of intestinal and oviduct origin were infected with C. muridarum or left uninfected and incubated for 20 h prior to inoculation with N. gonorrhoeae, which is the time point that coincides with active RB replication and the peak epithelial inflammatory cytokine response to chlamydial infection observed in vitro (65). Human cervical epithelial cells infected with C. trachomatis were tested similarly. We observed no difference in the percentage of cell-associated or invasive gonococci in either human or murine epithelial cells in the presence or absence of C. trachomatis or C. muridarum infection, respectively (Fig. 7). Interestingly, the level of gonococcal adherence to human and murine cell lines was similar despite the host restrictions associated with the human-specific pathogen N. gonorrhoeae (Fig. 7A). However, the level of gonococcal invasion into human cells was much greater than in either of the murine cell lines (Fig. 7B), underscoring the importance of host-restricted receptors in mediating gonococcal invasion of host cells.
FIG. 7.
Chlamydial coinfection does not result in increased association of gonococci with cultured epithelial cells. The percentage of cell-associated (A) or invasive (B) gonococci after inoculation of monolayers of ME180, IEC4.1, or BM1.11 cells with N. gonorrhoeae in the presence (white bars) or absence (gray bars) of a preexisting chlamydial infection is shown. ME180 human cervical epithelial cells were preinfected with C. trachomatis and the IEC4.1 and BM1.11 murine epithelial cells were preinfected with C. muridarum.
C. muridarum infection alters the immune environment.
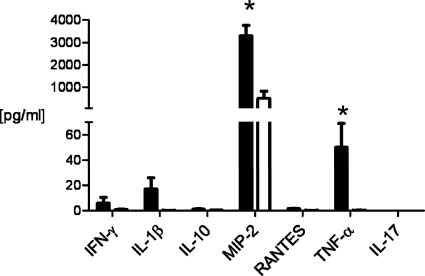
Because we observed a difference in gonococcal colonization as early as day 1 postinoculation with N. gonorrhoeae, we hypothesized that immunological differences may exist as a result of chlamydial infection that could confer a more hospitable environment for N. gonorrhoeae. We therefore measured the concentration of seven different cytokines and chemokines in vaginal swab suspensions that are reported to play a role in both gonococcal and chlamydial infection (MIP-2, IL-1β, TNF-α, IFN-γ, RANTES, IL-10, and IL-17) in C. muridarum-infected or mock-infected (control) mice at the time point that corresponds to inoculation with N. gonorrhoeae. Significant increases in the localized concentrations of the inflammatory mediators MIP-2 and TNF-α were detected in chlamydia-infected versus control mice (Fig. 8).
FIG. 8.
Vaginal levels of inflammatory mediators MIP-2 and TNF-α are increased in C. muridarum-infected mice on day 0, prior to inoculation with N. gonorrhoeae. Concentrations (pg/ml) in genital tract secretions of mice infected with C. muridarum (solid bars) and uninfected mice (white bars) are shown from a single experiment (n = 9 to 10 mice per group; *, P < 0.05).
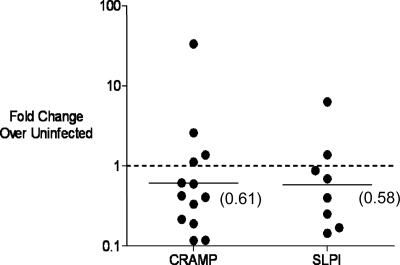
Several reports suggest that levels of MIP-2 and TNF-α can be kept in check by antimicrobial peptides, such as cathelicidin-related antimicrobial peptide (CRAMP) and secretory leukocyte peptidase inhibitor (SLPI), which also function as immunomodulatory molecules (30, 44, 51). Both CRAMP and SLPI are known to be effective against Gram-negative organisms, and thus their absence may help to explain an increase in gonococcal colonization (42). Therefore, we measured the expression of these two genes in chlamydia-infected and uninfected mice by RT-PCR. Transcripts for CRAMP and SLPI were ∼2-fold reduced in C. muridarum-infected mice at the time point that corresponds to inoculation with N. gonorrhoeae (Fig. 9). These data support the hypothesis that chlamydia-induced alterations in the immune environment of the lower genital tract make the genital tract more permissive for N. gonorrhoeae.
FIG. 9.
