Abstract
The currently available pneumococcal vaccines do not protect against all serotypes of Streptococcus pneumoniae. A shift toward nonvaccine serotypes causing colonization and invasive disease has occurred, and studies on protein-based vaccines have been undertaken. We assessed the association between specific antibodies against pneumococcal virulence proteins and colonization and respiratory tract infections (RTIs). Additionally, we assessed the extent to which colonization induces a humoral immune response. Nasopharyngeal swabs collected from children at 1.5, 6, 14, and 24 months of age were cultured for pneumococcus. Serum samples were obtained at birth and at 6, 14, and 24 months (n = 57 children providing 177 serum samples). Data were collected prior to the pneumococcal vaccine era. IgG, IgA, and IgM levels against 17 pneumococcal protein vaccine candidates were measured using a bead-based flow cytometry technique (xMAP; Luminex Corporation). Information regarding RTIs was questionnaire derived. Levels of IgG against all proteins were high in cord blood, decreased in the first 6 months and increased again thereafter, in contrast to the course of IgA and IgM levels. Specific antibodies were induced upon colonization. Increased levels of IgG against BVH-3, NanA, and SP1003 at 6 months, NanA, PpmA, PsaA, SlrA, SP0189, and SP1003 at 14 months, and SlrA at 24 months were associated with a decreased number of RTIs in the third year of life but not with colonization. Maternal antipneumococcal antibodies did not protect against pneumococcal colonization and infection. Certain antibodies against pneumococcal virulence proteins, some of which are induced by colonization, are associated with a decreased number of RTIs in children. This should be taken into account in future pneumococcal vaccine studies.
Streptococcus pneumoniae (pneumococcus) is a commensal organism but also a pathogen that plays an important role in the pathogenesis of respiratory tract infections (RTIs) such as pneumonia and otitis media in infants and young children. In addition, the pneumococcus may cause invasive diseases such as meningitis and sepsis (24). Morbidity and mortality in infants and young children worldwide are frequently caused by this pathogen. The World Health Organization generated estimates on child mortality due to invasive pneumococcal infection ranging from 700,000 to more than a million children per annum (24). Healthy children may be colonized with the pneumococcus in the nasopharynx; the frequency of this colonization increases in the first year of life from approximately 8% to 45% (20). This pathogen often presents as a commensal, causing no harm due to the adequate innate and adaptive immune reactions of the host. However, asymptomatic nasopharyngeal carriage is the primary source of pneumococcal infection (2).
More than 90 different pneumococcal serotypes have been identified on the basis of variability in the capsular polysaccharides. The current vaccines are based on antibodies against these polysaccharides; hence, only some these serotypes are covered. Presently, significant research is focused on improving pneumococcal vaccines in order to generate broader protection against pneumococcal disease. The current 7-valent pneumococcal conjugate vaccine (PCV-7), which has now been introduced in national immunization programs in most developed countries, is up to 90% effective in reducing vaccine serotype-specific invasive pneumococcal disease. However, the net vaccine benefit has been negatively affected by a 71% increased rate of invasive pneumococcal disease caused by nonvaccine serotypes (30). Recently, a 13-valent pneumococcal conjugate vaccine (PCV-13) was developed to further improve protection (9, 19) by covering the serotypes that most frequently cause infection and colonization. However, the increase in carriage of nonvaccine serotypes, and the associated increase in invasive disease, could ultimately outweigh the benefits of the current PCV (21). Since PCV is also quite expensive and therefore not extensively used in developing countries where it is needed the most, there is an urgent need to develop alternative pneumococcal vaccines to cover these gaps. Novel vaccines with expanded coverage and immunogenicity are urgently needed for optimal prevention of pneumococcal infections. Most promise is offered by the addition of protein-based vaccines to the current PCV, which may provide protection regardless of serotype (3). Several protein antigens, such as Ply, CbpA, PspA, PsaA, PiaA, PhtB, PhtE (BVH-3), and NanA, have been identified as vaccine candidates (1, 6, 8, 12, 14, 18, 22, 23, 26, 27, 29, 35). There is evidence that immunization with certain combinations of virulence proteins provides additive or even synergistic protection (5, 25). The combination of Ply, PspA, and CbpA provided protection in mouse models especially successfully (7, 11, 26). These murine studies demonstrated a protective effect of immunoglobulins directed against these primarily but not exclusively surface-located pneumococcal proteins. However, prospective studies on the protectiveness of antibodies against pneumococcal proteins in humans are lacking. Since infection with S. pneumoniae is supposed to start with colonization, it seems rational to aim for prevention of colonization and thus search for antipneumococcal antibodies providing protection against colonization (31).
Our primary objective was to assess, in children from the pre-pneumococcal-vaccine era, the level of protection provided by antibodies against 17 pneumococcal proteins for infant pneumococcal colonization and RTIs. Additionally, we assessed the extent to which colonization induces a humoral immune response.
