Abstract
Patients with atopic dermatitis (AD) are frequently colonized with Staphylococcus aureus, with one-third of isolates producing alpha-toxin. Moreover, S. aureus colonization is positively correlated with the severity of eczema. Interleukin-17A (IL-17A) has gained attention in diseases associated with chronic skin infections. The aim of this study was to investigate the effects of sublytic alpha-toxin concentrations on IL-17A production. Sublytic alpha-toxin concentrations strongly induced IL-17A in peripheral blood mononuclear cells (PBMCs), isolated CD4+ T cells, polarized Th17 cells, and Th17 clones from reactive atopy patch test lesions and blood from AD patients. Alpha-toxin induced IL-17A directly in T cells. The effect of alpha-toxin was further amplified by upregulation of IL-1 in monocytes. In conclusion, higher levels of IL-17A secretion induced by alpha-toxin in the skin partially explain how colonization with S. aureus can contribute to chronic skin inflammation.
Atopic dermatitis (AD) and psoriasis (PS) are the most common immune-mediated chronic inflammatory skin diseases, with an increasing prevalence, affecting approximately 1 to 4% of the population in industrial countries (8, 36, 39). One hallmark of AD is a striking susceptibility to colonization with Staphylococcus aureus: 80 to 100% of patients with AD are colonized with S. aureus (4, 27). In contrast, S. aureus can be isolated from the skin of only 5 to 30% of healthy individuals, mainly from intertriginous areas (27). Moreover, a positive correlation between disease severity and extent and S. aureus colonization of lesional and nonlesional skin has been noted (4), which may be due to IgE-mediated hypersensitivity (3, 23) or to production of exotoxins with superantigenic properties (28, 40, 46). For PS, one study detected S. aureus in nonlesional skin of 27% of patients and in 46% of skin lesions (26). Tomi et al. investigated 25 PS patients: the skin of 60% of patients was positive for S. aureus, and isolated S. aureus strains were toxigenic, carrying staphylococcal enterotoxins A to D (SEA to SED), in 36% of patients. Furthermore, the PASI score correlated significantly with the presence of enterotoxin-producing S. aureus strains (43).
Recently, the superantigen SEB was shown to enhance house dust mite-induced patch test reactions in patients with AD (20). Besides unspecific stimulation of T-cell receptor (TCR) Vβ chains, superantigens can augment an antigen-specific T-helper 1 (Th1) response via the induction of interleukin-12 (IL-12) in antigen-presenting cells (APCs), which might contribute to AD becoming chronic (7, 22). On the other hand, the severity of AD decreased in patients colonized with nontoxigenic S. aureus strains upon antimicrobial treatment as well, which suggests the involvement of pathogenic factors other than superantigens derived from S. aureus (5).
A distinct percentage of S. aureus strains are able to produce alpha-toxin, a potent 33-kDa cytolysin which does not belong to the group of enterotoxins (superantigens) (9). In a former study, we found a 30% rate of alpha-toxin-producing S. aureus strains isolated from the skin of untreated patients with AD (6). More recently, Wichmann et al. investigated 127 AD patients who were on standard anti-inflammatory and antiseptic treatment and found a skin colonization rate of 63% for alpha-toxin-producing S. aureus in these patients (47).
Recent findings have suggested that Th17 cells participate profoundly in the pathogenesis of psoriasis (44). Increased numbers of Th17 cells and enhanced expression of IL-17 and IL-23 have been shown in PS skin (24). IL-17 is thought to contribute to the distinct features of PS, such as neutrophil chemotaxis and increased expression of antimicrobial peptides (44). There is growing evidence that IL-17 plays a critical role in AD as well. Toda et al. showed by immunochemistry that IL-17 is enhanced in acute but not chronic lesions of AD skin compared with uninvolved skin of patients with AD (42). Koga et al. reproduced these findings and additionally showed an increase of Th17 cells in the peripheral blood of AD patients that correlated positively with disease severity (19). A recent study by Eyerich et al. showed an enhancement of IL-17 secretion upon stimulation with SEB in atopy patch test (APT) lesions from patients with AD. Moreover, IL-17 upregulated human β-defensin 2 (HBD-2) in human keratinocytes in vivo (10).
It still remains unclear whether the staphylococcal component alpha-toxin has functional effects on IL-17A production in Th17 T cells in patients with chronic inflammatory skin diseases. Our data indicate that alpha-toxin induces IL-17A directly and, even more strongly, in an IL-1-dependent manner.
MATERIALS AND METHODS
Isolation of monocytes and T cells and polarization and expansion of T cells as well as T-cell clones.
We used buffy coats that were disposed of by the blood bank after thrombocyte preparation from anonymous healthy blood donors. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation on Lymphoprep (Fresenius Kabi Norge AS, Halden, Norway). CD4+ T cells, CD45RA+ CD4+ naïve T cells, CD45RO+ CD4+ memory T cells, CCR6+ CD4+ T cells, and monocytes were isolated by using magnetic beads according to the manufacturer's instructions (Miltenyi Biotec Inc., Bergisch Gladbach, Germany). Monocytes and T cells were cultured in Iscove's medium (Biochrom, Berlin, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco, Eggestein, Germany), 2 mM glutamine (Seromed, Berlin, Germany), 1% nonessential salts (Biochrom, Berlin, Germany), 1% penicillin-streptomycin (Seromed, Berlin, Germany), and 0.5% gentamicin (Biochrom, Berlin, Germany) at 37°C in a humidified atmosphere at 5% CO2. Contaminating cells were evaluated by flow cytometry (FACScalibur; Becton Dickinson, Heidelberg, Germany) and included B lymphocytes (CD20) (<0.5%) and natural killer cells (CD56) (<3.0%). Granulocytes (CD16) were absent. Isolated T cells had a purity of at least 97%, and monocytes had a purity of 88%.
