Abstract
Cryptosporidiosis is an important diarrheal disease of humans and neonatal livestock caused by Cryptosporidium spp. that infect epithelial cells. Recovery from Cryptosporidium parvum infection in adult hosts involves CD4+ T cells with a strong Th1 component, but mechanisms of immunity in neonates are not well characterized. In the present investigation with newborn mice, similar acute patterns of infection were obtained in C57BL/6 wild-type (WT) and T and B cell-deficient Rag2−/− mice. In comparison with uninfected controls, the proportion of intestinal CD4+ or CD8+ T cells did not increase in infected WT mice during recovery from infection. Furthermore, infection in neonatal WT mice depleted of CD4+ T cells was not exacerbated. Ten weeks after WT and Rag2−/− mice had been infected as neonates, no patent infections could be detected. Treatment at this stage with the immunosuppressive drug dexamethasone produced patent infections in Rag2−/− mice but not WT mice. Expression of inflammatory markers, including gamma interferon (IFN-γ) and interleukin-12p40 (IL-12p40), was higher in neonatal WT mice than in Rag2−/− mice around the peak of infection, but IL-10 expression was also higher in WT mice. These results suggest that although CD4+ T cells may be important for elimination of C. parvum, these cells are dispensable for controlling the early acute phase of infection in neonates.
Intestinal cryptosporidiosis is a debilitating infectious diarrheal disease caused by apicomplexans of the genus Cryptosporidium that develop in epithelial cells (6, 8). Infection is transmitted in a fecal-oral manner by oocysts that release sporozoites in the intestine. Epithelial cells are invaded by the sporozoites, and asexual reproduction produces merozoites that infect new cells. Later generations of merozoites undergo sexual differentiation that leads to formation of new oocysts. Outbreaks of human cryptosporidiosis have been linked to contact with infected hosts or with oocyst contamination of water supplies or food (6, 8). Illness normally lasts a few days, but infection often persists in immunocompromised hosts, including AIDS patients, and may become fatal (6, 11).
Cryptosporidium parvum is a zoonotic pathogen that commonly infects humans and neonatal livestock (8). Immunological studies have shown that host resistance against C. parvum is established through both innate and adaptive immune responses. Recent studies indicated that NK cells were important in innate immunity, since Rag2−/− mice that lack T and B cells were more resistant to infection than alymphocytic Rag2−/− γc−/− mice (3). Gamma interferon (IFN-γ) is also important for innate immunity to infection (15, 23, 32), and although NK cells are a major source of IFN-γ, Rag2−/− γc−/− mice were found to have IFN-γ-dependent innate immunity against the parasite (3). Interleukin-12 (IL-12) was shown to be required for inducing IFN-γ-dependent immunity to C. parvum in SCID mice that lack T and B cells (32).
Studies suggest that in adaptive immunity to C. parvum, IFN-γ and IL-12 also have protective roles in adult mice, indicating the involvement of Th1 responses (5, 24). Ultimate control of infection in adult mice and humans is dependent on CD4+ T cells (2, 27), and IFN-γ expression by these cells could be a key factor in host defense, although in humans, this cytokine might be more important during secondary infection (12, 14, 36). In one report, CD8+ T cells in juvenile mice were reported to be involved in very early innate immunity (17), and antigen-specific human CD8+ T cells cytotoxic to C. parvum-infected cells have been developed in vitro (25), but numerous other studies suggest that there is no major role for CD8+ T cells in establishing immunity (2, 30). An investigation with γδ+ T cell-deficient mice suggested that γδ+ cells had a partial protective effect against infection in neonatal mice but not adult mice (35).
Adult immunocompetent animals are generally refractory to C. parvum infection (11). Neonatal animals, including cattle, sheep, deer, and mice, are highly susceptible to infection, although they usually survive (11). This vulnerability of neonates might be a result of defective T cell responses, as for example, newborn mice are lymphopenic and may be less able to develop Th1 responses (1). Recently, however, we observed that neonatal Rag2−/− and Rag2−/− γc−/− mice not only survived an early surge of C. parvum reproduction but also brought the infection under effective immunological control (3). This implied that T cells may not be essential for control of infection in neonatal hosts.
The aim of the present study was to investigate the respective contributions of innate and adaptive immunity in resistance to C. parvum infection of neonatal mice. Comparative studies with wild-type (WT) and Rag2−/− mice suggested that the early resistance that develops against infection in the neonatal host is not dependent on CD4+ T cells but on innate immunity.
MATERIALS AND METHODS
Animals.
The mice employed, WT C57BL/6 and Rag2−/− C57BL/6 mice (the latter developed at the Pasteur Institute), were specific pathogen free and bred and maintained in cages with filter lids. Animals had free access to food and water. Experiments were carried out under license from the United Kingdom Home office and with ethical approval of Queen Mary College, University of London.