MATERIALS AND METHODS
Study design and population.
This study was part of the Generation R Study. The Generation R Study is a population-based prospective cohort study following pregnant women and their children. Further details on this cohort study have been described previously (15, 16). The present study was performed in a subgroup of 1,079 Dutch women and their single-born children. Detailed assessments were conducted of this subgroup. All of these children were born between February 2003 and August 2005. This period was before the introduction of the pneumococcal vaccination in the Netherlands in 2006. The Medical Ethics Committee of the Erasmus Medical Center, Rotterdam, Netherlands, approved the study, and written informed consent was obtained.
Antipneumococcal antibodies.
A cord blood sample was obtained after delivery, and infant blood samples were obtained during visits at the research center at the ages of 6, 14, and 24 months. Of the 1,079 infants in the postnatal cohort of analyses, 57 were selected for this particular study. They were selected on the basis of the availability of biological samples. Seventeen pneumococcal proteins were selected based on importancek, as indicated by current scientific literature, their potential role in vaccine development, and availability. Antigens included putative protease maturation protein A (PpmA), pneumococcal surface adhesin A (PsaA), pneumococcal surface protein A (PspA), and choline binding protein A (PspC/CbpA), as well as neuraminidase (NanA), pneumolysin (PLY), a double mutant of pneumolysin (PdbD), the pneumococcal histidine triad (Pht) family (BVH-3), streptococcal lipoprotein rotamase A (SlrA), alpha-enolase (Eno), IgA1 protease, hyaluronidase (Hyl), and the Streptococcus pneumoniae proteins SP0189 (hypothetical protein), SP0376 (response regulator, intracellular location), SP1003 (PhtD/BVH11-2, histidine triad protein), SP1633 (response regulator, intracellular location), and SP1651 (thiol peroxidase, intracellular location). Isolation and purification methods were as described previously (33). Table 1 provides the functions of the studied proteins (17). The levels of IgG, IgA, and IgM against these proteins were measured using a recently described 17-plex assay based on pneumococcal proteins (28) with the (bead-based) flow cytometry technique (xMAP; Luminex Corporation, Austin, TX). Here we used this novel multiplex assay. The median fluorescence intensity (MFI) values, reflecting semiquantitative antibody levels, were averaged. Tests were performed in independent duplicates, and control beads (where no protein was coupled) were included to determine nonspecific binding. In cases of nonspecific binding, the nonspecific MFI values were subtracted from the antigen-specific results.
TABLE 1.
Functions of the 17 pneumococcal proteins
| Pneumococcal virulence protein | Main role |
|---|---|
| BVH-3 (PhtE) | Pneumococcal histidine triad; possibly a role in complement inhibition |
| PspC (CbpA) | Binds to human secretory component on a polymeric Ig receptor during the first stage of translocation across the epithelium |
| PdbD | Double mutant of PLY |
| Enolase (Eno) | Binds to plasminogen, which is subsequently activated to the serine protease plasmin by tissue or urokinase plasminogen activator |
| Hyaluronidase (Hyl) | Breaks down hyaluronan-containing extracellular matrix components |
| IgA-1protease | Cleaves human IgA1 |
| NanA | Removes sialic acids and cleaves terminal sugars from various glycoconjugates, which might reveal receptors for adherence |
| PLY | Pneumolysin; cytolytic toxin that also activates complement; an important determinant of virulence in in vivo models of disease; wide range of effects on host immune components at sublytic concentrations |
| PpmA | Induces opsonophagocytosis in vitro |
| PsaA | Component of the ABC transport system, which is involved in resistance to oxidative stress and transport of Mn2+ |
| PspA | Prevents binding of C3 onto pneumococcal surface; also binds lactoferrin |
| SlrA | Cyclophilin-type PPiase can catalyze the cis-trans isomerization of proline-containing tetrapeptides; modulates the biological function of important virulence proteins |
| SP0189 | Hypothetical protein |
| SP0376 | Response regulator (intracellular location) |
| SP1003 (BVH-11-2/PhtD) | PhtD (histidine triad protein) |
| SP1633 | Response regulator (intracellular location) |
| SP1651 | Thiol peroxidase (intracellular location) |
Streptococcus pneumoniae colonization.
During the visits at ages 1.5, 6, 14, and 24 months, nasopharyngeal swabs for the isolation of S. pneumoniae were obtained. Methods of sampling were as described previously (20).
RTIs.
