Abstract
Group B streptococcus (GBS) is an important cause of early- and late-onset sepsis in the newborn. Preterm infants have markedly increased susceptibility and worse outcomes, but their immunological responses to GBS are poorly defined. We compared mononuclear cell and whole-blood cytokine responses to heat-killed GBS (HKGBS) of preterm infants (gestational age [GA], 26 to 33 weeks), term infants, and healthy adults. We investigated the kinetics and cell source of induced cytokines and quantified HKGBS phagocytosis. HKGBS-induced tumor necrosis factor (TNF) and interleukin 6 (IL-6) secretion was significantly impaired in preterm infants compared to that in term infants and adults. These cytokines were predominantly monocytic in origin, and production was intrinsically linked to HKGBS phagocytosis. Very preterm infants (GA, <30 weeks) had fewer cytokine-producing monocytes, but nonopsonic phagocytosis ability was comparable to that for term infants and adults. Exogenous complement supplementation increased phagocytosis in all groups, as well as the proportion of preterm monocytes producing IL-6, but for very preterm infants, responses were still deficient. Similar defective preterm monocyte responses were observed in fresh whole cord blood stimulated with live GBS. Lymphocyte-associated cytokines were significantly deficient for both preterm and term infants compared to levels for adults. These findings indicate that a subset of preterm monocytes do not respond to GBS, a defect compounded by generalized weaker lymphocyte responses in newborns. Together these deficient responses may increase the susceptibility of preterm infants to GBS infection.
Streptococcus agalactiae (group B streptococcus [GBS]) is the leading infectious cause of death in the newborn (17), causing both early- and late-onset neonatal sepsis (EOS and LOS), characterized by septicemia, shock, and meningitis (16, 18, 31). The proportion of infants surviving extreme prematurity is increasing (11), and as a result preterm delivery is now the leading cause of neonatal morbidity and mortality. Preterm infants are exquisitely susceptible to both EOS and LOS; they suffer approximately a quarter of invasive GBS EOS cases and half of GBS LOS cases, with a >8-fold-greater mortality for EOS and 3-fold for LOS compared to term infants (29). While a proportion of the increased susceptibility to infection in preterm infants relates to the absence of passively acquired maternal IgG antibody, deficiencies in the early immune response to bacterial pathogens likely play a critical role (23). Several studies have addressed neonatal adaptive and humoral immune responses, such as complement activation and antibody production, but there has been little focus on innate immunity to bacterial pathogens and its relation to adaptive responses in this population (23, 39).
Innate immunity provides the first line of defense against bacterial infection and is therefore particularly important in the neonatal period (9). Additionally, the innate immune system, through activation of specific pattern recognition receptors engaging pathogen-associated molecular patterns, drives inflammation and in turn initiates and shapes the adaptive immune response (28). The relative inability of the neonatal immune system to mount an effective T helper 1 (Th1) response to allergic and polyclonal stimuli is well documented (12, 21, 41) and is suggested to represent an evolutionary adaptation that protects the placenta from the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) and the Th1 cytokine gamma interferon (IFN-γ) (43). However, most studies relate to the neonatal Th2 bias in response to allergic and polyclonal stimuli (30), with fewer data on lymphocyte-driven cytokine responses to bacterial stimuli. There is little information on innate and lymphocyte cytokine responses to GBS in preterm infants.
We therefore compared expression of monocyte- and lymphocyte-associated cytokines in response to heat-killed GBS (HKGBS) in preterm, term, and adult mononuclear cells and whole blood. We also examined the kinetics and cellular basis of these responses and examined whole-blood responses to live bacterial challenge.
MATERIALS AND METHODS
Study population and sample collection.
The study was approved by the King Edward Memorial Hospital ethics committee, and written informed consent was obtained from parents/guardians. Cord blood from preterm infants (gestational age [GA], 26 to 33 weeks) and term infants (GA, >37 weeks) was collected within a few minutes of delivery from mothers without histologic evidence of chorioamnionitis (32). Manual full-blood counts done at the time of collection showed similar total white cell and monocyte counts for all newborn groups (data not shown). Cord blood mononuclear cells (CBMC) were isolated by Lymphoprep gradient centrifugation (Nycomed Pharmacia, Norway) before cryopreservation as previously reported (42). Peripheral blood mononuclear cell (PBMC) samples were collected from healthy nonatopic adults, as previously described (38). Whole fresh cord/venous blood from an additional 6 extreme-preterm infants (GA, ≤30 weeks), 8 term infants (GA, >37 weeks), and 9 healthy adults was collected into heparinized tubes prior to use.
Preparation of live and heat-killed GBS.
A clinical isolate of GBS serotype 1a (strain M141) was kindly provided by Lyn Gilbert (Institute of Clinical Pathology and Medical Research, Sydney, Australia). Mid-log-phase cultures (optical density at 600 nm [OD600] = 0.8) grown in brain heart infusion broth (Pathwest Laboratories, Perth, Australia) were used for the experiments with live GBS. Bacteria were washed twice with phosphate-buffered saline (PBS) (Invitrogen, Australia) by centrifugation at 250 × g for 10 min and resuspended in fresh PBS at a density of 2.5 × 109 CFU/ml after direct counting using a Helber counting chamber. For generation of the HKGBS stock, M141 was grown to a final density of 2 × 1010 CFU/ml in Todd-Hewitt broth, heat killed in a water bath at 60°C for 45 min, and harvested by centrifugation. HKGBS was washed in PBS, aliquoted at 2.5 × 109 CFU/ml, and stored at −80°C. Bacterial killing was confirmed by lack of growth on agar at 48 h. HKGBS stock was determined to be free of lipopolysaccharide (LPS) and mycoplasma contamination by HEK-TLR4 bioassay and validated PCR (data not shown).
Stimulation of CBMC/PBMC.