Antimicrobial peptide gene expression is downregulated in mice with a preexisting chlamydial infection prior to inoculation with N. gonorrhoeae. CRAMP and SLPI gene expression levels were measured by RT-PCR on vaginal material collected from mice with or without a preexisting chlamydial infection 15 min prior to inoculation with N. gonorrhoeae. Each dot represents an individual C. muridarum-infected mouse compared to the average baseline value in uninfected mice at the same time point. The line is drawn at the geometric mean with the numerical value in parenthesis. The dashed line represents a fold change of one, which is the value that corresponds to no difference between mice with or without a preexisting chlamydial infection. Values less than 1 indicate downregulation of gene expression in C. muridarum-infected mice.
DISCUSSION
The incidence of chlamydia and gonorrhea coinfection in the young adult population in the United States is very high, as illustrated by a survey of 18- to 26-year-olds in which ca. 70% of young adults with gonorrhea were also infected with Chlamydia trachomatis (50). Despite these startling numbers, little is currently known about the pathogenesis or host response to coinfection. Advances are needed in this area to reduce both the costs associated with presumptive dual antibiotic treatment and the consequences of coinfection on reproductive health. Here, we describe the first small animal model of gonococcal and chlamydial coinfection for studying host-parasite interactions specific to coinfection and for developing products that are effective against both agents. While characterizing this model, we found that coinfection of female mice differs from infection with either pathogen alone in terms of gonococcal colonization load and host response to infection.
Successful coinfection of mice with N. gonorrhoeae and C. muridarum required that we first establish chlamydial infection in the absence of exogenous hormone treatment, as described previously (62), and then treat with 17β-estradiol to promote susceptibility to N. gonorrhoeae. This sequence of infection is likely to mimic a common scenario that leads to coinfection of women, as suggested by mathematical modeling of two cohort studies which showed a large number of women that were asymptomatically infected with C. trachomatis for 18 months or more in the absence of antibiotic treatment (24). The average length of gonococcal infection is thought to be much shorter (28). Thus, it is likely that most women with coinfection at diagnosis were either asymptomatically colonized with C. trachomatis for a long period of time prior to infection with N. gonorrhoeae or acquired both organisms simultaneously from a coinfected partner. An obvious limitation to this model is the need to treat mice with estradiol to promote susceptibility to infection with N. gonorrhoeae, which we showed reduces the inflammatory response to C. muridarum. The susceptibility of female mice to several human genital tract pathogens depends on the stage of the estrous cycle, and therefore mouse models of sexually transmitted infections frequently utilize hormone treatment, including models of N. gonorrhoeae (32), C. muridarum (3, 6), C. trachomatis (79), Mycoplasma genitalium (49), Candida albicans (71), and herpes simplex virus 2 (61) infections. We showed here that several cytokines and chemokines and vaginal PMNs were decreased in C. muridarum-infected mice following treatment with estradiol. This model therefore does not mimic the events that occur when women with symptomatic chlamydial infection encounter N. gonorrhoeae. Chlamydial infection of estradiol-treated mice may more closely mimic asymptomatic chlamydial infection in women, however (24). In support of this possibility, Agrawal et al. observed lower levels of several cytokines and chemokines in cervical washes from C. trachomatis-infected asymptomatic women compared to women who were symptomatic for infection (2).
Additional limitations to the model we describe here include the use of C. muridarum instead of C. trachomatis and host restrictions inherent to the use of the human-specific pathogen N. gonorrhoeae in a murine system. The host response and progression of infection and disease in mice infected with C. muridarum more closely mimics human disease, however, than does experimental infection of mice with C. trachomatis (3, 16), and the C. muridarum mouse model is commonly used to examine the immunobiology of chlamydial genital infection. Host restrictions that prevent murine infection with N. gonorrhoeae from fully mimicking human infection include the absence of colonization receptors for pili and opacity (Opa) proteins (13, 36, 52, 80) and differences in soluble complement regulatory proteins that bind the gonococcal surface to downregulate complement activation (54). N. gonorrhoeae also cannot use murine lactoferrin or transferrin as sources of iron (14, 43), and the gonococcal immunoglobulin A1 (IgA1) protease cannot cleave mouse IgA (40). Despite these host restrictions, studying gonococcal pathogenesis in the murine model has yielded considerable insight into the host response to infection (25, 31, 58, 76) and the role of certain gonococcal virulence factors in evasion of host defenses (34, 75, 81, 84, 85). The mouse model has also allowed the demonstration of hormonal influences on the selection of phase-variable Opa proteins in vivo (13, 32, 73), as well as the effect of certain antibiotic resistance mutations on microbial fitness (82). The increasing availability of transgenic mice in several of these host-restricted factors should allow for improved study of gonococcal chlamydial coinfection in vivo.