Parasite and animal infections.
Purified C. parvum oocysts (IOWA isolate obtained from Bunch Grass Farm, Deary, ID) were surface sterilized by being washed in phosphate-buffered saline (PBS), pH 7.2, with 10% domestic bleach and then being washed three times in PBS. Neonatal mice were infected with C. parvum by two oral inoculations, with 2.5 × 104 oocysts in 5 μl PBS with 5 min between doses. Infections were measured by microscopic counting of oocysts in acid-fast stained fecal smears. Parasites were counted in 50 random fields at a high magnification (×1,000).
Antibody treatment of mice.
CD4+ T cells in neonatal mice were depleted by three intraperitoneal (i.p.) injections of rat anti-mouse CD4 IgG monoclonal antibody GK1.5 (R&D Systems), as follows: 50 μg on the day of infection (day 0) and 75 μg on days 2 and 4. Depletion of NK1.1+ cells was achieved by i.p. injection of 100 μg murine anti-NK1.1 IgG monoclonal antibody PK136 (R&D Systems) on days 0 and 3. Control mice received similar amounts of normal rat or mouse IgG.
Preparation of lymphoid cell populations from mice and flow cytometry.
Spleens, mesenteric lymph nodes (MLN), and small intestines were excised and placed in cold isolation buffer (Hanks' balanced salt solution [HBSS] without calcium and magnesium [Invitrogen], 10% fetal calf serum [FCS], 0.09% sodium azide). Cell suspensions were prepared from spleens and MLN that were passed through 40-μm strainers and, after erythrocyte lysis using 8.3 g/liter NH4Cl in 0.01 M Tris-HCl, resuspended in FACS buffer (PBS, 1% FCS, 0.09% sodium azide). Intestines were flushed with isolation buffer, opened longitudinally, and cut into 0.3-cm pieces. After two washes, intestinal epithelial cells containing intraepithelial lymphocytes (IEL) were separated from the intestinal tissue by incubation for 20 min at 37°C, slow rotation in 10 mM EDTA-isolation buffer, and subsequent vortex mixing for 20 s. The remaining intestinal tissue was incubated three times in digestion buffer (0.5 mg/ml collagenase D [Roche] and 0.5 mg/ml DNase I [Sigma] in isolation buffer) for 20 min at 37°C and underwent slow rotation. After each incubation step, the suspension was vortexed for 20 s, and remaining pieces were allowed to settle. Supernatants containing lamina propria (LP) cells were pooled, and cell suspensions (epithelial cells with IEL or LP cells) were passed through a 40-μm strainer and washed. Cell pellets were resuspended in 40% isotonic Percoll (Sigma), layered on 80% isotonic Percoll, and centrifuged for 30 min at 900 × g. Lymphoid cells were collected at the interface and washed in FACS buffer. Cells were incubated with anti-CD16/CD32 antibodies to block Fc receptors, and after being labeled with fluorochrome-conjugated antibodies, cell surface expression of T cell markers was assessed using a FACSCanto II instrument (BD Biosciences). Anti-mouse CD8 fluorescein isothiocyanate (FITC; clone 53-6.7), anti-mouse CD3 PerCP-eFluor710 (clone 17A2), anti-mouse CD4 allophycocyanin (APC; clone GK1.5), and respective isotype-matched control antibodies were purchased from eBioscience.
Quantitative PCR.
Extraction of RNA from small intestinal samples, reverse transcription to cDNA, and real-time quantitative PCR were performed as described previously (3). The primer sequences used were as follows: β-2-microglobulin forward, GCA AGG ACT GGT CTT TCT ATA TCC T, and reverse, ATC ACA TGT CTC GAT CCC AGT AG (132 bp); IFN-γ forward, GCC AAG TTT GAG GTC AAC AAC, and reverse, ATC AGC AGC GAC TCC TTT TC (121 bp); IL-12p40 forward, TGT AAC CAG AAA GGT GCG TTC, and reverse, ACT TGC TGC ATG AGG AAT TGT (115 bp); CD69 forward, GAA GGA CCA TGG CAC CAG, and reverse, AGG ACG TGA TGA GGA CCA CT (101 bp); IL-2Rβ forward, CAGC AGA TCC CAT GAA GGA, and reverse, TAC CGG CAC TTG ACC AAA AT (115 bp); Klrb1b forward, AAG AAG AAC TGA GAT TCC TAC TGG AC, and reverse, TGA AAG CTG TGC CAT TTA TCC (117 bp); granzyme B forward, CTA AAG CTG AAG AGT AAG GCC AAG, and reverse, CCA GCC ACA TAG CAC ACA TC (104 bp); and IL-10 forward, ACT TTA AGG GTT ACT TGG GTT GC, and reverse, TCT CAC CCA GGG AAT TCA AA (126 bp).