Mononuclear cells (MNC) were thawed, centrifuged, and resuspended in AIM-V (Gibco, Life Technology, Paisley, Scotland) supplemented with 2-mercaptoethanol (2-ME) (Sigma, Castle Hill, Australia) at 2 × 106/ml. Cells were plated out in duplicate on 96 round-bottom well plates in 250-μl aliquots and stimulated with either HKGBS (1 × 108 CFU/ml) or phytohemagglutinin (PHA) (1 μg/ml) for 48 h at 37°C (with 5% CO2). The optimal dose and time chosen for HKGBS were based on optimization experiments using both term cord blood mononuclear cells and adult peripheral blood mononuclear cells. It should be noted that by “optimal” we are referring to maximal cytokine release for all cytokines measured, without evidence of cytotoxicity. Higher doses were tested but showed signs of cytotoxicity and even reduced cytokine production.
Stimulation of fresh cord and venous blood.
Fresh heparinized cord or adult venous blood was mixed 1:1 with RPMI medium supplemented with 2-ME and 5% (vol/vol) heat-inactivated fetal calf serum (FCS), and 100-μl aliquots were placed in a 96-well polypropylene tissue culture plate (Corning Inc., Australia). Live GBS were added at various doses (105 to 107 CFU/ml) for 18 h; then, plates were centrifuged at 250 × g for 4 min to pellet red blood cells, and supernatants were harvested and stored at −80°C.
Cytokine assays.
Cytokines (IFN-γ, interleukin 13 [IL-13], IL-6, IL-10, and TNF-α) from MNC cultures were measured by time-resolved fluorometry as previously described (33). Cytokine levels in supernatants of whole-blood cultures were determined using the BioPlex200 system (Bio-Rad Laboratories) in accordance with the manufacturer's instructions.
pHrodo labeling of HKGBS.
For phagocytosis and intracellular cytokine experiments, HKGBS were labeled with the pH-sensitive fluorescent dye pHrodo (Invitrogen) according to the manufacturer's instructions. Before use, pHrodo-labeled HKGBS were thoroughly dispersed by 30 s of rapid vortexing and 3 min in an ultrasonic water bath.
Phagocytosis assay.
C/PBMC (1.25 × 105/125 μl/well in a 96-well polypropylene plate) were cultured for 1 h at 37°C in the presence of pHrodo-labeled HKGBS (100 bacteria/cell) with or without 10% (vol/vol) rabbit complement (RbC) (Sigma) and then transferred to a 3-ml fluorescence-activated cell sorter (FACS) tube, and nonadherent bacteria were removed by washing twice with 2 ml of ice-cold PBS (250 × g, 5 min) before fixation in 2% (vol/vol) formalin solution (in PBS). Alternatively, 25 μl of fresh whole blood was mixed with 100 μl of RPMI plus 5% FCS and 2-ME and incubated at 37°C for 15 min with a previously optimized dose of pHrodo-labeled HKGBS (5 × 107/ml). Diluted blood was fixed, and red cells were lysed using BD FACS lysis solution (BD Biosciences) according to the manufacturer's instruction. Cells were washed twice with PBS and stored in 1× BD stabilizing fixative solution (BD Biosciences).
Intracellular cytokine (ICC) staining.
CBMC/PBMC were cultured with pHrodo-labeled HKGBS (as described previously) for 1 h. Fresh blood was cultured as described previously but for 30 min with various doses of live, unlabeled GBS. These time points were chosen from previous phagocytosis kinetic optimization experiments, which demonstrated that the maximum proportion of monocytes ingesting either HKGBS or live GBS occurred at 60 min or 30 min, respectively (data not shown). Cells were incubated for a further 4 h at 37°C in the presence of 6 μg/ml of brefeldin A (eBioscience, San Diego, CA) and transferred to a round-bottom polystyrene 96-well plate, washed twice with ice-cold PBS (250 × g, 5 min), and immediately fixed using BD FACS lysing solution. Cells were permeabilized using 1× BD Perm2 permeabilization buffer for 10 min, washed with PBS, and incubated with fluorescein isothiocyanate (FITC)- or allophycocyanin (APC)-labeled antibodies against TNF-α or IL-6 (eBioscience, San Diego, CA) or appropriate isotype controls for 30 min at 4°C in the dark before two final washes and fixation in 1× BD stabilizing fixative solution.
For the kinetic studies, PBMCs were stimulated for 0 to 48 h before addition of brefeldin A (6 μg/ml) for the final 4 h. Cells were harvested at each time point, counterstained with phycoerythrin (PE)-Cy5.5-labeled anti-CD3 and APC-labeled anti-HLA-DR antibodies (eBioscience), and identified based on granularity (side scatter) and CD3/HLA-DR expression (monocytes, medium side scatter, CD3−, HLA-DR+; T cells, low side scatter, CD3+, HLA-DR+/−; B cells, low side scatter, CD3−, HLA-DR+. Fixed and permeabilized cells were incubated with FITC-labeled anti-IL-6 and PE-labeled anti-IL-10 antibodies or FITC-labeled anti-TNF-α antibodies before washing and final fixation, as above.
For all assays, >2,000 monocytes, identified by their forward- and side-scatter characteristics, were acquired uncompensated using a FACSCalibur (BD Biosciences) flow cytometer along with BD CompBeads to allow post hoc compensation and data analysis with the FlowJo software program (Treestar, Ashland, OR). The proportion of monocytes ingesting GBS was determined using a threshold marker of 1.5% set on unstimulated monocytes. The degree of ingestion was determined from the mean fluorescence intensity (MFI) of the positive population or from the total population (phagocytic index). Cytokine-positive cells were identified from a threshold marker of 1.5% for each fluorophore set using the matching isotype.
Statistical analysis.
Data were summarized using medians, interquartile ranges, and ranges or using means and their standard errors (SEM). Two group comparisons of outcomes were performed using the Mann-Whitney test or Wilcoxon signed rank test for paired observations as appropriate. Group analysis was performed using two-way analysis of variance (ANOVA) with Bonferroni's adjustment for multiple comparisons. All analyses were conducted using the Prism 5 for Mac software program (GraphPad, La Jolla, CA). All P values were two sided and were considered significant at <0.05.
