Abstract
Staphylococcus aureus infections, particularly those from methicillin-resistant strains (i.e., MRSA), are reaching epidemic proportions, with no effective vaccine available. The vast number and transient expression of virulence factors in the infectious course of this pathogen have made the discovery of protective antigens particularly difficult. In addition, the divergent planktonic and biofilm modes of growth with their accompanying proteomic changes also demonstrate significant hindrances to vaccine development. In this study, a multicomponent vaccine was evaluated for its ability to clear a staphylococcal biofilm infection. Antigens (glucosaminidase, an ABC transporter lipoprotein, a conserved hypothetical protein, and a conserved lipoprotein) were chosen since they were found in previous studies to have upregulated and sustained expression in a biofilm, both in vitro and in vivo. Antibodies against these antigens were first used in microscopy studies to localize their expression in in vitro biofilms. Each of the four antigens showed heterogeneous production in various locations within the complex biofilm community in the biofilm. Based upon these studies, the four antigens were delivered simultaneously as a quadrivalent vaccine in order to compensate for this varied production. In addition, antibiotic treatment was also administered to clear the remaining nonattached planktonic cells since the vaccine antigens may have been biofilm specific. The results demonstrated that when vaccination was coupled with vancomycin treatment in a biofilm model of chronic osteomyelitis in rabbits, clinical and radiographic signs of infection significantly reduced by 67 and 82%, respectively, compared to infected animals that were either treated with vancomycin or left untreated. In contrast, vaccination alone resulted in a modest, and nonsignificant, decrease in clinical (34% reduction) and radiographic signs (9% reduction) of infection, compared to nonvaccinated animal groups untreated or treated with vancomycin. Lastly, MRSA biofilm infections were significantly cleared in 87.5% of vaccinated and antibiotic-treated animals, while antibiotics or vaccine alone could not significantly clear infection compared to controls (55.6, 22.2, and 33.3% clearance rates, respectively). This approach to vaccine development may lead to the generation of vaccines against other pathogenic biofilm bacteria.
While once only a hospital-acquired pathogen, methicillin-resistant Staphylococcus aureus (MRSA) infection has spread to the community and is now reaching epidemic proportions. A recent study has found that nearly 19,000 people per year die from MRSA infections in the United States, a death toll higher than that due to AIDS (16). In addition, up to 20% of patients who undergo surgery acquire at least one nosocomial infection (14), which is estimated to add $5 to $10 billion in costs to the U.S. healthcare system. S. aureus is one of the most common etiologic agents of these infections. These numbers of deaths, as well as the associated healthcare costs, do not even take into account the morbidity and mortality caused by methicillin-sensitive S. aureus (MSSA) strains that still cause the majority of staphylococcal infections. Therefore, the generation of a vaccine that is protective against S. aureus would have the potential to significantly reduce the morbidity and mortality associated with these infections. One of the major ways that S. aureus is able to persist is through growth as a biofilm, which is recalcitrant to clearance by antimicrobials, further limiting the efficacy of currently available antibiotics. With fewer appropriate means of treating the illnesses caused by this bacterium, the prevention of disease is essential.
There have been several approaches to designing an effective S. aureus vaccine. Whole live or killed S. aureus vaccines have proved to be largely ineffective in animal models (40). Thus, research has focused on using purified forms of either polysaccharide or protein from the bacterial surface. Much research has centered on the capsular polysaccharide types 5 and 8. One such vaccine, StaphVAX, demonstrated protective efficacy in animal models of infection; IgG produced as a result of vaccination showed high levels of opsonophagocytosis in vitro (10) and in a phase III clinical trial. However, protection waned with time and by 1 year postvaccination was <30% (34). Active or passive immunization with polysaccharide intracellular adhesin (PIA), the principal exopolysaccharide component of S. aureus and S. epidermidis biofilms, has been shown to be protective against S. aureus infection in a kidney infection model (25). However, recent research has illustrated that only one component of PIA is immunogenic, and responses to this antigen are variable (22). Deacetylation of PNAG, as well as conjugation to diphtheria toxin as a carrier protein, does help increase protection levels (23). However, not all clinical isolates of either S. aureus or S. epidermidis produce PIA (11, 27, 28, 31), making the relevance of such a vaccine questionable.
Protein-based vaccines have focused mainly on the microbial-surface-component-recognizing adhesive matrix molecules (MSCRAMM) subset of cell wall-associated proteins. Individual component vaccines consisting of clumping factor A (ClfA), ClfB, iron-regulated surface determinant B (IsdB), and fibronectin-binding protein (FnBBP) have all been tested. Recombinant ClfA was shown to be only partially protective when used in an animal model of septic arthritis (15). ClfA is also being investigated as a DNA vaccine candidate in mice and cattle. However, while injection of plasmid containing clfA increased clearance in a mastitis model, protection was not generated against infection in an intraperitoneal challenge (6). Immunization with rClfB led to lessened colonization of murine nares by S. aureus (33). Vaccination with IsdB led to increased survival rates of 20 to 40% in a murine sepsis model (18). A fusion protein consisting of the binding regions of Cna (collagen binding protein) and FnBP showed some protection in a mouse intraperitoneal model (41).