Statistical analyses.
In mouse experiments, infections were measured each day with 6 to 10 animals. Mean values ± standard errors of the means were calculated, and statistical examination was performed using Student's t test and the Mann-Whitney U test. The data shown are representative of replicate experiments.
RESULTS
Control of infections in Rag2−/− and WT mice.
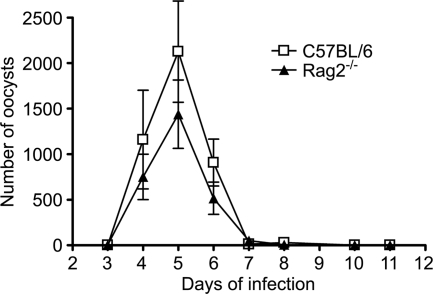
A comparison was made of the patterns of C. parvum infection in neonatal T and B cell-deficient C57BL/6 Rag2−/− and WT mice infected at 7 days of age. Figure 1 shows that for each mouse strain, a similar course of acute infection was obtained, and there was no significant difference in the level of oocyst excretion during the 11-day monitoring period of the acute infection.
FIG. 1.
Course of C. parvum infection in neonatal C57BL/6 and Rag2−/− mice. C57BL/6 and Rag2−/− animals were infected by oral gavage with C. parvum oocysts at 7 days of age, and microscopic measurements of oocyst shedding in Ziehl-Neelsen acid-fast stained fecal smears from mice at different times of infection were made. The values represent the mean numbers of oocysts per 50 fields ± standard errors for 6 to 10 animals per group.
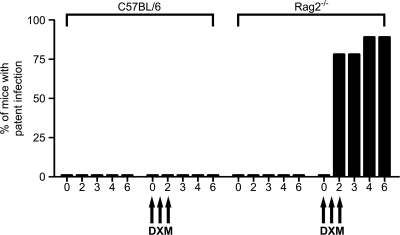
Oocyst excretion in WT and Rag2−/− mice could not be detected from 3 to 10 weeks postinfection (data not shown). At 10 weeks, the mice were immunosuppressed by i.p. treatment with 1 mg dexamethasone on three consecutive days. No infection relapses were observed in WT mice, but oocyst excretion was detected within a few days in Rag2−/− mice (Fig. 2). These results implied that following an early acute phase of infection during the neonatal period, Rag2−/− mice maintained a latent chronic infection, while WT mice eliminated the parasite.
FIG. 2.
Measurement of excretion of C. parvum oocysts in C57BL/6 and Rag2−/− mice immunosuppressed with dexamethasone (DXM) following recovery from neonatal infection. C57BL/6 and Rag2−/− mice were infected by oral gavage with C. parvum oocysts at 7 days of age. Ten weeks after recovery from acute infection, mice were immunosuppressed by i.p. injection with 1 mg dexamethasone on three consecutive days, and oocyst excretion was monitored for 6 days after the first injection. The percentage of mice that developed patent infection is shown. The values represent the mean numbers of oocysts per 50 fields ± standard errors for 9 animals per group.
Taken together, the above-mentioned results suggest that the early acute phase of infection in neonates is resolved as effectively in T cell-deficient mice as in immunocompetent mice, but later elimination of the parasite cannot be attained in the absence of T cells and adaptive immunity.
Role of CD4+ T cells in control of infection in WT neonates.
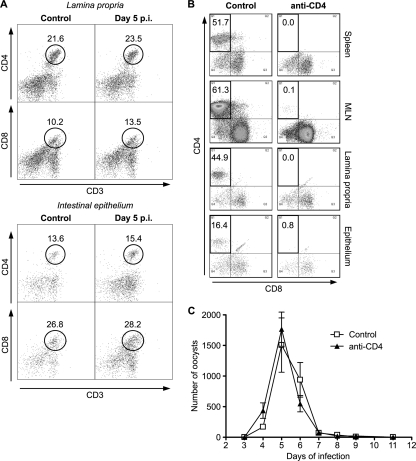
CD4+ T cells are essential in the resistance of adult hosts to cryptosporidial infection, but the protective role of these cells in neonates has not been thoroughly evaluated. In a flow cytometry study, measurements of the numbers of CD4+ and CD8+ T cells in the intestines from WT mice and age-matched uninfected mice were made on day 5, the point when recovery from infection begins. The relative numbers of CD4+ and CD8+ cells were similar to those reported previously in newborn mice of a similar age (28). No increase in the percentage of CD4+ or CD8+ T cells in infected mice or uninfected mice had occurred (Fig. 3A). Similar results were obtained when intestinal tissue was collected on day 8 (data not shown). As this may have suggested a lack of CD4+ T cell involvement in immunity, the effect of the depletion of CD4+ T cells on the course of infection in neonatal WT mice was examined. Treatment of neonatal WT mice with anti-mouse CD4 antibodies was shown to virtually ablate CD3+ CD4+ T cell numbers in the spleen, mesenteric lymph node, lamina propria, and intraepithelial lymphocyte populations (Fig. 3B). This loss of CD4+ cells, however, did not increase the susceptibility of the neonates to C. parvum infection, as the pattern of oocyst reproduction was similar to that in control mice (Fig. 3C). These results imply that CD4+ T cells in newborn mice do not play a key part in the control of C. parvum infection.