An important finding of the present study was the demonstration that higher numbers of N. gonorrhoeae colonized the murine genital tract when C. muridarum was present. This result was observed whether mice were infected with C. muridarum for a short period (2 to 4 days) or a longer interval (8 to 10 days) prior to challenge with N. gonorrhoeae. We interpret this finding as evidence that the factors responsible for increased gonococcal colonization are sustained during chlamydial infection. There are several possible explanations for the observed increased gonococcal colonization in C. muridarum-infected mice, including potential differences in the availability of nutrients, colonization receptors, or innate defenses. Experiments with tissue culture cells did not support the hypothesis that C. muridarum alters the number of gonococci that adhere to or invade epithelial cells. We also saw no difference in the number of gonococci required to infect mice with a preexisting chlamydial infection, as might be predicted if C. muridarum infection enhanced the capacity of gonococci to adhere to epithelial cells in the initial stages of infection (R. A. Vonck and A. E. Jerse, unpublished observation). We therefore examined the hypothesis that host responses to C. muridarum may alter the immune response to N. gonorrhoeae. In support of this hypothesis, we observed increased levels of the inflammatory mediators MIP-2 and TNF-α in chlamydia-infected mice prior to inoculation with N. gonorrhoeae, which occurred concurrently with reduced transcription of genes encoding the antimicrobial peptides CRAMP and SLPI. Decreased levels of antimicrobial peptides, one of the first lines of defense at the mucosal surface, could contribute to the increased gonococcal colonization that we observed during coinfection. The report that vaginal fluids from women with C. trachomatis had reduced levels of SLPI (21), and the demonstration that N. gonorrhoeae is susceptible to CRAMP and the human cathelicidin LL37 in vitro are consistent with this hypothesis (72, 82). This hypothesis is supported further by a growing body of evidence that IL-17 responses (25) and TLR4-mediated responses (59), both of which lead to the production of antimicrobial peptides (26, 86), are protective against N. gonorrhoeae. Optimization of methods to detect and measure antimicrobial peptide concentrations in genital tract secretions, which is under way in our laboratory, should facilitate more in-depth studies on the consequences of altered antimicrobial peptide responses during coinfection.
MIP-2 levels correlate with localized PMN influx in both N. gonorrhoeae (58) and C. muridarum (17) infection models and a second important difference between coinfection and infection with either pathogen alone was the higher vaginal levels of MIP-2 and greater PMN influx in coinfected mice. Interestingly, in a recent study of patients who were admitted to genitourinary medicine clinics in the United Kingdom, women that were coinfected with C. trachomatis and N. gonorrhoeae were more likely to be symptomatic than women infected with C. trachomatis alone (68). The mechanisms responsible for the induction of greater levels of inflammation in coinfected mice and humans remain to be elucidated. The simplest explanations are that higher concentrations of proinflammatory pathogen-derived ligands, due to the presence of both chlamydiae and gonococci, may have a cumulative effect or that the higher gonococcal colonization load in coinfected mice may influence the degree of PMN influx. It is also possible that a unique interplay occurs between distinct pathogen-specific signaling pathways, which results in greater inflammation. At this time, only the PMN response has been investigated, and the involvement of other inflammatory cell types and signaling pathways during coinfection warrant further investigation. Other potential consequences of coinfection that could be examined in this model include the possibility that N. gonorrhoeae infection may reactivate a latent chlamydia infection as suggested by Batteiger et al. (4) in a study of recurrent chlamydial infections. The coinfection mouse model should also facilitate the development of immunomodulatory therapies, such as Toll-like receptor (TLR) agonists and antagonists, which have been proposed to be used along with antimicrobial treatment to prevent the devastating effects of sexually transmitted infections on women's reproductive health (29).
