Abstract
In Escherichia coli, spatiotemporal control of cell division occurs at the level of the assembly/disassembly process of the essential cytoskeletal protein FtsZ. A number of regulators interact with FtsZ and modulate the dynamics of the assembled FtsZ ring at the midcell division site. In this article, we report the identification of an FtsZ stabilizer, ZapC (Z-associated protein C), in a protein localization screen conducted with E. coli. ZapC colocalizes with FtsZ at midcell and interacts directly with FtsZ, as determined by a protein-protein interaction assay in yeast. Cells lacking or overexpressing ZapC are slightly elongated and have aberrant FtsZ ring morphologies indicative of a role for ZapC in FtsZ regulation. We also demonstrate the ability of purified ZapC to promote lateral bundling of FtsZ in a sedimentation reaction visualized by transmission electron microscopy. While ZapC lacks sequence similarity with other nonessential FtsZ regulators, ZapA and ZapB, all three Zap proteins appear to play an important role in FtsZ regulation during rapid growth. Taken together, our results suggest a key role for lateral bundling of the midcell FtsZ polymers in maintaining FtsZ ring stability during division.
Cell division in the Gram-negative bacterium Escherichia coli is defined by polymerization and constriction of a cytokinetic ring formed by the protein FtsZ, a tubulin-like GTPase, at midcell (1, 13, 15). FtsZ plays a central role in division in most bacteria, in archaea, in chloroplasts, and in mitochondria of primitive eukaryotes. In E. coli, division involves at least 24 associated proteins, each dependent on FtsZ, either directly or indirectly, for midcell localization. Together this protein complex formed at midcell is referred to as the divisome.
Like tubulin, FtsZ is a self-activating GTPase (12, 32, 38), which in vitro assembles into protofilaments upon binding GTP (16). These protofilaments, depending on experimental conditions, can further associate to form higher-order structures, such as bundles, sheets, or rings (29, 39). GTP hydrolysis destabilizes FtsZ protofilaments, leading to disassembly (33). Besides a regulatory role, GTP hydrolysis has most recently been implicated in the generation of a constrictive force (15, 29, 35). The organization of the FtsZ ring in vivo, while not completely clear, is thought to be a discontinuous structure at the site of division consisting of short, randomly overlapping protofilaments (17, 27). Prior to cell division, FtsZ exists both as a cytosolic pool of monomers and short oligomers and as membrane-bound short protofilaments either separately or in overlapping bundles, and FtsZ molecules from these two pools are in dynamic exchange (20, 48).
In E. coli, the intracellular concentration of FtsZ remains essentially unchanged throughout the cell cycle (51). Hence, precise balance in FtsZ assembly dynamics is critical for FtsZ stability and the spatiotemporal regulation of division. A number of FtsZ regulatory proteins, some of which colocalize with FtsZ at midcell, promote the assembly/disassembly process of FtsZ in E. coli. While some regulatory proteins are essential for survival, many have functionally redundant roles, making them nonessential for viability. The critical role played by nonessential FtsZ regulators is revealed by the strong synergistic phenotypes: inhibition of division or synthetic lethality, observed when expression of two or more such regulatory proteins is altered (14, 18, 19).
Among these regulatory proteins, FtsZ stabilizers such as FtsA, ZipA, ZapA, and ZapB are predicted to promote FtsZ assembly in vivo by mechanisms that include concentrating FtsZ at the membrane, stabilizing lateral protofilament associations, cross-linking separate protofilaments, or preventing depolymerization through the inhibition of GTP hydrolysis. Structural studies and mutational analyses suggest that lateral association among FtsZ protofilaments is an important factor in the maintenance of the overall FtsZ ring integrity at midcell (8, 17, 34). The nonessential but widely conserved FtsZ regulator ZapA is thought to impart FtsZ ring stability by cross-linking adjacent FtsZ protofilaments (10, 28, 31, 53). Consistent with this role for ZapA in FtsZ bundling is its ability to antagonize the action of an FtsZ inhibitor, MinC, which destabilizes, among other means, by preventing lateral associations of FtsZ protofilaments (8, 19, 41, 44). ZapB, another nonessential FtsZ regulator, is thought to localize to the divisome via ZapA and primarily enhance the role of ZapA in FtsZ bundling (18). The other class of regulatory proteins, FtsZ ring assembly inhibitors such as MinC, SulA, SlmA, and ClpX, promotes destabilization by a variety of mechanisms, including preventing GTP binding, sequestering individual monomers, breaking or capping FtsZ polymers, inhibiting lateral interactions, and altering polymer assembly (3, 5, 8, 9, 43, 44, 50).
While the dynamics of the FtsZ ring in vivo is determined by a balance of stabilizing and destabilizing factors, the precise molecular interactions that maintain this balance are poorly understood. Given the complexity of bacterial FtsZ assembly and polymerization, we hypothesized that additional components that modulate FtsZ assembly exist, and we reasoned that a comprehensive understanding of FtsZ dynamics in cell division cannot be achieved without the identification of all such regulatory factors and characterization of their interactions with FtsZ. We report here the identification of a previously uncharacterized member of the FtsZ stabilizer family, YcbW, which we designate ZapC. Although ZapC does not share sequence identity to ZapA or ZapB, our in vivo and in vitro results indicate that, similar to the other Zap proteins, ZapC stabilizes FtsZ at midcell.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are listed in Table 1. E. coli strains were grown in Lennox broth (LB) and maintained on LB agar plates at 37°C unless otherwise stated. Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml for plasmids and 25 μg/ml for integrated chromosomal fusion strains; kanamycin, 50 μg/ml; chloramphenicol, 25 μg/ml; and tetracycline, 12.5 μg/ml. Isopropyl β-d-thiogalactoside (IPTG) was added to 1 μM, 2.5 μM, or 20 μM, and l-arabinose was added to 0.2% where appropriate. Strains with integrated chromosomal fusions or in ftsZ84(Ts) and ftsA12(Ts) backgrounds were maintained at 30°C. Plasmid DNAs from strains that contain a C-terminal green fluorescent protein (gfp) fused in frame to open reading frames (ORFs) in E. coli were used (ASKA clones) in the initial microscopy screen in the ftsZ84(Ts) background (23). Expression of E. coli ORFs was under the control of an IPTG-inducible P-T5/lac promoter of pCA24N (25). Strains containing plasmid DNA pBAD24-ZapC-GFP, pFtsZ-GFP, or integrated fusion pDSW208-ZapC-eYFP or -FtsZ-eCFP were used for imaging in this study.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or referencea |
|---|---|---|
| Strains | ||