FIG. 3.
Frequency of intestinal T cells in neonatal C57BL/6 mice at the peak of C. parvum infection and effect of CD4+ T cell depletion on the course of infection. (A) Neonatal C57BL/6 mice were infected by oral gavage with C. parvum oocysts, and the surface expression of CD3, CD4, and CD8 on CD45+ lamina propria cells (top) and on cells of the intestinal epithelium (bottom) on day 5 postinfection was analyzed by flow cytometry. Representative plots show the frequencies of CD4+ and CD8+ T cells among the CD3+ CD45+ cells. (B and C) Effect of CD4+ T cell depletion. (B) Neonatal C57BL/6 mice were infected by oral gavage with C. parvum oocysts. Animals were administered anti-CD4 antibody or control IgG on days 0, 2, and 4 postinfection. The efficiency of the ablation of CD4+ CD3+ CD45+ T cells in the spleen, mesenteric lymph nodes, lamina propria, and intestinal epithelium was assessed by flow cytometry on day 5 postinfection. Representative plots are shown. (C) The infection was followed by microscopic examination of fecal smears from mice at different times of infection and by quantification of oocyst shedding. The values shown represent the mean numbers of oocysts per 50 fields ± standard errors for at least 7 animals per group.
Involvement of NK1.1+ cells in control of infection in WT neonates.
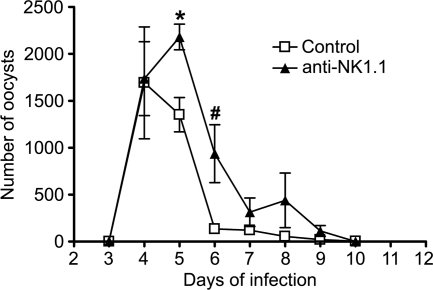
As T cells, including CD4+ T cells, appeared to not be important for establishing early resistance to infection in neonates, an investigation of the protective role of NK1.1+ cells, which include NK cells, in WT mice was made. Mice treated with anti-NK1.1 antibodies were slower in controlling the infection than controls, producing significantly higher numbers of oocysts on days 5 and 6 (Fig. 4). This suggests that NK1.1+ cells may play an important part in immunity against C. parvum in neonatal WT mice.
FIG. 4.
Effect of NK cell depletion on the course of C. parvum infection in neonatal C57BL/6 mice. Neonatal C57BL/6 animals were infected by oral gavage with C. parvum oocysts and administered either anti-NK1.1 antibodies or control IgG. The infection was followed by microscopic examination of fecal smears from mice at different times of infection and by quantification of oocyst shedding. The values represent the mean numbers of oocysts per 50 fields ± standard errors for 6 to 8 animals per group. Significant differences between mean values for control and antibody-treated mice are marked (*, P = 0.0286; #, P = 0.0476).
Intestinal expression of immunological mediators in infected WT and Rag2−/− neonatal mice.
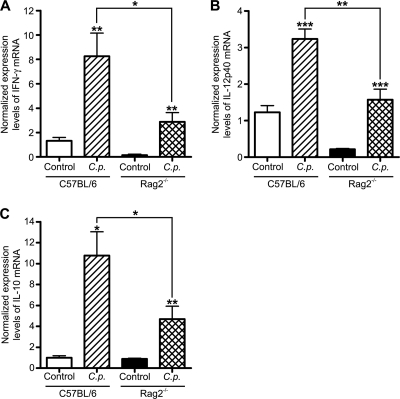
On day 5 of infection of WT and Rag2−/− mice, studies by quantitative PCR of intestinal expression of mRNA of cytokines known to be important in immunity to infection were done. There was a significant increase in expression of IFN-γ and IL-12p40 in infected WT and Rag2−/− mice (Fig. 5A and B), but Rag2−/− animals showed lower abundances of both cytokines than WT animals. Examination of the anti-inflammatory cytokine IL-10 also showed that there was enhanced expression in both strains of mice as a result of infection, but the level was greater in WT mice than in Rag2−/− mice (Fig. 5C).
FIG. 5.
