Abstract
Host cells use metabolic signaling through the LXRα nuclear receptor to defend against Listeria monocytogenes infection. 25-Hydroxycholesterol is a natural ligand of LXRs that is produced by the enzyme cholesterol 25-hydroxylase (CH25H). We found that expression of Ch25h is upregulated following L. monocytogenes infection in a beta interferon (IFN-β)-dependent fashion. Moreover, increased Ch25h expression promotes survival of L. monocytogenes-infected cells and increases sensitivity of the host to infection. We determined that expression of Cd5l, a prosurvival gene, is controlled by CH25H. In addition, we found that CD5L inhibits activation of caspase-1, promoting survival of infected macrophages. Our results reveal a mechanism by which an intracellular pathogen can prolong survival of infected cells, thus providing itself with a protected environment in which to replicate.
Signaling through liver X receptors (LXRs), members of the nuclear receptor family of transcription factors, maintains lipid homeostasis of mammalian cells (20, 46, 51). In macrophages, LXRs activate genes involved in the process of transporting cholesterol out of the cell and loading it onto carrier proteins for transport to the liver (11). There are two LXR isoforms, LXRα and LXRβ, encoded by two different genes (41, 48). Recently it has become apparent that LXR metabolic signaling also plays an important role in defense against bacterial infections (23, 51). Notably, the absence of LXRα, but not LXRβ, dramatically increases susceptibility of mice to Listeria monocytogenes infection (22). The Cd5l prosurvival gene has been identified as the downstream target of LXRα that is primarily responsible for control of host resistance to infection and atherosclerosis development (2, 32).
Oxidized intermediates of cholesterol metabolism, such as 25-hydroxycholesterol (25-HC), are natural endogenous ligands for LXRs (1, 12, 17, 21, 25). 25-HC is produced from cholesterol primarily by the action of cholesterol 25-hydroxylase (CH25H), which is the main enzyme in the cell that produces 25-HC. The ability to produce 25-HC from cholesterol was also reported for sterol 27-hydroxylase (27), a mitochondrial P450 enzyme, and cholesterol 24-hydroxylase (28).
25-HC is an important regulatory molecule that is also a substrate in bile synthesis. Its best-understood function is potent suppression of cholesterol biosynthesis (6, 24, 29). 25-HC inhibits the cleavage of sterol regulatory element binding proteins (SREBPs) (24) that control levels of key enzymes in cholesterol synthesis, such as 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and HMG-CoA synthase. In addition to controlling lipid homeostasis through inhibition of SREBPs, 25-HC acts as a natural endogenous ligand of several orphan nuclear receptors, including Nr5a1 (SF-1) and LXRs (25). Notably, activation of LXRs leads to upregulation of metabolic genes, such as the ABCA1 pump, and of the prosurvival genes Cd5l, Bcl-xl, and Birc1a (45). ABCA1 is capable of removing both cholesterols and 25-HC from the cell, providing an important negative feedback control of intracellular 25-HC activity.
The role of 25-HC in immune function is still poorly understood. Nevertheless, recent studies found that Ch25h mRNA is highly induced by treatment of bone marrow-derived macrophages (BMMs) with lipopolysaccharide (LPS) (15). It has also been shown that exposure of naïve B cells to nanomolar concentrations of 25-HC inhibited production of IgA by reducing B cell proliferation and class switch recombination (4). Our results presented below provide further evidence of the link between innate immune function and 25-HC biosynthesis in macrophages.
Upon infection with L. monocytogenes, macrophages detect bacterial cyclic di-AMP using an as-yet-unknown cytoplasmic receptor and activate IRF3 to rapidly induce type I interferons (IFNs) (42, 44, 49). This induction has a detrimental effect on resistance of the organism to infection (3, 33, 43, 54). This is in part because type I interferons sensitize lymphocytes to infection-induced death, which, in turn, suppresses innate immune responses (9). Deletion of lymphocyte proapoptotic factors such as TRAIL or inhibition of lymphocyte formation by scid or rag mutations results in a significant increase in early resistance of animals to L. monocytogenes infection (8, 53). Therefore, control of lymphocyte cell death is an important aspect of L. monocytogenes infection.
Bacterial pathogens overcome host immune defenses by either destroying host immune cells or using them to provide a protected environment. L. monocytogenes uses cytoplasmic replication as a strategy to avoid detection and destruction by host immune cells (13, 14, 18). Therefore, death of invaded host cells might promote resistance of the organism to infection with L. monocytogenes. This is especially the case for death by pyroptosis, a proinflammatory process in which self-destruction not only exposes pathogens to immune surveillance but also generates inflammatory signals to stimulate immune defenses (5, 16). The ability of cytoplasmic pathogens such as L. monocytogenes to prevent pyroptosis of host cells would allow for extended intracellular replication and benefit the pathogen. Our results describe a novel mechanism by which L. monocytogenes may achieve this goal.
MATERIALS AND METHODS
Reagents and plasmids.
25-Hydroxycholesterol (25-HC), T0901317, and 9-cis-retinoic acid were purchased from Sigma-Aldrich. The Ch25h expression vector was constructed by cloning the full-length C57BL/6J mouse cDNA in the pCS2 expression plasmid. The lentiviral construct (pGIPZ) carrying short hairpin RNA (shRNA) against C57BL/6J mouse Ch25h was obtained from Open Biosystems.
Animals.
Female mice, 10 to 14 weeks old, were used in all experiments. All mice were bred and maintained under specific-pathogen-free conditions in the animal facilities at the University of Massachusetts Medical School. All experiments involving live animals were carried out in accordance with the guidelines set forth by the University of Massachusetts Medical School Department of Animal Medicine and the Institute Animal Care and Use Committee.