The vaccines discussed above have several limitations, including incomplete protection and the differential expression of the component proteins among S. aureus isolates (26, 32). Use of a multicomponent vaccine has shown promise in promoting significant protection against S. aureus infection. When IsdA and IsdB, as well as SdrD and SdrE, were combined into a single vaccine, complete protection was afforded in a mouse renal abscess model, with bacterial levels being reduced below levels of detection and a lack of clinical signs of disease (37).
Even with the advancements being made in this field, the vast majority of research focuses on protection from acute, plankton-associated S. aureus infection. Also, the studies discussed above all make use of non-biofilm animal models of infection. Because we (4) and others (2, 29) have shown that gene expression and protein production between the two states of biofilm and planktonic modes of growth differ greatly, the vaccine candidates that prevent infection in acute, plankton-associated models (for example, sepsis, intraperitoneal infection, and renal abscess models) may not be effective against biofilm infections such as osteomyelitis, endocarditis, or prosthetic implant infections.
Previous work in our laboratory identified several cell wall and membrane-associated proteins that are immunogenic during S. aureus biofilm infection and whose genes are upregulated during biofilm growth (4). In the present work, recombinant forms of four of these proteins were combined in a quadrivalent vaccine and tested for their ability to provide protection against challenge with an S. aureus biofilm infection that is normally recalcitrant to antimicrobial clearance. An antibiotic, while not effective against biofilm communities, was also used in conjunction with vaccination for the clearance of any remaining planktonic staphylococci. The quadrivalent vaccine with antibiotic therapy was effective in clearing the infection, while either vaccination or antibiotic treatment alone were not. This study is the first to acknowledge, and overcome, the differences of protein expression within biofilms and, as such, suggests a possible alternative in rational vaccine design for other biofilm-mediated infections.
MATERIALS AND METHODS
Bacterial strains.
MRSA strain MRSA-M2 was isolated from a patient with osteomyelitis at the University of Texas Medical Branch. Escherichia coli TOP10 and BL21 cells were utilized for protein production experiments.
Cloning, expression, and purification of proteins.
Candidate antigens selected in Brady et al. (4) were amplified using the primers listed in Table 1 . The PCR products were cloned into pBAD-Thio/TOPO (SACOL0037) or pASK-IBA14 (SACOL0486, SACOL0688, and glucosaminidase), transformed into TOP10 E. coli, and sequenced. The clones were then expressed using either arabinose induction (SACOL0037) or anhydrotetracycline induction (all others). SACOL0037 was purified via nickel affinity chromatography, while all other antigens were purified by using Strep-Tactin Superflow columns (IBA, Göttingen, Germany). The purity was confirmed by resolving each protein on SDS-15% PAGE, and quantities were determined by using BCA (Pierce, Rockford, IL).
TABLE 1.
Primers and plasmids utilized in this study
| Primer or plasmid | Sequence (5′-3′) or genotype/characteristicsa | Product or source |
|---|---|---|
| Primers | ||
| 5′SA0037 | ATGAATACAATCAAAACTACGAAA | Conserved hypothetical protein (519 bp) |
| 3′SA0037 | CTTCTCATCGTCATCTGATTTCAAAATCCATTTTTGA | |
| 5′Lipase | ACTCTAGGTCTCACTCCCATCTGAAACAACATTATGACCAAAT | Lipase (966 bp) |
| 3′Lipase | ATGGTAGGTCTCATATCATAAAGGATTTAACGGTAATTCATTACT | |
| 5′SA0688 | ATGGTAGGTCTCACTCCGATAAGTCAAATGGCAAACTAAAAGT | ABC transporter lipoprotein (860 bp) |
| 3′SA0688 | ATGGTAGGTCTCATATCATTTCATGCTTCCGTGTACAGTT | |
| 5′Glucosaminidase | ATGGTAGGTCTCACTCCGCTTATACTGTTACTAAACCACAAAC | Glucosaminidase (1,443 bp) |
| 3′Glucosaminidase | ATGGTAGGTCTCATATCATTTATATTGTGGGATGTCGAAGTATT | |
| 5′SA0486 | ACTCTAGGTCTCACTCCAAAGAAGATTCAAAAGAAGAACAAAT | Hypothetical lipoprotein (683 bp) |
| 3′SA0486 | ATGGTAGGTCTCATATCAGCTATCTTCATCAGACGGCCCA | |
| Plasmids | ||
| pBAD-Thio/TOPO | 4,454 bp; pUC ori, Ampr, pBAD promoter, for arabinose-inducible expression of PCR product | Invitrogen Life Technologies |
| pASK-IBA14 | 3,001 bp; pUC ori, Ampr, tetA promoter, for tetracycline-inducible expression of PCR product | IBA, Göttingen, Germany |
BsaI sites are underlined in the primer sequences. Ampr, ampicillin resistance.
Evaluation of antigen expression in S. aureus biofilms in vitro.