Intestinal cytokine expression in neonatal C57BL/6 and Rag2−/− mice during acute C. parvum infection (C.p.). Neonatal C57BL/6 and Rag2−/− animals were infected by oral gavage with C. parvum oocysts, and intestinal tissue samples from the small intestine were collected on day 5 postinfection. The amounts of intestinal IFN-γ (A), IL-12p40 (B), and IL-10 (C) mRNA were quantified by real-time quantitative PCR using the threshold cycle (ΔΔCT) method, with β-2-microglobulin as the reference gene and samples collected from uninfected animals as calibrators. The examples shown represent at least 6 animals per group (*, P < 0.05; **, P ≤ 0.01; ***, P < 0.001).
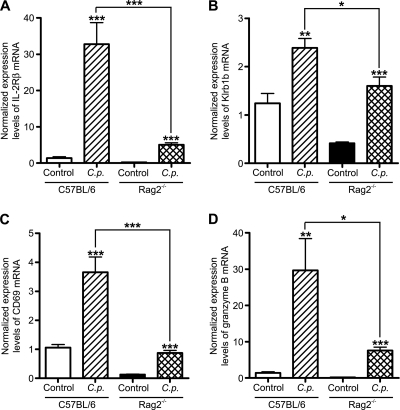
Further studies showed that in the small intestine of infected WT and Rag2−/− mice, there was a significant increase on day 5 in the abundance of mRNA encoding the β subunit of the IL-2 receptor (Fig. 6A), which is constitutively expressed on the majority of NK cells and upregulated on activated T cells, and in the abundance of mRNA coding for the NK/NK T (NKT) cell-specific receptor Klrb1b (NK1.1 in animals on a C57BL/6 background) (Fig. 6B). Similarly, in both mouse strains, there was significantly elevated expression of the lymphocyte activation marker CD69 and granzyme B, a serine protease important in NK cell- and T cell-mediated cytotoxicity (Fig. 6C and D). As with IFN-γ and IL-12p40, the levels of expression of Klrb1b, CD69, and granzyme B mRNA in infected mice were more pronounced in WT mice than in Rag2−/− mice. Collectively, these findings indicate that infection with C. parvum leads to an increase in the small intestine of markers of NK cells and T lymphocytes and their activation.
FIG. 6.
Intestinal expression of NK cell and T cell markers in neonatal C57BL/6 and Rag2−/− mice during acute C. parvum infection. Neonatal C57BL/6 and Rag2−/− animals were infected by oral gavage with C. parvum oocysts, and intestinal tissue samples from the small intestine were collected 5 days postinfection. The amounts of intestinal IL-2Rβ (A), Klrb1b (B), CD69 (C), and granzyme B (D) mRNA were quantified by real-time quantitative PCR using the ΔΔCT method, with β-2-microglobulin as the reference gene and samples collected from uninfected animals as calibrators. The examples shown represent at least 6 animals per group (*, P < 0.05; **, P ≤ 0.01; ***, P < 0.001).
DISCUSSION
In neonatal animals such as cattle and mice infected with C. parvum, the intestine becomes heavily parasitized within several days, but the host is then usually able to mount a protective immune response that leads to the infection becoming subclinical in a few days (11). The immunological basis underlying the control of parasite reproduction by neonates has not been well described, however. In adult hosts, CD4+ T cells, but not CD8+ T cells, are required for resistance to infection by C. parvum or Cryptosporidium muris (reviewed in reference 22). The findings of the present investigation, however, suggest that for neonatal mice, innate immune mechanisms are sufficient to block the early surge of parasite reproduction and that CD4+ T cells may not have a significant protective role during this period.
A key observation in this investigation was that newborn Rag2−/− mice were able to control infection as efficiently as WT mice of the same age. This indicated that in the neonates, innate responses could be as effective as adaptive immunity in establishing control of infection. After the initial phase of infection, neither mouse strain showed evidence of relapse by microscopy at up to 10 weeks postinfection. At this time, dexamethasone treatment produced patent infections in Rag2−/− mice but not WT mice. This suggested that the WT mice but not the Rag2−/− mice probably had eliminated the parasite. Without dexamethasone treatment, many Rag2−/− mice (but not WT mice) infected as neonates eventually demonstrated patent infections that became progressive and fulminant (data not shown), but the immunological basis for this loss of innate resistance is currently unknown. Hence, adaptive immunity is required to achieve sterile immunity against C. parvum, but neonates appear to be able to activate potent innate immune mechanisms that alone are proficient for surmounting a severe C. parvum infection.
In the only similar study of which we are aware in which the early courses of C. parvum infection in WT and mutationally T cell-deficient neonatal mice were compared, Heine et al. (16) observed that the recovery of neonates was less pronounced in athymic BALB/c nude mice than in WT mice and that the T cell-deficient mice soon developed fulminant infections. The variation in results between the two studies may have been due to differences in mouse strain used, parasite isolate used, numbers of oocysts used for infection, or animal maintenance conditions used. However, we observed an infection pattern similar to that seen in Rag2−/− mice with BALB/c SCID mice infected as neonates with the IOWA or Moredun isolates of C. parvum (our unpublished data).