For polarization of T cells into Th1 and Th2 cells and for polarization of T cells into Th17 cells, 1 × 106 isolated naïve CD4+ T cells/ml and 1 × 106 memory T cells/ml, respectively, were resuspended in 24-well flat-bottom plates (1 ml/well) in modified Iscove's medium supplemented with 5% human AB serum, 2 mM glutamine (Seromed, Berlin, Germany), 1% nonessential salts (Biochrom, Berlin, Germany), 1% penicillin-streptomycin (Seromed, Berlin, Germany), and 0.5% gentamicin (Biochrom, Berlin, Germany) (IAB medium) and then stimulated with activating anti-CD3 (450 ng/ml) and anti-CD28 (200 ng/ml) (Pelicluster, Amsterdam, Netherlands) antibodies as well as with IL-2 (20 ng/ml; Tebu Peprotech, Offenbach, Germany). For polarization of Th1 cells, 25 ng/ml IL-12 (Tebu Peprotech) and neutralizing anti-IL-4 antibody (1 μg/ml; R&D Systems, Minneapolis, MN) were added. For polarization of Th2 cells, IL-4 (20 ng/ml) (R&D Systems) and neutralizing anti-gamma interferon (anti-IFN-γ) antibody (1 μg/ml) (R&D Systems) were used. For polarization of Th17 cells, 250 ng/ml IL-6 (ImmunoTools, Friesoythe, Germany) and 10 ng/ml transforming growth factor beta (TGF-β; Promocell, Heidelberg, Germany) were used. Every 2 days, cells were split, IAB medium was changed, and activating anti-CD3 and anti-CD28 antibodies, as well as IL-2, were added. After 14 days, cells were used for experiments. For control of proper polarization, a portion of cells was restimulated with phorbol 12-myristate 13-acetate and ionomycin (Sigma Aldrich, Deisenhofen, Germany) for 6 h, followed by intracellular flow cytometric staining of IFN-γ, IL-4, and IL-17 as well as cell surface staining of IL-18 receptor, CCR4, and CCR6. Polarized Th1 cells showed clear expression of IL-18 receptor and IFN-γ, Th2 cells expressed high levels of CCR4 and IL-4, and Th17 cells were highly positive for CCR6 and IL-17.
Th17 cell clones (TCC) derived from 48-h positive APT lesions and PBMCs reactive to Mala s 13 from Malassezia sympodialis (a kind gift from R. Crameri, Swiss Institute of Allergy and Asthma Research, Davos, Switzerland) from AD patients were isolated as previously described (38, 45). AD was assessed by the diagnostic criteria of Hanifin and Rajka (16).
Cloning was performed by limiting dilution (0.3 cell/well) in 96-well round-bottom microplates in IAB medium supplemented with 20 U/ml IL-2 and 10 U/ml phytohemagglutinin (PHA; Sigma Aldrich) on a feeder layer of irradiated allogeneic PBMCs (55 Gy of 37Cs). Fresh medium containing IL-2 and irradiated feeder PBMCs were added every second week. Mala s 13 reactivity was determined by using a tritiated thymidine assay. For phenotyping, TCC (1 × 106/ml) were cultured in Iscove's medium supplemented with 10% FCS and concanavalin A (ConA) (2 μg/ml; Sigma) in 24-well plates. Supernatants were collected at 72 h for assessment of IL-17 (R&D Systems, Minneapolis, MN). TCC were designated Th17 TCC based on a cytokine secretion profile for IL-17 of >500 pg/ml. They were negative for IFN-γ, IL-4, IL-5, IL-13, and IL-22 as assessed by enzyme-linked immunosorbent assay (ELISA).
The study was approved by the local ethics committee and was performed in accordance with the protocols of the Declaration of Helsinki.
Stimulation and blocking of monocytes and T cells.
Monocytes or T cells were either left unstimulated or stimulated in a time- and dose-dependent manner with alpha-toxin (Sigma Aldrich, Deisenhofen, Germany) at sublytic concentrations or with SEB (Toxin Technology Inc., Sarasota, FL), as indicated. Lipopolysaccharide (LPS) was not detected in any reagent, as determined by the Limulus amebocyte lysate assay (Haemochrom Diagnostika, Essen, Germany). For blocking experiments, anti-IL-1 receptor (anti-IL-1R) (Kineret; Biovitrum, Stockholm, Sweden) and/or neutralizing anti-human IL-6 (anti-hIL-6; R&D Systems) was added.
Flow cytometric analysis.
Flow cytometric analysis was performed as described previously (15, 33, 34). Cells were washed and resuspended in phosphate-buffered saline (PBS) containing 0.2% gelatin, 20 mM sodium azide, and 10 μg/ml heat-aggravated human immunoglobulin G (IgG) (Sigma, Deisenhofen, Germany) to block the Fc receptor (FcR) for 15 min. Subsequently, cells were incubated with the indicated monoclonal antibodies or with isotype-matched controls on ice for 30 min (Sigma, Deisenhofen, Germany). Stained cells were washed three times, fixed in PBS with 1% paraformaldehyde, and analyzed by flow cytometry (FACScalibur; Becton Dickinson, Heidelberg, Germany).
Intracellular cytokine staining was performed according to the protocol and with the reagents provided by BD Biosciences (Becton Dickinson, Heidelberg, Germany). Briefly, cells were stimulated with PMA and ionomycin for 6 h in the presence of monensin. After 2 h, brefeldin A was added. After cell surface staining, cells were fixed, permeabilized with Cytofix/Cytoperm, and incubated with antibodies. Acquisition and analysis were done with a FACSCalibur flow cytometer (Becton Dickinson).
Cell viability staining with 7-AAD.
Cell viability was determined with 7-amino-actinomycin D (7-AAD). In brief, 7-AAD intercalates double-stranded nucleic acids. It is excluded by viable cells but can penetrate cell membranes of apoptotic cells. Since cell lysis due to pore-forming abilities is a well-established function of alpha-toxin, cell viability was assessed with 7-AAD (Becton Dickinson, Heidelberg, Germany) by flow cytometry following the manufacturer's instructions. PBMCs were stimulated with 50 ng/ml and 100 ng/ml alpha-toxin for 3 days (n = 4). Cell viability was not affected in either T cells or monocytes (data not shown). Therefore, we chose concentrations of 50 ng/ml and 100 ng/ml for our investigations.
Cytokine assessment by ELISA.
Cell-free culture supernatants were harvested and analyzed for IL-1α, IL-1β, IL-17, TGF-β (Duo sets; R&D Systems, Minneapolis, MN), IL-6, and IL-23 (p19/p40) (Ready-Set-Go; eBioscience, San Diego, CA), using a commercially available ELISA system following the manufacturer's instructions.
IL-17 cytokine secretion assay.
The IL-17 cytokine secretion assay was performed according to the protocol and with reagents provided by Miltenyi Biotec Inc. Briefly, cells were either left unstimulated or stimulated with alpha-toxin (50 ng/ml) or SEB (1 μg/ml) for 16 h or with 20 μg/ml Cytostim for 6 h. Cells were incubated with the indicated antibodies. Acquisition and analysis were done with a FACSCalibur flow cytometer (Becton Dickinson).
RNA isolation, RT, and real-time PCR.
RNA isolation, reverse transcription (RT), and quantitative real-time PCR were performed as previously described (15, 33). In brief, RNA was isolated using an RNA High purification kit (Roche Molecular Biochemicals, Mannheim, Germany) according to the supplier's instructions. The cDNA was synthesized, with integrated removal of genomic DNA in the samples, by using a QuantiTect reverse transcription kit (Qiagen, Hilden, Germany). Quantitative real-time RT-PCR (qRT-PCR) was performed on a LightCycler PCR machine (Roche Molecular Biochemicals, Mannheim, Germany), using a Quantitect SYBR green PCR kit (Qiagen, Hilden, Germany) and Quantitect primers for IL-1β, IL-17, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (housekeeping gene). Purified PCR products were used as external calibrators. For quantitative analysis, standard curves for IL-1β and IL-17 were created. These standard curves describing the PCR efficiencies of the target and the reference gene (GAPDH) allowed an efficiency-corrected quantification using Relative Quantification software (Roche Molecular Biochemicals, Mannheim, Germany).