Purified proteins were used to develop polyclonal antibodies through a commercial source (Lampire, Inc., Everett, PA). Antibodies were purified from the serum and used to probe 14-day-old S. aureus biofilms grown in vitro as described previously (4, 5).
Vaccination of animals.
To prepare the purified recombinant proteins for vaccination, the appropriate amounts of SA0037, SA0486, SA0688, and glucosaminidase were combined. Because we noticed faint extraneous bands in our SA0037 preparation following purification on the Probond column, we took a further step of resolving rSA0037 using SDS-PAGE and cutting out the proper band. This band was then resuspended in 250 μl of phosphate-buffered saline (PBS), homogenized, and the mixture was used to rehydrate the trichloroacetic acid precipitation. The rehydrated protein was combined with an equal volume of Titermax Gold adjuvant (Titermax USA, Norcross, GA) and mixed via sonication.
Eight-week-old female New Zealand White rabbits (2-3 kg each) were used in the present study. All procedures were performed as per humane criteria set forth by University of Maryland Baltimore Animal Care and Use Committee. Animals were divided at random into groups. Groups received glucosaminidase, the quadrivalent vaccine, or PBS as a control. For the initial testing of glucosaminidase alone or the quadrivalent vaccine, animals were immunized with 10 μg of each antigen intramuscularly at days 0, 10, and 20, with challenge following on day 30. In all of the remaining other vaccine studies, animals were immunized with either 75 μg each of antigen intramuscularly, or the PBS control, at days 0 and 10. Antibody titers to vaccine antigens were measured by enzyme-linked immunosorbent assay (see supplemental materials and methods). Titers against antigens increased 10 days after vaccination and continued to rise until day 35 (see supplemental material and Table S1).
Production of osteomyelitis.
Animals were challenged 10 days following the last vaccination with intratibial inoculation of MRSA-M2 as described previously (21). All procedures were performed as per humane criteria set forth by University of Maryland Baltimore Animal Care and Use Committee. The infection was allowed to progress for 14 days, at which time the animals were evaluated and euthanized for the first study with glucosaminidase alone and the quadrivalent vaccine. In the subsequent study for testing the adjunctive effects of antimicrobial therapy, animals were either left alone or treated for 14 days via a twice daily subcutaneous injection of vancomycin (40 mg/kg) as previously described (20).
Analysis of vaccine efficacy.
Vaccine efficacy was evaluated in three ways. At 14 days following MRSA inoculation into the tibia, rabbits were monitored for clinical signs of infection (i.e., non-weight bearing on the affected leg). Animals were then anesthetized and radiographically examined to determine the radiologic score of osteomyelitis, according to Fig. 1 and described previously (21). Scores were evaluated as shown in Table 2. Rabbits were then sacrificed by an intravenous injection of sodium pentobarbital. Both tibias were removed, dissected free of all soft tissue, and processed for bacterial cultures. Using a 5.0-mm, single-action rongeur, the bones were split into small pieces, and the marrow was removed. The whole bone was then pulverized, combined with the marrow, and suspended in 3 ml of sterile 0.85% saline per g of tissue. Tenfold serial dilutions were performed in triplicate and spotted onto a tryptic soy agar blood plate supplemented with oxacillin (40 μg/ml) to determine the presence or absence of S. aureus in the bone tissue. The CFU per gram of bone were then calculated after overnight incubation of the plates at 37°C. For more details concerning rabbit tibial culture, refer to the supplemental materials and methods.
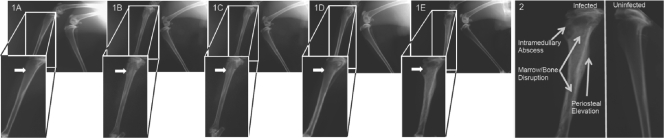
FIG. 1.
Radiographic differences between infected and uninfected tibias. (Panels 1A to 1E) Left tibiae are shown exhibiting radiologic scores of 0 in a representative animal (1A), with increasing scores being shown to a maximum radiologic score of 4 in a representative control animal (1E) where arrows designate areas of S. aureus injection sites. In each panel, the right (noninoculated) tibiae are also shown and serve as internal controls for scoring. (Panel 2) Expanded view of a radiographic image of an infected (left) tibia demonstrating areas of abscess, marrow and bone disruption, and periosteal elevation in an infected tibia compared to an uninfected tibia (right). For complete descriptions of the scoring parameters, please refer to Table 2 and the supplemental materials and methods.
TABLE 2.
Radiographic staging guidelines
| Radiological score | Characteristics of infected bone |
|---|---|
| 0 | Normal, no lytic changes around needle stick |
| 1+ | Lytic changes around the needle stick, <5% disruption of normal bone architecture |
| 2+ | 5-15% disruption of normal bone architecture |
| 3+ | 15-40% disruption of normal bone architecture |
| 4+ | >40% disruption of normal bone architecture |
Statistical analysis.
The statistical significance between experimental groups and controls, as well as between the various experimental groups, was calculated by using a Student t test for radiologic and CFU data and the Fisher exact test for clinical symptoms and infection clearance rates. A P value of ≤0.05 was considered statistically significant, while P values between 0.1 and 0.005 were considered to show a trend.