We next studied the involvement of CD4+ T cells in the recovery of WT neonatal mice from infection. An examination of T cells present in the intestine during the recovery period showed that the percentages of intestinal CD4+ T cells and CD8+ T cells were not significantly greater than those in uninfected mice. This was in agreement with the finding in a previous report that the proportions of CD4+ and CD8+ T cells in Peyer's patches of C. parvum-infected neonatal mice did not rise significantly until late in recovery (21). Others observed no C. parvum antigen-specific activation of splenic T cells from neonatal mice at the peak of infection (13). The integrin α4β7 is important for migrating activated mucosal T and B cells to reenter the gut, but recovery from C. parvum infection was not impaired in β7-deficient neonatal mice (20). Studies with calves at the peak of C. parvum infection have shown increases in the percentage of CD4+ T cells in the epithelium but not in the lamina propria (10, 37). Significantly, in the present study, it was found that anti-CD4 antibodies were highly effective in depleting CD4+ cells from the intestine as well as other immune organs, but the loss of CD4+ T cells did not increase the susceptibility of the neonatal mice to infection. It had previously been shown that neonatal WT mice given weekly injections with the same anti-CD4 antibodies failed to clear the infection, but the early acute phase of infection we described was not monitored in that study (30). Adult WT mice continuously administered this antibody developed low-grade chronic infections that persisted for weeks after antibody treatment was terminated (31). Clearly, therefore, CD4+ T cells are important for clearance of the parasite. However, the evidence obtained from the present study suggests that CD4+ T cells are not required in the vital early immune response that protects neonates from C. parvum infection.
While depleting CD4+ cells in neonatal WT mice had no effect on infection, depleting NK1.1+ cells, which include NK cells, hampered recovery. We had previously shown a protective role for NK cells in neonatal and adult T cell-deficient mice (3). However, NKT cells may also express NK1.1 (9), and thus, could be involved in immunity, although as we observed, Rag2−/− mice that lack NKT cells and other T cells were as effective in controlling infection as WT mice.
At the peak of infection in WT and Rag2−/− mice, measurements made by quantitative PCR showed increased levels of expression of IFN-γ, IL-12p40, the lymphocyte markers CD69 and IL-2Rβ, NK1.1, and granzyme B. The observations for IFN-γ and IL-12p40 were expected, as they are important in the development of immunity to C. parvum. Increased percentages of activated T cells in the intestines of C. parvum-infected calves have been found (10, 37). The enhanced expression of NK1.1 is commensurate with the result showing that treatment with anti-NK1.1 increased the level of infection. Granzyme B is important for cytotoxic activity of T cells and NK cells, and increased expression may be an indication that cytolysis of infected epithelial cells is involved in immunity. Cytolysis of C. parvum-infected human intestinal epithelial cell lines by human antigen-specific CD8+ T cells or IL-15-activated NK cells has been described, although these cytotoxic cells were developed in vitro (7, 25). Interestingly, the intestinal levels of all these markers of inflammation were higher in WT mice than in Rag2−/− mice, and yet, there was no variation in the courses of infection between the two mouse strains. This difference for some of the markers (e.g., CD69, IL2Rβ, and granzyme B) is likely to be due to the absence of adaptive immune cells in Rag2−/− mice. Importantly, it was also observed that the level of IL-10, which downregulates inflammation, was significantly higher in WT mice than in Rag2−/− mice. The cellular sources of IL-10 in these mice are not known, but two possibilities are that regulatory T cells and IL-10-secreting B cells are more readily induced in neonatal mice than in adult mice (33, 34).
The lack of T cell involvement in immunity to C. parvum in newborn mice may be explained by a paucity of T cells in neonates (1), a propensity of neonatal Th1 cells to undergo apoptosis (19), a high level of regulatory T cell activation (34), or delayed maturation of IL-12-producing dendritic cells (18). The transfer of adult T cells into newborn mice has increased resistance to microbial pathogens such as influenza virus, suggesting that resident neonatal T cells are less efficiently activated (39). Neonatal mice or goats may compensate for impaired adaptive immune responses by developing more potent innate immune responses than adults, and this may result from increased inflammatory activation through Toll-like receptors (TLRs) (28, 29, 38). Evidence suggests that neonatal mice may overcome bacterial sepsis independently of adaptive immunity by mechanisms involving TLR ligation (38). Neonates are capable of generating strong innate immune responses to the extent that inducing inflammation via TLRs could lead to greater morbidity and mortality than in adults because of reduced regulation of the TLR activation pathways in the newborn animals (40). Studies suggest that TLRs might play an important part in immunity to C. parvum: neonatal mice treated with the TLR9 ligand CpG were found to be strongly resistant to infection (4), and juvenile mice lacking MyD88, a key adaptor molecule in TLR activation, had increased susceptibility to infection (26).