Parentally retrieved questionnaires were obtained at 12, 24, 36, and 48 months. Questions regarding doctor visits (never, once or twice, three or four times, more than four times) because of fever and respiratory tract complaints were used to assess the burden of RTIs. We defined three subgroups: (i) child has not been to a doctor with fever and cough/runny or blocked nose/earache in the preceding year, (ii) child has been to a doctor with fever and cough/runny or blocked nose/earache once or twice in the preceding year, and (iii) child has been to a doctor with fever and cough/runny or blocked nose/earache three times or more in the preceding year. For the analyses, we compared children who frequently visited the doctor (at least three times) with the children who never visited the doctor for RTIs. Children scoring three or four times or four times or more on number of doctor visits were classified as visiting the doctor at least three times. Additionally, children scoring one or two times on doctor visits for at least two different symptoms (e.g., once or twice for fever with earache and once or twice for fever with a cough) were scored as visiting the doctor at least three times as well. The latter category may comprise children with only two doctor visits since we cannot distinguish between two and three or four doctor visits in this group.
Statistical analysis.
Wilcoxon signed rank tests were used to compare antipneumococcal antibody levels in the groups of children at the four different ages. We compared IgG, IgM, and IgA levels between 0 and 6 months, 6 and 14 months, 14 and 24 months, and overall between 0 and 24 months.
Mann-Whitney U tests were used to compare differences in maternal antibody levels for colonized and noncolonized infants in the first year of life and to compare differences in maternal antibody levels for children with and without frequent RTIs in the first year of life.
Moreover, to assess whether levels of antibodies protect against later colonization, we used the Mann-Whitney U test to compare differences in antibody levels at the ages of 6, 14, and 24 months for children who were later colonized or were noncolonized. Additionally, we used this test to compare differences in antibody levels at the ages of 6, 14, and 24 months for children with and without frequent RTIs in the 3rd year of life. At age 14 months and older, the results will not be blurred by maternal antibodies.
Finally, Mann-Whitney U tests were used to compare differences in antibody levels between previously colonized and noncolonized children at the different measurement moments to assess whether these specific antibodies are induced upon colonization. The Wilcoxon signed rank tests and Mann-Whitney U tests were used for the same type of analyses described previously (34).
The results are presented as MFI values. P values of <0.05 were considered statistically significant. The statistical analyses were performed using the Statistical Package of Social Sciences version 17.0 for Windows (SPSS Inc., Chicago, IL).
RESULTS
Of the 57 children included for this study, 51 provided three serum samples and four serum samples were obtained from 6 children, for a total of 177 serum samples. Of these 177 samples, 54 (31%) were cord blood samples, 32 samples (18%) were obtained at 6 months, 46 (26%) at 14 months, and 45 (25%) at 24 months. Nasopharyngeal swabs were available for 40 children (70%) at 1.5 months, for 49 (86%) at 6 months, for 50 (88%) at 14 months, and for 48 (89%) at 24 months. At 1.5 months, 17.5% of the children (n = 7) were colonized with S. pneumoniae, which increased at 6, 14, and 24 months to 28.6% (n = 14), 36.0% (n = 18), and 39.6% (n = 19), respectively. In the first year of life, 7 infants (12.3%) visited a doctor at least three times for putative RTIs; this number increased to 13 children (22.8%) in the second year of life and decreased thereafter to 5 children (9.4%) in the third year of life. General population characteristics are presented in Table 2.
TABLE 2.
Population characteristicsa
| Population characteristic | Valueb |
|---|---|
| Gestational age (wk) | 40.2 (1.39) |
| Birth wt (g) | 3,677 (489) |
| Gender female | 28 (49.1%) |
| Positive for colonization at: | |
| 1.5 mo | 7 (17.5%) |
| 6 mo | 14 (28.6%) |
| 14 mo | 18 (36.0%) |
| 24 mo | 19 (39.6%) |
| 36 mo | 8 (19.5%) |
| Frequent RTIs (>3 times) | |
| 1st yr | 7 (12.3%) |
| 2nd yr | 13 (22.8%) |
| 3rd yr | 5 (9.4%) |
Data are given for the 57 children included in the study. Data are missing for colonization status at 1.5 months (n = 17), at 6 months (n = 8), at 14 months (n = 7), at 24 months (n = 9), at 36 months (n = 16) and for RTIs in 2nd (n = 1) and 3rd year of life (n = 4).
Values are given as means (standard deviation) or absolute numbers (%).
Dynamics of antipneumococcal antibodies.