RESULTS
Biofilm upregulated immunogens are produced heterogeneously within the biofilm.
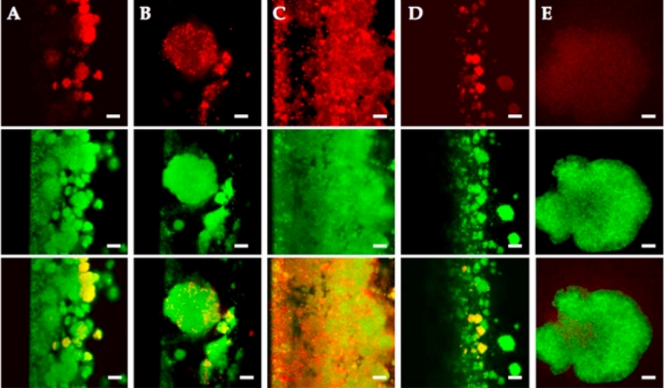
Differential protein production within the biofilm may allow certain portions of the biofilm to escape immune recognition and clearance. Therefore, we chose to study a subset of candidate antigens. We used IgG samples from animals vaccinated against individual antigens, with confocal immunofluorescence microscopy on S. aureus biofilms grown in vitro, to determine the relative areas of production a number of other biofilm upregulated antigens, glucosaminidase, SA0317 (lipase), SA0486 (a hypothetical lipoprotein), SA0037 (a conserved hypothetical protein of unknown function), SA0688 (an ABC transporter lipoprotein), and SA. As seen in previous studies (5), there was heterogeneous production of proteins within the biofilm community (Fig. 2). IgG against each antigen (with the exception of lipase) appear to bind to S. aureus biofilms differently. Although anti-glucosaminidase and anti-SA0688 IgGs bind to individual microcolonies, anti-SA0486 IgG reacts with smaller bacterial flocs within the entire biofilm, and anti-SA0037 IgG binds to individual cells within microcolonies. In addition, lipase, which is secreted by S. aureus in a biofilm mode of growth, serves as a negative control. Because this enzyme is produced and released into the flowing media, IgGs against lipase did not bind to the biofilm. Thus, it was evident that these antigens are not expressed homogeneously throughout the biofilm and that antibodies to a single antigen may not provide adequate immunological recognition of the biofilm, leaving some areas that were not recognized and thus persist. Therefore, we further tested a single component and quadrivalent vaccine in subsequent studies (see below).
FIG. 2.
Biofilm-upregulated immunogens are produced heterogeneously. Immunofluorescence microscopy was performed using IgG antibodies against biofilm upregulated immunogens, followed by goat anti-rabbit F(ab′)2 secondary antibody (red, top panels) and SYTO-9 stain to visualize the entire biofilm (green, center panels; merge, bottom panels). (A) Glucosaminidase; (B) SA0037; (C) SA0486; (D) SA0688; (E) lipase, a secreted protein not found in large quantities within the biofilm (negative control). Magnification bars, 20 μm.
Vaccination with a single biofilm antigen or quadrivalent vaccine.
In previous work we identified 22 cell wall and membrane-associated immunogens that were upregulated during biofilm growth (4). Among these antigens, autolysin (AtlA) was one of the most immunoreactive. Because of its reported role in biofilm formation (3) and its upregulation during early biofilm growth (when an immune response could theoretically eradicate the biofilm), we chose to test a component of autolysin alone and a combined quadrivalent set of biofilm upregulated antigens as potential vaccines. The quadrivalent vaccine included SA0486, SA0037, SA0688, and glucosaminidase. We chose these antigens because we showed in earlier work (4) that they are cell wall associated, biofilm upregulated, and immunogenic in rabbits. Purified recombinant glucosaminidase (one of the two protein components of AtlA) or the quadrivalent vaccine was injected into rabbits (two doses of 10 μg of each antigen, 10 days apart), and then animals were challenged using a tibial osteomyelitis infection. This vaccination did not lead to significant differences in bacteriological signs of infection compared to PBS-vaccinated controls but did yield significantly lower radiological scores (Table 3 and Fig. 3).
TABLE 3.
Radiological and clinical vaccine scores for glucosaminidase and quadrivalent vaccines
| Parameter | Glucosaminidase | Quadrivalent | PBS control |
|---|---|---|---|
| No. of rabbits | 4 | 5 | 7 |
| Mean radiological scorea | 0.37* | 1.10* | 2.71 |
| Rabbits (%) showing clinical signs of infection | 0 | 0 | 57 |
| No. of rabbits cleared | 1 | 0 | 0 |
*, P < 0.05 glucosaminidase versus PBS control and quadrivalent versus PBS control.
FIG. 3.