RESULTS
Impaired preterm cytokine responses to GBS.
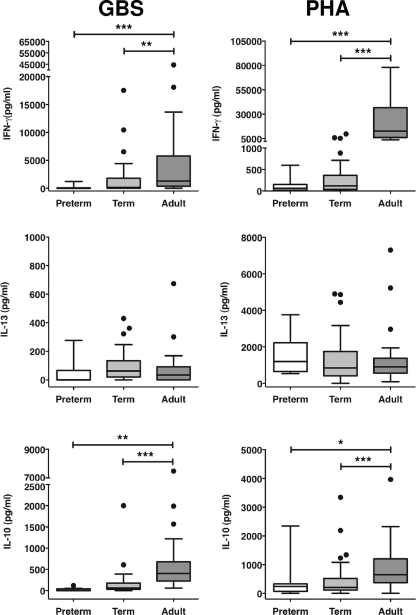
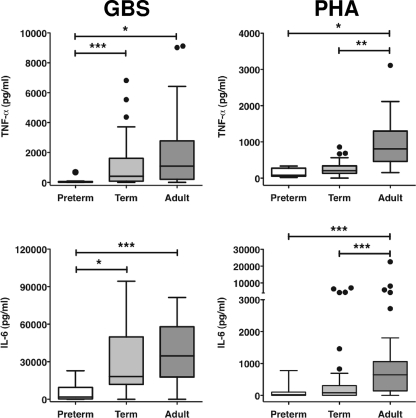
We compared the cytokine responses (TNF-α, IL-6, IFN-γ, IL-13, and IL-10) of preterm, term, and adult MNC after stimulation with either HKGBS or PHA. Two distinct stimulus-dependent patterns of responses were observed. First, the levels of all cytokines induced by PHA, except IL-13, were significantly lower for both the preterm and term infants in comparison to those for adult cells, but responses did not differ between the neonatal groups (Fig. 1 and 2). Responses to HKGBS were more complex. The levels of TNF-α and IL-6 were not significantly different between term infants and adults but were significantly lower for the preterm infants than for both term infants and adults (Fig. 2). As with PHA, levels of IFN-γ and IL-10 were significantly and equivalently lower following HKGBS stimulation for both preterm and term infants than for adults (Fig. 1).
FIG. 1.
Neonatal and adult MNC lymphocyte cytokine responses to PHA or GBS. CBMC or PBMC were incubated for 48 h with optimal doses of heat-killed GBS (1 × 108 CFU/ml) or PHA. Data shown are box-whisker plots with outliers (Tukey); n = 8 to 10 for preterm (26- to 33-week GA), 43 for term (<37-week GA), and 37 for adult. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
FIG. 2.
Neonatal and adult MNC innate/inflammatory cytokine responses to PHA or GBS. CBMC or PBMC were incubated for 48 h with optimal doses of heat-killed GBS (1 × 108 CFU/ml) or PHA. Data shown are box-whisker plots with outliers (Tukey); n = 8 to 10 for preterm (26- to 33-week GA), 43 for term (<37-week GA), and 37 for adult. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Source and kinetics of PHA- and GBS-induced cytokines.
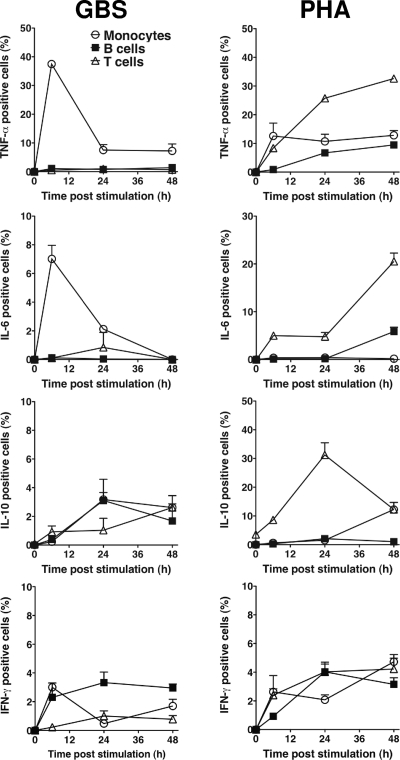
The distinct cytokine responses observed between preterm and term infants to GBS and PHA suggested that these stimuli might differentially activate specific MNC cell types. We therefore examined the kinetics of monocyte-, B cell- and T cell-specific cytokine responses to both HKGBS and PHA (Fig. 3). Adult MNC were used in order to define a normal “competent” response to GBS and because of the larger cell numbers required. T cells were the major producers of TNF-α, IL-6, and IL-10 in response to PHA stimulation, with peak responses between 24 and 48 h and with 20 to 30% of all T cells responding. In contrast, <5% of all cell types produced IFN-γ in response to PHA. The kinetics and cellular source of cytokine responses to HKGBS varied with the type of cytokine. HKGBS-induced IL-10 was detected from 24 h to 48 h in <5% of all cell types, whereas B cells were the major producers of IFN-γ. In contrast, HKGBS-induced TNF-α and IL-6 were produced exclusively by monocytes, with peak responses after 6 h and ∼40% of monocytes producing TNF-α but <10% producing IL-6. After 6 h, expression of both cytokines diminished rapidly. Dual staining confirmed that HKGBS-induced IL-6 and TNF-α were coexpressed (data not shown).
FIG. 3.
Cell-specific intracellular cytokine production by adult MNC in response to GBS or PHA. PBMC were stimulated for 0, 6, 24, and 48 h with either PHA or HKGBS (1 × 108 CFU/ml), brefeldin A was added for the last 4 h of stimulation at each time point, and cells were stained for cytokine and cell lineage markers. Data shown are means ± SEM (n = 4 donors).
Normal phagocytosis of GBS by preterm monocytes.