Perhaps the largest potential consequence of the unique characteristics of coinfection that we describe here is on transmission. Studies performed by Cohen et al. demonstrated a dose response for experimental urethral infection of male volunteers in which the percentage of infected volunteers increased with increasing doses of N. gonorrhoeae (12). Thus, increased gonococcal colonization due to chlamydial infection may lead to increased transmission from a coinfected female to a male partner. In addition, the spread of Chlamydia both to the upper reproductive tract of the infected host and to an uninfected partner may be facilitated by the host PMN response, as proposed by Rank et al. from studies with C. caviae in a guinea pig genital tract infection model (64). The increased PMN influx observed in coinfected female mice might then be expected to lead to increased transmission from a coinfected female to a male partner. Three published studies have attempted to determine whether there is a difference in the rate N. gonorrhoeae and C. trachomatis transmission to an uninfected partner in the context of either single or dual infection, and no clear difference in transmission of either organism in the case of coinfection was identified (45, 46, 48). However, investigators in these studies were unable to appropriately identify index cases and several patients included as index cases were actually negative for an infection for which their partner tested positive. Thus, as concluded by Matondo et al. in 1995, we believe this question requires further study (48).
In summary, the female mouse model of gonococcal and chlamydial coinfection described here is easy to manipulate and can be used to answer questions about the pathogenesis and host response of coinfection due to the availability of mouse-specific reagents and genetically defined mouse strains. Furthermore, due to the extensive historical data on experimental murine infection with either single pathogen, this model should allow for detailed studies on differences during coinfection. Continuing studies with this model are likely to further inform the field of gonococcal immunology and increase our knowledge of polymicrobial infections in general. In addition, this model should be a useful system for testing new antimicrobial and immunomodulatory therapies in the context of dual infection.
Acknowledgments
This study was supported by National Institutes Health grants RO1 AI42053 and U19 AI31496 (A.E.J.) and R01AI54624 and U19 AI84024 (T.D.) and USUHS Concept grant RO73-RC (A.E.J.).
Editor: J. N. Weiser
Footnotes
Published ahead of print on 18 January 2011.
REFERENCES
- 1.Abdelrahman, Y. M., and R. J. Belland. 2005. The chlamydial developmental cycle. FEMS Microbiol. Rev. 29:949-959. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal, T., V. Vats, S. Salhan, and A. Mittal. 2009. Determination of chlamydial load and immune parameters in asymptomatic, symptomatic and infertile women. FEMS Immunol. Med. Microbiol. 55:250-257. [DOI] [PubMed] [Google Scholar]
- 3.Barron, A. L., H. J. White, R. G. Rank, B. L. Soloff, and E. B. Moses. 1981. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J. Infect. Dis. 143:63-66. [DOI] [PubMed] [Google Scholar]
- 4.Batteiger, B. E., J. Fraiz, W. J. Newhall, B. P. Katz, and R. B. Jones. 1989. Association of recurrent chlamydial infection with gonorrhea. J. Infect. Dis. 159:661-669. [DOI] [PubMed] [Google Scholar]
- 5.Belay, T., et al. 2002. Chemokine and chemokine receptor dynamics during genital chlamydial infection. Infect. Immun. 70:844-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berti, M., G. P. Candiani, and V. Arioli. 1989. A new mouse model of Chlamydia trachomatis MoPn genital infection. J. Chemother. 1:44-45. [PubMed] [Google Scholar]
- 7.Brodeur, B. R., et al. 1985. Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody. Infect. Immun. 50:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2008. 2006 disease profile. Centers for Disease Control and Prevention, Atlanta, GA.
