Abstract
Natural habitats are often characterized by a low availability of phosphate. In plants and many bacteria, phosphate deficiency causes different physiological responses, including the replacement of phosphoglycerolipids in the membranes with nonphosphorous lipids. We describe here a processive glycosyltransferase (Pgt) in Mesorhizobium loti (Rhizobiales) involved in the synthesis of di- and triglycosyldiacylglycerols (DGlycD and TGlycD) during phosphate deprivation. Cells of the corresponding Δpgt deletion mutant are deficient in DGlycD and TGlycD. Additional Pgt-independent lipids accumulate in Mesorhizobium after phosphate starvation, including diacylglyceryl trimethylhomoserine (DGTS) and ornithine lipid (OL). The accumulation of the nonphosphorous lipids during phosphate deprivation leads to the reduction of phosphoglycerolipids from 90 to 50%. Nodulation experiments of Mesorhizobium wild type and the Δpgt mutant with its host plant, Lotus japonicus, revealed that DGlycD and TGlycD are not essential for nodulation under phosphate-replete or -deficient conditions. Lipid measurements showed that the Pgt-independent lipids including OL and DGTS accumulate to higher proportions in the Δpgt mutant and therefore might functionally replace DGlycD and TGlycD during phosphate deprivation.
Phospho- and glycoglycerolipids are the prevalent lipid classes in biological membranes. Phosphoglycerolipids are found in almost all procaryotic and eucaryotic membranes, whereas with a few exceptions, the occurrence of glycoglycerolipids is restricted to chloroplasts of plants, cyanobacteria, and some anoxygenic photosynthetic and Gram-positive bacteria (15). Some glycoglycerolipid-containing bacteria are found among the Proteobacteria (purple bacteria and relatives), which comprise anoxygenic photosynthetic species such as Blastochloris viridis (Rhizobiales) or Rhodobacter sphaeroides (Rhodobacterales) (3, 9, 19), or nonphotosynthetic bacteria, e.g., Rhizobium and Sinorhizobium (Rhizobiaceae, Rhizobiales) (6, 24). The latter bacteria are known for their symbiosis with legumes during nitrogen fixation. The bacterial glycoglycerolipids serve as surrogate lipids for phosphoglycerolipids when the bacteria suffer from phosphate deprivation. Under phosphate deficiency, bacteria respond with an accumulation of glycoglycerolipids and other nonphosphorous lipids. One of the phosphorus-free lipids is ornithine lipid (OL). It consists of the amino acid ornithine bound to a hydroxy fatty acid, with a second fatty acid esterified to the hydroxy group of the hydroxy fatty acid. OL is found in Gram-negative bacteria (21). Another nonphosphorous lipid is the betaine lipid diacylglyceryl trimethylhomoserine (DGTS). DGTS is a widespread membrane lipid among lower plants and green algae and is found in a few bacteria (12, 31). It is believed that DGTS and PC, both zwitterionic lipids with similar head group structures, are interchangeable not only in structural but also in functional aspects. In Rhodobacter, for example, the amounts of sulfoquinovosyldiacylglycerol (SQD) and OL and of the two lipids α-glucosyl-(1→4)-β-galactosyl-diacylglycerol and DGTS increase during phosphate starvation. The latter two lipids are absent from cells grown under high-phosphate conditions (2, 3). Sinorhizobium responds with an accumulation of SQD, OL, and DGTS but is devoid of DGlycD and TGlycD (20, 34). A more specific function of a bacterial glycoglycerolipid was revealed for α-glucosyl-(1→3)-α-mannosyl-diacylglycerol produced in subnanomolar concentrations by Rhizobium leguminosarum. This lipid was suggested to be important for the induction of symbiosis-related processes (24, 25).