| MC4100 | F−araD139 ΔlacU169 relA1 rpsL150 thi33 mot flb5301 deoC7 ptsF25 rbsR | Lab stock |
| AG1 | recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+) supE44 relA1 | 25 |
| BL21(DE3)/pLysS | F−ompT hsdSB (rB− mB−) dcm gal λ(DE3) pLysS | Lab stock |
| EC307 | MC4100 leu::Tn10 ftsZ84(Ts) | 24 |
| EC290 | MG1655 leu::Tn10 ftsA12(Ts) | J. Beckwith |
| MDG148 | MC4100 araD+leu::Tn10(Tetr) ftsA12(Ts) | 7 |
| JOE534 | MC4100Δ(araD)AH33 attφ80::pJC73 (pDSW208-ftsZ-ecfp) | 7 |
| JD10 | MC4100 pBAD24-zapC-gfp | |
| JD11 | EC307 pBAD24-zapC-gfp | |
| JD16 | MC4100 Δ(λattL-lom)::bla lacIq pDSW208-zapC-eyfp | |
| JD18 | MC4100 ΔzapC::kan | |
| JD20 | EC307 Δ(λattL-lom)::bla lacIq pDSW208-zapC-eyfp | |
| JD21 | MDG148 Δ(λattL-lom)::bla lacIq pDSW208-zapC-eyfp | |
| JD29 | BL21(DE3) pLysS pET21a-zapC-his | |
| JD45 | JD16 attφ80::pJC73 (pDSW208-ftsZ-ecfp) | |
| JD48 | EC290 pBAD24-zapC-gfp | |
| JD59 | JD18 pNG162-zapC-his | |
| AJ28 | MC4100 ΔminCDE::kan | 24 |
| HY1-21 | MC4100 ΔzapC | |
| HY1-31 | MC4100 ΔzapA::Cm | |
| HY1-32 | MC4100 ΔzapB::Cm | |
| HY2-04 | MC4100 ΔzapB | |
| HY1-33 | MC4100 ΔclpX724::kan | |
| HY1-20 | MC4100 ΔslmA::Cm | |
| HY1-24 | MC4100 ΔminCDE::kan ΔzapC | |
| HY1-30 | MC4100 ΔslmA::Cm ΔzapC::kan | |
| HY1-34 | MC4100 ΔclpX::kan ΔzapC | |
| HY1-28 | MC4100 ΔzapA::Cm ΔzapC::kan | |
| HY1-29 | MC4100 ΔzapB::Cm ΔzapC::kan | |
| HY2-12 | MC4100 ΔzapA::Cm ΔzapB | |
| BL21(DE3)/pET11b-FtsZ | F−ompT hsdSB (rB− mB−) dcm gal λ(DE3) pET11b-FtsZ | L. Romberg |
| Plasmids | ||
| pJC58 | pDSW208(eYFP) MCS-eyfp cloning vector | 7 |
| pJHK5 | pBAD24-GFP, Ampr | M. Goldberg |
| pKD46 | pSC101 oriTs pBAD gam bet exo, Ampr | 11 |
| pCP20 | pSC101 oriTs cI857 λPRflp, Ampr Cmr | 11 |
| pZG | pBC(SK+) ftsZ-gfp, Cmr | W. Margolin |
| pNG162 | pSC101, Spcr | J. Beckwith |
This study unless otherwise noted.
Plasmid and strain construction. (i) Plasmid construction.
Plasmid pBAD24-ZapC-GFP, expressing a hybrid protein under the control of the arabinose promoter of pBAD24, was constructed by amplifying zapC using the P5YcbW-U and P6YcbW-D primers from the ASKA plasmid clone (JW5125; http://www.shigen.nig.ac.jp/ecoli/strain/top/top.jsp). The PCR product was digested by PstI and cloned into pJHK5 (a kind gift of Marcia Goldberg) to create a C-terminal GFP fusion protein. To generate a zapC-eyfp fusion in single copy, zapC was PCR amplified using the SalI ycbW 5P and AJ ycbW RP primers, digested with SalI, and cloned into the SalI site of pDSW208-eYFP (a kind gift of Jon Beckwith) to create a ZapC-enhanced yellow fluorescent protein (eYFP) fusion protein. Sequence analysis was performed to verify each construct. pDSW208-zapC-eyfp was integrated into the lambda attachment site of the wild-type MC4100 strain as described previously (4). The zapC-eyfp integrated fusion was P1 transduced into the strain of interest (45). To generate a zapC-his complementing vector, zapC-his was PCR amplified using the ycbW 5P GW and ycbW 3P GW primers from the pET21a-zapC-his (JD29) vector into the pDONR221 plasmid (Invitrogen) to create a Gateway entry clone. After sequence verification, the entry clone was recombined into an IPTG-inducible pNG162 destination vector to create the pNG162-zapC-his expression clone by Gateway. A list of the primers used in the study is included in Table S1 in the supplemental material.
(ii) Construction of strains carrying deletions in genes of interest.
Chromosomal deletions in genes of interest were obtained from the Keio Collection (2) for zapC and clpX and transduced into our wild-type strain background by P1 transduction to create isogenic strains. Deletions in zapA, zapB, and slmA were created using the λ Red recombination method using the following primer pairs: ZapA-PS1 and ZapA-PS2, PS1-ZapB and PS2-ZapB, and SlmA-PS1 and SlmA-PS2. Where necessary, the antibiotic resistance gene was eliminated with the aid of an FLP expression plasmid essentially as described previously (11).
(iii) Construction of double mutants for synergistic assays.
Isogenic strains were constructed by P1-mediated transduction of each mutation into the wild-type strain or mutant strain of interest as described previously (45).
(iv) Construction of strains for protein purification.
Using the primers pET YcbW NdeI 5P and pET YcbW XhoI no stop 3P, zapC was amplified by PCR, and the product was digested by NdeI and XhoI and cloned into the same sites of the pET21a vector (Novagen). Sequence was verified, and the correct clone was transformed into BL21(λDE3)/pLysS cells to express the protein of interest. The pET11b-FtsZ plasmid was obtained (a kind gift of Laura Romberg) and transformed into the BL21(DE3) strain to express FtsZ essentially as described previously (40).
Microscopy.
Microscopy was performed using a 100× oil immersion objective on a Nikon Eclipse Ti microscope with Chroma filters. Images were captured digitally using a Nikon DigiSight monochrome charge-coupled-device (CCD) camera and analyzed using Nikon Elements Basic software. Color images were pseudocolored using the same software and assembled using the Adobe Photoshop software program.
Live bacterial cell imaging.
Screening of the ZapC-eYFP fusion protein in various strains was performed individually on glass slides as described previously (6). Briefly, wild-type cells carrying a fusion plasmid were grown to an optical density at 600 nm (OD600) of 0.2 to 0.3, at which point the fusion was induced with 2.5 μM IPTG for 30 min at 30°C. Where used for the filamentation of cells, aztreonam was added to 1 μg/ml at an OD600 of 0.1 to 0.2 prior to induction of fusion protein expression, and incubation with aztreonam was continued for 60 min, at which point the fusion protein was induced at an OD600 of 0.3 to 0.4 as described above. In the ftsZ84(Ts) and ftsA12(Ts) backgrounds, cells were grown to an OD600 of 0.2 to 0.3 at the permissive temperature (30°C), at which time one-half of the culture was shifted to the restrictive temperature (42°C) and the other half was maintained at the permissive temperature for an additional 60 min. At this point, fusions were induced with 2.5 μM IPTG for an additional 10 min at 42°C or 30 min at 30°C. Cells grown at 30°C were mounted on a 1% agarose pad on a 15-well slide (ICN) and visualized. Cells grown at the restrictive temperature were fixed with paraformaldehyde glutaraldehyde fixation essentially as described previously (7). For colocalization studies with the ZapC-eYFP and FtsZ-enhanced cyan fluorescent protein (eCFP) integrated fusion strains, cells were grown to an OD600 of ∼0.3, at which point expression was induced using 1 μM IPTG for 30 min at 30°C. Cells were mounted on a 1% agarose pad on a 15-well slide and visualized. To visualize FtsZ-GFP in cells, plasmid DNA pZG (a kind gift of Bill Margolin) was transformed into wild-type and zapC deletion strains. Strains were grown at 37°C to an OD600 of 0.3 to 0.4, at which point FtsZ-GFP expression was induced with 20 μM IPTG for 30 min. Cells were mounted on a 1% agarose pad on a 15-well slide and visualized.