- 10.Centers for Disease Control and Prevention. 2002. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm. Rep. 51:1-78. [PubMed] [Google Scholar]
- 11.Cohen, M. S., and J. G. Cannon. 1999. Human experimentation with Neisseria gonorrhoeae: progress and goals. J. Infect. Dis. 179(Suppl. 2):S375-S379. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, M. S., et al. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. [DOI] [PubMed] [Google Scholar]
- 13.Cole, J. G., N. B. Fulcher, and A. E. Jerse. Opacity proteins increase Neisseria gonorrhoeae fitness in the female genital tract due to a factor under ovarian control. Infect. Immun. 78:1629-1641. [DOI] [PMC free article] [PubMed]
- 14.Cornelissen, C. N., G. D. Biswas, and P. F. Sparling. 1993. Expression of gonococcal transferrin-binding protein 1 causes Escherichia coli to bind human transferrin. J. Bacteriol. 175:2448-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalal, S. J., J. S. Estep, I. E. Valentin-Bon, and A. E. Jerse. 2001. Standardization of the Whitten Effect to induce susceptibility to Neisseria gonorrhoeae in female mice. Contemp. Top. Lab. Anim. Sci. 40:13-17. [PubMed] [Google Scholar]
- 16.Darville, T., et al. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 65:3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darville, T., C. W. Andrews, Jr, J. D. Sikes, P. L. Fraley, and R. G. Rank. 2001. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect. Immun. 69:3556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 2000. Host cell invasion by pathogenic neisseriae. Subcell. Biochem. 33:61-96. [DOI] [PubMed] [Google Scholar]
- 19.de la Maza, L. M., S. Pal, A. Khamesipour, and E. M. Peterson. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect. Immun. 62:2094-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dicker, L. W., D. J. Mosure, S. M. Berman, and W. C. Levine. 2003. Gonorrhea prevalence and coinfection with chlamydia in women in the United States, 2000. Sex. Transm. Dis. 30:472-476. [DOI] [PubMed] [Google Scholar]
- 21.Draper, D. L., et al. 2000. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am. J. Obstet. Gynecol. 183:1243-1248. [DOI] [PubMed] [Google Scholar]
- 22.Duncan, J. A., et al. 2009. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J. Immunol. 182:6460-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards, J. L., and M. A. Apicella. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17:965-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairley, C. K., L. Gurrin, J. Walker, and J. S. Hocking. 2007. “Doctor, how long has my Chlamydia been there?” Answer: “years”. Sex. Transm. Dis. 34:727-728. [DOI] [PubMed] [Google Scholar]
- 25.Feinen, B., A. E. Jerse, S. L. Gaffen, and M. W. Russell. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal. Immunol. 3:312-321. [DOI] [PMC free article] [PubMed]
- 26.Fukata, M., and M. T. Abreu. 2007. TLR4 signalling in the intestine in health and disease. Biochem. Soc. Trans. 35:1473-1478. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert, D. N., R. C. Moellering, G. M. Ellopoulos, and M. A. Sande. 2007. The Sanford guide to antimicrobial therapy 2007, 37th ed. Antimicrobial Therapy, Inc., Sperryville, VA.
- 28.Hook, E. W., and H. H. Handsfield. 1999. Gonococcal infections in the adult, p. 451-466. In K. K. Holmes, et al. (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill Book Co., New York, NY.
- 29.Horne, A. W., S. J. Stock, and A. E. King. 2008. Innate immunity and disorders of the female reproductive tract. Reproduction 135:739-749. [DOI] [PubMed] [Google Scholar]
- 30.Huang, L. C., R. Y. Reins, R. L. Gallo, and A. M. McDermott. 2007. Cathelicidin-deficient (Cnlp−/−) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 48:4498-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imarai, M., et al. 2008. Regulatory T cells are locally induced during intravaginal infection of mice with Neisseria gonorrhoeae. Infect. Immun. 76:5456-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerse, A. E. 1999. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 67:5699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jerse, A. E., et al. 1994. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 179:911-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jerse, A. E., et al. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 71:5576-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson, A. P., M. Tuffrey, and D. Taylor-Robinson. 1989. Resistance of mice to genital infection with Neisseria gonorrhoeae. J. Med. Microbiol. 30:33-36. [DOI] [PubMed] [Google Scholar]
- 36.Kallstrom, H., M. K. Liszewski, J. P. Atkinson, and A. B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25:639-647. [DOI] [PubMed] [Google Scholar]