Transient transgenesis and infections.
Mice were injected in the tail vein with 1.5 ml Dulbecco's phosphate-buffered saline (D-PBS) solution containing 5 μg of pCS2-Ch25h or Ch25h frameshift mutant expression construct. After 48 h, mice were infected intravenously with 5 × 104 L. monocytogenes strain 10403s in 0.3 ml of PBS. After 24 h, infected animals were sacrificed by CO2 asphyxiation. Harvested livers and spleens were weighed and homogenized in 0.02% Triton X-100. Aliquots of serial 5-fold dilutions in sterile water were plated in duplicate on tryptic soy broth (TSB) agar plates containing 10 μg/ml streptomycin. The number of bacteria per milligram of tissue was determined by counting colonies at the appropriate dilution.
All cell lines and primary macrophages were infected ex vivo at a multiplicity of infection (MOI) of 5.
Cell culture and generation of stable cell lines.
The macrophage-like ZBM2 cell line was generated from C57BL/6J bone marrow macrophages as previously described (34). ZBM2 cells and primary bone marrow macrophages were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) (HyClone) and 10% L-cell-conditioned medium (LCCM).
To produce recombinant lentiviruses, subconfluent HEK293T cells were cotransfected with 15 μg shRNA plasmid, 6 μg pMD2G, and 10 μg pCMVD 8.2 using Fugene HD (Roche). At 72 h after transfection, the medium containing lentiviral particles was collected and stored at −80°C.
Stable cell lines producing Ch25h shRNA were generated by transduction of ZBM2 cells with lentiviral particles containing Ch25h shRNA or control shRNA sequence. Individual clones were isolated following selection with 2 μg/ml puromycin. The efficiency of knockdown was determined by quantitative reverse transcriptase PCR (qRT-PCR) analysis.
Stable cell lines expressing full-length Ch25h cDNA were generated by transfection of ZBM2 cells with pCS2-Ch25h plasmid using Fugene HD. Individual clones were selected as described above. Levels of Ch25h expression were analyzed by qRT-PCR.
Cell viability assays.
Cell viability was analyzed using CellTiter-Fluor cell viability assays (Promega). ZBM2 cells (4 × 104) were seeded in 96-well tissue culture plates, and following overnight incubation, the cells were infected with L. monocytogenes at defined MOIs. After 1 h, 10 μg/ml of gentamicin was added to kill extracellular bacteria. At defined time points, cellular viability assays were performed according to the manufacturer's protocol. The relative cell viability was calculated as follows: % of relative cellular viability = (ODe × 100)/ODc, where ODe means the experimental absorbance and ODc means the absorbance of untreated control. The means and standard deviations (SD) were calculated based on results from three independent experiments.
The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling (TUNEL) staining assay was carried out using the In Situ Cell Death Detection kit (Roche). The percentage of TUNEL-positive cells was calculated as follows: % of TUNEL-positive cells = number of TUNEL-positive cells × 100/total number of DAPI-positive cells. The means and standard deviations were calculated based on results from three independent experiments.
RNA isolation and real-time PCR.
Total RNA was isolated from cultured cells or tissue using Trizol reagent according to the manufacturer's instructions. Relative mRNA levels were quantified by qRT-PCR on an ABI 7300 real-time PCR system utilizing SYBR green chemistry. Ribosomal protein s17 (Rps17) was used as a loading control. The following primers were used: Ch25h-F, 5′-GAC CTT CTT CGA CGT GCT GA-3′; Ch25h-R, 5′-CCA CCG ACA GCC AGA TGT TA-3′; Cd5l-F, 5′-CCT TCG GTC TTG CCT TTT GA-3′; Cd5l-R, 5′-GTG TCT CCT CCC ACC AGC TT-3′; Abca1-F, 5′-GAG CAT CGT GGA CCT CTT CC-3′; Abca1-R, 5′-GGA CAC ACA GGC AGC ATC TT-3′; Rps17-F, 5′-TGT CGG GAT CCA CCT CAA TG-3′; Rps17-R, 5′-CGC CAT TAT CCC CAG CAA G-3′. Each experiment included at least two biological and three experimental replicates.
Western blot assays.
Combined cell and supernatant lysates were separated on a 10% acrylamide gel, transferred on polyvinylidene difluoride (PVDF) membranes, and probed with primary antibodies against caspase-1 p10 (1:200 dilution, SC 514; Santa Cruz), followed by appropriate secondary HRP-conjugated antibodies. In all cases, the blots were stripped and reprobed with anti-tubulin antibody (EMD).
Statistical analysis.
All data, expressed as means ± SD, were analyzed with the Student t test. Differences were considered statistically significant at P < 0.05.
RESULTS
Ch25h is an IFN-β-inducible gene.
Type I interferons have pleiotropic effects, as evidenced by their ability to induce the expression of a large number of genes (26). Existing inbred mouse strains differ in their susceptibilities to L. monocytogenes and in their abilities to induce IFN-β in response to infection (19). To gain insight into the function of IFN-β in the course of L. monocytogenes infection, we examined differences in infection-induced gene expression in macrophages from several inbred mouse strains. Using this approach, we identified the Ch25h gene as a candidate IFN-β-inducible gene that affects the response to L. monocytogenes.