Indirect Immunofluorescence.
For visualizing of FtsZ in cells overexpressing ZapC, cells containing pBAD24-zapC-gfp were grown to an OD600 of 0.3 to 0.4 at 37°C, at which point expression of ZapC-GFP was induced with 0.2% l-arabinose for 30 min at 37°C. Wild-type and zapC cells were harvested at an OD600 of 0.3 to 0.4 at 37°C. Next, cells were fixed in methanol-acetone and the presence of FtsZ was probed with an anti-FtsZ rabbit polyclonal antibody at 1:10,000 (a kind gift of Debu RayChaudhuri) and a Texas-Red-conjugated secondary antibody as described previously (24).
Transmission electron microscopy.
To visualize the morphology of the FtsZ polymers under conditions identical to those used for FtsZ sedimentation studies (described below), aliquots from experimental and control reactions were removed, negatively stained with 5% ammonium molybdate and 1% trehalose (pH 7.2, adjusted with 5 M NaOH), and examined by conventional transmission electron microscopy (TEM) as described previously (3, 22, 33, 37). Images were collected on a Jeol 2100F microscope operated at 200 kV, recorded on a 2k-by-2k CCD camera at 2.5 μm underfocus and at a nominal magnification of ×30,000 or ×50,000.
Protein interaction platform (PIP) assays in yeast.
Each bacterial gene of interest was PCR amplified with and without a stop codon into pDONR223 (Invitrogen) plasmids to create Gateway entry clones. After sequence verification, yeast expression plasmids for each ORF were created using site-specific recombination (Gateway) to generate fusion proteins that are expressed under the control of a regulatable GAL1 promoter. pAG413-egfp-X and pAG415-X-eyfp clones were used to transform the yeast S288C MATa strain, and pAG416-μNS-Y expression plasmids were used to transform the S288C MATα strain (with X and Y representing bacterial genes of interest). The strains that conditionally express the fluorophore fusion plasmid were mated with those that conditionally express the μNS fusion plasmid, and diploids were selected. Visualization of live yeast diploid strains expressing the fluorophore and μNS fusions was conducted in 96-well glass-bottom plates as described previously (42).
Protein purification.
One-liter bacterial cultures were grown and induced to express FtsZ. FtsZ was purified as described previously (40), and the concentration was determined by bicinchoninic acid (BCA) assay. ZapC-His was purified by growing 1-liter bacterial cultures with the fusion plasmid to an OD600 between 0.3 and 0.4, at which point ZapC-His expression was induced with 0.5 mM IPTG, followed by growth for 3 h. Cells were sonicated and centrifuged at 40,000 × g in a Beckman type 60 Ti rotor, and supernatants were loaded into Talon columns (Clontech) and washed and the protein was eluted and dialyzed against storage buffer (50 mM Na-morpholinepropanesulfonic acid [MOPS] [pH 7.2], 50 mM KCl, 5% glycerol, and 20 mM EDTA). The eluate was separated on a 12.5% SDS-PAGE gel to obtain estimates of purification, and the concentration was determined by A280 measurements.
FtsZ sedimentation assay.
Purified FtsZ (5 μM) was added to purified ZapC-His (5 μM) in FtsZ polymerization buffer (50 mM HEPES [pH 7.2], 50 mM KCl, 10 mM MgCl2), and GDP or GTP (1 mM) was added last. Reactions (100-μl mixtures) were carried out at room temperature and spun down using a TLA100.2 rotor at 100,000 rpm for 15 min. At this point, 80 μl of supernatant was carefully removed, and 4× loading dye was added to a final concentration of 1×. The rest of the supernatant was discarded, and the pellets were resuspended in original reaction volume buffer plus 1× loading dye, final concentration. Five microliters of the supernatants and pellets were resolved in a 12.5% SDS-PAGE gel.
Analysis of proteins.
Western blot analysis was performed using standard protocols. Proteins were prepared from cell pellets as described previously (23). Densities of protein bands were determined by Image J analysis (NIH).
Synergistic studies.
Isogenic single and double mutant strains were constructed by P1 transduction as described above. Bacterial overnight cultures were subcultured at 1:100 in LB or in M9 minimal medium supplemented with 0.2% glucose, 1 μg/ml thiamine, 0.1% Casamino Acids, and 1 mM MgSO4 with appropriate antibiotics when necessary at 37°C, and growth was monitored over an 8-h period every hour. Cells at 2-h time points during the growth curve analysis in LB were visualized by phase microscopy, and cell lengths were measured using Nikon Basic Elements Imaging software. Cells lacking the chromosomal copy of zapC but carrying a complementing zapC-his plasmid were analyzed for cell length measurements similarly. Mutants were also characterized by plate viability assays. Overnight cultures were serially diluted, and 10-μl aliquots were spotted on LB agar plates and incubated at 37°C overnight. The relative plating efficiency was determined by comparison with control strains on the same plate.
RESULTS
Identification of ZapC (YcbW).
We identified YcbW, a previously uncharacterized protein, as localizing to midcell in an FtsZ-dependent manner in a recently described protein localization screen conducted with E. coli (23). As detailed below, the ycbW gene product is a Z ring-associated protein, and we will henceforth refer to it as ZapC. ZapC is predicted to be a small (20.6-kDa), α-β, cytoplasmic protein (PredictProtein). It is conserved in a subgroup of gammaproteobacteria, such as Shigella, Salmonella, Yersinia, and Vibrio, and is nonessential for viability (2).
ZapC colocalizes with FtsZ at midcell.
Since all cell division proteins characterized to date require FtsZ for localization to midcell, we investigated the possibility that ZapC may be an as yet unidentified component of the division apparatus at midcell. The ZapC-GFP fusion plasmid used in the microscopy localization screen (23) is cloned into the high-copy pCA24N vector under the control of a leaky T5/lac IPTG inducible promoter (25). Expression of ZapC-GFP (see Fig. S1 in the supplemental material) or ZapC alone from this plasmid produced a mix of filaments and normal-length cells, suggesting that the phenotypes we were observing were due to overexpression of this fusion protein. To rule out potential localization artifacts, we examined localization of ZapC by introducing a zapC-eyfp fusion whose expression was driven by an IPTG-inducible promoter into the wild-type chromosome at the lambda att site in single copy (4). A zapC-eyfp fusion localized eYFP to midcell (Fig. 1 a and b), and the cell morphology and cell lengths were comparable to those of the wild type. The frequency of ZapC-eYFP localization at midcell in the wild-type background was 83.1% (138 out of 166 cells counted), suggesting that ZapC may be an early recruit to the divisome. We next determined whether the midcell site in which ZapC-eYFP is seen is defined by the presence of FtsZ rings. We integrated an ftsZ-ecfp fusion into a strain containing the zapC-eyfp fusion integrated at the λ attachment site. Upon induction of the fusion proteins, ZapC and FtsZ were observed to colocalize at midcell (Fig. 1c and d).