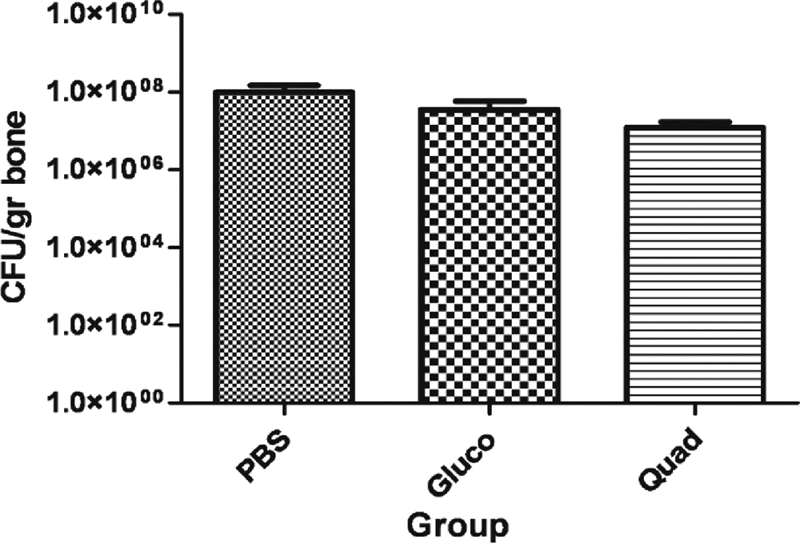
Vaccination with the single antigen glucosaminidase and the quadrivalent vaccine. The mean CFU/gram of bone are shown for PBS controls, glucosaminidase-vaccinated (Gluco) and quadrivalent-vaccinated groups. Animals were vaccinated with three doses of the quadrivalent vaccine (10 μg of each) or PBS.
The failure of the single and quadrivalent vaccine alone to promote effective bacterial clearance may have been due to the inability of the immune system to clear planktonic cells, since the antigen was a biofilm upregulated protein. Although the single antigen (i.e., glucosaminidase) and the quadrivalent vaccine showed similar clinical and radiological reductions in infection, we did not test this single antigen in subsequent studies for a number of reasons. First, there have been a number of generated, as well as naturally spontaneous, mutants arising in this particular gene (12, 13, 38). Therefore, a vaccine composed of this single component may be short-lived in usefulness due to null mutant infecting strains that could escape clearance by the immune response. Also, the well-described heterogeneous antigenic nature of biofilms in our studies and many others supports the idea that a multicomponent vaccine may be needed for protection against biofilm-mediated disease (1, 5, 36). Lastly, a multivalent vaccine strategy is a standard method in many currently approved vaccines, including the pneumococcus and the acellular pertussis component of the DTaP vaccine (1, 17, 19).
Vaccination with biofilm-upregulated antigens, and subsequent antibiotic therapy, leads to clearance of biofilm infection.
The ultimate goal of complete bacterial clearance was not realized with the single antigen and quadrivalent vaccine. Because there was an obvious, albeit statistically insignificant trend to reduced infection upon challenge with the quadrivalent vaccine, we hypothesized that vaccination with these biofilm upregulated antigens may reduce the number of bacterial cells with a biofilm phenotype. As a result, the bacterial populations remaining in the vaccinated group postchallenge may be due to the planktonic subset of S. aureus within the tibia not being effectively cleared by the host immune response. Planktonic S. aureus cells possess a number of immuno-avoidance strategies, including protein A, leukotoxins, an antiphagocytic capsule, and the recently described phenol soluble modulins and nitric oxide-inducible lactate dehydrogenase system (30, 39) that enable persistence. However, they are sensitive to effective antimicrobial agents compared to their biofilm-embedded counterparts. To this end, at 14 days after challenge we treated both vaccinated and nonvaccinated animals with 40 mg of vancomycin/kg twice daily for 10 days and compared the efficacy of the dual treatment to untreated and unvaccinated, vaccinated but untreated, and unvaccinated but treated controls. As in the studies described above, the vaccine alone group showed no significant effect on infection clearance or concentrations of bacteria in the tibia (Fig. 4 B). However, both the vancomycin alone group and the group treated with vancomycin therapy following vaccination with the quadrivalent vaccine were able to significantly reduce bacterial counts in the affected tibia (P < 0.05) (Table 4 and Fig. 4). When clearance rates were observed, vaccination and antibiotic treatment afforded only this group significantly lower rates, as well as scores for clinical and radiological signs of disease (P < 0.05) (Table 4 and Fig. 4). Thus, this combination therapy is able to abrogate all signs of S. aureus osteomyelitis infection and, in the rare case clearance is not achieved, bacterial levels (as well as severity of disease) are markedly decreased. Therefore, the combined use of a prophylactic, biofilm-directed vaccine, plus antibiotic treatment aimed at planktonic growth, leads to the prevention of biofilm-mediated osteomyelitis infection in a rabbit model.
FIG. 4.