- 37.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and D. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly, K. A., et al. 2001. Chlamydia trachomatis infection does not enhance local cellular immunity against concurrent Candida vaginal infection. Infect. Immun. 69:3451-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly, K. A., E. A. Robinson, and R. G. Rank. 1996. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect. Immun. 64:4976-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilian, M., J. Mestecky, and M. W. Russell. 1988. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol. Rev. 52:296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kita, E., H. Matsuura, and S. Kashiba. 1981. A mouse model for the study of gonococcal genital infection. J. Infect. Dis. 143:67-70. [DOI] [PubMed] [Google Scholar]
- 42.Kolls, J. K., P. B. McCray, Jr., and Y. R. Chan. 2008. Cytokine-mediated regulation of antimicrobial proteins. Nat. Rev. Immunol. 8:829-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, B. C., and A. B. Schryvers. 1988. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol. Microbiol. 2:827-829. [DOI] [PubMed] [Google Scholar]
- 44.Lentsch, A. B., H. Yoshidome, R. L. Warner, P. A. Ward, and M. J. Edwards. 1999. Secretory leukocyte protease inhibitor in mice regulates local and remote organ inflammatory injury induced by hepatic ischemia/reperfusion. Gastroenterology 117:953-961. [DOI] [PubMed] [Google Scholar]
- 45.Lin, J. S., et al. 1998. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J. Infect. Dis. 178:1707-1712. [DOI] [PubMed] [Google Scholar]
- 46.Lycke, E., G. B. Lowhagen, G. Hallhagen, G. Johannisson, and K. Ramstedt. 1980. The risk of transmission of genital Chlamydia trachomatis infection is less than that of genital Neisseria gonorrhoeae infection. Sex. Transm. Dis. 7:6-10. [DOI] [PubMed] [Google Scholar]
- 47.Makepeace, B. L., P. J. Watt, J. E. Heckels, and M. Christodoulides. 2001. Interactions of Neisseria gonorrhoeae with mature human macrophage opacity proteins influence production of proinflammatory cytokines. Infect. Immun. 69:1909-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matondo, P., I. Johnson, and S. Sivapalan. 1995. Epidemiology and transmission patterns of concomitant genital chlamydial and gonococcal infections. Genitourin. Med. 71:266-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGowin, C. L., R. A. Spagnuolo, and R. B. Pyles. Mycoplasma genitalium rapidly disseminates to the upper reproductive tracts and knees of female mice following vaginal inoculation. Infect. Immun. 78:726-736. [DOI] [PMC free article] [PubMed]
- 50.Miller, W. C., et al. 2004. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 291:2229-2236. [DOI] [PubMed] [Google Scholar]
- 51.Morioka, Y., K. Yamasaki, D. Leung, and R. L. Gallo. 2008. Cathelicidin antimicrobial peptides inhibit hyaluronan-induced cytokine release and modulate chronic allergic dermatitis. J. Immunol. 181:3915-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mrkic, B., et al. 1998. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 72:7420-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murakami, M., T. Ohtake, R. A. Dorschner, and R. L. Gallo. 2002. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J. Dent. Res. 81:845-850. [DOI] [PubMed] [Google Scholar]
- 54.Ngampasutadol, J., et al. 2008. Species-specificity of Neisseria gonorrhoeae infection: do human complement regulators contribute? Vaccine 26(Suppl. 8):I62-I66. [DOI] [PubMed] [Google Scholar]
- 55.Nsuami, M., C. L. Cammarata, B. N. Brooks, S. N. Taylor, and D. H. Martin. 2004. Chlamydia and gonorrhea co-occurrence in a high school population. Sex. Transm. Dis. 31:424-427. [DOI] [PubMed] [Google Scholar]
- 56.O'Connell, C. M., R. R. Ingalls, C. W. Andrews, Jr., A. M. Scurlock, and T. Darville. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J. Immunol. 179:4027-4034. [DOI] [PubMed] [Google Scholar]
- 57.O'Connell, C. M., and K. M. Nicks. 2006. A plasmid-cured Chlamydia muridarum strain displays altered plaque morphology and reduced infectivity in cell culture. Microbiology 152:1601-1607. [DOI] [PubMed] [Google Scholar]
- 58.Packiam, M., S. J. Veit, D. J. Anderson, R. R. Ingalls, and A. E. Jerse. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect. Immun. 78:433-440. [DOI] [PMC free article] [PubMed]
- 59.Packiam, M., S. J. Veit, N. Mavrogiorgos, A. E. Jerse, and R. R. Ingalls. 2010. Protective and immunoregulatory role of Toll-like receptor 4 in experimental gonococcal infection of female mice. International Pathogenic Neisseria Conference, Banff, Alberta, Canada.