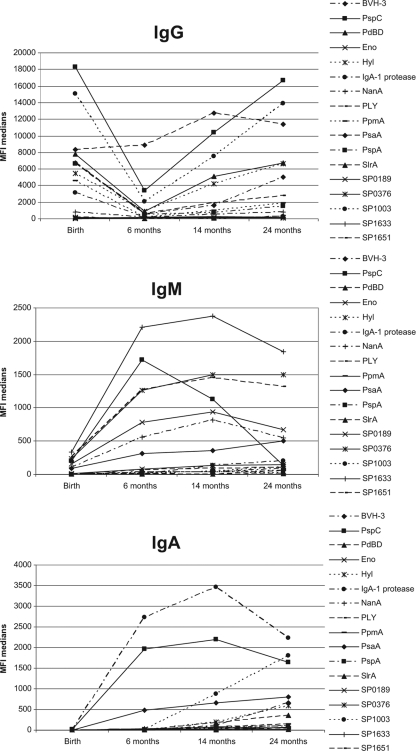
The levels of IgG, IgM, and IgA directed against pneumococcal proteins showed a dynamic process over the first 2 years of life (Fig. 1). There was extensive variability in serum responsiveness over time for each pneumococcal protein. Overall, IgG levels against pneumococcal proteins tended to be high in cord blood, but these levels decreased significantly in the first 6 months of life. This holds true for all antipneumococcal antibodies except for anti-PsaA, anti-SP0189, anti-SP1633, and anti-SP1651 antibodies. The latter anti-S. pneumoniae antibodies were low at birth and showed neither a significant increase nor a notable decrease. Apparently, these proteins are poorly immunogenic. A significant increase was observed after the first 6 months for all proteins except for Eno, Hyl, PspA, and the S. pneumoniae proteins. Low values were obtained for IgA and IgM in the cord blood samples, increasing significantly in the first 2 years of life (P values, <0.001 for all proteins except for IgM against PspC).
FIG. 1.
Dynamics of IgG, IgM, and IgA levels in the first 2 years of life. Median MFI values, averaged for all children (n = 57), are presented by age (1.5, 6, 14, and 24 months). High levels of placentally transferred IgG are observed at 1.5 months and decrease in the first 6 months of life. After 6 months, an increase in the IgG levels is observed. Low levels of IgM and IgA in serum were observed after birth, but these clearly increased during the first year of life.
Maternal antipneumococcal antibodies.
For 54 infants, cord blood samples for analyses of maternal antipneumococcal antibodies were available. Antipneumococcal IgA and IgM levels in cord blood were low because maternal IgA and IgM are not transported across the placenta. Hence, we studied maternal IgG levels only in relation to infant colonization and infection. Maternal IgG levels in cord blood were on average higher in children with higher colonization prevalence in the first year of life. Elevated levels of maternal anti-BVH-3, anti-NanA, and anti-SP1651 IgG were significantly associated with enhanced child colonization rates at 1.5 months (BVH-3, P = 0.003) and 14 months (BVH-3, P = 0.049, and NanA, P = 0.047). IgG levels against BVH-3 were also significantly increased in children frequently colonized with S. pneumoniae in the first year of life (P = 0.003). This indicates that these antibodies are not able to protect the child against colonization. In contrast, these antibodies seem to facilitate colonization. There were no maternal IgG antibodies found to provide protection against colonization. Moreover, we did not observe a protective effect of maternal IgG antibody levels and RTIs in the first year of life.
Antipneumococcal antibodies and colonization.
To study whether antibodies in the child protect against future colonization with S. pneumoniae, we focused on antibody levels at 14 and 24 months since the samples at 6 months may still contain maternal antibodies. At the age of 24 months, IgG levels against PspC were significantly increased in children who were noncolonized at the age of 36 months compared to the IgG levels in children colonized at this same age. No other protective associations between protein-specific antipneumococcal antibody levels and colonization were observed.
However, pneumococcal colonization does induce an antibody response close to the time nearest to the colonization moment, as can be seen in Table 3. This may be due to colonization itself or to clinical or subclinical infections.
TABLE 3.
Levels of antipneumococcal antibodies following pneumococcal colonizationa
| Colonization moment | Antibody detected atb: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 mo |
14 mo |
24 mo |
|||||||
| IgG | IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | |
| 1.5 mo | BVH-3, NanA, PpmA, PsaA, SlrA | PspC, Eno, NanA, SP0189, SP0376 | PsaA | SP0189 | NA | NA | NA | ||
| 6 mo | BVH-3, Hyl, PpsA, SP1003 | PsaA | Hyl, PsaA, PspA, SP1003 | PspC, Eno | PspCc, SlrA | BVH-3, NanA, PsaA, SP1003 | NA | NA | NA |
| 14 mo | NA | NA | NA | BVH-3, PspC, PdbD, NanA, PLYc, PspA, SP1003 | PspCc | PspC, PdbD, NanA, PLY, PspA, SP1003 | NanA | PLYc | |
Antipneumococcal antibodies in this table were significantly (P < 0.05) increased until 1 year after colonization.
The antibody levels at 6 months might be obscured by the presence of maternal antibodies. NA, not applicable, either because the determinant occurs after the outcome (colonization at 14 months and antibody levels at 6 months) or because of a long time span between the determinant and the outcome (colonization at 1.5 and 6 months and antibody levels at 24 months).
This antibody was at a significantly decreased level following colonization (P value < 0.05).