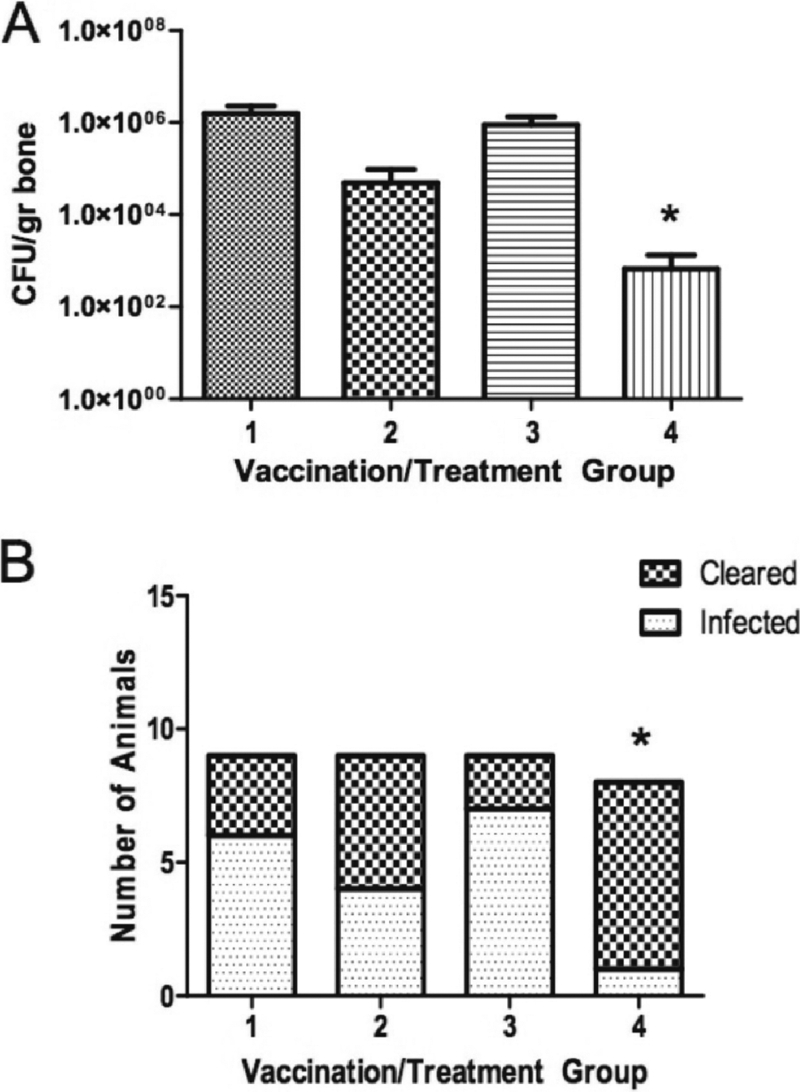
Vaccination with quadrivalent vaccine and adjunctive vancomycin treatment. (A) Animals vaccinated with PBS only (column 1), PBS and subsequent treatment with vancomycin (column 2), the quadrivalent vaccine only (column 3), or the vaccine plus vancomycin (column 4). The mean ± the standard error of the mean for CFU/gram of bone is shown for each group. *, Significant difference from group 1, the PBS control (P < 0.05, Student t test). (B) Animals in each group that were completely cleared of infection. *, Significant difference from group 1, the PBS control (P < 0.05, Fisher exact test).
TABLE 4.
Radiological and clinical scores for quadrivalent vaccine with vancomycin therapy
| Parameter | Group 1 (PBS) | Group 2 (PBS + vancomycin) | Group 3 (vaccine) | Group 4a (vaccine + vancomycin) |
|---|---|---|---|---|
| No. of rabbits | 9 | 9 | 9 | 8 |
| Mean radiological score | 2.3 | 2.1 | 2.0 | 0.4* |
| Rabbits (%) with clinical signs of infection | 100 | 100 | 66 | 38* |
| No. of rabbits cleared | 3 | 5 | 2 | 7* |
*, P < 0.05 of vaccine plus vancomycin group 4 versus PBS control group 1.
DISCUSSION
S. aureus has re-emerged as a major human pathogen, and there are currently no vaccines that afford consistent, long-term protection against S. aureus infections. While infections, particularly those with MRSA, are often nosocomial in origin, community acquired infections associated with this microbial species have reached epidemic levels. One of the ways in which S. aureus is able to persist in the host and remain recalcitrant to clearance by the immune system or antibiotics is through a biofilm mode of growth. Therefore, the need for an effective vaccine and/or treatment modality that could prevent the establishment of biofilm-mediated chronic infections by S. aureus is necessary. This study is the first to demonstrate protection against biofilm-associated infection through the use of a multicomponent vaccine and subsequent antibiotic therapy. When administered to New Zealand White rabbits, the combination of biofilm-directed vaccination and antibiotic treatment was able to significantly lessen the radiological and clinical signs of infection, and afforded complete clearance to 87.5% of animals, reducing bacterial loads overall by over 3 logs.
A number of vaccines have been evaluated for their protective efficacy against staphylococcal infections, in particular against a primary planktonic bacteremia, pneumonia, septic arthritis, and intraperitoneal staphylococcal infection (6, 15, 18, 24, 25, 34, 37). Although this mode of growth is important and can often end in septicemia and death, it can be cleared by antimicrobial therapy and may be the transient intermediary step between inoculation and rapid dissemination to distal sites of biofilm infection. Therefore, a long-term T-cell-mediated memory response and antibody production against staphylococcal antigens for this transient and antibiotic sensitive bacteremia may take up to 10 days for full activation. This may not be rapid enough to clear the infection prior to the development of a secondary biofilm infection that can occur within several days postinoculation and will resist clearance by antimicrobial agents (35).