The findings of the current investigation suggest that the relative importance of innate and adaptive immunity in control of C. parvum might alter with the age of the host. If T cells are not essential for the development of resistance to C. parvum in neonates, as the current results indicate, it will be important to better characterize the nature of the protective innate responses of these hosts during infection. Elucidation of these responses could open the way for designing novel approaches in immunotherapy.
Acknowledgments
This work was funded by the Biotechnology and Biological Sciences Research Council of the United Kingdom (grant BB/F006179/1) and the Institut Pasteur, INSERM, and the Ligue Nationale Contre le Cancer (J.P.D.S.).
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre, S. A., P. H. Mason, and L. E. Perryman. 1994. Susceptibility of major histocompatibility complex (MHC) class I- and MHC class II-deficient mice to Cryptosporidium parvum infection. Infect. Immun. 62:697-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barakat, F. M., V. McDonald, J. P. Di Santo, and D. S. Korbel. 2009. Roles for NK cells and an NK cell-independent source of intestinal gamma interferon for innate immunity to Cryptosporidium parvum infection. Infect. Immun. 77:5044-5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrier, M., et al. 2006. Oral and intraperitoneal administration of phosphorothioate oligodeoxynucleotides leads to control of Cryptosporidium parvum infection in neonatal mice. J. Infect. Dis. 193:1400-1407. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, L. D., J. N. Stewart, and J. R. Mead. 2002. Susceptibility to Cryptosporidium parvum infections in cytokine- and chemokine-receptor knockout mice. J. Parasitol. 88:1014-1016. [DOI] [PubMed] [Google Scholar]
- 6.Chen, X. M., J. S. Keithly, C. V. Paya, and N. F. LaRusso. 2002. Cryptosporidiosis. N. Engl. J. Med. 346:1723-1731. [DOI] [PubMed] [Google Scholar]
- 7.Dann, S. M., et al. 2005. Interleukin-15 activates human natural killer cells to clear the intestinal protozoan Cryptosporidium. J. Infect. Dis. 192:1294-1302. [DOI] [PubMed] [Google Scholar]
- 8.Davies, A. P., and R. M. Chalmers. 2009. Cryptosporidiosis. BMJ 339:b4168. [DOI] [PubMed] [Google Scholar]
- 9.Emoto, M. 2008. Liver invariant NKT cells and listeriosis. Microbes Infect. 10:1036-1040. [DOI] [PubMed] [Google Scholar]
- 10.Fayer, R., et al. 1998. Cryptosporidium parvum infection in bovine neonates: dynamic clinical, parasitic and immunologic patterns. Int. J. Parasitol. 28:49-56. [DOI] [PubMed] [Google Scholar]
- 11.Fayer, R., and B. L. Ungar. 1986. Cryptosporidium spp. and cryptosporidiosis. Microbiol. Rev. 50:458-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez Morales, M. A., G. La Rosa, A. Ludovisi, A. M. Onori, and E. Pozio. 1999. Cytokine profile induced by Cryptosporidium antigen in peripheral blood mononuclear cells from immunocompetent and immunosuppressed persons with cryptosporidiosis. J. Infect. Dis. 179:967-973. [DOI] [PubMed] [Google Scholar]
- 13.Harp, J. A., and R. E. Sacco. 1996. Development of cellular immune functions in neonatal to weanling mice: relationship to Cryptosporidium parvum infection. J. Parasitol. 82:245-249. [PubMed] [Google Scholar]
- 14.Harp, J. A., W. M. Whitmire, and R. Sacco. 1994. In vitro proliferation and production of gamma interferon by murine CD4+ cells in response to Cryptosporidium parvum antigen. J. Parasitol. 80:67-72. [PubMed] [Google Scholar]
- 15.Hayward, A. R., K. Chmura, and M. Cosyns. 2000. Interferon-gamma is required for innate immunity to Cryptosporidium parvum in mice. J. Infect. Dis. 182:1001-1004. [DOI] [PubMed] [Google Scholar]
- 16.Heine, J., H. W. Moon, and D. B. Woodmansee. 1984. Persistent Cryptosporidium infection in congenitally athymic (nude) mice. Infect. Immun. 43:856-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leav, B. A., et al. 2005. An early intestinal mucosal source of gamma interferon is associated with resistance to and control of Cryptosporidium parvum infection in mice. Infect. Immun. 73:8425-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, H. H., et al. 2008. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J. Exp. Med. 205:2269-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, L. Q., et al. 2004. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity 20:429-440. [DOI] [PubMed] [Google Scholar]
- 20.Mancassola, R., et al. 2004. Increased susceptibility of β7-integrin-deficient neonatal mice in the early stage of Cryptosporidium parvum infection. Infect. Immun. 72:3634-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariotte, D., E. Comby, P. Brasseur, and J. J. Ballet. 2004. Kinetics of spleen and Peyer's patch lymphocyte populations during gut parasite clearing in Cryptosporidium parvum infected suckling mice. Parasite Immunol. 26:1-6. [DOI] [PubMed] [Google Scholar]
- 22.McDonald, V. 2008. Immune responses, p. 209-233. In R. Fayer R, and L. Xiao (ed.), Cryptosporidium and Cryptosporidiosis, 2nd ed. CRC Press, Taylor and Francis Group, Boca Raton, FL.