Statistical analysis.
For statistical analyses, the software Sigma Stat for Windows (Systat Software, San Jose, CA) was used. Statistical analyses were performed using Student's t test.
RESULTS
Induction of IL-17 by alpha-toxin in human PBMCs and CD4+ T cells.
IL-17 expression and secretion upon alpha-toxin stimulation were investigated on the mRNA level by quantitative RT-PCR as well as on the protein level by ELISA and on the single-cell level by flow cytometry (cytokine secretion assay).
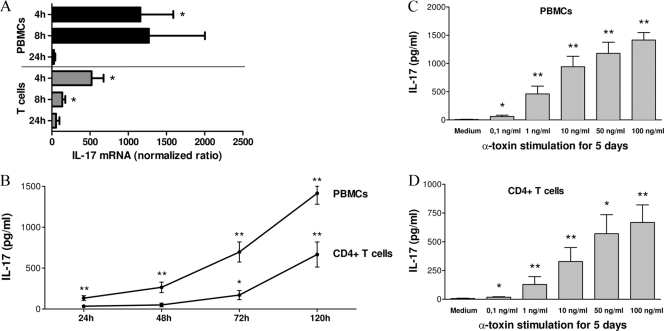
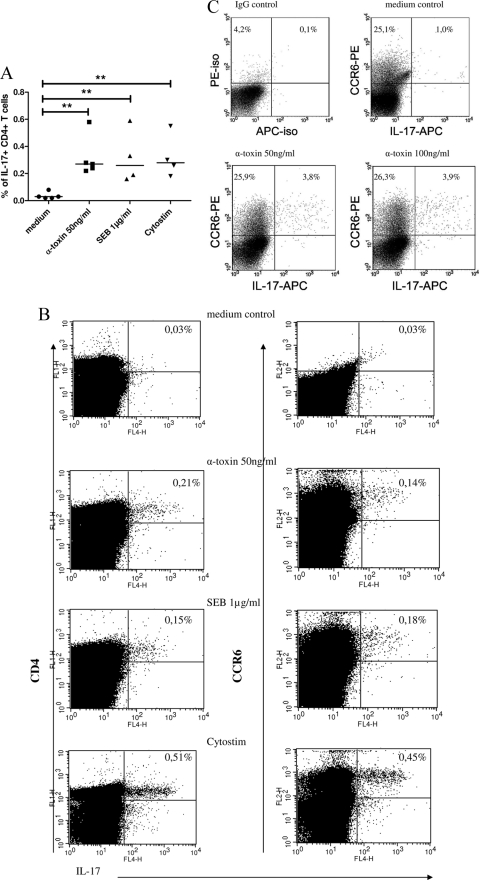
PBMCs and CD4+ T cells were either left unstimulated or stimulated with alpha-toxin (50 ng/ml) for 4, 8, and 24 h. Stimulation with alpha-toxin yielded a significant upregulation of IL-17 mRNA in PBMCs as well as in CD4+ T cells (Fig. 1A), as expected, with an increase upon 4 to 8 h of alpha-toxin stimulation and a decline after 24 h. Alpha-toxin induced a higher increase of IL-17 mRNA expression in PBMCs than in CD4+ T cells. This could also be demonstrated on the protein level by ELISA, in a time-dependent (24 h, 48 h, 72 h, and 120 h) (Fig. 1B) and dose-dependent (0.1 to 100 ng/ml) (Fig. 1C and D) manner. In contrast, no IL-17 secretion was detectable upon stimulation with lytic concentrations of alpha-toxin (≥1 μg/ml) (data not shown). IL-1β concentrations in isolated CD4+ T cells were below the detection level (4 pg/ml), as assessed by ELISA (data not shown). PBMCs as well as CD4+ T cells secreted significantly increased IL-17 upon stimulation with alpha-toxin compared to the medium control level. Consistent with our mRNA data, PBMCs secreted approximately two times more IL-17 upon alpha-toxin stimulation than did T cells. In a former study, we described an induction of T-cell proliferation upon alpha-toxin stimulation (6). To rule out the possibility that the higher IL-17 concentrations in cell culture supernatants were due to larger numbers of proliferated T cells in alpha-toxin-stimulated cultures than in the medium control, we performed a cytokine secretion assay (Fig. 2A and B) as well as intracellular IL-17 staining (Fig. 2C) by flow cytometry. Both techniques allow quantification of IL-17 on the single-cell level. We showed an induction of IL-17 secretion upon stimulation with alpha-toxin (50 ng/ml) in CD4+ T cells in general and in the CCR6+ compartment by the IL-17 cytokine secretion assay. SEB (1 μg/ml) was used as a positive control (Fig. 2A and B). Moreover, we demonstrated an intracellular upregulation of IL-17 secretion upon alpha-toxin stimulation (50 ng/ml and 100 ng/ml) in CCR6+ T cells by flow cytometry (Fig. 2C). The combination of SEB (100 ng/ml) plus alpha-toxin (100 ng/ml) failed to synergistically augment IL-17 induction in CD4+ T cells, as assessed by ELISA in 3 independent experiments (data not shown).
FIG. 1.
IL-17 is induced upon alpha-toxin stimulation in PBMCs and CD4+ T cells. (A) IL-17 mRNA was upregulated on PBMCs (n = 7) and isolated CD4+ T cells (n = 4) upon stimulation with alpha-toxin (50 ng/ml) for 4, 8, and 24 h, as determined by qRT-PCR. (B) PBMCs (n = 10) and T cells (n = 8) were either left unstimulated or stimulated with 50 ng/ml alpha-toxin for 24 h, 48 h, 72 h, and 120 h. Cell-free supernatants were analyzed for IL-17 secretion by ELISA. (C and D) PBMCs (C; n = 10) and T cells (D; n = 8) were either left unstimulated or stimulated with alpha-toxin in a dose (0.1 to 100 ng/ml)-dependent manner for 5 days, and cell-free supernatants were analyzed for IL-17 secretion by ELISA. Data are shown as mean IL-17/GAPDH ratios ± standard errors of the means (SEM) (A) or as mean IL-17 values (pg/ml) ± SEM (B to D). *, P < 0.05; **, P < 0.01.
FIG. 2.