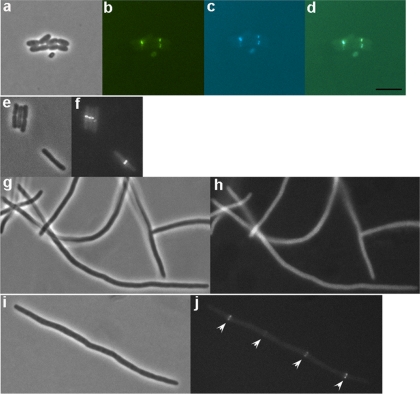
FIG. 1.
Localization analyses of a ZapC-eYFP fusion in various strain backgrounds. Phase and fluorescein isothiocyanate (FITC) channels are shown. (a to d) Colocalization of ZapC-eYFP and FtsZ-eCFP in wild-type cells (JD45). (b) ZapC-eYFP localized at midcell. (c) FtsZ-eCFP localized at midcell. (d) Overlay of ZapC-eYFP and FtsZ-eCFP. (e to h) ZapC-eYFP localization in ftsZ84(Ts) background (JD20). A ZapC-eYFP fusion localizing to midcell at 30°C is shown (f); a ZapC-eYFP fusion shows diffuse localization at 42°C (h). (i and j) ZapC-eYFP localization in wild-type cells (JD16) treated with aztreonam to inactivate FtsI. (j) Fluorescent image showing a ZapC-eYFP fusion localizing at periodic intervals along the length of an aztreonam-induced filament. Scale = 5 μm.
ZapC depends on FtsZ for localization to midcell and is an early divisome protein.
Next we examined whether the midcell localization of a zapC-eyfp fusion depends on FtsZ using a thermolabile FtsZ mutant. At the permissive temperature (30°C), the zapC-eyfp fusion localizes eYFP to midcell in an ftsZ84(Ts) strain (Fig. 1e and f). However, at the restrictive temperature (42°C), in the absence of functional FtsZ, ZapC-eYFP fails to localize to potential division sites (Fig. 1g and h). To further examine whether ZapC is an early assembly protein, we tested whether the localization of the ZapC-eYFP fusion protein was perturbed in cells that were filamented with aztreonam. Aztreonam inhibits FtsI, a transpeptidase, causing cells to become filamented without disrupting the localization of early division proteins (52). FtsI is recruited later in the division cycle and is involved in septal remodeling and synthesis. ZapC-eYFP localized at periodic intervals along the length of aztreonam-induced filaments (Fig. 1i and j). These periodic interval sites represent potential division sites along the length of the filaments, indicating that ZapC is not dependent on the catalytic activity of FtsI to be targeted to midcell. ZapC-eYFP also localized at periodic intervals along the length of filaments in a thermolabile ftsA12(Ts) mutant at the restrictive temperature (see Fig. S2 in the supplemental material). FtsA is an essential division protein that helps tether FtsZ to the membrane and also stabilizes FtsZ protofilaments at midcell (1). Consistent with the identification of ZapC in the previously described protein localization screen (23), these data indicate that ZapC is recruited to the cytokinetic ring at an early stage of its assembly, dependent on FtsZ.
Cells lacking or overexpressing ZapC are slightly elongated.
Given that ZapC localizes to midcell in an FtsZ-dependent manner and is likely to be an early divisome protein, we next examined the cell morphologies and growth patterns of cells lacking zapC. A zapC mutant is not essential for viability when cells are grown in rich or minimal medium. However, we observed that zapC mutants are slightly elongated compared to wild-type cells during exponential growth in rich medium (Table 2). Our data are consistent with cell morphology phenotypes reported for mutations in zapA and zapB, both nonessential FtsZ regulators (18, 30), and suggest that ZapC may play a role in FtsZ regulation.
TABLE 2.
Morphologies of cells lacking or overexpressing ZapCa
| Strain descriptionb | Cell lengthc ± SD | No. of cells measured |
|---|---|---|
| Wild type | 2.88 ± 0.71 | 200 |
| pZapC-GFP (uninduced) | 2.43 ± 0.70 | 410 |
| pZapC-GFP (induced) | 3.99 ± 1.49 | 401 |
| zapC | 3.29 ± 1.18 | 679 |
Phase images of cells from fixed immunolabeling experiments were used for cell length measurements. Fixation did not appear to have significant effects on cell length based on a comparison to live cell length measurements for wild-type and zapC cells as reported in Fig. 5 and in Table 3.
Strain JD10 contains the pBAD24-ZapC-GFP plasmid overexpressing ZapC.
Cell length in μm.
Cells overexpressing ZapC are elongated compared to wild-type cells and uninduced controls, suggesting a role for ZapC in FtsZ ring stability (Table 2). We used an arabinose-inducible ZapC-GFP overexpression construct that upon induction with arabinose forms loose helices at midcell (see Fig. S3a to h in the supplemental material) similar to those reported for FtsZ in cells overexpressing FtsZ (36, 49). In addition, upon induction, ZapC-GFP localizes GFP to midcell in an FtsZ-dependent manner (see Fig. S3i to l in the supplemental material).
FtsZ ring morphology in cells lacking or overexpressing ZapC.
Since inactivation or overexpression of FtsZ regulatory proteins can often cause changes in both cell morphology and/or FtsZ ring presence or morphology (missing rings, diffuse arcs, spirals, doublets, and ectopic rings), indicating a role for these proteins in FtsZ assembly and dynamics, we examined septal FtsZ ring morphologies in cells lacking or overexpressing ZapC. As mentioned above, cells lacking ZapC are slightly elongated and reveal varied FtsZ ring morphologies compared to wild-type cells (Fig. 2 a to d). Longer zapC cells showed missing or ectopic FtsZ rings, indicating that the loss of ZapC affected FtsZ ring formation under the experimental conditions tested (Fig. 2d). Native FtsZ levels remained essentially unchanged in the absence of ZapC compared to results for the wild type (Fig. 2k).