Because of the complicated multicellular architecture of a biofilm, various sites within these communities can, and do, express different proteins necessary for survival under various respiratory conditions and areas of stress (7-9). When we tested our single antigen and quadrivalent vaccine in our chronic osteomyelitis model, we noted that both the clinical and radiological signs of disease were significantly decreased in the vaccinated group compared to controls; however, a lessened, but nonsignificant, number of bacteria were still found in the bones of vaccinated animals. This suggests that the vaccine is working against biofilm bacteria and decreasing the manifestations of osteomyelitis but is not clearing out all of the S. aureus present in the bone. Previous work by our laboratory demonstrated that antibodies against biofilm-upregulated proteins could be used to study biofilm architecture in vitro (5); here, we expanded on this work to more closely examine the expression states of our potential vaccine candidates within an intact biofilm. We noted that there is heterogeneous expression of each of the four candidate antigens in a mature biofilm. This could lead to vaccine-based selection of those areas of the biofilm that do not express the candidate antigen to persist and spread infection. Other vaccine studies (37) have suggested a multicomponent vaccine may afford more complete protection against S. aureus challenge in a planktonic model of infection.
A mature biofilm is recalcitrant to clearance by both the host immune response and antimicrobial therapies (7-9). Therefore, use of antibiotics against a biofilm infection is generally not effective. The benefit of a biofilm-directed vaccine would be to generate a memory response that can be elicited quickly upon challenge with the etiologic organism, generating a protective response that can work against early biofilm microbes that are still in a clearable state. Because the antigens chosen for this vaccine were those that were significantly upregulated during biofilm growth, we postulated that the vaccine was not effective against planktonic bacteria and, thus, the response elicited through vaccination and subsequent challenge may not have been sufficient to eradicate all populations. We therefore added an antibiotic treatment arm to our study, comparing the effectiveness of prophylactic vaccination combined with postchallenge vancomycin therapy to vaccination alone, and also of vancomycin treatment without prior vaccination. While vaccination alone was not able to significantly decrease the levels of S. aureus in the infected bone, both vancomycin treatment alone and the combination of vaccination and antibiotic therapy significantly reduced S. aureus numbers. Although reductions in bacterial populations are important, significantly increased infection clearance rate are essential for a potential treatment or prevention strategy since any remaining bacteria can regrow to produce a fulminant infection. When clearance rates were compared, only the vaccination combined with antibiotic treatment was able to significantly eliminate the S. aureus infection from the host.
This vaccine holds significant promise for those with identified risk factors for S. aureus biofilm infection. Although these patients may still acquire the S. aureus infection, an anti-biofilm vaccine could allow these previously untreatable infections to be prevented by vaccination in combination with antimicrobial therapy, whereas the only reliable therapy at present is surgical intervention. These data give new perspectives on means to limit and eradiate S. aureus biofilm infections that could help to prevent the onset of chronic disease, saving patients from significant morbidity and mortality. As well, the methodology used here, where the entire microbial community is considered for its in vivo expression and differential protein production in the various niches of the biofilm, has implications for how future biofilm vaccines should be designed. This suggests a potential alternative in how antigens are rationally chosen for these infections.
Supplementary Material
Acknowledgments
This study was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant R01 AI69568-01A2).
Editor: A. Camilli
Footnotes
Published ahead of print on 10 January 2011.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Anetzberger, C., T. Pirch, and K. Jung. 2009. Heterogeneity in quorum sensing-regulated bioluminescence of Vibrio harveyi. Mol. Microbiol. 73:267-277. [DOI] [PubMed] [Google Scholar]
- 2.Beenken, K. E., et al. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas, R., et al. 2006. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 259:260-268. [DOI] [PubMed] [Google Scholar]
- 4.Brady, R. A., J. G. Leid, A. K. Camper, J. W. Costerton, and M. E. Shirtliff. 2006. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect. Immun. 74:3415-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady, R. A., J. G. Leid, J. Kofonow, J. W. Costerton, and M. E. Shirtliff. 2007. Immunoglobulins to surface-associated biofilm immunogens provide a novel means of visualization of methicillin-resistant Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 73:6612-6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouillette, E., et al. 2002. DNA immunization against the clumping factor A (ClfA) of Staphylococcus aureus. Vaccine 20:2348-2357. [DOI] [PubMed] [Google Scholar]
- 7.Brown, M. R., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-780. [DOI] [PubMed] [Google Scholar]
- 8.Brown, M. R., and P. Gilbert. 1993. Sensitivity of biofilms to antimicrobial agents. J. Appl. Bacteriol. 74(Suppl.):87S-97S. [DOI] [PubMed] [Google Scholar]