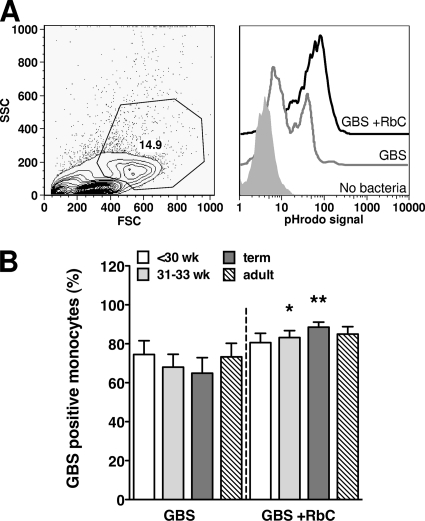
To determine if the lower levels of TNF-α and IL-6 in response to HKGBS for preterm infants might reflect an impaired capacity of monocytes to phagocytose HKGBS, we assessed nonopsonic and opsonic uptake of HKGBS. The preterm group was further differentiated as either extremely (<30 weeks) or moderately (31 to 33 weeks) preterm, to determine if there was a GA-dependent phagocytic deficiency. FACS analysis of MNC revealed that monocytes were the only cell phagocytosing HKGBS, regardless of opsonization status (data not shown). Phagocytosis of nonopsonized HKGBS by monocytes from all groups was efficient and equivalent, with at least 60% of the monocyte population ingesting HKGBS on average (Fig. 4B). Opsonization of HKGBS with complement increased monocyte phagocytosis in all groups, significantly so for the moderate preterm and term groups, with 10 to 20% more monocytes ingesting bacteria on average (Fig. 4B). There was no significant difference between the groups in the number of HKGBS phagocytosed per monocyte (determined by MFI; data not shown).
FIG. 4.
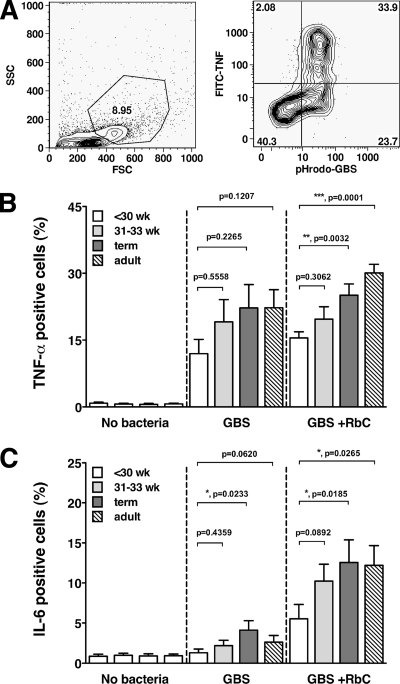
Nonopsonic and opsonic phagocytosis of GBS by neonatal and adult monocytes. CBMC or PBMC were incubated for 1 h with pHrodo dye-labeled HKGBS (1 × 108 CFU/ml) with our without RbC. (A) Left panel, representative forward-scatter (FSC) versus side-scatter (SSC) FACS plot showing inclusion gate for monocytes; right panel, representative histograms showing pHrodo-specific fluorescence in monocytes without bacteria or with GBS plus or minus RbC. (B) Percentage of monocytes positive for phagocytosed GBS (means ± SEM). n = 11 for the <30-week group, 15 for the 31- to 33-week and term groups, and 18 for adults. *, P < 0.0151; **, P < 0.0084; Wilcoxon signed rank test comparing phagocytosis of GBS with and without RbC.
Reduced responsiveness of preterm monocytes to GBS.
To investigate whether there was an intrinsic defect in preterm monocyte cytokine production, we examined HKGBS uptake and cytokine production on a per-cell basis. In all groups, monocytes were the sole source of both TNF-α and IL-6 (data not shown) and all cytokine-positive monocytes were also positive for internalized HKGBS (Fig. 5A). Notably, not all monocytes with internalized HKGBS secreted cytokine. Significantly fewer GBS-containing monocytes in the extreme preterm group were also IL-6 positive than was the case for term infants, and there was a trend toward fewer TNF-α-producing cells as well (Fig. 5B and C). The TNF-α and IL-6 responses of CBMCs from moderately preterm infants did not differ significantly from those for term infants or adults, although there was also a trend toward lower IL-6 production in this group. Opsonization of HKGBS did not increase the proportion of moderately preterm, term, or adult monocytes producing TNF-α but did significantly increase the proportion producing IL-6, 2- to 3-fold (Fig. 5B and C) (P < 0.03 for all groups, comparing nonopsonic to opsonic, using the Wilcoxon signed rank test). Importantly, opsonization of HKGBS failed to “normalize” cytokine production by monocytes from extreme preterm infants to levels comparable to those of other groups, with the significantly fewer monocytes producing either TNF-α or IL-6 in comparison to term infants or adults. There were no differences between the groups in the magnitude of the cytokines response per monocyte (as determined by MFI of positive cells; data not shown).
FIG. 5.
Monocyte-specific TNF-α and IL-6 production with respect to GBS uptake. CBMC and PBMC were exposed to 1 × 108 CFU/ml pHrodo-HKGBS (plus or minus RbC) for 1 h and then washed and incubated for a further 4 h in the presence of brefeldin A before staining for intracellular cytokine. (A) Left panel, representative forward-scatter versus side-scatter FACS plot showing inclusion gate for monocytes; right panel, representative FACS plot showing monocyte-specific TNF-α production in association with ingestion of pHrodo-GBS. (B and C) Percentage of HKGBS-positive monocytes producing TNF-α or IL-6 (means ± SEM). n = 10 for the <-30-week group, 13 for the 31- to 33-week and term groups, and 14 for adults. P values (Mann-Whitney test) are as displayed.