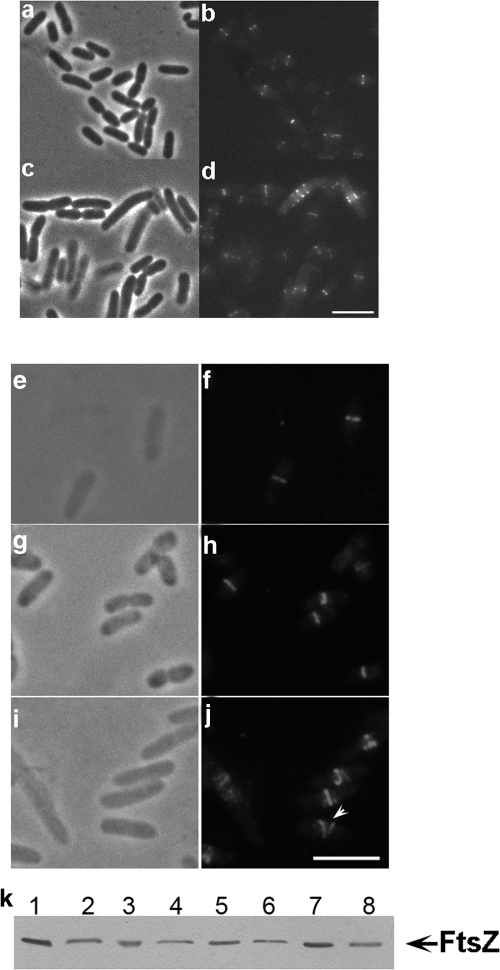
FIG. 2.
FtsZ ring morphologies in cells lacking or overexpressing ZapC. FtsZ-GFP (pZG) localization in wild-type (MC4100) or zapC (JD18) cells. Phase, FITC, or Texas Red channels are shown. (a and b) FtsZ-GFP in wild-type cells. (c and d) FtsZ-GFP in zapC cells. (e to j) FtsZ morphologies as detected by immunolabeling with anti-FtsZ antibodies in cells overexpressing ZapC-GFP (JD10). (e and f) FtsZ in wild-type cells. (g and h) FtsZ containing the uninduced ZapC-GFP plasmid. (i and j) Helical FtsZ ring morphologies in cells overexpressing ZapC-GFP. Scale = 5 μm. (k) Immunoblotting analysis of levels of FtsZ expression in cells lacking ZapC (JD18) or overexpressing ZapC-GFP (JD10), performed using anti-FtsZ antibodies. Lanes 1 and 2, wild type (MC4100); lanes 3 and 4, uninduced ZapC-GFP cells; lanes 5 and 6, ZapC-GFP overexpressed; lanes 7 and 8, zapC. Lanes 2, 4, 6, and 8 were loaded with one-half of the sample loaded in lanes 1, 3, 5, and 7.
ZapC-GFP-overexpressing cells form loose FtsZ helices compared to wild-type cells and uninduced controls (Fig. 2j), similar to those described previously (14, 49). No defects in growth, nucleoid replication, or nucleoid segregation are seen upon ZapC-GFP overexpression (see Fig. S4 in the supplemental material). Additionally, immunoblotting of extracts from ZapC-GFP-overexpressing cells shows that FtsZ levels remain unchanged, indicating that the observed changes in FtsZ ring morphology are not due to changes in steady-state cellular FtsZ levels (Fig. 2k). Our data showing aberrantly organized FtsZ rings in concert with cell morphology phenotypes in cells lacking ZapC or overexpressing a ZapC fusion protein suggest a role for ZapC in regulating FtsZ assembly dynamics at midcell.
ZapC interacts with FtsZ in vivo in yeast.
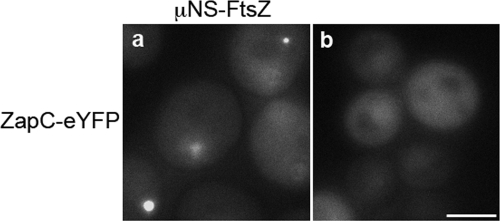
Since ZapC colocalizes with FtsZ at midcell and appears to regulate FtsZ, we next identified ZapC interaction with FtsZ using the protein interaction platform (PIP) assay, a method for detecting interacting proteins in yeast (42). The PIP assay relies on the reoviral scaffolding protein μNS, which forms large focal inclusions in yeast. When a query protein is fused to μNS and an interaction partner is fused to a fluorophore, interactions can be identified by screening for yeast that include fluorescent foci. Yeast containing μNS-FtsZ and μNS-ZapC were mated with yeast expressing both N-terminal and C-terminal fluorophore fusions to FtsZ and ZapC. Cells expressing μNS-FtsZ and ZapC-eYFP formed fluorescent foci, indicating that ZapC interacts with FtsZ and this interaction is likely to be direct since these yeast did not express any other bacterial protein (Fig. 3 a). In a control experiment, ZapC-eYFP when expressed alone in yeast was diffuse (Fig. 3b). We also observed that only a C-terminal ZapC-eYFP fusion yielded a positive interaction with FtsZ while an N-terminal fusion to ZapC did not. These data suggest that the N terminus of ZapC likely interacts with FtsZ. While GFP-FtsZ was diffuse, FtsZ-eYFP formed rings or radiating arcs on its own as described previously for fission yeast (47) and hence was not suitable for further interpretations in this assay. As positive controls for the PIP assay, we identified previously characterized FtsZ, ZapA, and ZapB interactions, indicating that protein interactions as detected by PIP are likely to be physiologically relevant (data not shown). The protein interaction data demonstrate that ZapC interacts with FtsZ.
FIG. 3.
ZapC-FtsZ interaction in yeast as detected in PIP assays. (a) ZapC-eYFP (pAG415 GAL1 ZapC-eYFP) and μNS-FtsZ (pAG416 GAL1 μNS-FtsZ) fusions form discrete fluorescent foci when coexpressed in yeast. (b) A ZapC-eYFP fusion shows diffuse localization in yeast when expressed alone. Scale = 5 μM.
ZapC increases FtsZ bundling.
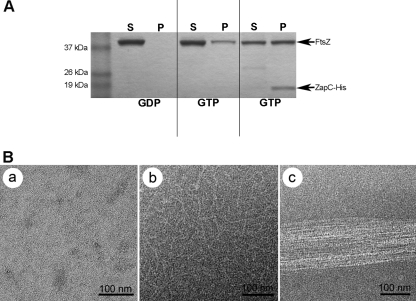
Our in vivo data suggest that ZapC directly interacts with FtsZ and likely enhances FtsZ ring stability. Since FtsZ stabilizers such as ZapA and ZipA promote assembly by increasing polymer bundling in vitro (21, 28, 37, 46), we investigated whether ZapC enhanced FtsZ polymerization in vitro using an FtsZ pelleting assay that was visualized via electron microscopy. Purified FtsZ (≥90% purity) was incubated with purified ZapC-His (∼65 to 70% purity) (see Fig. S5 in the supplemental material), and amounts of polymeric FtsZ in the pellet were determined. In the presence of GDP, FtsZ was found mostly in the supernatant and no bundles were visualized upon examination with TEM (Fig. 4). In the presence of GTP, FtsZ was found in the pellet and characteristic FtsZ polymers were seen in electron microscopy images (Fig. 4). However, upon addition of a 1:1 molar ratio of purified FtsZ and ZapC-His, there was a 2.6-fold ± 0.4-fold increase in FtsZ in the pellet compared to FtsZ alone and ZapC-His cosedimented with FtsZ (Fig. 4A). TEM images of FtsZ plus ZapC-His reactions in the presence of GTP show increased lateral bundling of FtsZ polymers (Fig. 4B). In control reactions, in the presence of GTP, addition of ZapC-His suspension buffer alone did not change the amount of FtsZ found in the pellet compared to that of FtsZ alone (see Fig. S6 in the supplemental material). Also, in the absence of FtsZ, ZapC-His was found in the supernatant and did not self-assemble into polymeric structures as visualized by TEM (see Fig. S6 in the supplemental material). Taken together, our in vitro data indicate that ZapC promotes FtsZ bundling through specific stabilizing interactions. In addition, the expression of a zapC-his fusion was able to rescue (average cell length = 2.7 μm ± 0.94 μm) the cell elongation phenotype observed during exponential growth in rich medium in a zapC background, indicating that the zapC-his fusion is functional (Fig. 5).