Induction of IL-17 by staphylococcal exotoxins (alpha-toxin and SEB) on the single-cell level, as assessed by flow cytometry. PBMCs were either left unstimulated (medium control) or stimulated for 16 h with alpha-toxin (50 ng/ml) or SEB (1 μg/ml). Cytostim (20 μg/ml) was used as a positive control as recommended by the manufacturer. (A and B) IL-17 was assessed by an IL-17 secretion assay via flow cytometry. (A) Summary of 4 or 5 experiments, showing percentages of IL-17+ CD4+ T cells. (B) Data for one representative experiment, showing percentages of IL-17+ CD4+ as well as IL-17+ CCR6+ T cells. (C) Representative intracellular IL-17 FACS staining after restimulation with PMA/ionomycin (n = 7 independent experiments). **, P < 0.01.
Effects of alpha-toxin on Th17-associated cytokine induction in monocytes and PBMCs.
Since we observed approximately 2-fold more IL-17 secretion upon alpha-toxin stimulation in PBMCs than in purified CD4+ T cells (Fig. 1B), we investigated if secreted mediators from APCs are involved in this reaction.
To explore which mediators with known influence on IL-17 secretion may be induced by alpha-toxin, we investigated the expression and secretion of IL-1, IL-6, IL-23, and TGF-β in monocyte and PBMC fractions. These cells were either left unstimulated or stimulated with 50 ng/ml alpha-toxin for 4 h, 8 h, and 24 h. Stimulation of monocytes (Fig. 3A) and PBMCs (data not shown) with alpha-toxin induced IL-1β expression as determined by qRT-PCR. Stimulation of PBMCs with 50 ng/ml alpha-toxin for 72 h yielded a significant enhancement of IL-1β secretion as determined by ELISA (Fig. 3B). Stimulation of monocytes with alpha-toxin for 72 h also induced IL-6 secretion (stimulation index [SI], 24 ± 4.3) but did not influence the production of IL-23 or TGF-β (data not shown).
FIG. 3.
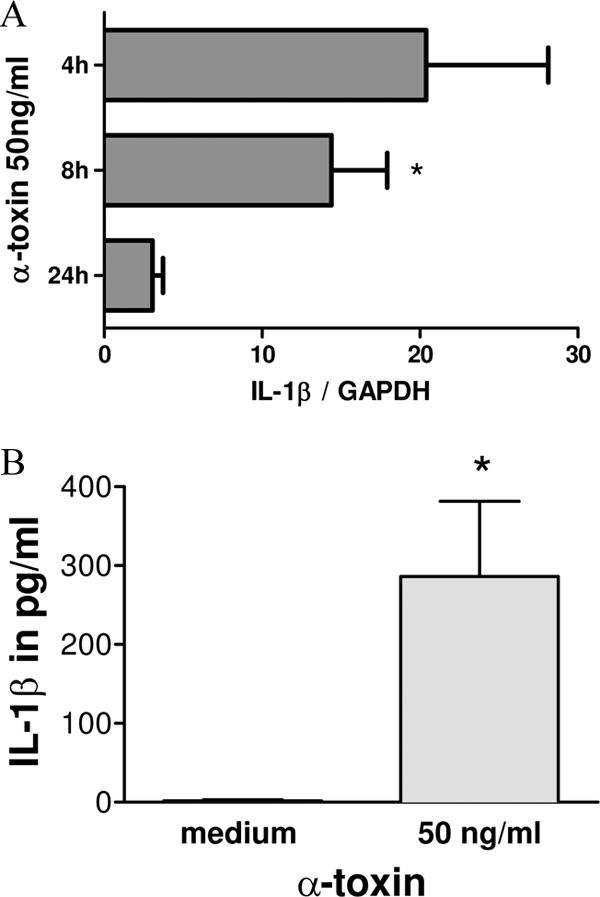
IL-1β is induced in monocytes and PBMCs upon stimulation with alpha-toxin. (A) Monocytes (n = 4) were either left unstimulated or stimulated with alpha-toxin (50 ng/ml) for 4 h, 8 h, and 24 h. IL-1β mRNA levels were determined by qRT-PCR. Stimulation of monocytes with alpha-toxin induced IL-1β mRNA expression. Data are shown as mean IL-17/GAPDH ratios ± SEM. *, P < 0.05. (B) Stimulation of PBMCs with 50 ng/ml alpha-toxin for 72 h yielded a significant enhancement of IL-1β secretion. The mean values plus SEM for 5 independent experiments are shown. *, P < 0.05.
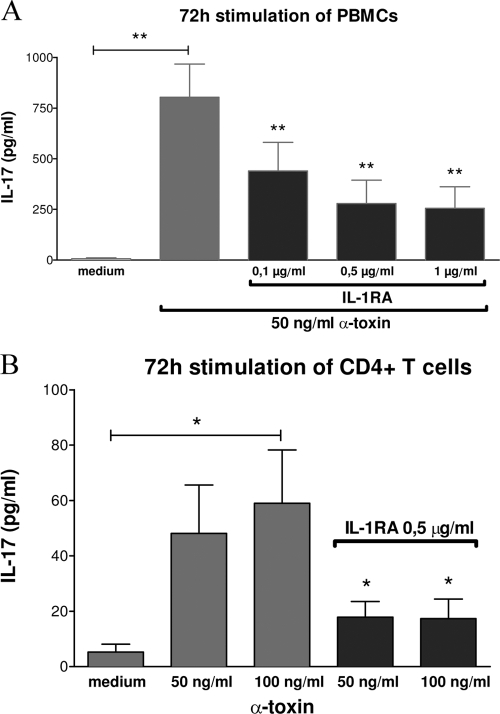
Alpha-toxin-induced IL-17 secretion in PBMCs and CD4+ T cells can be blocked with IL-1RA but not with anti-IL-6.
We next wanted to elucidate whether the proinflammatory cytokines IL-1β and IL-6 are critically involved in the 2-fold higher IL-17 secretion level upon alpha-toxin stimulation in PBMCs than that in purified CD4+ T cells (Fig. 1C). Therefore, we performed blocking experiments with an IL-1R antagonist (IL-1RA) (Kineret) and with neutralizing antibodies to IL-6. PBMCs were stimulated for 24 h (data not shown), 48 h (data not shown), and 72 h (Fig. 4A) with 50 ng/ml alpha-toxin and simultaneously stimulated dose dependently with IL-1RA (0.1 μg/ml, 0.5 μg/ml, and 1 μg/ml) to block IL-1 receptors on T cells. Alpha-toxin-induced IL-17 secretion in PBMCs could be blocked partially with all tested IL-1RA concentrations, with significance after 48 h (data not shown) and 72 h (Fig. 4A).
FIG. 4.