- 9.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fattom, A. I., G. Horwith, S. Fuller, M. Propst, and R. Naso. 2004. Development of StaphVAX, a polysaccharide conjugate vaccine against Staphylococcus aureus infection: from the lab bench to phase III clinical trials. Vaccine 22:880-887. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick, F., H. Humphreys, and J. P. O'Gara. 2005. Evidence for icaADBC-independent biofilm development mechanism in methicillin-resistant Staphylococcus aureus clinical isolates. J. Clin. Microbiol. 43:1973-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana, C., L. Cellini, and B. Dainelli. 1993. Twelve aberrant strains of Staphylococcus aureus subsp. aureus from clinical specimens. J. Clin. Microbiol. 31:2105-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster, S. J. 1995. Molecular characterization and functional analysis of the major autolysin of Staphylococcus aureus 8325/4. J. Bacteriol. 177:5723-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horan, T. C., et al. 1993. Nosocomial infections in surgical patients in the United States, January 1986-June 1992. National Nosocomial Infections Surveillance (NNIS) Syst. Infect. Control Hosp. Epidemiol. 14:73-80. [DOI] [PubMed] [Google Scholar]
- 15.Josefsson, E., O. Hartford, L. O'Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572-1580. [DOI] [PubMed] [Google Scholar]
- 16.Klevens, R. M., et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 17.Kretsinger, K., et al. 2006. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm. Rep. 55:1-37. [PubMed] [Google Scholar]
- 18.Kuklin, N. A., et al. 2006. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 74:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyaw, M. H., et al. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354:1455-1463. [DOI] [PubMed] [Google Scholar]
- 20.Mader, J. T., and K. Adams. 1989. Comparative evaluation of daptomycin (LY146032) and vancomycin in the treatment of experimental methicillin-resistant Staphylococcus aureus osteomyelitis in rabbits. Antimicrob. Agents Chemother. 33:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mader, J. T., and M. E. Shirtliff. 1999. The rabbit model of bacterial osteomyelitis of the tibia. Academic Press, Ltd., London, England.
- 22.Maira-Litran, T., et al. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maira-Litran, T., A. Kropec, D. A. Goldmann, and G. B. Pier. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-β-(1-6)-glucosamine. Infect. Immun. 73:6752-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenney, D., et al. 2000. Vaccine potential of poly-1-6 β-d-N-succinylglucosamine, an immunoprotective surface polysaccharide of Staphylococcus aureus and Staphylococcus epidermidis. J. Biotechnol. 83:37-44. [DOI] [PubMed] [Google Scholar]
- 25.McKenney, D., et al. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 26.Ni, E. D., et al. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 27.Nilsdotter-Augustinsson, A., A. Koskela, L. Ohman, and B. Soderquist. 2007. Characterization of coagulase-negative staphylococci isolated from patients with infected hip prostheses: use of phenotypic and genotypic analyses, including tests for the presence of the ica operon. Eur. J. Clin. Microbiol. Infect. Dis. 26:255-265. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill, E., et al. 2007. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J. Clin. Microbiol. 45:1379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resch, A., et al. 2006. Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics 6:1867-1877. [DOI] [PubMed] [Google Scholar]
- 30.Richardson, A. R., S. J. Libby, and F. C. Fang. 2008. A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science 319:1672-1676. [DOI] [PubMed] [Google Scholar]
- 31.Rohde, H., et al. 2007. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 28:1711-1720. [DOI] [PubMed] [Google Scholar]
- 32.Ryding, U., J. I. Flock, M. Flock, B. Soderquist, and B. Christensson. 1997. Expression of collagen-binding protein and types 5 and 8 capsular polysaccharide in clinical isolates of Staphylococcus aureus. J. Infect. Dis. 176:1096-1099. [DOI] [PubMed] [Google Scholar]
- 33.Schaffer, A. C., et al. 2006. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect. Immun. 74:2145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinefield, H., et al. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491-496. [DOI] [PubMed] [Google Scholar]
- 35.Shirtliff, M. E., and J. T. Mader. 2000. Osteomyelitis: clinical features and molecular aspects of persistence. ASM Press, Washington, DC.
- 36.Stewart, P. S., and M. J. Franklin. 2008. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6:199-210. [DOI] [PubMed] [Google Scholar]
- 37.Stranger-Jones, Y. K., T. Bae, and O. Schneewind. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 103:16942-16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi, J., et al. 2002. Molecular characterization of an atl-null mutant of Staphylococcus aureus. Microbiol. Immunol. 46:601-612. [DOI] [PubMed] [Google Scholar]
- 39.Wang, R., et al. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13:1510-1514. [DOI] [PubMed] [Google Scholar]
- 40.Watson, D. L. 1987. Serological response of sheep to live and killed Staphylococcus aureus vaccines. Vaccine 5:275-278. [DOI] [PubMed] [Google Scholar]
- 41.Zhou, H., Z. Y. Xiong, H. P. Li, Y. L. Zheng, and Y. Q. Jiang. 2006. An immunogenicity study of a newly fusion protein Cna-FnBP vaccinated against Staphylococcus aureus infections in a mice model. Vaccine 24:4830-4837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.