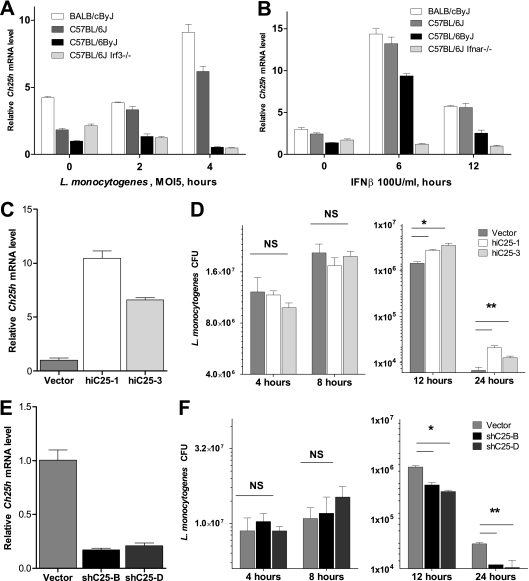
Basal Ch25h mRNA expression was highest in macrophages from the BALB/cByJ mouse strain (Fig. 1A). BALB/c mice are very sensitive to L. monocytogenes infection and exhibit uniform mortality after intravenous infection with as little as 1 × 104 CFU, whereas all infected C57BL/6J mice survive this dose. Mouse strains that have either a mutation in Irf3 (C57BL/6ByJ) or a deletion of Irf3 (C57BL/6J Irf3−/−) are hyperresistant and can survive infection with as many as 1 × 106 L. monocytogenes cells. Macrophages from all C57BL/6 lines had similar basal Ch25h mRNA levels. However, at 2 h postinfection, Ch25h expression began to increase in cells from C57BL/6J mice but not in cells from C57BL/6ByJ or C57BL/6J Irf3−/− mice. By 4 h postinfection, Ch25h levels had increased 3.5-fold in C57BL/6J macrophages and 2-fold in BALB/cByJ macrophages. There was no detectable increase in macrophages from C57BL/6ByJ and C57BL/6J Irf3−/− mice. This experiment revealed that basal levels of Ch25h RNA are inversely correlated with resistance to L. monocytogenes. Furthermore, Ch25h expression is induced in response to infection, and this induction is dependent on IRF3.
FIG. 1.
Levels of Ch25h expression impact the course of L. monocytogenes infection. Differences in Ch25h expression observed in vivo are sufficient to alter the course of bacterial infection of macrophages. (A) Mouse strain-specific differences in Ch25h expression following L. monocytogenes infection of bone marrow-derived macrophages (BMMs) from sensitive BALB/cByJ, resistant C57BL/6J, and superresistant C57BL/6ByJ and C57BL/6J IRF3−/− mice (n = 3 ± SD). (B) Treatment of BMMs with 100 U/ml of recombinant IFN-β leads to rapid induction of Ch25h expression in BMMs from all strains except those lacking the alpha/beta interferon receptor (Ifnar−/−) (n = 3 ± SD). (C and E) C57BL/6J-derived ZBM2 macrophage-like cell lines that express either Ch25h cDNA (C) or Ch25h shRNA (E) model differences in Ch25h expression found in inbred mouse strains. By 12 h following infection of these cell lines with L. monocytogenes (MOI, 5) there were significant differences in the numbers of viable intracellular bacteria (D and F). Lower Ch25h mRNA levels corresponded to a lower number of recovered bacteria (D), while a higher level of expression of Ch25h led to a increase in the number of recovered bacteria (F). (n = 3 ± SD, representative of results of three independent experiments. NS, no significant difference. *, P < 0.05; **, P < 0.01.)
The dependence of Ch25h induction on IRF3 suggested that it is an IFN-β-inducible gene. To test this, we treated macrophages from BALB/cByJ, C57BL/6J, and C57BL6/ByJ mice with recombinant IFN-β for up to 12 h. We used cells from C57BL/6J Ifnar−/− mice which lack the IFN-I receptor as a negative control. We found that Ch25h was significantly upregulated in macrophages that have an intact type I interferon signaling cascade (Fig. 1B) whereas there was no induction in cells from C57BL/6J Ifnar−/− mice. Together, these observations demonstrate that Ch25h expression is induced by L. monocytogenes and this induction is mediated by type I interferons such as IFN-β. A recent study of macrophage and dendritic cell (DC) response to Toll-like receptor (TLR) ligands provides an independent confirmation of our results (35).
Ch25h affects L. monocytogenes replication in infected macrophages.
To investigate the role of Ch25h in macrophage function, we generated a series of mouse macrophage-like cell lines that mimic the differences in Ch25h expression observed in the inbred strains of mice. To elevate Ch25h mRNA levels, we stably introduced a C57BL/6 Ch25h cDNA under the control of the constitutive cytomegalovirus (CMV) promoter into the C57BL/6J-derived ZBM2 macrophage cell line. To reduce Ch25h expression levels, we stably introduced Ch25h shRNA (Open Biosystems). qRT-PCR analysis of two independent overexpressing cell lines (hiC25-1 and hiC25-3) revealed a 5- to 10-fold increase in Ch25h expression in these lines compared to that in vector-transfected cells (Fig. 1C). In two independent Ch25h knockdown cell lines (shC25-B, shC25H-D) Ch25h mRNA levels were reduced to ∼20% of levels in cells transduced with lentivirus expressing scrambled control shRNA (Fig. 1E).
To elucidate the role of Ch25h in control of L. monocytogenes infection we used our cell lines to determine the number of viable intracellular bacteria at various times after infection. Knockdown, overexpression, and respective control cell lines were infected with L. monocytogenes at an MOI of 5. At early (4- and 8-h) time points there was no significant difference in recovered bacterial numbers among all cell lines examined (Fig. 1D and F). This observation indicates that there is no effect of Ch25h expression levels on the uptake of L. monocytogenes by macrophages. At 12-h and 24-h time points there were significant differences in numbers of recovered bacteria that correlated with Ch25h expression levels. Cells that had higher levels of Ch25h had more bacteria than the vector-transfected controls (Fig. 1D). Conversely, there were fewer L. monocytogenes cells recovered from cell lines with reduced Ch25h mRNA levels (Fig. 1F). Therefore, we conclude that changes in Ch25h expression levels affect the course of L. monocytogenes infection of macrophages.