Alpha-toxin-induced IL-17 secretion in PBMCs and CD4+ T cells can be blocked partially with the IL-1 receptor antagonist Kineret. (A) PBMCs were stimulated for 72 h with 50 ng/ml alpha-toxin and simultaneously dose dependently with IL-1RA, as indicated, to block IL-1 receptors on T cells. (B) Highly purified CD14+ monocytes were stimulated for 24 h with alpha-toxin. Afterwards, alpha-toxin was washed out very carefully and cells were incubated in fresh culture medium for 72 h to produce cytokines. Supernatants were harvested and used for stimulation of allogeneic CD4+ T cells for 72 h. One hour prior to stimulation with these supernatants, T cells were incubated with 0.5 μg/ml IL-1RA. The mean values plus SEM are shown for 7 (T cells) and 9 (PBMCs) independent experiments. *, P < 0.05; **, P < 0.01. P values are for comparison to alpha-toxin if not indicated with brackets.
Next, we investigated the direct effect of IL-1R blocking on T cells upon stimulation with supernatants from alpha-toxin-stimulated purified monocytes. We stimulated highly purified CD14+ monocytes for 24 h with alpha-toxin in a dose-dependent manner. Afterwards, alpha-toxin was washed out and monocytes were incubated in fresh culture medium for 72 h. Supernatants from these monocytes were harvested and used for stimulation of allogeneic CD4+ T cells for 72 h. One hour prior to stimulation with these supernatants, T cells were incubated with 0.5 μg/ml IL-1RA to block IL-1 receptors. Alpha-toxin-induced IL-17 secretion in purified CD4+ T cells could be blocked significantly with 0.5 μg/ml IL-1RA (Fig. 4B). Blocking of IL-6 with neutralizing antibodies (50 ng/ml to 100 ng/ml) which had been proven to be biologically active had no effect on alpha-toxin-induced IL-17 secretion in PBMCs or in CD4+ T cells (data not shown).
Alpha-toxin-induced IL-17 secretion can be augmented by stimulation with IL-1β in CD4+ T cells.
Since alpha-toxin-induced IL-17 secretion in PBMCs and CD4+ T cells could be blocked partially with an IL-1 receptor antagonist, we next wanted to investigate whether stimulation with IL-1β can augment alpha-toxin-induced IL-17 secretion. Isolated CD4+ T cells were stimulated with alpha-toxin in a dose-dependent manner, with and without IL-1β. Medium and IL-1β stimulations without alpha-toxin were used as negative controls. Alpha-toxin-induced IL-17 secretion was augmented by stimulation with 50 pg/ml IL-1β in CD4+ T cells (P = 0.06 for 10 ng/ml alpha-toxin and P = 0.07 for 100 ng/ml alpha-toxin) (data not shown).
IL-17 induction upon alpha-toxin stimulation is restricted to polarized Th17 cells and leads to enhanced IL-17 secretion in T-cell clones from PBMCs and reactive atopy patch test lesions.
We polarized CD4+ CD45RA+ CD45RO− naïve T cells into Th1 or Th2 cells and CD4+ CD45RO+ CD45RA− memory cells into Th17 cells as described in Materials and Methods. As expected, polarized Th1 cells showed clear expression of IL-18 receptor and IFN-γ, Th2 cells expressed high levels of CCR4 and IL-4, and Th17 cells were positive for CCR6 and IL-17, as assessed by flow cytometry (data not shown). Th17 cells were stimulated for 5 h with 50 ng/ml alpha-toxin or 1 μg/ml SEB, and IL-17 mRNA was assessed by qRT-PCR. Stimulation with alpha-toxin significantly enhanced IL-17 mRNA expression (Fig. 5A). SEB was used as a positive control and induced an approximately 4-fold increase of IL-17 mRNA (SI, 4.45 ± 1.04) (data not shown). Stimulation with 50 ng/ml as well as 100 ng/ml alpha-toxin induced a slight increase of retinoid acid receptor-related orphan (RORC) expression in Th17 cells compared to that in the medium control (n = 7 independent experiments). However, this observation was not significant (data not shown). Th1, Th2, and Th17 cells were stimulated for 24 h, 48 h, and 72 h with alpha-toxin (50 ng/ml and 100 ng/ml), and IL-17 secretion in cell-free culture supernatants was quantified by ELISA. IL-17 secretion was detectable in Th17 cells only. Alpha-toxin significantly enhanced IL-17 secretion in Th17 cells but not in Th1 or Th2 cells after 24 h, 48 h (data not shown), and 72 h (Fig. 5B).
FIG. 5.
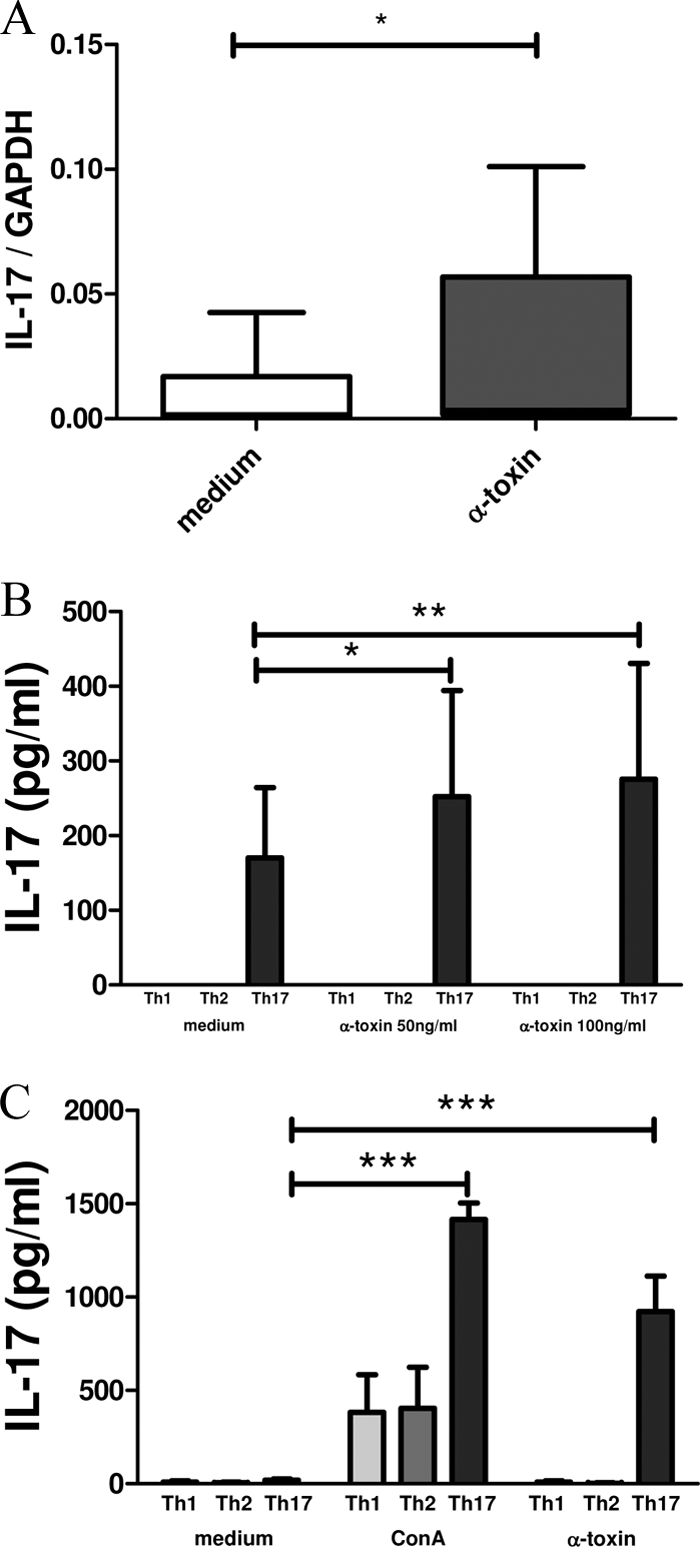
