Investigation of microbial mineral respiration remains an experimental challenge. In this issue of Journal of Bacteriology, Rollefson et al. (11) present a foundational study on the functionality of the biofilm matrix in Geobacter sulfurreducens, a model dissimilatory metal respiring bacterium (DMRB). In this study, the investigators identify an extracellular polysaccharide scaffold or network that entraps redox-active proteins, thus positioning these proteins for optimal electron transfer from the membrane-bound respiratory supercomplexes to a mineral phase electron acceptor. The distinguishing feature of this study is the perspective, in that the team examined specifically exopolysaccharide formation and how it enables entrapment and tethering of redox proteins in the vicinity of the cell. Previous studies on Geobacter (10) and Shewanella (4) have focused primarily on the presence and functionality of conductive pili and nanowires, proteinaceous structures that also enable and enhance extracellular electron transfer. Rollefson et al. remind investigators in this field that many microbial systems have redundancy in essential functions, and in the case of DMRB, it is clearly critical that more than one mechanism exists to ensure vectoral electron transport to mineral phase electron acceptors.
The major findings of Rollefson et al. (11) were (i) identification of a biofilm locus in G. sulfurreducens that harbors exopolysaccharide synthesis and export genes; (ii) detection of c-type cytochromes in exopolysaccharide materials; (iii) genetic mutation of a gene (xapD [GSU1501], an ATP-dependent ABC transporter) in the locus which results in reduced functionality of the biofilm matrix, i.e., reduced agglutination and attachment, and less matrix-bound cytochrome; and (iv) microscopic confirmation of exopolysaccharide materials in the biofilm matrix. The research employed a combination of traditional microbiological techniques, genetic manipulation and mutation, and fluorescence and electron microscopy to obtain an integrated view of biofilm matrix composition and functionality in G. sulfurreducens. The elegance of the study is in its relative simplicity. For example, while agglutination has been examined in bacteria for decades, basic assessment of this phenomenon has not been made in many DMRB models, thus showing that classical microbiology still can reveal a great deal about DMRB biofilms. For example, Fig. 2 and 3 of the Rollefson et al. paper clearly reveal both macroscopic and microscopic differences in culture and biofilm morphology. Differential extraction procedures purify pili and exopolysaccharide and demonstrate binding of redox proteins and positioning of an extracellular biomolecular conductive network, and although one can argue the efficacy and resolution of these methods, the results seem to suggest that protein-exopolysaccharide complexes can be successfully isolated from biofilm cultures, and these approaches can be reliably used on other model DMRB systems. Unique experiments using polysaccharide binding stains coupled with scanning electron microscopy (SEM) reveal that exopolysaccharide is a key component for attachment and colonization of mineral and electrode surfaces. Indeed, the exopolysaccharide appears as a network of strands wiring cells together and to the substrate. Blocking techniques with safranin O work to strengthen the interpretation of the SEM images by better visualization of the extracellular matrix material.
IMPACTS OF THE RESEARCH
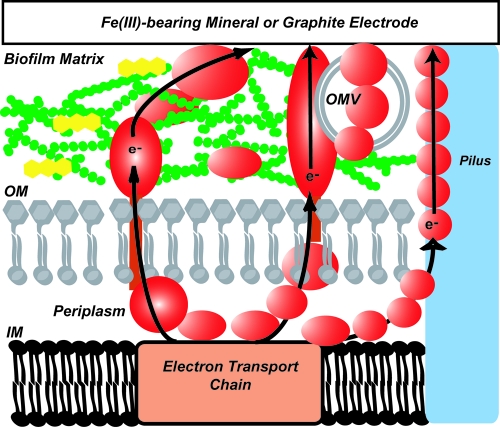
Over 20 genera across multiple phyla of bacteria and archaea transform minerals via oxidation and reduction, and Geobacter has been a useful and fascinating model of DMRB for study. Geobacter has been shown to produce biofilms containing exopolysaccharides (7) as well as proteinaceous structures (pili) (4, 10). A biofilm wired with redox-active proteins (e.g., reference 6) could display increased electron flux between cells and higher capacity for electron transfer to solid-phase electron acceptors. The biofilm matrix or extracellular polymeric substance (EPS) is a perfect medium for entrapment of redox proteins for short- and long-range electron transfer (9). Put differently, a collection of redox-active organisms would encounter the same fundamental electrical problems that human cities do in the sense that electrical isolation of differently metabolizing organisms could lead to local variations in potential that could be damaging to individuals and potentially to the group as a whole. The collection of organisms might find it advantageous to connect to a common ground. Biofilms provide a structural matrix on which redox-active enzymes can be situated in an ideal spatial relationship, both with each other and with metal and mineral substrates. This arrangement solves the problem of respiration using a solid-phase electron acceptor by providing an electrically conductive link to the mineral and can even solve the long-distance electron transfer issue by building an electrically dynamic biofilm matrix (Fig. 1).
FIG. 1.