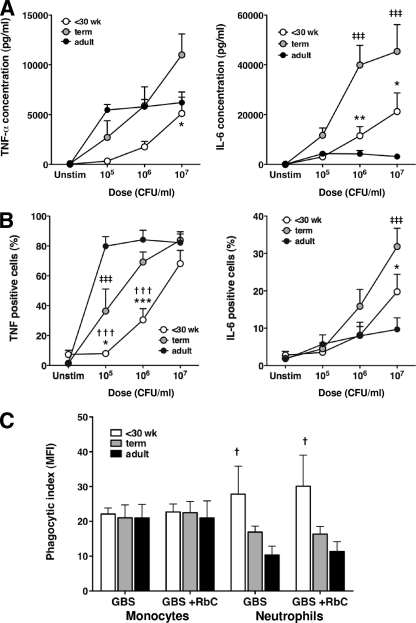
Cord blood responses to live GBS are impaired in preterm infants.
We investigated whether the deficiency in preterm infants’ monocytic cytokine production in response to GBS was present in the most clinically relevant model: whole blood challenged with live GBS. Term cord blood and adult peripheral blood were responsive to all doses of live GBS, producing significant levels of TNF-α and IL-6 (Fig. 6A). However, the level of IL-6 produced by term cord blood was significantly higher (≤2.5-fold) than that produced by adult peripheral blood at all live GBS doses. TNF-α production was higher in adult blood at the lowest dose of live GBS (105/ml) but plateaued thereafter, whereas production by term blood was dose dependent (Fig. 6A). Preterm cord blood (<30 weeks) was less responsive than adult and term blood, requiring a log higher inoculum of live GBS to elicit detectable TNF-α production. Preterm infant TNF-α and IL-6 levels were lower than those of term infants, with differences reaching significance at higher GBS inocula (Fig. 6A). Preterm TNF-α levels were less than those from adult blood at all inocula, but IL-6 levels appeared to be higher than those for adult blood at doses of ≥106/ml, although this did not achieve significance (Fig. 6A). The monocyte-specific responses to live GBS, assessed by intracellular cytokine staining, mirrored the cytokine secretion data (Fig. 6B). As with HKGBS (Fig. 5), the proportion of preterm monocytes producing either TNF-α or IL-6 in response to live GBS was significantly lower than that in term infants. However, this effect was dose dependent, with the differences in TNF-α production significant at live GBS doses of ≤106/ml, whereas the significant differences in IL-6 were observed only at 107/ml. An examination of phagocytosis in whole blood (assessed by pHrodo-labeled heat-killed GBS) showed no difference in the capacity of monocytes from preterm infants to ingest HKGBS. Notably, preterm neutrophils showed significantly more uptake of HKGBS than adult neutrophils (P < 0.05) but not term neutrophils (Fig. 6C). The addition of RbC to whole-blood cultures did not alter phagocytic uptake by either monocytes or neutrophils from any group.
FIG. 6.
Fresh cord-blood responses to live GBS. (A and B) Fresh cord/venous blood IL-6 and TNF responses to live GBS determined by Bioplex assay of culture supernatants or by intracellular cytokine staining (of monocytes), respectively. Data shown are means ± SEM; n = 6 for preterm infants (<30-week GA), 8 for term infants, and 9 for adults. *, †, ‡, P < 0.05; **, ††, ‡‡, P < 0.01; ***,†††, ‡‡‡, P < 0.001, comparing responses between preterm and term infants (*), preterm infants and adults (†), or term infants and adults (‡) using two-way ANOVA with Bonferroni's adjustment for multiple comparisons. (C) Phagocytosis of HKGBS by neutrophils or monocytes in freshly isolated cord blood from preterm infants (<30-week GA; n = 4) and term infants (n = 5) or venous blood from adults (n = 7), as determined by flow cytometry using pHrodo-labeled HKGBS (5 × 107 CFU/ml). Data shown are means ± SEM. †, P < 0.05, comparing uptake by preterm cells to that by adults using two-way ANOVA with Bonferroni's adjustment for multiple comparisons.
DISCUSSION
Preterm infants are particularly susceptible to GBS infection, with worse outcomes, but the underlying mechanisms remain unclear. We investigated the cellular and molecular competence of monocyte and lymphocyte responses to live and heat-killed GBS in preterm and term infants and adults. Our data indicate the following: (i) innate immune responses to GBS are driven by monocytes and are deficient only in preterm infants, particularly those with a GA of <30 weeks; (ii) the deficient monocyte cytokine response of preterm infants to GBS is present in whole blood but is not limited by the degree of opsonic uptake of the bacteria; and (iii) newborn lymphocyte responses to GBS may be impaired in comparison to adult responses, regardless of GA. Our findings suggest a major impairment in immune responses to GBS in preterm infants, which is likely to contribute to their increased susceptibility.
Deficient preterm monocytic responses to GBS.
The rapid onset of GBS sepsis in newborns suggests that the early control of GBS infection requires adequate engagement of host innate immune defenses (16). Our kinetic studies using adult cells demonstrated that innate cytokine responses were activated within 6 h of exposure to HKGBS with monocyte-driven proinflammatory cytokine production. Notably, production of these cytokines by CBMC from term infants was not impaired relative to that for adults, and whole-blood responses exceeded adult responses. This suggests that the blood monocyte compartment of healthy, term infants is functionally competent in recognizing and responding to GBS. Several studies have demonstrated equivalent or greater inflammatory production by term MNC (in CBMC or whole blood) after GBS stimulation (27, 47), with cytokine production predominantly monocyte derived (35, 40, 44). Our data, derived using live bacteria over a range of inocula, are therefore consistent with GBS exposure resulting in an overt inflammatory response in the newborn (16).
In contrast to term infant responses, TNF and IL-6 responses of preterm infants with a GA of <30 weeks were significantly impaired due to a reduced proportion of cytokine-producing monocytes. Similarly, preterm monocyte responses in whole cord blood stimulated with live GBS were consistently weaker than those of term infants. However, IL-6 production remained greater than adult responses at the highest inocula, consistent with the inflammatory nature of GBS sepsis even in the preterm infant (16). Thus, the trend toward equivalent or higher cytokine responses in the very preterm infants in comparison to adult responses when high doses of inocula were used could explain how an early failure in recognition/control of GBS still leads to inflammatory sequelae in this vulnerable population.