Ch25h mRNA levels modulate survival of infected macrophages.
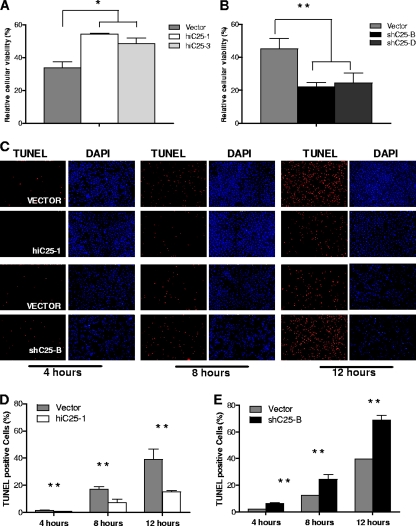
Our cell culture assay of L. monocytogenes infection measures numbers of intracellular bacteria that are protected from gentamicin in the medium. Host cells that die as a result of infection expose intracellular bacteria to the antibiotic. Therefore, differences in the number of recovered bacteria can reflect either a difference in the rate of bacterial replication or differences in the viability of host cells. To differentiate between these alternative explanations, we analyzed the viability of our cell lines following L. monocytogenes infection. We used a CellTiter-Fluor assay to establish a direct correlation between the levels of Ch25h expression and the viability of infected cells. This assay is not affected by the presence of intracellular bacteria since it measures a conserved and constitutive protease activity present within live mammalian cells. We found that cell lines expressing high levels of Ch25h mRNA had a higher rate of survival than the control cell line (Fig. 2A). Furthermore, the lines carrying Ch25h shRNA were very sensitive to L. monocytogenes infection (Fig. 2B). This indicates that an increase in Ch25h mRNA levels promotes survival of infected macrophages. This conclusion is further supported by our analysis of infection-induced DNA fragmentation that is indicative of either apoptotic or pyroptotic cell death. TUNEL staining of infected cells revealed that an increase in Ch25h levels resulted in a significant reduction in the number of TUNEL-positive cells from 17% in control cell lines to 7% as early as 8 h after infection (Fig. 2C and D). Conversely, in the shC25-B cell line that carried Ch25h shRNA there were significantly more (24% versus 12% in vector controls) TUNEL-positive cells at the 8-h time point (Fig. 2C and E). Significant differences in the number of TUNEL-positive cells were maintained throughout the 12-h course of infection examined. At the 12-h time point Ch25h overexpression reduced by more than 2-fold the percentage of TUNEL-positive cells, from the 40% found in vector controls to 15%, while reduction in Ch25h expression led to an increase in the percentage of TUNEL-positive cells to 69%. These results establish that changes in Ch25h expression modulate survival of infected cells.
FIG. 2.
Increase in Ch25h expression promotes survival of L. monocytogenes-infected macrophages. Macrophages that express higher levels of Ch25h (A) have improved survival after 12 h of L. monocytogenes infection (MOI, 5) as measured by the CellTiter-Fluor assay. Reduction in Ch25h expression leads to an increase in infection-induced death (B). The extent of infection-induced DNA fragmentation was evaluated by TUNEL staining (C). Cells that overexpress Ch25h (HiC25-1) have fewer TUNEL-positive cells than the control cell lines or cell lines that express Ch25h shRNA (shC25-B). The ratio of TUNEL-positive to DAPI-stained cells (D and E) was determined by counting 200 DAPI-positive cells in each group. Objective magnification is 100×. (n = 3 ± SD, representative of results of three independent experiments. *, P < 0.05; **, P < 0.01.)
Increased Ch25h expression leads to increased susceptibility to L. monocytogenes infection in vivo.
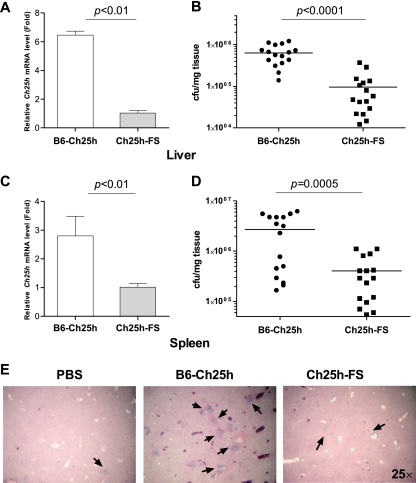
To address the role of changes in Ch25h expression in the resistance of the whole organism to L. monocytogenes infection, we used transient transgenesis to overexpress Ch25h in mice. This approach uses hydrodynamic delivery of recombinant DNA mainly to liver and spleen cells, which are the primary sites of L. monocytogenes infection (7, 52). We compared the effect of injection of a Ch25h cDNA expression construct to that of the same construct containing a frameshift mutation. In five independent experiments we found that a transient increase in Ch25h expression levels for 48 h prior to infection with L. monocytogenes (Fig. 3A and C) resulted in a 7-fold increase in bacterial loads in livers of infected animals (Fig. 3B). Spleens of animals that overexpressed Ch25h also had 7-fold-higher bacterial counts (Fig. 3D). Histological examination revealed an increased number of infectious foci in liver sections from animals that had higher levels of Ch25h expression (Fig. 3E). All of these observations serve to support our hypothesis that elevated Ch25h expression prolongs survival of infected cells and therefore contributes to increased bacterial burdens.
FIG. 3.