Colonization at 1.5 months induces both an IgG and an IgM response against several pneumococcal proteins at 6 months and an IgA response against PsaA. IgG levels at 6 months are elevated in the colonized children compared to the children who were noncolonized at 1.5 months (median MFI values for colonized versus noncolonized children, respectively: for BVH-3, 942 versus 392; NanA, 287 versus 112; PpmA, 1,076 versus 180; PsaA, 13,000 versus 6,406; SlrA, 176 versus 16). At 14 months, only the IgG level against SP0189 was significantly elevated in children earlier colonized with the pneumococcus at 1.5 months (Table 3). Colonization at 6 months is correlated with elevated levels of IgG and IgA against several pneumococcal proteins at the time of colonization and later at 14 months but is barely correlated to IgM levels. Children who were colonized at 14 months had higher levels of IgG and IgA to several pneumococcal proteins at 14 months than noncolonized children. Few differences between colonized and noncolonized children at 14 months were noted in the antibody levels at 24 months (Table 3).
Children with frequent colonization in the first 14 months (at least twice) barely showed differences in antibody levels at 24 months compared to the children with no colonization in the first 14 months. Only the level of IgG against PpmA antigens at 24 months was elevated in children with frequent colonization in the first 14 months (data not shown). This may be due to a long time span between colonization and antibody level at 24 months.
Antipneumococcal antibodies and RTIs.
Besides colonization, we studied the correlation between systemic antibody levels and the number of doctor visits for RTIs. Table 4 shows all correlations between levels of specific antipneumococcal antibodies and RTIs in the third year of life with P values below 0.08.
TABLE 4.
Correlation between protein-specific IgG, IgM, and IgA antibody levels and RTIs in children
| Time of measurement and antibody | MFI level in children with indicated no. of RTIs in 3rd year of lifea |
||
|---|---|---|---|
| Never (n = 29) | ≥3 times (n = 5) | P value | |
| 6 mo | |||
| IgG | |||
| BVH-3 | 779 (42-1,931) | 133 (99-168) | 0.050b |
| SP1003 | 2,138 (344-15,291) | 826 (448-1,205) | 0.050b |
| NanA | 173 (38-654) | 40 (37-43) | 0.038b |
| IgA | |||
| Eno | 10 (0-217) | 1 (0-2) | 0.063 |
| IgA1 protease | 2,859 (846-6,454) | 1,190 (1,061-1,319) | 0.064 |
| NanA | 21 (0-146) | 0 (0-0) | 0.022b |
| SP0376 | 18 (1-329) | 3 (2-3) | 0.049b |
| SP1003 | 11 (0-1,102) | 0 (0-0) | 0.061 |
| 14 mo | |||
| IgG | |||
| NanA | 701 (46-3,019) | 252 (9-865) | 0.045b |
| PpmA | 985 (0-12,962) | 0 (0-296) | 0.015b |
| PsaA | 13,531 (833-16,944) | 4,091 (769-11,533) | 0.021b |
| SlrA | 58 (0-2,550) | 9 (3-20) | 0.032b |
| SP0189 | 127 (31-1,512) | 58 (22-96) | 0.030b |
| SP1003 | 9,823 (53-18,717) | 522 (27-7,307) | 0.021b |
| IgM | |||
| IgA1 protease | 202 (0-2,690) | 0 (0-97) | 0.026b |
| BVH-3 | 67 (0-1,187) | 0 (0-9) | 0.041b |
| IgA | |||
| PpmA | 14 (0-1,454) | 0 (0-62) | 0.074 |
| SP1003 | 870 (1-7,807) | 298 (0-571) | 0.021b |
| 24 mo | |||
| IgG | |||
| PpmA | 2,047 (0-8,113) | 518 (0-1,101) | 0.065 |
| SlrA | 335 (15-3,975) | 53 (34-74) | 0.049b |
| SP1003 | 14,161 (2,018-18,743) | 8,007 (81-11,630) | 0.075 |
| IgM | |||
| PspA | 40 (0-1,817) | 154 (98-234) | 0.066c |
| IgA | |||
| SlrA | 20 (0-979) | 0 (72-712) | 0.059 |
Values are median MFI levels (5 to 95% range), reflecting antigen-specific IgG, IgM, or IgA levels. All correlations with a P value of <0.08 are shown.
P value < 0.05.
Increased levels occurred in children with frequent RTIs.