Generalized arrangement of redox components in dissimilatory iron-respiring bacteria. Electron routes are indicated by arrows. The biofilm matrix, composed of exopolysaccharides (green) and redox proteins (red ovals), entraps the redox molecules, where they can interact with both the cell and mineral surface. Outer membrane vesicles (OMV) may also be entrapped, while small-mass flavins and other small-mass redox compounds (yellow) diffuse through the matrix. This model is based on work presented in Coursolle et al. (1), Inoue (5), Gorby et al. (3), and Magnuson et al. (8).
Many geomicrobiology investigators have long suspected that the biofilm matrix is electrically active. This activity is necessary due to the solid-phase, extracellular nature of iron- and manganese-bearing electron-accepting minerals. Even if cells are distal to the mineral surface, they can still convey electrons to the bulk matrix, wherein a cascade of redox reactions can move electrons through the conductive material and eventually to the mineral. Another significant implication of the work is the ability to now identify similar biofilm loci in other DMRB. As an example, a cursory search of microbial genomes reveals that homologs of GSU1501 are present in several other Geobacter genomes, associated with exopolysaccharide synthesis and export genes. Additionally, other DMRB have homologs as well, associated with type II and type IV secretion systems. This suggests that phylogenetically distinct DMRB share a theme for synthesis of a functional exopolysaccharide-cytochrome matrix, as all Geobacter species produce a plethora of c-type cytochromes as part of their respiratory machinery. With additional investigation, similar loci may be discovered in many other genera of DMRB.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
Because c-type cytochromes move electrons from one place to another, we can speculate that their presence in the EPS allows them to provide environmental information to cells, facilitate communication between cells, or provide electron storage capacity outside the cell (in the absence of available electron acceptors) and conduct electrons (in the presence of electron acceptors). Given the complexity of the biofilm matrix (2), it is highly unlikely that c-type cytochromes would occupy this key real estate if there were no advantage inherent to their presence. Biofilm matrix material may be simply a junkyard of molecular components released upon cell lysis or may have a unique composition carefully synthesized and regulated, a potential conclusion from the Rollefson et al. work. Regardless, the matrix is electrocatalytically active, and it may very well be that mineral-transforming microbes have evolved several parallel means for extracellular electron transport. Based on the results of this study, we can now pose additional questions. How common are xap genes in DMRB across phylogenetic and physiologic boundaries? Can we demonstrate the presence of c-type cytochromes in exopolysaccharide materials from a variety of DMRB? Do protein-polysaccharide interactions actually govern the directionality of electron transport via conformational gating? Is the composition of the extracellular matrix carefully orchestrated genomically and biochemically? Pursuit of these questions will guide investigators toward a more unified theory of microbial extracellular electron transport and will aid in better design and application of novel bioenergy devices for green electricity generation.
Acknowledgments
I am grateful to the National Science Foundation and the Department of Energy for support of this work and to Michael W. Swenson and Carrick Eggleston for valuable research, discussions, and input.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Published ahead of print on 23 December 2010.
REFERENCES
- 1.Coursolle, D., D. B. Baron, D. R. Bond, and J. A. Gralnick. 2010. The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J. Bacteriol. 192:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flemming, H. C., T. R. Neu, and D. J. Wozniak. 2007. The EPS matrix: the “house of biofilm cells.” J. Bacteriol. 189:7945-7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorby, Y., et al. 2008. Redox-reactive membrane vesicles produced by Shewanella. Geobiology 6:232-241. [DOI] [PubMed] [Google Scholar]
- 4.Gorby, Y. A., et al. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. U. S. A. 103:11358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue, K., et al. 2010. Purification and characterization of OmcZ, an outer-surface, octaheme c-type cytochrome essential for optimal current production by Geobacter sulfurreducens. Appl. Environ. Microbiol. 76:3999-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leang, C., X. Qian, T. Mester, and D. R. Lovley. 2010. Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl. Environ. Microbiol. 76:4080-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnuson, T. S., A. L. Neal, and G. G. Geesey. 2004. Combining in situ reverse transcriptase polymerase chain reaction, optical microscopy, and X-ray photoelectron spectroscopy to investigate mineral surface-associated microbial activities. Microb. Ecol. 48:388-398. [DOI] [PubMed] [Google Scholar]
- 8.Magnuson, T. S., et al. 2010. Proteogenomic and functional analysis of chromate reduction in Acidiphilium cryptum JF-5, an Fe(III)-respiring acidophile. Biometals 23:1129-1138. [DOI] [PubMed] [Google Scholar]
- 9.McLean, J. S., et al. 2010. Quantification of electron transfer rates to a solid phase electron acceptor through the stages of biofilm formation from single cells to multicellular communities. Environ. Sci. Technol. 44:2721-2727. [DOI] [PubMed] [Google Scholar]
- 10.Reguera, G., et al. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098-1101. [DOI] [PubMed] [Google Scholar]
- 11.Rollefson, J. B., C. S. Stephen, M. Tien, and D. R. Bond. 2011. Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J. Bacteriol. 193:1023-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]