IL-17 induction upon alpha-toxin stimulation is restricted to polarized Th17 cells and Th17 T-cell clones. CD4+ CD45RA+ CD45RO− naïve T cells were polarized into Th1 or Th2 cells, and CD4+ CD45RO+ CD45RA− memory cells were polarized into Th17 cells. (A) IL-17 mRNA was upregulated on Th17 cells (n = 10) upon stimulation with alpha-toxin (50 ng/ml) for 5 h, as determined by qRT-PCR. Th1, Th2, and Th17 cells (n = 8 to 10 each) (B) or predefined Th1, Th2, and Th17 TCC which had been generated from atopy patch test lesions and PBMCs from AD patients by limiting dilution (n = 12) (C) were stimulated for 72 h with 50 ng/ml alpha-toxin. ConA (10 μg/ml) was used as a positive control. Supernatants were quantified for IL-17 secretion by ELISA. Data are shown as mean IL-17/GAPDH ratios ± SEM (A) or as mean IL-17 values (pg/ml) plus SEM (B and C). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To investigate the direct effects of alpha-toxin-induced IL-17 secretion in T cells, we generated Th1, Th2, and Th17 TCC from Mala s 13-reactive atopy patch test lesions and PBMCs from AD patients by limiting dilution and stimulated them for 72 h with 50 ng/ml alpha-toxin. ConA (2 μg/ml) was used as a positive control. IL-17 secretion in cell-free culture supernatants was quantified by ELISA. IL-17 secretion was detected in Th17 TCC which did not secrete IFN-γ or IL-4/IL-13. Moreover, alpha-toxin significantly enhanced IL-17 secretion in Th17 TCC after 72 h but not that in Th1 or Th2 TCC (Fig. 5C). IL-1β concentrations in polarized Th17 cells as well as T-cell clones were below the detection level (4 pg/ml), as assessed by ELISA (data not shown).
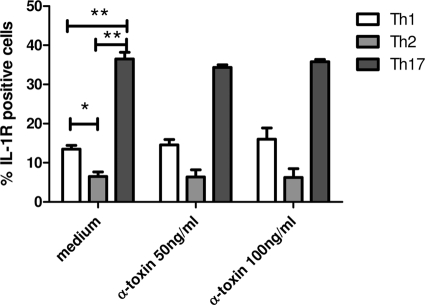
Polarized Th17 cells express higher levels of IL-1 receptor than do Th1 and Th2 cells.
IL-1R+ and IL-1R− CD4+ T cells were described recently that differ in the capacity to produce IL-17: IL-1R+ CD4+ memory T cells produce higher levels of IL-17 than do IL-1R− CD4+ memory T cells, as described by Lee et al. (21). Therefore, we next examined whether there were differences in IL-1R expression in polarized Th1, Th2, and Th17 cells. Th17 cells expressed significantly more IL-1R than did Th1 and Th2 cells. However, alpha-toxin had no effect on IL-1R expression in either Th1, Th2, or Th17 cells (Fig. 6).
FIG. 6.
IL-1R expression on Th1, Th2, and Th17 cells upon stimulation with alpha-toxin (50 ng/ml) for 72 h (n = 3), as determined by flow cytometry. Data are shown as mean percentages of IL-1R-positive cells plus SEM. *, P < 0.05; **, P < 0.01.
DISCUSSION
Alpha-toxin-producing S. aureus strains can be isolated from the skin of AD patients and are able to invade the skin and directly interact with T cells (6). At higher concentrations, alpha-toxin may lead to necrosis in a wide range of human cells by forming small pores (1 to 2 nm in diameter) in cell membranes (9). In keratinocytes, tumor necrosis factor alpha (TNF-α) is released simultaneously with lysis (12). Besides this well-described mechanism of lysis, alpha-toxin may induce apoptosis in T lymphocytes (17) and is able to induce proinflammatory effects in a variety of cells involved in inflammatory processes, e.g., monocytes (2), polymorphonuclear cells (41), and endothelial cells (13). In our former study, we showed that sublytic concentrations of alpha-toxin were able to effectively activate T lymphocytes, which led to proliferation and induction of IFN-γ at both the mRNA and protein levels (6). This indicates that besides pore-forming abilities, sublytic concentrations of alpha-toxin have immunological properties and therefore may serve as a pathogenic factor in chronic inflammatory skin diseases.
Since evidence that IL-17 secretion is critical for clearing infections of the skin is increasing (30) and IL-17 has been described as present in the most common inflammatory skin diseases, PS (29) and AD (19, 42), we investigated whether the microbial product staphylococcal alpha-toxin could be an adequate stimulus for IL-17 secretion in chronic inflammatory skin diseases.
We showed that alpha-toxin is indeed a strong inducer of IL-17 in Th17 T cells. This effect was direct upon stimulation with alpha-toxin as well as IL-1 dependent, as shown by blocking experiments with IL-1RA and stimulation of CD4+ T cells with supernatants from alpha-toxin-stimulated monocytes. This is in line with the work of Rao et al., who found with a model of late graft failure that IL-1 was able to increase the frequency of alloreactive memory CD4+ T cells that produced IL-17 but not that of those that produced IFN-γ. Conversely, IL-1RA was able to block IL-17 secretion in cocultures of epithelial cells with allogeneic CD4+ T cells (37). IL-1 induction in monocytes by alpha-toxin was already published by Bhakdi et al. in 1989 (2). Moreover, the ability of alpha-toxin to induce inflammasome-mediated caspase-1 activation, which is required for IL-1β cleavage and secretion, has also been reported (14, 32). Therefore, alpha-toxin-induced activation of the NLRP-3 inflammasome, with consecutive IL-1 secretion, could be the underlying mechanism responsible for the 2-fold-enhanced IL-17 secretion in PBMCs compared to that in CD4+ T cells upon alpha-toxin stimulation in our study. However, we cannot exclude the possibility that other small cell types in the PBMC fraction, such as γδ T cells, can contribute to the augmentation of IL-17 in PBMCs compared to isolated CD4+ T cells upon alpha-toxin stimulation. To date, γδ T cells are well established as an IL-17 source in mice but not in the human system (11).