Mesorhizobium, a member of the Phyllobacteriaceae (Rhizobiales), lacks glycoglycerolipids when grown under optimal conditions. Its genome contains an open reading frame (ORF; mlr5650) that encodes a polypeptide with sequence similarity to glucosylceramide synthases from fungi, animals, and plants (16). An orthologous sequence was found in Agrobacterium. The eukaryotic glucosylceramide synthases transfer glucose from UDP-glucose onto ceramide to form glucosylceramide (33). However, Mesorhizobium and Agrobacterium lack ceramides and glucosylceramides. The corresponding enzymes were characterized in a previous work after heterologous expression as processive glycosyltransferases using UDP-glucose or UDP-galactose as sugar donors and ceramide or diacylglycerol (DAG) as primary acceptors (16). The main glycoglycerolipids formed after heterologous expression of the Mesorhizobium ORF mlr5650 were glucosylgalactosyldiacylglycerol (GGD), digalactosyldiacylglycerol (DGD), and different molecular species of triglycosyldiacylglycerol (TGlycD). The TGlycDs contained glucose and/or galactose in different combinations. All of the sugars were in β-configuration and (1→6)-linked to each other. The bacterial DGD is structurally different from the plant DGD, which is characterized by a terminal α-galactose bound to the inner β-galactose (15).
The lipid composition of Mesorhizobium comprises the phospholipids phosphatidylethanolamine (PE), monomethyl-PE (MMPE), dimethyl-PE (DMPE), phosphatidylglycerol (PG), phosphatidylcholine (PC), diphosphatidylglycerol, or cardiolipin (CL), and the nonphosphorouslipid OL (7). There have been no lipid studies of Mesorhizobium grown under phosphate starvation. To characterize the in vivo function of the gene mlr5650, we generated a deletion mutant (Δpgt) and measured the lipid composition and the capability of nodulation of the wild type and the Δpgt mutant under conditions of high and low phosphate. We show that the ORF mlr5650 encodes a processive glycosyltransferase, which is functionally active in Mesorhizobium and leads to the accumulation of DGlycD and TGlycD during phosphate starvation. Furthermore, our results demonstrate that phosphate deprivation in Mesorhizobium results in the accumulation of further nonphosphorous lipids like DGTS, OL, and additional glycolipids, which are independent of the mlr5650-dependent glycosyltransferase Pgt. These nonphosphorous lipids are presumably mutually exchangeable and thus equivalent in their functions to replace phosphoglycerolipids during phosphate deprivation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strain Mesorhizobium loti R7a was received from University of Dunedin, Dunedin, New Zealand. M. loti strains were grown in complex tryptone-yeast (TY) medium containing 4.5 mM CaCl2 (4) or AB minimal medium (30), including 25 mM TES [N-Tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] and 0.1% sucrose, with shaking at 28°C. For phosphate starvation experiments, M. loti strains were cultivated for 3 days in AB minimal medium containing 1.25 mM inorganic phosphate (Pi). Cells were centrifuged, and the cell pellet was washed and resuspended in the 2-fold volume of the previous volume of AB minimal medium containing 1.25 mM Pi (for control) or lacking Pi (i.e., inorganic phosphate was replaced with KCl and NaCl). Growth rates in AB minimal medium containing either high (1.25 mM) or low (0.6 μM) concentrations of Pi were determined as follows. Precultures from the M. loti strains were grown in phosphate containing AB minimal medium to an optical density at 600 nm (OD600) of 0.4 to 0.6. The cells were centrifuged, and the cell pellet washed and resuspended in the appropriate medium. Three different cultures of 60 ml of AB minimal medium were inoculated with wild-type or mutant strains to an OD600 of 0.05. The exact OD600 was determined at t = 0. Growth curves were determined for 5 days. Gentamicin was added to a final concentration of 20 μg ml−1 for the cultivation of the M. loti Δpgt mutant. For salt stress and nitrogen starvation experiments, the cells were grown in AB minimal medium containing NaCl (300 mM) or KCl (instead of NH4Cl). For low-oxygen experiments, cells were grown in AB minimal medium in air-tight 50-ml Falcon tubes.
Construction of an M. loti deletion mutant by inactivation of the pgt+ locus using a gentamicin resistance cassette.
The construct pBmeso-Gm contains 557 bp of the 5′ end and 542 bp of the 3′ end from the pgt+ ORF flanking a gentamicin resistance cassette. This construct was introduced into M. loti R7a via electroporation, and transformants were selected on solidified AB minimal medium containing 0.1% sucrose and 20 μg of gentamicin ml−1. The M. loti Δpgt mutant strain was identified by PCR using specific oligonucleotides (PD601 [CTGCAGCGGCGTTGTGACAATTTAC], PD627 [GCGCTATAGAAGGTCTTCCAG], PD626 [TGACACATGACAATGGTGGT], and PD1048 [ACATAGCTGGGACCATCAAGTC]).