- 60.Pal, S., T. J. Fielder, E. M. Peterson, and L. M. de la Maza. 1993. Analysis of the immune response in mice following intrauterine infection with the Chlamydia trachomatis mouse pneumonitis biovar. Infect. Immun. 61:772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parr, M. B., et al. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Invest. 70:369-380. [PubMed] [Google Scholar]
- 62.Rank, R. G. 1994. Animal models for urogenital infections. Methods Enzymol. 235:83-93. [DOI] [PubMed] [Google Scholar]
- 63.Rank, R. G., K. H. Ramsey, E. A. Pack, and D. M. Williams. 1992. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect. Immun. 60:4427-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rank, R. G., J. Whittimore, A. K. Bowlin, S. Dessus-Babus, and P. B. Wyrick. 2008. Chlamydiae and polymorphonuclear leukocytes: unlikely allies in the spread of chlamydial infection. FEMS Immunol. Med. Microbiol. 54:104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rasmussen, S. J., et al. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Invest. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reddy, B. S., et al. 2004. Cytokine expression pattern in the genital tract of Chlamydia trachomatis positive infertile women: implication for T-cell responses. Clin. Exp. Immunol. 137:552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robertson, J. N., M. E. Ward, D. Conway, and E. O. Caul. 1987. Chlamydial and gonococcal antibodies in sera of infertile women with tubal obstruction. J. Clin. Pathol. 40:377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenvinge, M. M., and R. Lau. 2009. Screening for asymptomatic chlamydia in women: how often would gonorrhoea be missed? Int. J. STD AIDS 20:571-572. [DOI] [PubMed] [Google Scholar]
- 69.Roshick, C., H. Wood, H. D. Caldwell, and G. McClarty. 2006. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect. Immun. 74:225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell, M., et al. 2010. Infectivity acts as in vivo selection for maintenance of the chlamydial “cryptic” plasmid. Infect. Immun. 79:98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryley, J. F., and S. McGregor. 1986. Quantification of vaginal Candida albicans infections in rodents. J. Med. Vet. Mycol. 24:455-460. [PubMed] [Google Scholar]
- 72.Shafer, W. M., X. Qu, A. J. Waring, and R. I. Lehrer. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. U. S. A. 95:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simms, A. N., and A. E. Jerse. 2006. In vivo selection for Neisseria gonorrhoeae opacity protein expression in the absence of human carcinoembryonic antigen cell adhesion molecules. Infect. Immun. 74:2965-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soboll, G., T. M. Schaefer, and C. R. Wira. 2006. Effect of Toll-like receptor (TLR) agonists on TLR and microbicide expression in uterine and vaginal tissues of the mouse. Am. J. Reprod. Immunol. 55:434-446. [DOI] [PubMed] [Google Scholar]
- 75.Soler-Garcia, A. A., and A. E. Jerse. 2007. Neisseria gonorrhoeae catalase is not required for experimental genital tract infection despite the induction of a localized neutrophil response. Infect. Immun. 75:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song, W., et al. 2008. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17β-estradiol-treated mice. Vaccine 26:5741-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sonoda, Y., et al. 1998. Physiologic regulation of postovulatory neutrophil migration into vagina in mice by a C-X-C chemokine(s). J. Immunol. 160:6159-6165. [PubMed] [Google Scholar]
- 78.Stamm, W. E. 1999. Chlamydia trachomatis infections of the adult, p. 407-422. In K. K. Holmes, et al. (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill Book Co., New York, NY.
- 79.Tuffrey, M., P. Falder, and D. Taylor-Robinson. 1982. Genital-tract infection and disease in nude and immunologically competent mice after inoculation of a human strain of Chlamydia trachomatis. Br. J. Exp. Pathol. 63:539-546. [PMC free article] [PubMed] [Google Scholar]
- 80.Virji, M., et al. 1999. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol. Microbiol. 34:538-551. [DOI] [PubMed] [Google Scholar]
- 81.Warner, D. M., J. P. Folster, W. M. Shafer, and A. E. Jerse. 2007. Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J. Infect. Dis. 196:1804-1812. [DOI] [PubMed] [Google Scholar]
- 82.Warner, D. M., W. M. Shafer, and A. E. Jerse. 2008. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol. Microbiol. 70:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolf, K., G. V. Plano, and K. A. Fields. 2009. A protein secreted by the respiratory pathogen Chlamydia pneumoniae impairs IL-17 signaling via interaction with human Act1. Cell. Microbiol. 11:769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu, H., and A. E. Jerse. 2006. Alpha-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect. Immun. 74:4094-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu, H., A. A. Soler-Garcia, and A. E. Jerse. 2009. A strain-specific catalase mutation and mutation of the metal-binding transporter gene mntC attenuate Neisseria gonorrhoeae in vivo but not by increasing susceptibility to oxidative killing by phagocytes. Infect. Immun. 77:1091-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu, J. J., and S. L. Gaffen. 2008. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front. Biosci. 13:170-177. [DOI] [PubMed] [Google Scholar]