Interestingly, Lee et al. showed that CD4+ CD45RO+ memory T cells expressing IL-1R secreted more IL-17 than did IL-1R-negative CD4+ CD45RO+ memory T cells. Moreover, IL-1β promoted IL-17 production from both IL-1R+ and IL-1R− memory CD4+ T cells (21). We extended this observation and showed that polarized Th17 cells (generated from CD4+ CD45+ memory T cells) expressed higher levels of IL-1R than did Th1 and Th2 cells. Alpha-toxin stimulation did not influence IL-1R expression on Th17 cells. However, alpha-toxin stimulation enhanced IL-1R mRNA expression on CD4+ T cells, which again points to an involvement of alpha-toxin in the positive-feedback loop of IL-1-induced IL-17 secretion.
Further insights about immune aberrations might come from studies of patients with immunodeficiency and an AD-like phenotype, such as hyper-IgE syndrome, which is a primary immunodeficiency characterized by atopic eczema and enhanced susceptibility to skin infections with S. aureus and Candida albicans. Recent studies showed an impaired Th17 axis but normal production of other proinflammatory cytokines, including IL-1β. However, human keratinocytes and bronchial epithelial cells were dependent on the synergistic action of Th17 cytokines and classical proinflammatory cytokines for their production of antistaphylococcal factors, including antimicrobial peptides (31). These results point to a link between IL-17 and IL-1 as well.
Eyerich et al. (10) used Dermatophagoides pteronyssinus (Der p 1)-specific TCC from atopy patch test lesions obtained from AD patients to show that SEB strongly promoted IL-17 release by T cells in dendritic cell (DC)-T-cell cocultures. We reproduced these data with CD4+ CCR6+ T cells and showed for the first time that stimulation with alpha-toxin induced a similar IL-17 release in CD4+ T cells and CD4+ CCR6+ T cells to that induced by SEB.
In a previous study, we showed that stimulation of PBMCs and CD4+ T cells with alpha-toxin induced a Th1 response by enhancing IFN-γ, whereas alpha-toxin failed to induce IL-4 in Th2-polarized cells (unpublished data). Therefore, a combined Th1-Th17 immune response would most likely appear upon alpha-toxin stimulation.
Next, we wanted to elucidate the effects of alpha-toxin stimulation on IL-17 production in AD patients. Therefore, we investigated IL-17A secretion upon alpha-toxin stimulation in T-cell clones from Mala s 13-reactive atopy patch test lesions and peripheral blood from patients with AD and showed IL-17 induction by alpha-toxin stimulation as well.
Toda et al. showed by immunochemistry that IL-17A was enhanced in acute but not chronic lesions of AD skin compared with uninvolved skin of AD patients (42). Koga et al. reproduced these findings and additionally showed an increase of Th17 cells in the peripheral blood of AD patients, positively correlating with disease severity (19). Moreover, they showed that the percentage of IL-17+ CD4+ T cells in the peripheral blood of AD patients was only slightly lower than that for psoriatic patients. Nograles et al. investigated skin collected from patients with chronic AD and psoriasis as well as peripheral blood from these patients. They could show no differences in IL-17A, IL-22, IL-4, and IFN-γ secretion in CD4+ and CD8+ T cells obtained from the peripheral blood of PS patients and AD patients (35). Kagami et al. found elevated Th17 levels and, to a lesser extent, elevated Th1 and Th22 levels in the peripheral blood of psoriasis patients (18). We demonstrated IL-17A secretion in Th17 cell clones from Mala s 13-reactive atopy patch test lesions and peripheral blood from patients with AD which was significantly upregulated upon stimulation with alpha-toxin. Since alpha-toxin is present on the skin of 30% of untreated AD patients (6) and 63% of AD patients treated with antimicrobials (47), IL-17A may have a high clinical impact in the pathogenesis, flare-ups, and maintenance of eczema. Further investigations are necessary to explore the role of IL-17A and, in this context, IL-1RA in chronic inflammatory skin diseases, as they could represent interesting targets for drug development.
Acknowledgments
We thank Gabriele Begemann and Kathrin Baumert for their excellent technical assistance and R. Crameri (Swiss Institute of Allergy and Asthma Research, Davos, Switzerland) for the donation of Mala s 13.
This study was supported by grants SFB 566 and GRK 1441 from the Deutsche Forschungsgemeinschaft.
The authors have no financial conflicts of interest.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 18 January 2011.
REFERENCES
- 1.Reference deleted.