Elevated levels of Ch25h increases susceptibility to L. monocytogenes infection in vivo. Hydrodynamic delivery of the pCS2-CH25H construct led to a 6-fold increase in Ch25h expression in livers (A) and a 3-fold increase in spleens (C) of C57BL/6J mice 48 h after plasmid injection. At 24 h after infection with L. monocytogenes, mice injected with intact transgene had 7-fold higher numbers of bacteria in their livers (B) and similarly higher bacterial loads in their spleens (D) (total of 16 animals per treatment, considering the combined results of five independent experiments, each with 3 or 4 animals per treatment). Histological analysis (E) of tissue sections identified substantially more infectious foci (arrows) in livers of animals injected with the intact transgene than in livers of animals injected with the vehicle or the mutant construct.
CH25H controls expression of Cd5l.
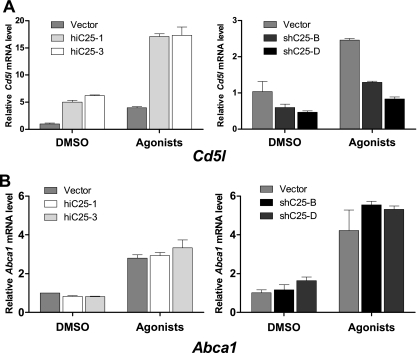
CH25H converts cholesterol to 25-HC, which can serve as an endogenous ligand for LXR nuclear receptors. CD5L, an LXRα target gene, is a soluble protein belonging to group B of the scavenger receptor cysteine-rich (SRCR) superfamily. It is expressed by macrophages present in lymphoid tissues (spleen, lymph node, thymus, and bone marrow), and it binds to myelomonocytic and lymphoid cells, which suggests that it may play an important role in the regulation of the innate immune system and in control of survival of infected macrophages (22, 38). We therefore examined the effect of differential Ch25h expression on the levels of Cd5l mRNA in our cell lines. Cells that overexpressed Ch25h (Fig. 4A) had significantly higher levels of Cd5l transcripts that were further increased by treatment with LXR/retinoid X receptors (RXR) agonists. Conversely, in cells that have less Ch25h we observed reduced levels of Cd5l mRNA. This reduction was also evident when cells were treated with synthetic LXR/RXR agonists. In contrast to these effects of Ch25h expression on Cd5l levels, we observed no effect of Ch25h expression on either basal or induced levels of Abca1, which is induced by either LXRα or LXRβ (Fig. 4B). Because Cd5l is a target of LXRα (22), our observation suggests that 25-hydroxycholesterol acts as an endogenous ligand of LXRα. Thus, it appears that induction of IFN-β after L. monocytogenes infection leads to an increase in CD5L production that ultimately promotes macrophage survival.
FIG. 4.
Changes in Ch25h expression result in changes in mRNA levels of the Cd5l prosurvival gene. (A) Increase (left panel) or suppression (right panel) of Ch25h expression results in corresponding changes in Cd5l mRNA levels as measured by qRT-PCR. This effect was additive to the effect of 24-h treatment with RXR (1 μM 9-cRA) and LXR (5 μM T091317) ligands. (B) Neither increase (left panel) nor reduction (right panel) in Ch25h mRNA levels had an effect on expression of Abca1 in either vehicle (DMSO) or ligand-stimulated cells.
Caspase-1 activation is controlled by CD5L.
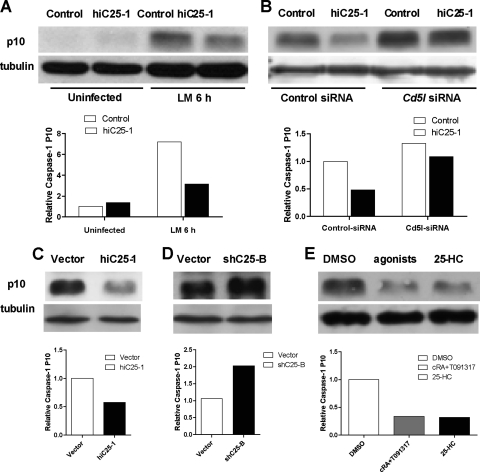
CD5L has been previously shown to inhibit apoptosis of L. monocytogenes-infected macrophages by interfering with activation of caspase-3 (22). However, L. monocytogenes has also been reported to induce macrophage pyroptosis (10, 39), which is a caspase-3-independent, caspase-1-dependent proinflammatory mode of macrophage cell death (37, 47). To test the role of CD5L in inhibition of pyroptosis, we transfected control or Ch25h-overexpressing cell lines with either Cd5l siRNA or a scrambled small interfering RNA (siRNA) control. Following infection with L. monocytogenes, we measured the amount of the p10 cleavage product of caspase-1. In the absence of infection there was almost no detectable caspase-1 processing in our cell lines (Fig. 5A). Infection resulted in caspase-1 maturation but to a significantly reduced extent in the hiC25-1 cell line that overexpresses Ch25h (Fig. 5B, control siRNA-treated samples). Treatment of either the control or hiC25-1 cell line with Cd5l-siRNA resulted in enhanced caspase-1 processing (Fig. 5B), indicating that CD5L inhibits L. monocytogenes-induced macrophage death in part by inhibiting caspase-1 cleavage and pyroptosis. Comparison of infection-induced caspase-1 cleavage in cells overexpressing Ch25h (Fig. 5C) and cells with reduced Ch25h expression (Fig. 5D) indicates that levels of Ch25h define the extent of caspase-1 cleavage by L. monocytogenes infection (Fig. 5C and D).
FIG. 5.