Isolation and separation of lipids.
Cultures (50 to 200 ml) of the Mesorhizobium wild-type or mutant strain were cultivated as described above under phosphate-sufficient or phosphate-depleted conditions to late logarithmic phase. Cells were harvested by centrifugation (15 min, 8,000 × g), washed and boiled for 10 min in water, and then centrifuged again. Lipids were isolated from the cell pellet (16). Poly-β-hydroxybutyrate, which accumulates in phosphate-deprived cells, was removed by addition of 4 volumes of n-hexane, centrifugation, and discarding of the pellet (18). Lipids were concentrated by evaporation of the solvent under N2 gas and dissolved in chloroform-methanol (2:1). Separation of the lipids by one- or two-dimensional thin-layer chromatography (TLC) was carried out on Baker Si 250 plates (J. T. Baker, Phillipsburg, NJ) or on TLC silica 60 plates (Merck, Darmstadt, Germany), respectively. For an improved one-dimensional separation, the Baker Si 250 plates were submerged in 0.15 M ammonium sulfate. The plates were dried at room temperature and activated for 2.5 h at 120°C prior to use. The solvent used for one-dimensional TLC was acetone-toluene-water (91:30:8). For the two-dimensional separation, the solvents were chloroform-methanol-water (65:25:4) for the first dimension and chloroform-methanol-glacial acidic acid-water (85:15:10:3.5) for the second dimension. Lipids were visualized with iodine vapor, α-naphthol-H2SO4, ninhydrin, and Dragendorff's reagent or under UV light after being sprayed with aniline naphthalene-sulfonic acid solution (1, 16). Commercial lipid standards were obtained from Supelco (Munich, Germany).
Lipid analysis by GC and GC-MS.
Lipids were separated by two-dimensional TLC as described above, fatty acid methyl esters (FAMEs) were prepared from each lipid (30 min at 80°C in 1 N methanolic HCl) and quantified by gas chromatography (GC)-liquid chromatography with a flame ionization detector (Agilent HP 6890 Plus GC) using pentadecanoic acid (15:0) as an internal standard (5). The mol% fraction of the polar lipids was calculated.
FAMEs were separated by GC-MS on an Agilent HP 6890 plus GC with mass selective detector 5973inert (Agilent Technologies, Böblingen, Germany), equipped with an HP-5MS column (30m; 0.25-mm diameter; 0.25-μm film; Agilent) using a temperature gradient of 140°C (2 min), followed by heating to 250°C (4 min) at 10°C/min, followed by cooling to 140°C at 20°C/min. Identification of the FAMEs was done using a bacterial fatty acid methyl ester mix (BAME-Mix; Supelco, Munich, Germany) or mass spectrometric (MS) analysis. During the analysis of mesorhizobial lipids, different 19:0 methoxy fatty acids were detected by GC-MS that were derived from 19:0-cyclo by ring opening during the derivatization (methylation) reaction (8, 26). Therefore, these fatty acids were included in the calculation of the 19:0-cyclo content. Furthermore, the amide linked hydroxy fatty acids which are only present in OL were not hydrolyzed under the methylation conditions and therefore not detected by GC-MS (7). Therefore, quantification of OL is based on the ester-linked fatty acid only.
Analysis of glycolipid head group composition by GC.
For the determination of the sugar composition of glycolipid head groups, the lipids were isolated from phosphate starved M. loti R7a by TLC. To eliminate glucose contamination when using ammonium sulfate-treated silica plates, the glycolipids were washed and extracted with chloroform-methanol-0.9% NaCl solution (2:1:0.75). Glycolipids were hydrolyzed, and the monosaccharides were converted into alditol acetates (28). Alditol acetates were separated by GC on an Agilent HP 6890 Plus GC system with a flame ionization detector (FID), equipped with a 30-m SP-2380 column (Supelco, Munich, Germany) using a temperature gradient of 160°C (2 min), followed by heating to 200°C (5 min) at 20°C/min, followed by additional heating to 245°C (12 min) at 20°C/min, followed by cooling to 160°C at 20°C/min.