The reduced responsiveness observed in preterm CBMC inversely correlated with GA, consistent with data on preterm responses to LPS and a range of live bacteria, including GBS (47). This study did not examine the cellular source of cytokine, but the authors speculated that the impaired response resulted from inherently defective monocyte function, as we have now demonstrated. Similar GA-dependent responses are reported following LPS stimulation (6), with infants with a GA of <32 weeks having a lower proportion of IL-6-producing monocytes (35). A recent study examining intracellular cytokine responses to GBS and other bacterial stimuli in preterm infants did not find a significant impairment in IL-6, -8, -10, and -12 production in comparison to that of term infants or adults, although responses were monocyte driven (40). However, the levels of secreted cytokines produced by each group were not measured, and the study did not distinguish between extreme and moderate preterm infants. Additionally, differences in the stimulation protocols used (prolonged brefeldin A exposure versus only a 4-h exposure in our study) complicates any comparison.
Normal opsonic uptake of HKGBS by preterm infants.
The relative impairment in inflammatory cytokine monocyte responses of very preterm infants did not result from a reduced capacity to bind and phagocytose nonopsonized or opsonized HKGBS, despite the requirement for bacterial internalization to induce cytokine. There is limited information on the effects of prematurity on monocyte phagocytosis, but studies on preterm neutrophils and preterm rabbit alveolar macrophages report impaired bacterial uptake (13, 20). The uptake of preopsonized (pooled adult sera) Escherichia coli by peripheral blood neutrophils from stressed and well-preterm infants (GA of <32 weeks) was found to be modestly impaired in comparison to that of adults and healthy term infants but not related directly to expression levels of complement or immunoglobulin receptors (8). Similarly, Bektas et. al. (4) found that neutrophils from preterm infants (GA, 28 to 36 weeks) were less able to phagocytose pooled-serum opsonized Candida albicans than those from adults (4). However, they also noted that the levels of opsonic S. aureus uptake and induction of O2− were comparable between the groups, suggesting that any impairment in phagocytosis may be pathogen specific. In contrast, Fujiwara et. al. (10) found that peripheral blood neutrophil uptake of S. aureus was lower for preterm infants than for term infants and adults but could be increased by opsonization with adult plasma (10).
Impaired opsonic uptake of GBS in whole preterm blood might result from low levels of maternal GBS-specific antibody (15, 25) or deficient complement levels and activation (24). However, we found comparable levels of monocytic phagocytosis and apparently higher levels of neutrophil phagocytosis in fresh cord blood from extreme-preterm infants. Bialek and Bartmann (4a) examined phagocytosis of preopsonized GBS by neutrophils in the peripheral blood of preterm infants with a GA of <32 weeks and adults and found that phagocytosis (measured as a percentage of phagocytosing cells) was equivalent if pooled adult serum was used as opsonin but deficient in preterm infants if a pool of preterm serum was used. The opsonic effects seen with the adult and preterm serums used were heat sensitive and were not responsive to addition of GBS-specific antibodies, implicating complement as a major limiting factor. The fact that we could detect no defect in GBS uptake in preterm cord blood when it was added in unopsonized form and that addition of rabbit complement did not increase GBS uptake in any group suggests that there are sufficient opsonins available, at least in cord blood, to mediate initial GBS ingestion by the preterm infant. A detailed examination of preterm infant neutrophil and monocyte phagocytic capacity (for GBS and other pathogens) in cord and peripheral blood would be informative.
Overall, our findings, in keeping with published data on GBS and/or other bacterium-derived stimuli (6, 47), indicate that monocytes from preterm infants have an intrinsic, GA-related deficiency in their ability to recognize and respond to GBS. This may reflect fundamental impairment in the innate recognition systems broadly involved in host defense, such as the Toll-like receptors (TLR) or Nod-like receptors (NLR) (16). TLR and NLR function in preterm infants is poorly understood, although recent data suggest this group has deficient responses to TLR2 and TLR4 agonists, possibly due to reduced MyD88 expression and p38 mitogen-activated protein kinase (MAPK) induction (34). Consistent with our findings, the greatest degree of impairment was seen in those born at a <30-week GA.
Our data would also suggest that this intrinsic defect is not common to all monocytes but instead resides in a specific subset that is capable of phagocytosis but not response to bacterial stimuli. Human monocytes consist of at least two functionally distinct subsets (CD14dim/CD16+ and CD14hi/CD16−) which differ in TLR expression and cytokine production (5, 37), although there is likely to be more complex functional heterogeneity (7). We did not find differences in the proportions of CD16+ and CD16− monocytes in unstimulated cord blood from preterm and term infants (data not shown). Further investigation of the development of the newborn monocyte system and the response to neonatal pathogens and bacterial ligands is warranted.
Impaired newborn lymphocytic responses to HKGBS.
There is a general consensus that newborn lymphocyte/adaptive responses are impaired or immature compared to those of adults (3). A relative reduction in Th1 responses (IFN-γ) with concomitant Th2 polarization in the newborn is often emphasized (1, 2, 26), although more recent studies also suggest an impairment in newborn lymphocyte-derived regulatory cytokines (such as IL-10) and regulatory T-cell function as well (14, 36). We did find that IFN-γ and IL-10 production was lower in newborn CBMC than in adult PBMC following HKGBS and PHA stimulation, consistent with previous data (19, 45, 46). Our adult kinetic studies confirmed that both cytokines were produced in large part by T and B cells (especially after PHA stimulation), and we were able to detect lymphocytic proliferation in response to GBS that was impaired in CBMC. Reduced lymphocyte IFN-γ may reflect impaired IL-12 and IL-18 production induced by GBS (22) and, together with reduced IL-10, might suggest a bias toward Th2 responses to GBS in the newborn. IL-13 was indeed induced by GBS in our system, but the levels were relatively low and did not vary between newborns and adults. PHA-induced IL-13 responses were similar between groups, consistent with studies that demonstrate no inherent Th2 bias in newborn lymphocyte responses following mitogen stimulation (14). Our data would therefore suggest a general impairment in lymphocyte responses to GBS at birth, consistent with its role as a major pathogen in the first few days and weeks of life.