Activation of caspase-1 is regulated by the LXR signaling pathway. (A) The mature p10 form of caspase-1 is virtually undetectable in uninfected ZBM2 cells transfected with either vector or Ch25h cDNA. After 6 h infection with L. monocytogenes at an MOI of 5 there was more processed caspase-1 in vector-transfected cells than in cells that overexpress Ch25h. (B) Treatment of macrophage-like cell lines with siRNA directed against Cd5l, a downstream target of LXRs, enhances infection-induced processing of caspase-1 as measured by Western blot detection of mature p10 at 5 h postinfection. The effect of Ch25h overexpression on processing of caspase-1 is negated by Cd5l siRNA. Higher Ch25h levels (hiC25-1) suppress L. monocytogenes-induced caspase-1 maturation (C), while reduction in Ch25h expression led to enhanced processing of caspase-1 (D). (E) Prestimulation of BMM with RXR (1 μM 9-cRA) and LXR (5 μM T091317) ligands or with 25 μM 25-HC for 24 h prior to infection with L. monocytogenes reduces activation of caspase-1. Results are representative of three independent experiments. Graphs below all images represent NIH ImageJ densitometry quantification of tubulin-normalized caspase-1 p10 levels.
Activation of the LXR pathway suppresses caspase-1 processing.
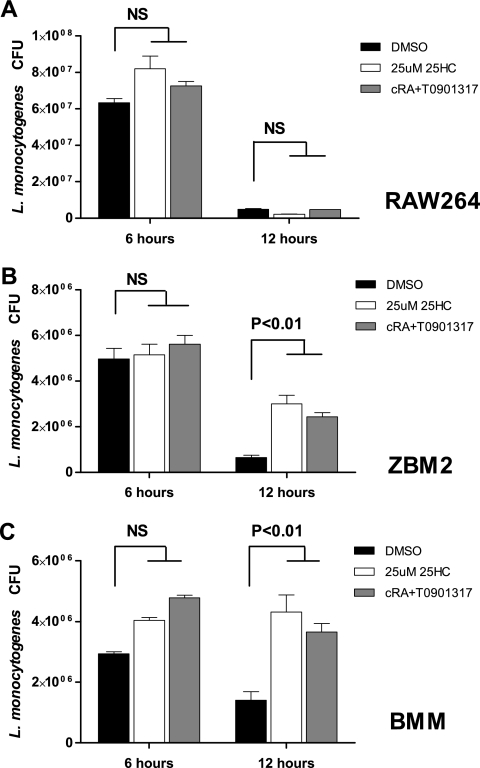
The effect of Ch25h expression on the activation of caspase-1 could be specific to 25-HC, or it could reflect the effect of the activation of LXRs. We therefore analyzed the effect of treatment of primary macrophages with either 25-hydroxycholesterol or synthetic LXR ligands. Cells treated with 25-HC or a mixture of RXR and LXR ligands displayed a similar reduction in the maturation of caspase-1 following infection with L. monocytogenes (Fig. 5E). There was no effect of pretreatment on survival of RAW264 macrophage-like cells that were reported to lack LXRα and not induce Cd5l in response to LXR/RXR agonists (Fig. 6A) (22). However, consistent with their role in promoting survival of infected macrophages, pretreatment of ZBM2 cells or BMMs with either LXR/RXR ligands or 25-HC resulted in a higher number of bacteria recovered at 12 h postinfection (Fig. 6B and C). Therefore, our data indicate that activation of LXRα by either endogenous or exogenous stimulation results in inhibition of L. monocytogenes-induced activation of caspase-1.
FIG. 6.
Activation of the LXR signaling pathway promotes bacterial replication. Prestimulation with RXR (9-cRA) and LXR (T091317) ligands or with 25-HC for 24 h did not alter the course of infection of RAW264 macrophage-like cells that lack LXRα. On the other hand, pretreatment with the same agonists of ZBM2 cells (B) or BMMs (C) increases the number of recovered live bacteria 12 h postinfection (MOI, 5).
DISCUSSION
LXRα has been recently shown to play a significant role in resistance to L. monocytogenes infection by controlling survival of macrophages (22). Therefore, the identification of Ch25h as a differentially expressed IFN-β-inducible gene was striking because CH25H converts cholesterol to 25-HC, a natural ligand of LXRs. Results from earlier gene knockout studies indicated a requirement for LXRα signaling for resistance to infection and attributed the effects of LXRα disruption to impaired Cd5l expression. Our data similarly demonstrate that upregulation of Cd5l at the time of infection promotes survival of infected macrophages. However, rather than promoting resistance to infection, signaling events that lead to CD5L induction, such as overexpression of Ch25h, result in increased recovery of L. monocytogenes from infected macrophages in vitro and in greater bacterial loads in livers and spleens of infected mice in vivo. We suggest that the different consequences for the host observed in LXRα-deficient mice and in mice transiently overexpressing Ch25h may reflect the different effects of a transient induction of macrophage survival at the time of infection compared to the constitutive reduction of a macrophage survival factor for the life of the animal. Steady-state viability of macrophages must be maintained to ensure detection of invading pathogens, but increased survival of L. monocytogenes-infected macrophages could intensify the disease. A detrimental effect of prolonged macrophage survival is not unique to L. monocytogenes pathogenesis. For example, myeloid-specific overexpression of Cd5l leads to increased numbers of macrophages, neutrophils, and dendritic cells, resulting in systemic inflammation and adenocarcinoma in the lung (36). Therefore, our study not only reveals new points of intersection of metabolic and inflammatory signaling but also underscores the delicate balance of cell survival that has to be maintained by the host to resist infection.