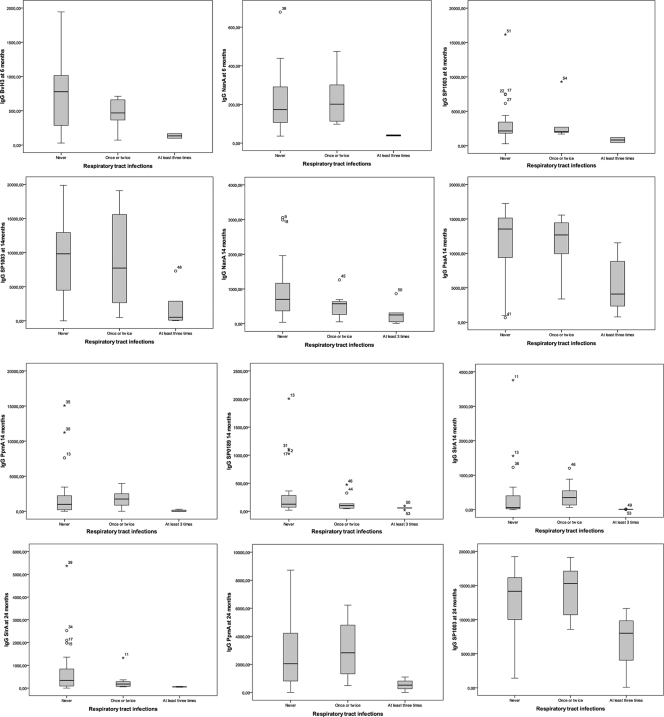
Increased levels of IgG against BVH-3, NanA, and SP1003 at 6 months, IgG directed against NanA, PpmA, PsaA, SlrA, SP0189, and SP1002 at 14 months, and IgG against SlrA at 24 months were observed in children with a lack of doctor visits for RTIs in the 3rd year of life compared to children with at least three visits (Table 4 and Fig. 2). The same significant effect was observed for increased levels of IgA against NanA and SP0376 at 6 months and against SP1003 at 14 months. IgM levels at 6 months were not associated with RTIs in the 3rd year of life. At 14 months, however, IgM levels against IgA1 protease and BVH-3 were significantly increased in children with a lack of doctor visits for RTIs in the 3rd year (Table 4).
FIG. 2.
Association between specific antipneumococcal IgG levels and RTIs in the third year of life. IgG levels at 6, 14, and 24 months are compared with the numbers of doctor visits for RTIs in the 3rd year of life. Higher levels of IgG against certain pneumococcal proteins were correlated with a lack of doctor visits for RTIs (first box in every box plot). The median level of IgG against certain pneumococcal proteins was lower in children with at least 3 doctor visits for RTIs (third box in every box plot). This was statistically significant for all pneumococcal proteins presented in these box plots, except for PpmA and SP1003 at 24 months (P = 0.065 and 0.075, respectively). The analyses were conducted for the group of children with at least three doctor visits versus children with a lack of doctor visits. Results for children with one or two doctor visits were included in this figure but not analyzed. P values are presented in Table 4. Values are median MFI levels, with an interquartile (25 to 27%) box, a 5 to 95% range, outliers (○), and extreme outliers (*). Numbers represent child identification numbers.
DISCUSSION
Our study demonstrates that several antipneumococcal protein antibodies are induced upon colonization, possibly due to clinical or subclinical infection during colonization, and that some of these specific antibody levels are also associated with reduced number of doctor visits for RTIs. Because of the change in pneumococcal serotypes causing colonization and infection following the implementation of the polysaccharide-based vaccine, novel protein-based vaccines are needed for prevention of pneumococcal infections. However, data on antibodies against pneumococcal virulence proteins in relation to human colonization and infection are lacking. Our observations are relevant in the context of future pneumococcal protein vaccine development.
We describe that IgG antibodies against BVH-3, NanA, PpmA, PsaA, SlrA, SP0189, and SP1003 are increased in children who suffered fewer respiratory infections in the third year of life, suggesting that these antibodies are either protective or markers of other protective agents. This is in line with data presented by Bogaert et al., who also found anti-PpmA IgG antibodies to significantly protect against RTIs (4).
We did not find evidence for protection against pneumococcal colonization in young children by any of our antipneumococcal antibodies (except for IgG levels against PspC), which is in line with experimental studies conducted in mice (36).
However, colonization does induce a humoral immune response against several pneumococcal proteins, some of which are also associated with a lack of doctor visits for RTIs. This suggests a potential protective role of colonization against RTIs in the long run. Pneumococcal colonization may increase the risk of clinical or subclinical pneumococcal infection, inducing an immune response which protects against RTIs in the long run.
Some studies document that maternal antipolysaccharide antibodies prevent colonization and infection in infants and thus propose active immunization of pregnant women (10, 13). The largest effect of such maternal antibodies would be expected to occur at a young age. We did not find any short-term effects of protection by maternal antipneumococcal virulence protein antibodies against both nasopharyngeal colonization and RTIs in children. Although it is known that maternal IgG can cross epithelial barriers and can reach significant levels at the nasopharyngeal mucosal surface, these specific antibodies are not capable of preventing colonization and infection in youngsters, as was previously described for antipolysaccharide antibodies.