FIG. 4.
In vitro characterization of ZapC-FtsZ interactions. Both FtsZ and ZapC-His were at 5 μM concentrations when added to a total polymerization reaction volume of 100 μl. (A) Relative concentrations of FtsZ in supernatant (S) and pellet (P) from sedimentation assays were visualized by SDS-PAGE analysis. (B) Transmission electron microscopy images of reactions detailed in the sedimentation assays in part A with FtsZ in the presence of GDP (a), characteristic FtsZ protofilaments in the presence of GTP (b), or FtsZ bundles seen in the presence of ZapC-His (c).
FIG. 5.
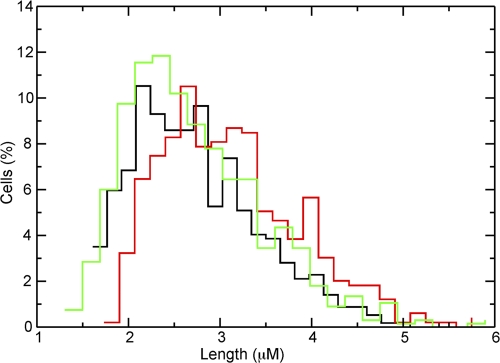
Complementation of zapC cell length phenotype by a zapC-his fusion (JD59). Histograms of cell length in μm plotted against the percentage of cells for the wild type (MC4100, black; n = 570) or the zapC (JD18, red; n = 469) or zapC-his (green; n = 676) mutant, where n equals the total number of cells measured.
Synergistic studies of a zapC mutation with mutations in other nonessential FtsZ regulators.
Since it appears that ZapC is an FtsZ stabilizer, we combined a zapC mutation with mutations in other FtsZ bundlers, ZapA and ZapB, to determine synergistic phenotypes. We examined cell morphologies and growth patterns of zapA zapC and zapB zapC double mutants. Cell lengths of both zapA zapC and zapB zapC double mutants were essentially unchanged from those of the single mutants (Table 3). We also compared growth patterns of double mutants to each other, those of the single mutants, and that of the wild type. Cells lacking ZapC and either ZapA or ZapB showed a slight lag in growth compared to the single mutants (see Fig. S7 in the supplemental material). We confirmed that a zapA zapB double mutant showed longer cells than the zapA and zapB single mutants and the wild type, with no changes in mass doubling times as reported previously (18). Plate viability assays with overnight cultures showed no changes in relative growth in any of the strains tested. Also, growth curves conducted in M9 minimal medium showed no differences between any of the strains tested (data not shown). Deletion of both zapC and the FtsZ inhibitors minC, slmA, and clpX did not show any significant changes in overnight plate viability assays (data not shown). Hence, the elongated cell morphologies of cells lacking the Zap proteins either singly or in combination during exponential growth in rich medium suggest that ZapC functionally overlaps with ZapA and ZapB and plays a role in FtsZ regulation at midcell.
TABLE 3.
Morphologies of cell division mutants during growth in rich medium
| Mutated gene(s) | OD600a | Cell lengthb ± SD | No. of cellsc |
|---|---|---|---|
| Noned | 0.63 ± 0.11 | 2.68 ± 0.69 | 570 |
| zapA | 0.52 ± 0.10 | 3.21 ± 0.94 | 347 |
| zapB | 0.45 ± 0.10 | 3.29 ± 0.91 | 342 |
| zapCd | 0.46 ± 0.07 | 3.05 ± 0.82 | 469 |
| zapA zapC | 0.35 ± 0.08 | 3.35 ± 1.41 | 350 |
| zapB zapC | 0.34 ± 0.10 | 3.13 ± 0.85 | 344 |
| zapA zapB | 0.48 ± 0.13 | 3.67 ± 1.64 | 344 |
Average OD600 measurements from the numbers of colonies tested. Overnight cultures were subcultured at 1:100 and grown for 8 h in LB at 37°C. Cells were harvested at the 2-h time point; OD600 and cell lengths were measured at this point.
Average cell lengths shown in μm, with SD.
Number of individual cells measured from at least 2 different CFU determinations for each strain.
The same cell length measurements for zapC are reported in Fig. 5.
DISCUSSION
This work has identified a previously unknown regulator of FtsZ assembly, ZapC. Our experiments indicate that ZapC helps to stabilize FtsZ protofilaments in the ring in a manner that may be similar to that of ZapA and ZapB. ZapC does not share any sequence identity to ZapA or ZapB but does appear to share some phenotypic and functional characteristics of the other Zap proteins. Like ZapA and ZapB, ZapC is an early assembly protein and colocalizes with FtsZ at midcell, and like ZapA it localizes to the divisome in an FtsZ-dependent manner. Also in similarity to ZapA, ZapC interacts with FtsZ in vivo and increases FtsZ bundling in vitro. While ZapA and ZapB are oligomeric in solution, ZapC is monomeric (see Fig. S8 in the supplemental material). However, results from an FtsZ pelleting assay at approximately a 1:1 molar ratio of FtsZ and ZapC-His show most of ZapC-His to be in the pellet while only half of FtsZ is found in the pellet, suggestive of oligomerization of ZapC, perhaps in the presence of FtsZ. Preliminary PIP assays also reveal a weak interaction of ZapC with itself. Based on these data and the FtsZ bundling role of ZapC, we cannot discount the possibility that like ZapA and ZapB, ZapC may be oligomeric in the cell.
Cells lacking ZapC show increases in cell length, and as reported for zapA cells (30), we observed that cell length standard deviations between the wild type and those with deletions in zapA, zapB, and zapC overlap in our study. However, there is a reproducible increase in average cell lengths in the single mutants compared to results for the wild type. While the cell length increases we observed (20% and 23% for zapA and zapB single mutants and 38% for the zapA zapB double mutant compared to the wild-type length) were less than what has been reported (40% for zapA and zapB single mutants and 70% for the zapA zapB double mutant), our results replicate the trends seen in the prior study (18). The differences in cell length values between our study and the prior work may be attributed to strain backgrounds and perhaps differences in the optical densities at which cell length measurements were made. Increased cell lengths in zapA and zapB cells are often accompanied by abnormal or missing FtsZ rings as determined by localization of an FtsZ-GFP fusion (18). Similar to findings for deletions in zapA and zapB, a zapC mutant exhibited changes in gross FtsZ morphologies. In cells overexpressing ZapC, we observe changes in native FtsZ ring morphology to loose helices. The cell length increases taken together with FtsZ patterns in cells lacking or overexpressing ZapC are consistent with a role for ZapC in modulating FtsZ ring assembly and stability.