Our cell culture experiments revealed that changes in Ch25h expression lead to 2-fold differences in the viability of infected cells (Fig. 2A and B) and the percentage of TUNEL-positive cells (Fig. 2D and E). While it could be difficult to appreciate the significance of these differences, similar changes have been observed in other cell culture analyses of infected macrophages. For example, an analysis of the role of the Mcl-1 proapoptotic gene in Mycobacterium tuberculosis infection revealed that a reduction in Mcl-1 levels by transfection with antisense oligonucleotides that led to 2-fold differences in the viability of infected MDMs at day 1 and a 1.5-fold difference at day 4 had a significant effect on intracellular growth of the pathogen (40). Follow-up studies of pneumococcal infections provided further support for the importance of Mcl-1-controlled macrophage survival in the course of infectious diseases (30). In addition, a recent study demonstrated how macrophage cell death promotes resistance to Salmonella enterica serovar Typhimurium. Approximately 2-fold differences in macrophage viability translated into differences between life and death of infected animals (31). Similarly, in our study the importance of the effects observed ex vivo was confirmed by in vivo experiments.
Hydrodynamic delivery of recombinant DNA targets transient expression of transgenes mainly to livers and spleens of experimental animals (7, 52). Because these organs are primary sites of bacterial replication in the murine model of listeriosis, we used this approach to demonstrate that an increase in Ch25h expression increases the bacterial burdens of infected animals. This observation is consistent with our hypothesis that increased survival of infected macrophages promotes bacterial replication. However, considering that 25-HC can be secreted by macrophages, we cannot exclude the possibility that changes in Ch25h expression have paracrine effects that exacerbate the course of infection (15). In either case, our data clearly demonstrate a detrimental effect of increased Ch25h expression on resistance to L. monocytogenes infection.
Our results demonstrate that L. monocytogenes infection upregulates Ch25h expression through IFN-β signaling. This results in enhanced macrophage survival, presumably due to reduced levels of active caspase-1. Because caspase-1 is rapidly activated in response to infection (50), before Ch25h expression reaches its peak, it appears that Ch25h levels affect processing of this enzyme. We propose that this is due to a Ch25h-mediated increase in CD5L production, since Cd5l siRNA is capable of reversing the effects of Ch25h overexpression on the processing of caspase-1 (Fig. 5). It is possible that these events are a part of a strategy evolved by the pathogen to maintain a protected intracellular environment for its replication and prevent immune activation by pyroptotic death of macrophages. However, CD5L is a survival factor for lymphocytes as well as macrophages. Given the profound detrimental effect of L. monocytogenes-induced splenocyte apoptosis on host resistance (8), the induction of CD5L could be an attempt by the host to enhance survival of lymphocytes.
The control of infectious disease relies on the integration of a multitude of host cellular signaling pathways. Cross talk between inflammatory and metabolic pathways has recently been recognized as an important interaction that shapes the immune response to infection (23, 51). Our studies reveal new points of intersection of metabolic and inflammatory signaling and define differences in Ch25h expression that affect both pathways. These findings are based on our analysis of L. monocytogenes infection in inbred strains of mice that we use to model the phenotypic diversity of mammalian populations. Therefore, our findings underscore the importance of genetically defined variation in gene expression as a factor in the control of infectious diseases.
Acknowledgments
We thank Ashild Vik and Paul Kaufman for helpful discussions of the manuscript.
This work was supported by an NIAID grant AI060991 to V.L.B.
Editor: S. M. Payne
Footnotes
Published ahead of print on 24 January 2011.
REFERENCES
- 1.Albers, M., et al. 2006. A novel principle for partial agonism of liver X receptor ligands. Competitive recruitment of activators and repressors. J. Biol. Chem. 281:4920-4930. [DOI] [PubMed] [Google Scholar]
- 2.Arai, S., et al. 2005. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 1:201-213. [DOI] [PubMed] [Google Scholar]
- 3.Auerbuch, V., D. G. Brockstedt, N. Meyer-Morse, M. O'Riordan, and D. A. Portnoy. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200:527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauman, D. R., et al. 2009. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. U. S. A. 106:16764-16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergsbaken, T., S. L. Fink, and B. T. Cookson. 2009. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, M. S., and J. L. Goldstein. 1974. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J. Biol. Chem. 249:7306-7314. [PubMed] [Google Scholar]
- 7.Budker, V. G., et al. 2006. Mechanism of plasmid delivery by hydrodynamic tail vein injection. II. Morphological studies. J. Gene Med. 8:874-888. [DOI] [PubMed] [Google Scholar]
- 8.Carrero, J. A., B. Calderon, and E. R. Unanue. 2006. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J. Exp. Med. 203:933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrero, J. A., B. Calderon, and E. R. Unanue. 2004. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 200:535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervantes, J., T. Nagata, M. Uchijima, K. Shibata, and Y. Koide. 2008. Intracytosolic Listeria monocytogenes induces cell death through caspase-1 activation in murine macrophages. Cell. Microbiol. 10:41-52. [DOI] [PubMed] [Google Scholar]
- 11.Chawla, A., et al. 2001. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 7:161-171. [DOI] [PubMed] [Google Scholar]
- 12.Chen, W., G. Chen, D. L. Head, D. J. Mangelsdorf, and D. W. Russell. 2007. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 5:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242-248. [DOI] [PubMed] [Google Scholar]
- 14.Cossart, P., and A. Toledo-Arana. 2008. Listeria monocytogenes, a unique model in infection biology: an overview. Microbes Infect. 10:1041-1050. [DOI] [PubMed] [Google Scholar]
- 15.Diczfalusy, U., et al. 2009. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 50:2258-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink, S. L., and B. T. Cookson. 2007. Pyroptosis and host cell death responses during Salmonella infection. Cell. Microbiol. 9:2562-2570. [DOI] [PubMed] [Google Scholar]