Conclusion.
We have demonstrated that monocyte and lymphocyte cytokine responses of the preterm infant are functionally impaired, with the degree of monocyte impairment inversely correlating with GA. Failure to mount a coordinated and timely immune response to GBS in preterm infants is likely to contribute to increased susceptibility and worse outcomes. Future strategies that augment innate function and/or adaptive immunity (including regulatory cytokine induction) may reduce the burden of GBS disease, particularly in preterm infants and other high-risk groups.
Acknowledgments
We thank the research assistants of the Vaccine Trials Group at the Children's Clinical Research Facility, Perth, WA, for excellent technical assistance. We also thank the obstetric, midwifery and neonatal staff at King Edward Memorial Hospital for all of their assistance. We are very grateful to Lyn Gilbert, Director, Centre for Infectious Diseases and Microbiology, Institute of Clinical Pathology and Medical Research, Westmead Hospital, Sydney, for the original clinical GBS isolate and helpful preliminary discussions. Furthermore, we thank Anthony Keil, Peter Campbell, Cristina Farrar, and David Atlas (Department of Microbiology, PathWest Laboratory Medicine, Princess Margaret Hospital for Children, Child and Adolescent Health Services, Western Australia) for their assistance with bacterial cultures.
A. J. Currie is supported by a fellowship from the BrightSpark Foundation. T. Strunk was supported by a Research Fellowship of the Deutsche Forschungsgemeinschaft (STR1022/1-1) and by an International Postgraduate Research Scholarship of the University of Western Australia. This study was supported by Princess Margaret Hospital, Women and Infants Research Foundation (Perth, Australia), the Clive and Vera Ramaciotti Medical Research Foundation, University of Western Australia, Rebecca Cooper Medical Research Foundation, Channel 7 Telethon, European Society for Pediatric Infectious Diseases, and the National Health and Medical Research Council, Australia.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 7 February 2011.
REFERENCES
- 1.Adkins, B. 1999. T-cell function in newborn mice and humans. Immunol. Today 20:330-335. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B. 2000. Development of neonatal Th1/Th2 function. Int. Rev. Immunol. 19:157-171. [DOI] [PubMed] [Google Scholar]
- 3.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. [DOI] [PubMed] [Google Scholar]
- 4.Bektas, S., B. Goetze, and C. P. Speer. 1990. Decreased adherence, chemotaxis and phagocytic activities of neutrophils from preterm neonates. Acta Paediatr. Scand. 79:1031-1038. [DOI] [PubMed] [Google Scholar]
- 4a.Bialek, R., and P. Bartmann. 1998. Is there an effect of immunoglobulins and G-CSF on neutrophil phagocytic activity in preterm infants? Infection 26:375-378. [DOI] [PubMed] [Google Scholar]
- 5.del Fresno, C., et al. 2009. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J. Immunol. 182:6494-6507. [DOI] [PubMed] [Google Scholar]
- 6.Dembinski, J., D. Behrendt, R. Martini, A. Heep, and P. Bartmann. 2003. Modulation of pro- and anti-inflammatory cytokine production in very preterm infants. Cytokine 21:200-206. [DOI] [PubMed] [Google Scholar]
- 7.Diercks, A., H. Kostner, and A. Ozinsky. 2009. Resolving cell population heterogeneity: real-time PCR for simultaneous multiplexed gene detection in multiple single-cell samples. PLoS One 4:e6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falconer, A. E., R. Carr, and S. W. Edwards. 1995. Impaired neutrophil phagocytosis in preterm neonates: lack of correlation with expression of immunoglobulin or complement receptors. Biol. Neonate 68:264-269. [DOI] [PubMed] [Google Scholar]
- 9.Fleer, A., and T. G. Krediet. 2007. Innate immunity: toll-like receptors and some more. A brief history, basic organization and relevance for the human newborn. Neonatology 92:145-157. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara, T., T. Kobayashi, J. Takaya, S. Taniuchi, and Y. Kobayashi. 1997. Plasma effects on phagocytic activity and hydrogen peroxide production by polymorphonuclear leukocytes in neonates. Clin. Immunol. Immunopathol. 85:67-72. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg, R. L., J. F. Culhane, J. D. Iams, and R. Romero. 2008. Epidemiology and causes of preterm birth. Lancet 371:75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goriely, S., et al. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 13.Hall, S. L., and M. P. Sherman. 1992. Intrapulmonary bacterial clearance of type III group B streptococcus is reduced in preterm compared with term rabbits and occurs independent of antibody. Am. Rev. Respir. Dis. 145:1172-1177. [DOI] [PubMed] [Google Scholar]
- 14.Halonen, M., et al. 2009. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J. Immunol. 182:3285-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemming, V. G., R. T. Hall, P. G. Rhodes, A. O. Shigeoka, and H. R. Hill. 1976. Assessment of group B streptococcal opsonins in human and rabbit serum by neutrophil chemiluminescence. J. Clin. Invest. 58:1379-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henneke, P., and R. Berner. 2006. SIRS and group-B streptococcal sepsis in newborns: pathogenesis and perspectives in adjunctive therapy. Semin. Fetal Neonatal Med. 11:333-342. [DOI] [PubMed] [Google Scholar]
- 17.Johri, A. K., et al. 2006. Group B streptococcus: global incidence and vaccine development. Nat. Rev. Microbiol. 4:932-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan, H. T., et al. 2008. Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis. Pediatr. Infect. Dis. J. 27:1057-1064. [DOI] [PubMed] [Google Scholar]