Intriguingly, the cell elongation phenotype of zapC cells is seen only during exponential growth in rich medium. Both zapA and zapB are also reported to exhibit longer cells during exponential growth in rich medium (18, 30). While the significance of these observations is not completely understood, these data, taken together with increased FtsZ bundling in the presence of ZapA or ZapC in vitro, raise the possibility of a critical role for FtsZ lateral associations during exponential growth. In vivo, assembly of the FtsZ ring is thought to progress as follows: localization to midcell, a likely formation of a helical intermediate collapsing into a tightly pitched ring, followed by constriction of the FtsZ ring (31, 49). A critical role for FtsZ lateral associations in vivo has been recently proposed, including a role for ZapA in promoting the transition of the FtsZ helical intermediate to the midcell ring structure. These interpretations are based on Z ring structures visualized by photoactivated localization microscopy (PALM) (17), the ability of a known FtsZ destabilizer, MinC, to prevent FtsZ lateral associations (8), the ability of Bacillus ZapA to rescue an ftsZ mutant that is trapped as a helical intermediate incapable of making lateral associations (31), and the ability of ZapA to counteract the role of MinC (19, 41). Although ZapA is widely conserved and ZapB and ZapC are restricted to a subgroup of gammaproteobacteria, each is dispensable for cell viability. It is therefore tempting to speculate that each has functionally overlapping roles in bundling of FtsZ during exponential growth. Perhaps the intrinsic affinity of FtsZ protofilaments alone is unable to establish stabilizing lateral interactions in rapidly growing cells. Instead, FtsZ relies on accessory FtsZ regulators, such as ZapA, ZapB, and ZapC, to aid in transitioning and stabilizing a likely helical intermediate to a compact ring.
Our genetic analysis of zapC points to a functional overlap with zapA and zapB. A zapA zapC double mutant shows a marginal increase in cell length over that with the single mutations in zapA and zapC. This modest increase in cell length is accompanied by a large standard deviation compared to results for the wild type and single mutants, indicating that the zapA zapC cell population exhibits greater variability in cell length. We do not observe such cell length increases in a zapB zapC double mutant. These data would imply that ZapA, ZapB, and ZapC interact to promote FtsZ ring stability. If indeed ZapC interacts with both ZapA and ZapB, the slightly increased cell length population exhibited only by a zapA zapC double mutant would suggest that in addition to an overlapping role with ZapA, ZapC perhaps serves a distinct role in FtsZ ring stability. In support of the interaction model, preliminary PIP assays in yeast have revealed weak interactions of ZapC with ZapA and ZapB. However, at the moment, we cannot discount the possibility of ZapC promoting FtsZ bundling independent of ZapA and ZapB. It is possible that while ZapC may act redundantly in modulating FtsZ, under specific environmental conditions, its role may become essential. Further studies will be needed to verify potential interactions of ZapC with ZapA and ZapB and to tease apart any overlapping and/or distinct roles of the Zap proteins in enhancing FtsZ polymerization.
There is accumulating evidence that lateral bundling is required for normal FtsZ formation in vivo and perhaps even FtsZ constriction (26). Given the emerging molecular details of the Z ring-associated proteins in enhancing FtsZ bundling, it will be of interest to understand the extent to which the Zap family plays a role in imparting dynamism to the FtsZ ring during exponential growth. Finally, our study also underscores the need to identify other members of the FtsZ regulatory network to gain a comprehensive understanding of FtsZ structure, assembly, and kinetics at midcell.
Supplementary Material
Acknowledgments
We thank Laura Romberg, Bill Margolin, Debu RayChaudhuri, Marcia Goldberg, and Jonathan Beckwith for strains and reagents, Andrea Piserchio, Deniz Temel, and Kaushik Dutta for assistance in protein purification, Bill Rice for assistance with TEM, Cindy Liang for technical assistance, Petra Levin and Seth Goldman for critical reading of the manuscript, and Piet de Boer for communication of results prior to publication.
This work was supported by the following grants: AHA SDG 0735538T and NIH SC2 GM082336 (to A.J.), RO1 AI078445 (to C.F.L.), and NIH/NCRR/RCMI G12-RR03060 to City College of New York.
Footnotes
Published ahead of print on 7 January 2011.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Adams, D., and J. Errington. 2009. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat. Rev. Microbiol. 7:642-653. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhardt, T., and P. A. de Boer. 2005. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol. Cell 18:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, D., D. S. Weiss, J. C. Chen, and J. Beckwith. 2000. Towards single-copy gene expression systems making gene cloning physiologically relevant: lambda InCh, a simple Escherichia coli plasmid-chromosome shuttle system. J. Bacteriol. 182:842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camberg, J. L., J. R. Hoskins, and S. Wickner. 2009. ClpXP protease degrades the cytoskeletal protein, FtsZ, and modulates FtsZ polymer dynamics. Proc. Natl. Acad. Sci. U. S. A. 106:10614-10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles, M., M. Pérez, J. H. Kobil, and M. B. Goldberg. 2001. Polar targeting of Shigella virulence factor IcsA in Enterobacteriacae and Vibrio. Proc. Natl. Acad. Sci. U. S. A. 98:9871-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, J. C., and J. Beckwith. 2001. FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 42:395-413. [DOI] [PubMed] [Google Scholar]
- 8.Dajkovic, A., G. Lan, S. X. Sun, D. Wirtz, and J. Lutkenhaus. 2008. MinC spatially controls bacterial cytokinesis by antagonizing the scaffolding function of FtsZ. Curr. Biol. 18:235-244. [DOI] [PubMed] [Google Scholar]
- 9.Dajkovic, A., A. Mukherjee, and J. Lutkenhaus. 2008. Investigation of regulation of FtsZ assembly by SulA and development of a model for FtsZ polymerization. J. Bacteriol. 190:2513-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dajkovic, A., S. Pichoff, J. Lutkenhaus, and D. Wirtz. 2010. Cross-linking FtsZ polymers into coherent Z rings. Mol. Microbiol. 78:651-668. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer, P., R. Crossley, and L. Rothfield. 1992. The essential bacterial cell-division protein FtsZ is a GTPase. Nature 359:254-256. [DOI] [PubMed] [Google Scholar]
- 13.de Boer, P. A. 2010. Advances in understanding E. coli cell fission. Curr. Opin. Microbiol. 13:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebersbach, G., E. Galli, J. Møller-Jensen, J. Löwe, and K. Gerdes. 2008. Novel coiled-coil cell division factor ZapB stimulates Z ring assembly and cell division. Mol. Microbiol. 68:720-735. [DOI] [PubMed] [Google Scholar]
- 15.Erickson, H. P., D. E. Anderson, and M. Osawa. 2010. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol. Mol. Biol. Rev. 74:504-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson, H. P., D. W. Taylor, K. A. Taylor, and D. Bramhill. 1996. Bacterial cell division protein FtsZ assembles into protofilament sheets and minirings, structural homologs of tubulin polymers. Proc. Natl. Acad. Sci. U. S. A. 93:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu, G., T. Huang, J. Buss, C. Coltharp, Z. Hensel, and J. Xiao. 2010. In vivo structure of the E. coli FtsZ-ring revealed by photoactivated localization microscopy (PALM). PLoS One 5:e12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galli, E., and K. Gerdes. 2010. Spatial resolution of two bacterial cell division proteins: ZapA recruits ZapB to the inner face of the Z-ring. Mol. Microbiol. 76:1514-1526. [DOI] [PubMed] [Google Scholar]
- 19.Gueiros-Filho, F. J., and R. Losick. 2002. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 16:2544-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haeusser, D. P., R. L. Schwartz, A. M. Smith, M. E. Oates, and P. A. Levin. 2004. EzrA prevents aberrant cell division by modulating assembly of the cytoskeletal protein FtsZ. Mol. Microbiol. 52:801-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hale, C. A., A. C. Rhee, and P. A. de Boer. 2000. ZipA-induced bundling of FtsZ polymers mediated by an interaction between C-terminal domains. J. Bacteriol. 182:5153-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris, J. R., and R. W. Horne. 1991. Negative staining, p. 203-228. In J. R. Harris (ed.), Electron microscopy in biology: a practical approach. IRL Press, Oxford, United Kingdom.