It has been shown that pneumococcal elimination by vaccines may lead to elevated colonization levels for other bacterial species. For example, it was demonstrated that prevention of pneumococcal otitis media was counterbalanced by increasing numbers of cases caused by Staphylococcus aureus (32). It is unlikely that antiprotein vaccines against the pneumococcus will be different in this respect. However, in our study, we present antipneumococcal antibodies associated with a decreased RTI rate but not associated with protection against colonization. If colonization with S. pneumoniae persisted, while RTIs were prevented, other organisms may remain excluded from colonization.
Some limitations of our study should be discussed. Information about RTIs in the children was obtained through parentally reported questionnaires, which may represent an over-report of complaints; parents with high concerns may report infections that are contrary to a doctor's diagnosis. Furthermore, we do not have specific information on the exact timing of RTIs in the children, and as described in Materials and Methods, a misclassification may have occurred due to the grouping of children by number of RTIs. However, if such a misclassification did occur, it would most likely lead to an underestimation of the effect. Another possible limitation is that we studied antibodies in serum. A suggestion for future studies would be to study IgA levels in saliva, which may play an important role in colonization as well. Moreover, we cannot distinguish whether serum antibodies protect directly or indirectly as part of a more extensive immune response in which protection can be generated by other immunological factors. Alternatively, there may be diffusion to mucosal surfaces or the antibodies may be produced locally following colonization. In other words, the antipneumococcal antibodies may protect directly, they may be markers of alternative responses, or they may result from a combination of these possibilities. In addition, it is possible that the findings presented here simply reflect immune system maturation. Those children with more mature immune systems may have high antibody levels and may remain healthier than those with less mature immune systems. Unfortunately, based on the current study, we are unable to choose one scenario over another. Finally, our study was conducted using only 57 children; the results should be confirmed in a larger sample size.
In conclusion, levels of antibodies against diverse pneumococcal virulence proteins are correlated with a reduced frequency of doctor visits for RTIs in children. This was not due to protection of the specific antibodies against asymptomatic colonization; rather, antibodies resulted from colonization. This study adds to the discussion on improvement of the current preventive strategies against pneumococcal disease. Future studies should aim toward developing protein-based vaccines. In particular, the effect of a combination of several pneumococcal proteins and their correlate of protection in humans should be studied.
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center, Rotterdam, Netherlands, in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of general practitioners, hospitals, midwives, and pharmacies in Rotterdam. We thank Ad Luijendijk for technical supervision at the Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam. Additionally, we thank Sten Willems for his advice on the statistical analyses.
The first phase (until the last-enrolled children turned 4 years) of the Generation R Study was made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam, and the Netherlands Organization for Health Research and Development (Zon MW). Additionally, an unrestricted grant from the ECT-Rotterdam funded this project.
None of the authors hold any financial or commercial conflict of interests.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1.Adrian, P. V., et al. 2004. Development of antibodies against pneumococcal proteins alpha-enolase, immunoglobulin A1 protease, streptococcal lipoprotein rotamase A, and putative proteinase maturation protein A in relation to pneumococcal carriage and otitis media. Vaccine 22:2737-2742. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert, D., R. De Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert, D., P. W. Hermans, P. V. Adrian, H. C. Rumke, and R. de Groot. 2004. Pneumococcal vaccines: an update on current strategies. Vaccine 22:2209-2220. [DOI] [PubMed] [Google Scholar]
- 4.Bogaert, D., et al. 2006. Development of antibodies against the putative proteinase maturation protein A in relation to pneumococcal carriage and otitis media. FEMS Immunol. Med. Microbiol. 46:166-168. [DOI] [PubMed] [Google Scholar]