- 23.McDonald, V., and G. J. Bancroft. 1994. Mechanisms of innate and acquired resistance to Cryptosporidium parvum infection in SCID mice. Parasite Immunol. 16:315-320. [DOI] [PubMed] [Google Scholar]
- 24.Mead, J. R., and X. You. 1998. Susceptibility differences to Cryptosporidium parvum infection in two strains of gamma interferon knockout mice. J. Parasitol. 84:1045-1048. [PubMed] [Google Scholar]
- 25.Pantenburg, B., et al. 2010. Human CD8+ T cells clear Cryptosporidium parvum from infected intestinal epithelial cells. Am. J. Trop. Med. Hyg. 82:600-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers, K. A., et al. 2006. MyD88-dependent pathways mediate resistance to Cryptosporidium parvum infection in mice. Infect. Immun. 74:549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt, W., et al. 2001. Rapid increase of mucosal CD4 T cells followed by clearance of intestinal cryptosporidiosis in an AIDS patient receiving highly active antiretroviral therapy. Gastroenterology 120:984-987. [DOI] [PubMed] [Google Scholar]
- 28.Steege, J. C. A., W. A. Buurman, and P. P. Forget. 1997. The neonatal development of intraepithelial and lamina propria lymphocytes in the murine small intestine. Dev. Immunol. 5:121-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tourais-Esteves, I., N. Bernardet, S. Lacroix-Lamande, S. Ferret-Bernard, and F. Laurent. 2008. Neonatal goats display a stronger TH1-type cytokine response to TLR ligands than adults. Dev. Comp. Immunol. 32:1231-1241. [DOI] [PubMed] [Google Scholar]
- 30.Ungar, B. L., J. A. Burris, C. A. Quinn, and F. D. Finkelman. 1990. New mouse models for chronic Cryptosporidium infection in immunodeficient hosts. Infect. Immun. 58:961-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ungar, B. L. P., T.-C. Kao, J. A. Burris, and F. D. Finkelman. 1991. Cryptosporidium infection in an adult mouse model. Independent roles for IFN-γ and CD4+ T lymphocytes in protective immunity. J. Immunol. 147:1014-1022. [PubMed] [Google Scholar]
- 32.Urban, J. F., Jr., et al. 1996. IL-12 protects immunocompetent and immunodeficient neonatal mice against infection with Cryptosporidium parvum. J. Immunol. 156:263-268. [PubMed] [Google Scholar]
- 33.Walker, W. E., and D. R. Goldstein. 2007. Neonatal B cells suppress innate toll-like receptor immune responses and modulate alloimmunity. J. Immunol. 179:1700-1710. [DOI] [PubMed] [Google Scholar]
- 34.Wang, G. H., et al. 2010. “Default” generation of neonatal regulatory T cells. J. Immunol. 185:71-78. [DOI] [PubMed] [Google Scholar]
- 35.Waters, W. R., and J. A. Harp. 1996. Cryptosporidium parvum infection in T-cell receptor (TCR)-alpha- and TCR-delta-deficient mice. Infect. Immun. 64:1854-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White, A. C., et al. 2000. Interferon-gamma expression in jejunal biopsies in experimental human cryptosporidiosis correlates with prior sensitization and control of oocyst excretion. J. Infect. Dis. 181:701-709. [DOI] [PubMed] [Google Scholar]
- 37.Wyatt, C. R., E. J. Brackett, and J. Savidge. 2001. Evidence for the emergence of a type-1-like immune response in intestinal mucosa of calves recovering from cryptosporidiosis. J. Parasitol. 87:90-95. [DOI] [PubMed] [Google Scholar]
- 38.Wynn, J. L., et al. 2008. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112:1750-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You, D. H., et al. 2008. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J. Immunol. 181:3486-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao, J., et al. 2008. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc. Natl. Acad. Sci. U. S. A. 105:7528-7533. [DOI] [PMC free article] [PubMed] [Google Scholar]