- 19.Joyner, J. L., N. H. Augustine, K. A. Taylor, T. R. La Pine, and H. R. Hill. 2000. Effects of group B streptococci on cord and adult mononuclear cell interleukin-12 and interferon-gamma mRNA accumulation and protein secretion. J. Infect. Dis. 182:974-977. [DOI] [PubMed] [Google Scholar]
- 20.Kallman, J., J. Schollin, C. Schalen, A. Erlandsson, and E. Kihlstrom. 1998. Impaired phagocytosis and opsonisation towards group B streptococci in preterm neonates. Arch. Dis. Child Fetal Neonatal Ed. 78:F46-F50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langrish, C. L., J. C. Buddle, A. J. Thrasher, and D. Goldblatt. 2002. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin. Exp. Immunol. 128:118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Pine, T., J. Joyner, N. Augustine, S. D. Kwak, and H. Hill. 2003. Defective production of IL-18 and IL-12 by cord blood mononuclear cells influences the T helper-1 interferon gamma response to group B streptococci. Pediatr. Res. 54:276-281. [DOI] [PubMed] [Google Scholar]
- 23.Levy, O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7:379-390. [DOI] [PubMed] [Google Scholar]
- 24.Levy, O., et al. 2003. Critical role of the complement system in group B streptococcus-induced tumor necrosis factor alpha release. Infect. Immun. 71:6344-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, F.-Y. C., et al. 2004. Level of maternal IgG anti-group B streptococcus type III antibody correlated with protection of neonates against early-onset disease caused by this pathogen. J. Infect. Dis. 190:928-934. [DOI] [PubMed] [Google Scholar]
- 26.Marodi, L. 2002. Down-regulation of Th1 responses in human neonates. Clin. Exp. Immunol. 128:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed, M. A., S. Cunningham-Rundles, C. R. Dean, T. A. Hammad, and M. Nesin. 2007. Levels of pro-inflammatory cytokines produced from cord blood in-vitro are pathogen dependent and increased in comparison to adult controls. Cytokine 39:171-177. [DOI] [PubMed] [Google Scholar]
- 28.Palm, N. W., and R. Medzhitov. 2009. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 227:221-233. [DOI] [PubMed] [Google Scholar]
- 29.Phares, C. R., et al. 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA 299:2056-2065. [DOI] [PubMed] [Google Scholar]
- 30.Prescott, S. L., et al. 1998. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J. Immunol. 160:4730-4737. [PubMed] [Google Scholar]
- 31.Pulver, L., et al. 2009. Continued early onset group B streptococcal infections in the era of intrapartum prophylaxis. J. Perinatol. 29:20-25. [DOI] [PubMed] [Google Scholar]
- 32.Redline, R. W. 2004. Placental inflammation. Semin. Neonatol. 9:265-274. [DOI] [PubMed] [Google Scholar]
- 33.Rowe, J., et al. 2004. High IFN-gamma production by CD8+ T cells and early sensitization among infants at high risk of atopy. J. Allergy Clin. Immunol. 113:710-716. [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi, K., et al. 2007. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 195:296-302. [DOI] [PubMed] [Google Scholar]
- 35.Schultz, C., et al. 2002. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr. Res. 51:317-322. [DOI] [PubMed] [Google Scholar]
- 36.Schultz, C., T. Strunk, P. Temming, N. Matzke, and C. Härtel. 2007. Reduced IL-10 production and -receptor expression in neonatal T lymphocytes. Acta Paediatr. 96:1122-1125. [DOI] [PubMed] [Google Scholar]
- 37.Skinner, N. A., C. M. MacIsaac, J. A. Hamilton, and K. Visvanathan. 2005. Regulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16+ monocytes in response to sepsis-related antigens. Clin. Exp. Immunol. 141:270-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strunk, T., M. Coombs, A. Currie, P. Richmond, D. Golenbock, L. Stoler-Barak, L. Gallington, M. Otto, D. Burgner, and O. Levy. 2010. TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLoS One 5:e10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strunk, T., et al. 2007. Neonatal immune responses to coagulase-negative staphylococci. Curr. Opin. Infect. Dis. 20:370-375. [DOI] [PubMed] [Google Scholar]
- 40.Tatad, A. M. F., et al. 2008. Cytokine expression in response to bacterial antigens in preterm and term infant cord blood monocytes. Neonatology 94:8-15. [DOI] [PubMed] [Google Scholar]
- 41.Trivedi, H. N., et al. 1997. Analysis of neonatal T cell and antigen presenting cell functions. Hum. Immunol. 57:69-79. [DOI] [PubMed] [Google Scholar]
- 42.van den Biggelaar, A. H. J., et al. 2009. Neonatal innate cytokine responses to BCG controlling T-cell development vary between populations. J. Allergy Clin. Immunol. 124:544-550, 550.e1-2. [DOI] [PubMed] [Google Scholar]
- 43.Wegmann, T. G., H. Lin, L. Guilbert, and T. R. Mosmann. 1993. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today 14:353-356. [DOI] [PubMed] [Google Scholar]
- 44.Williams, P. A., et al. 1993. Production of tumor necrosis factor by human cells in vitro and in vivo, induced by group B streptococci. J. Pediatr. 123:292-300. [DOI] [PubMed] [Google Scholar]
- 45.Wilson, C. B., et al. 1986. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J. Clin. Invest. 77:860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winter, H. S., S. E. Gard, T. J. Fischer, Y. J. Bryson, and E. R. Stiehm. 1983. Deficient lymphokine production of newborn lymphocytes. Pediatr. Res. 17:573-578. [DOI] [PubMed] [Google Scholar]
- 47.Yachie, A., et al. 1992. Defective production of interleukin-6 in very small premature infants in response to bacterial pathogens. Infect. Immun. 60:749-753. [DOI] [PMC free article] [PubMed] [Google Scholar]