- 5.Briles, D. E., et al. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briles, D. E., S. K. Hollingshead, G. S. Nabors, J. C. Paton, and A. Brooks-Walter. 2000. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine 19(Suppl. 1):S87-S95. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., et al. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 8.Darrieux, M., et al. 2007. Fusion proteins containing family 1 and family 2 PspA fragments elicit protection against Streptococcus pneumoniae that correlates with antibody-mediated enhancement of complement deposition. Infect. Immun. 75:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito, S., et al. 2010. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin. Vaccine Immunol. 17:1017-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis, J. P., et al. 2009. Maternal antibodies to pneumolysin but not to pneumococcal surface protein A delay early pneumococcal carriage in high-risk Papua New Guinean infants. Clin. Vaccine Immunol. 16:1633-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gor, D. O., X. Ding, D. E. Briles, M. R. Jacobs, and N. S. Greenspan. 2005. Relationship between surface accessibility for PpmA, PsaA, and PspA and antibody-mediated immunity to systemic infection by Streptococcus pneumoniae. Infect. Immun. 73:1304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamel, J., et al. 2004. Prevention of pneumococcal disease in mice immunized with conserved surface-accessible proteins. Infect. Immun. 72:2659-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmlund, E., B. Quiambao, J. Ollgren, H. Nohynek, and H. Kayhty. 2006. Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine 24:57-65. [DOI] [PubMed] [Google Scholar]
- 14.Holmlund, E., et al. 2007. Serum antibodies to the pneumococcal surface proteins PhtB and PhtE in Finnish infants and adults. Pediatr. Infect. Dis. J. 26:447-449. [DOI] [PubMed] [Google Scholar]
- 15.Jaddoe, V. W., et al. 2007. The Generation R Study Biobank: a resource for epidemiological studies in children and their parents. Eur. J. Epidemiol. 22:917-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaddoe, V. W., et al. 2008. The Generation R Study: design and cohort update until the age of 4 years. Eur. J. Epidemiol. 23:801-811. [DOI] [PubMed] [Google Scholar]
- 17.Kadioglu, A., J. N. Weiser, J. C. Paton, and P. W. Andrew. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288-301. [DOI] [PubMed] [Google Scholar]
- 18.Katsurahara, T., M. Hotomi, K. Yamauchi, D. S. Billal, and N. Yamanaka. 2008. Protection against systemic fatal pneumococcal infection by maternal intranasal immunization with pneumococcal surface protein A (PspA). J. Infect. Chemother. 14:393-398. [DOI] [PubMed] [Google Scholar]
- 19.Kieninger, D. M., et al. 2010. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine 28:4192-4203. [DOI] [PubMed] [Google Scholar]
- 20.Labout, J. A., et al. 2008. Factors associated with pneumococcal carriage in healthy Dutch infants: the generation R study. J. Pediatr. 153:771-776. [DOI] [PubMed] [Google Scholar]
- 21.Melegaro, A., et al. 2010. Dynamic models of pneumococcal carriage and the impact of the heptavalent pneumococcal conjugate vaccine on invasive pneumococcal disease. BMC Infect. Dis. 10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melin, M. M., et al. 2008. Distribution of pneumococcal surface protein A families 1 and 2 among Streptococcus pneumoniae isolates from children in Finland who had acute otitis media or were nasopharyngeal carriers. Clin. Vaccine Immunol. 15:1555-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melin, M. M., et al. 2008. Development of antibodies to PspA families 1 and 2 in children after exposure to Streptococcus pneumoniae. Clin. Vaccine Immunol. 15:1529-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien, K. L., et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893-902. [DOI] [PubMed] [Google Scholar]
- 25.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogunniyi, A. D., M. Grabowicz, D. E. Briles, J. Cook, and J. C. Paton. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajam, G., J. M. Anderton, G. M. Carlone, J. S. Sampson, and E. W. Ades. 2008. Pneumococcal surface adhesin A (PsaA): a review. Crit. Rev. Microbiol. 34:131-142. [DOI] [PubMed] [Google Scholar]
- 28.Shoma, S., et al. 18 May 2010. Development of a multiplexed bead-based immunoassay for the simultaneous detection of antibodies to 17 pneumococcal proteins. Eur. J. Clin. Microbiol. Infect. Dis. doi: 10.1007/s10096-010-1113-x. [DOI] [PMC free article] [PubMed]
- 29.Simell, B., et al. 2006. Serum antibodies to pneumococcal neuraminidase NanA in relation to pneumococcal carriage and acute otitis media. Clin. Vaccine Immunol. 13:1177-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valsecchi, F., et al. 2010. Complex I disorders: causes, mechanisms, and development of treatment strategies at the cellular level. Dev. Disabil. Res. Rev. 16:175-182. [DOI] [PubMed] [Google Scholar]
- 31.Van Effelterre, T., et al. 2010. A dynamic model of pneumococcal infection in the United States: implications for prevention through vaccination. Vaccine 28:3650-3660. [DOI] [PubMed] [Google Scholar]
- 32.Veenhoven, R. H., et al. 2004. Nasopharyngeal pneumococcal carriage after combined pneumococcal conjugate and polysaccharide vaccination in children with a history of recurrent acute otitis media. Clin. Infect. Dis. 39:911-919. [DOI] [PubMed] [Google Scholar]
- 33.Verkaik, N., E. Brouwer, H. Hooijkaas, A. van Belkum, and W. van Wamel. 2008. Comparison of carboxylated and Penta-His microspheres for semi-quantitative measurement of antibody responses to His-tagged proteins. J. Immunol. Methods 335:121-125. [DOI] [PubMed] [Google Scholar]
- 34.Verkaik, N. J., et al. 2010. Induction of antibodies by Staphylococcus aureus nasal colonization in young children. Clin. Microbiol. Infect. 16:1312-1317. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, Q., et al. 2006. Serum and mucosal antibody responses to pneumococcal protein antigens in children: relationships with carriage status. Eur. J. Immunol. 36:46-57. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, Z., T. B. Clarke, and J. N. Weiser. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 119:1899-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]