- 17.Forman, B. M., B. Ruan, J. Chen, G. J. Schroepfer, Jr., and R. M. Evans. 1997. The orphan nuclear receptor LXRalpha is positively and negatively regulated by distinct products of mevalonate metabolism. Proc. Natl. Acad. Sci. U. S. A. 94:10588-10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garifulin, O., and V. Boyartchuk. 2005. Listeria monocytogenes as a probe of immune function. Brief Funct. Genomic. Proteomic. 4:258-269. [DOI] [PubMed] [Google Scholar]
- 19.Garifulin, O., et al. 2007. Irf3 polymorphism alters induction of interferon beta in response to Listeria monocytogenes infection. PLoS Genet. 3:1587-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, X., S. Li, J. Wu, C. Xia, and D. S. Lala. 2003. Liver X receptors interact with corepressors to regulate gene expression. Mol. Endocrinol. 17:1019-1026. [DOI] [PubMed] [Google Scholar]
- 21.Janowski, B. A., P. J. Willy, T. R. Devi, J. R. Falck, and D. J. Mangelsdorf. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383:728-731. [DOI] [PubMed] [Google Scholar]
- 22.Joseph, S. B., et al. 2004. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119:299-309. [DOI] [PubMed] [Google Scholar]
- 23.Joseph, S. B., A. Castrillo, B. A. Laffitte, D. J. Mangelsdorf, and P. Tontonoz. 2003. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9:213-219. [DOI] [PubMed] [Google Scholar]
- 24.Kandutsch, A. A., and H. W. Chen. 1974. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J. Biol. Chem. 249:6057-6061. [PubMed] [Google Scholar]
- 25.Lala, D. S., et al. 1997. Activation of the orphan nuclear receptor steroidogenic factor 1 by oxysterols. Proc. Natl. Acad. Sci. U. S. A. 94:4895-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, B., et al. 2004. PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nat. Immunol. 5:891-898. [DOI] [PubMed] [Google Scholar]
- 27.Lund, E., I. Bjorkhem, C. Furster, and K. Wikvall. 1993. 24-, 25- and 27-hydroxylation of cholesterol by a purified preparation of 27-hydroxylase from pig liver. Biochim. Biophys. Acta 1166:177-182. [DOI] [PubMed] [Google Scholar]
- 28.Lund, E. G., J. M. Guileyardo, and D. W. Russell. 1999. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. U. S. A. 96:7238-7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lund, E. G., T. A. Kerr, J. Sakai, W. P. Li, and D. W. Russell. 1998. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J. Biol. Chem. 273:34316-34327. [DOI] [PubMed] [Google Scholar]
- 30.Marriott, H. M., et al. 2005. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J. Clin. Invest. 115:359-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao, E. A., et al. 2010. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11:1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazaki, T., Y. Hirokami, N. Matsuhashi, H. Takatsuka, and M. Naito. 1999. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J. Exp. Med. 189:413-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connell, R. M., et al. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa, Y., et al. 1991. Generation of functional murine macrophage lines employing a helper-free and replication-defective SV40-retrovirus: cytokine-dependent growth. Cell Struct. Funct. 16:467-474. [DOI] [PubMed] [Google Scholar]
- 35.Park, K., and A. L. Scott. 2010. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 88:1081-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu, P., H. Du, Y. Li, and C. Yan. 2009. Myeloid-specific expression of Api6/AIM/Sp alpha induces systemic inflammation and adenocarcinoma in the lung. J. Immunol. 182:1648-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathinam, V. A., et al. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11:395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarrias, M. R., et al. 2005. A role for human Sp alpha as a pattern recognition receptor. J. Biol. Chem. 280:35391-35398. [DOI] [PubMed] [Google Scholar]
- 39.Sauer, J. D., et al. 2010. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7:412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sly, L. M., S. M. Hingley-Wilson, N. E. Reiner, and W. R. McMaster. 2003. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J. Immunol. 170:430-437. [DOI] [PubMed] [Google Scholar]
- 41.Song, C., J. M. Kokontis, R. A. Hiipakka, and S. Liao. 1994. Ubiquitous receptor: a receptor that modulates gene activation by retinoic acid and thyroid hormone receptors. Proc. Natl. Acad. Sci. U. S. A. 91:10809-10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stetson, D. B., and R. Medzhitov. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93-103. [DOI] [PubMed] [Google Scholar]
- 43.Stockinger, S., et al. 2002. Production of type I IFN sensitizes macrophages to cell death induced by Listeria monocytogenes. J. Immunol. 169:6522-6529. [DOI] [PubMed] [Google Scholar]
- 44.Stockinger, S., et al. 2004. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J. Immunol. 173:7416-7425. [DOI] [PubMed] [Google Scholar]
- 45.Valledor, A. F., et al. 2004. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc. Natl. Acad. Sci. U. S. A. 101:17813-17818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner, B. L., et al. 2003. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol. Cell. Biol. 23:5780-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warren, S. E., D. P. Mao, A. E. Rodriguez, E. A. Miao, and A. Aderem. 2008. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J. Immunol. 180:7558-7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willy, P. J., et al. 1995. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 9:1033-1045. [DOI] [PubMed] [Google Scholar]
- 49.Woodward, J. J., A. T. Iavarone, and D. A. Portnoy. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328:1703-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, J., T. Fernandes-Alnemri, and E. S. Alnemri. 2010. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J. Clin. Immunol. 30:693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zelcer, N., and P. Tontonoz. 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116:607-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, G., et al. 2004. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 11:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng, S. J., J. Jiang, H. Shen, and Y. H. Chen. 2004. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J. Immunol. 173:5652-5658. [DOI] [PubMed] [Google Scholar]
- 54.Zwaferink, H., S. Stockinger, P. Hazemi, R. Lemmens-Gruber, and T. Decker. 2008. IFN-beta increases listeriolysin O-induced membrane permeabilization and death of macrophages. J. Immunol. 180:4116-4123. [DOI] [PubMed] [Google Scholar]