- 2.Bhakdi, S., M. Muhly, S. Korom, and F. Hugo. 1989. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect. Immun. 57:3512-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breuer, K., M. Wittmann, B. Bösche, A. Kapp, and T. Werfel. 2000. Severe atopic dermatitis is associated with sensitization to staphylococcal enterotoxin B (SEB). Allergy 55:551-555. [DOI] [PubMed] [Google Scholar]
- 4.Breuer, K., A. Kapp, and T. Werfel. 2001. Bacterial infections and atopic dermatitis. Allergy 56:1034-1041. [DOI] [PubMed] [Google Scholar]
- 5.Breuer, K., S. Haussler, A. Kapp, and T. Werfel. 2002. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br. J. Dermatol. 147:55-61. [DOI] [PubMed] [Google Scholar]
- 6.Breuer, K., et al. 2005. Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin. Exp. Allergy 35:1088-1095. [DOI] [PubMed] [Google Scholar]
- 7.Bright, J. J., Z. Xin, and S. Sriram. 1999. Superantigens augment antigen-specific Th1 responses by inducing IL-12 production in macrophages. J. Leukoc. Biol. 65:665-670. [DOI] [PubMed] [Google Scholar]
- 8.Cork, M. J., et al. 2006. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J. Allergy Clin. Immunol. 118:3-21. [DOI] [PubMed] [Google Scholar]
- 9.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyerich, K., et al. 2009. IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. J. Allergy Clin. Immunol. 123:59-66. [DOI] [PubMed] [Google Scholar]
- 11.Eyerich, S., K. Eyerich, A. Cavani, and C. Schmidt-Weber. 2010. IL-17 and IL-22: siblings, not twins. Trends Immunol. 31:354-361. [DOI] [PubMed] [Google Scholar]
- 12.Ezepchuk, Y. V., et al. 1996. Staphylococcal toxins and protein A differentially induce cytotoxicity and release of tumor necrosis factor-a from human keratinocytes. J. Invest. Dermatol. 107:603-609. [DOI] [PubMed] [Google Scholar]
- 13.Grimminger, F., F. Rose, and U. Sibelius. 1997. Human endothelial cell activation and mediator release in response to bacterial exotoxins Escherichia coli hemolysin and staphylococcal alpha-toxin. J. Immunol. 159:1909-1969. [PubMed] [Google Scholar]
- 14.Gurcel, L., L. Abrami, S. Girardin, J. Tschopp, and F. Gisou van der Goot. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135-1145. [DOI] [PubMed] [Google Scholar]
- 15.Gutzmer, R., et al. 2009. The histamine H4 receptor is functionally expressed on Th2 cells. J. Allergy Clin. Immunol. 123:619-625. [DOI] [PubMed] [Google Scholar]
- 16.Hanifin, J. M., and G. Rajka. 1980. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 92:44-47. [Google Scholar]
- 17.Jonas, D., et al. 1994. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect. Immun. 62:1304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagami, S., H. L. Rizzo, J. J. Lee, Y. Koguchi, and A. Blauvelt. 2010. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Invest. Dermatol. 130:1373-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga, C., K. Kabashima, N. Shiraishi, M. Kobayashi, and Y. Tokura. 2008. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Invest. Dermatol. 128:2625-2630. [DOI] [PubMed] [Google Scholar]
- 20.Langer, K., K. Breuer, A. Kapp, and T. Werfel. 2007. Staphylococcus aureus derived enterotoxins enhance house dust mite induced patch test reactions in atopic dermatitis. Exp. Dermatol. 16:124-129. [DOI] [PubMed] [Google Scholar]
- 21.Lee, W. W., et al. 2010. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood 115:530-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung, D. Y., et al. 1995. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin-12 production. J. Exp. Med. 181:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung, D. Y. M., et al. 1993. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. J. Clin. Invest. 92:1374-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowes, M. A., et al. 2008. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Invest. Dermatol. 128:1207-1211. [DOI] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Marples, R. R., C. L. Heaton, and A. M. Kligman. 1973. Staphylococcus aureus in psoriasis. Arch. Dermatol. 107:568-570. [PubMed] [Google Scholar]
- 27.Matsui, K., A. Nishikawa, H. Suto, R. Tsuboi, and H. Ogawa. 2000. Comparative study of Staphylococcus aureus isolated from lesional and non-lesional skin of atopic dermatitis. Microbiol. Immunol. 44:945-947. [DOI] [PubMed] [Google Scholar]
- 28.McFadden, J. P., W. C. Noble, and R. D. R. Camp. 1993. Superantigenic exotoxin-secreting potential of staphylococci isolated from atopic eczematous skin. Br. J. Dermatol. 128:631-632. [DOI] [PubMed] [Google Scholar]
- 29.McKenzie, B. S., R. A. Kastelein, and D. J. Cua. 2006. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27:17-23. [DOI] [PubMed] [Google Scholar]
- 30.Milner, J. D., et al. 2008. Impaired Th17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:733-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minegishi, Y., et al. 2009. Molecular explanation between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J. Exp. Med. 206:1291-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz-Planillo, R., L. Franchi, L. S. Miller, and G. Nunez. 2009. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the NLRP3 inflammasome. J. Immunol. 183:3942-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niebuhr, M., et al. 2008. Dysregulation of TLR-2 induced effects in monocytes from patients with atopic dermatitis: impact of the TLR-2 R753Q polymorphism. Allergy 63:728-734. [DOI] [PubMed] [Google Scholar]
- 34.Niebuhr, M., C. Lutat, S. Sigel, and T. Werfel. 2009. Impaired TLR-2 expression and TLR-2 mediated cytokine secretion in macrophages from patients with atopic dermatitis. Allergy 64:1580-1587. [DOI] [PubMed] [Google Scholar]
- 35.Nograles, C. E., et al. 2009. IL-22-producing T22 T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing Th17 T cells. J. Allergy Clin. Immunol. 123:1244-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proksch, E., R. Fölster-Holst, and J. M. Jensen. 2006. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 43:159-169. [DOI] [PubMed] [Google Scholar]
- 37.Rao, D. A., K. J. Tracey, and J. S. Pober. 2007. IL-1α and IL-1β are endogenous mediators linking cell injury to the adaptive alloimmune response. J. Immunol. 179:6536-6546. [DOI] [PubMed] [Google Scholar]
- 38.Sager, N., A. Feldmann, G. Schilling, P. Kreitsch, and C. Neumann. 1992. House dust mite-specific T cells in the skin of subjects with atopic dermatitis: frequency and lymphokine profile in the allergen patch test. J. Allergy Clin. Immunol. 89:801-810. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt, J., and G. Wozel. 2009. Targeted treatment of psoriasis with adalimumab: a critical appraisal based on a systematic review of the literature. Biologics 3:303-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skov, L., et al. 2000. Application of staphylococcal enterotoxin B on normal and atopic skin induces up-regulation of T-cells by a superantigen-mediated mechanism. J. Allergy Clin. Immunol. 105:820-826. [DOI] [PubMed] [Google Scholar]
- 41.Suttorp, N., W. Seeger, J. Zucker-Reimann, L. Roka, and S. Bhakdi. 1987. Mechanism of leukotriene generation in polymorphonuclear leukocytes by staphylococcal alpha-toxin. Infect. Immun. 55:104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toda, M., et al. 2003. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J. Allergy Clin. Immunol. 111:875-881. [DOI] [PubMed] [Google Scholar]
- 43.Tomi, N. S., B. Kränke, and E. Aberer. 2005. Staphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythrodermia, and in healthy control subjects. J. Am. Acad. Dermatol. 53:67-72. [DOI] [PubMed] [Google Scholar]
- 44.van Beelen, A., M. B. M. Teunissen, M. L. Kapsenberg, and E. C. de Jong. 2007. Interleukin-17 in inflammatory skin disorders. Curr. Opin. Allergy Clin. Immunol. 7:374-381. [DOI] [PubMed] [Google Scholar]
- 45.Werfel, T., M. Hentschel, A. Kapp, and H. Renz. 1997. Dichotomy of blood- and skin-derived IL-4-producing allergen-specific T cells and restricted V beta repertoire in nickel-mediated contact dermatitis. J. Immunol. 158:2500-2505. [PubMed] [Google Scholar]
- 46.Werfel, T. 2009. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J. Invest. Dermatol. 129:1878-1891. [DOI] [PubMed] [Google Scholar]
- 47.Wichmann, K., et al. 2009. Isolation of alpha-toxin-producing Staphylococcus aureus from the skin of highly sensitized adult patients with severe atopic dermatitis. Br. J. Dermatol. 161:300-305. [DOI] [PubMed] [Google Scholar]