- 23.Janakiraman, A., K. R. Fixen, A. N. Gray, H. Niki, and M. B. Goldberg. 2009. A genome-scale proteomic screen identifies a role for DnaK in chaperoning of polar autotransporters in Shigella. J. Bacteriol. 191:6300-6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janakiraman, A., and M. B. Goldberg. 2004. Evidence for polar positional information independent of cell division and nucleoid occlusion. Proc. Natl. Acad. Sci. U. S. A. 101:835-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitagawa, M., et al. 2005. Complete set of ORF clones of Escherichia coli ASKA library (A Complete Set of E. coli K-12 ORF Archive): unique resources for biological research. DNA Res. 12:291-299. [DOI] [PubMed] [Google Scholar]
- 26.Lan, G., B. R. Daniels, T. M. Dobrowsky, D. Wirtz, and S. X. Sun. 2009. Condensation of FtsZ filaments can drive bacterial cell division. Proc. Natl. Acad. Sci. U. S. A. 106:121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Z., M. Trimble, Y. Brun, and G. Jensen. 2007. The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J. 26:4694-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Low, H. H., M. C. Moncrieffe, and J. Lowe. 2004. The crystal structure of ZapA and its modulation of FtsZ polymerization. J. Mol. Biol. 341:839-852. [DOI] [PubMed] [Google Scholar]
- 29.Mingorance, J., G. Rivas, M. Vélez, P. Gómez-Puertas, and M. Vicente. 2010. Strong FtsZ is with the force: mechanisms to constrict bacteria. Trends Microbiol. 18:348-356. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi, T., et al. 2009. The GTPase activity of Escherichia coli FtsZ determines the magnitude of the FtsZ polymer bundling by ZapA in vitro. Biochemistry 48:11056-11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monahan, L., A. Robinson, and E. Harry. 2009. Lateral FtsZ association and the assembly of the cytokinetic Z ring in bacteria. Mol. Microbiol. 74:1004-1017. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee, A., K. Dai, and J. Lutkenhaus. 1993. Escherichia coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc. Natl. Acad. Sci. U. S. A. 90:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee, A., and J. Lutkenhaus. 1998. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 17:462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliva, M. A., D. Trambaiolo, and J. Lowe. 2007. Structural insights into the conformational variability of FtsZ. J. Mol. Biol. 373:1229-1242. [DOI] [PubMed] [Google Scholar]
- 35.Osawa, M., D. E. Anderson, and H. P. Erickson. 2008. Reconstitution of contractile FtsZ rings in liposomes. Science 320:792-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters, P. C., M. D. Migocki, C. Thoni, and E. J. Harry. 2007. A new assembly pathway for the cytokinetic Z ring from a dynamic helical structure in vegetatively growing cells of Bacillus subtilis. Mol. Microbiol. 64:487-499. [DOI] [PubMed] [Google Scholar]
- 37.RayChaudhuri, D. 1999. ZipA is a MAP-Tau homolog and is essential for structural integrity of the cytokinetic FtsZ ring during bacterial cell division. EMBO J. 18:2372-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.RayChaudhuri, D., and J. T. Park. 1992. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature 359:251-254. [DOI] [PubMed] [Google Scholar]
- 39.Romberg, L., and P. A. Levin. 2003. Assembly dynamics of the bacterial cell division protein FtsZ: poised at the edge of stability. Annu. Rev. Microbiol. 57:125-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romberg, L., M. Simon, and H. P. Erickson. 2001. Polymerization of FtsZ, a bacterial homolog of tubulin, is assembly cooperative? J. Biol. Chem. 276:11743-11753. [DOI] [PubMed] [Google Scholar]
- 41.Scheffers, D. J. 2008. The effect of MinC on FtsZ polymerization is pH dependent and can be counteracted by ZapA. FEBS Lett. 582:2601-2608. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz, A., M. Morrison, A. Agunwamba, M. L. Nibert, and C. Lesser. 2009. Protein interaction platforms: visualization of interacting proteins in yeast. Nat. Methods 6:500-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen, B., and J. Lutkenhaus. 2011. Differences in MinC/MinD sensitivity between polar and internal Z rings in Escherichia coli. J. Bacteriol. 193:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen, B., and J. Lutkenhaus. 2010. Examination of the interaction between FtsZ and MinCN in E. coli suggests how MinC disrupts Z rings. Mol. Microbiol. 75:1285-1298. [DOI] [PubMed] [Google Scholar]
- 45.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Genetic transduction using P1vir, p. 111-112. In Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 46.Small, E., et al. 2007. FtsZ polymer-bundling by the Escherichia coli ZapA orthologue, YgfE, involves a conformational change in bound GTP. J. Mol. Biol. 369:210-221. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasan, R., M. Mishra, L. Wu, Z. Yin, and M. K. Balasubramanian. 2008. The bacterial cell division protein FtsZ assembles into cytoplasmic rings in fission yeast. Genes Dev. 22:1741-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stricker, J., P. Maddox, E. D. Salmon, and H. P. Erickson. 2002. Rapid assembly dynamics of the Escherichia coli FtsZ-ring demonstrated by fluorescence recovery after photobleaching. Proc. Natl. Acad. Sci. U. S. A. 99:3171-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thanedar, S., and W. Margolin. 2004. FtsZ exhibits rapid movement and oscillation waves in helix-like patterns in Escherichia coli. Curr. Biol. 14:1167-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tonthat, N. K., et al. 2011. Molecular mechanism by which the nucleoid occlusion factor, SlmA, keeps cytokinesis in check. EMBO J. 30:154-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weart, R. B., and P. A. Levin. 2003. Growth rate-dependent regulation of medial FtsZ ring formation. J. Bacteriol. 185:2826-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss, D. S., J. C. Chen, J. M. Ghigo, D. Boyd, and J. Beckwith. 1999. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J. Bacteriol. 181:508-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida, Y., et al. 2009. The bacterial ZapA-like protein ZED is required for mitochondrial division. Curr. Biol. 19:1491-1497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.