Structural analysis of lipids by Q-TOF mass spectrometry.
Lipids were isolated as described above, and mass spectra were recorded using a quadrupole time-of-flight (Q-TOF) mass spectrometer (Q-TOF 6530; Agilent). The lipids were dissolved in chloroform-methanol-ammonium acetate (300:665:35) and directly infused at a flow rate of 1 μl min−1 using a chip-based nanospray ion source (HPLC Chip/MS 1200 with infusion chip; Agilent). Samples were analyzed in positive mode with a fragmentor voltage of 270 V. Molecular ions were selected in the quadrupole and then fragmented in the collision cell with nitrogen gas and a collision energy of 12 or 20 V (glycolipids, U1), 40 V (U2), 0.50 V (DGTS), 20 V (PE, MMPE, and DMPE), 33 V (PC), 30 V (OL), or 55 V (CL). The data were processed with the Mass Hunter Workstation software (version B.02.00; Agilent).
Nodulation experiments.
Lotus japonicus plants were grown from cuttings with 16 h of light and 8 h of darkness with day and night temperatures of 21 and 17°C, respectively. After 3 weeks, the plants were transferred to silica sand. The plants were watered twice a week with complete mineral mix without KNO3 (27), including 2.5 mM KH2PO4 (pH 6.8) for phosphate-sufficient or 2.5 mM KCl for phosphate-deficient conditions. The M. loti strains were grown in AB minimal medium with or without phosphate as described above to an OD600 of 0.5 and diluted (1:10) with water. After 3 weeks on sand, the plants were inoculated with M. loti R7a or M. loti Δpgt mutant. At 4 weeks after infection, the nodules per plant were counted.
Nodulation assays of M. loti in the presence of antibiotics were done according to a previously described method (23). The plants were grown on square plates (11 by 11 cm) on 1/4 B&D medium [1 liter of medium containing 14 g of agar and 250 μM CaCl2, 125 μM KH2PO4 or KCl, 2.5 μM iron(III) citrate, 62.5 μM MgSO4, 62.5 μM K2SO4, 0.25 μM MnSO4, 0.5 μM H3BO3, 0.125 μM ZnSO4, 0.25 μM CuSO4, 0.025 μM CoSO4, and 0.025 μM Na2MoO4]. Lotus seedlings were grown for 2.5 weeks under axenic conditions with or without phosphate. M. loti strains were grown to an OD600 of 0.02 to 0.05 in the presence or absence of phosphate. A culture volume of 50 μl per plant root was used for the infection. After 48 h, the roots of each plant were treated with 200 μl of tetracycline (10 μg ml−1). Nodules were counted 3 weeks after infection.
RESULTS
Inactivation of the gene mlr5650 in Mesorhizobium.
We previously characterized the ORF mlr5650 after recombinant expression as a gene coding for a processive glycosyltransferase with broad substrate specificity transferring glucose or galactose onto diacylglycerol, monoglycosyldiacylglycerol, and diglycosyldiacylglycerol. Heterologous expression led to the detection of a series of glycoglycerolipids. The main products were DGlycDs (GGD, DGD) and TGlycDs with different combinations of glucose and galactose in their head groups (16). Because of the processivity and the specificity for different sugar donors, the gene was denoted pgt+ (processive glycosyltransferase). The function of pgt+ remained unknown because the different DGlycDs and TGlycDs were not detectable in Mesorhizobium, and there were no reports on the occurrence of glycolipids in this organism (7, 21). Therefore, a deletion mutant was generated to study the physiological role of pgt+. The deletion mutant was obtained by insertion of a gentamicin resistance cassette into the mlr5650 locus by homologous recombination. The presence of the gene disruption in Δpgt was confirmed by PCR (Fig. 1). The Δpgt mutant did not show any difference in growth or lipid pattern compared to wild type when grown under optimal conditions. Therefore, the pgt+ gene has no essential function under optimal growth conditions.
FIG. 1.
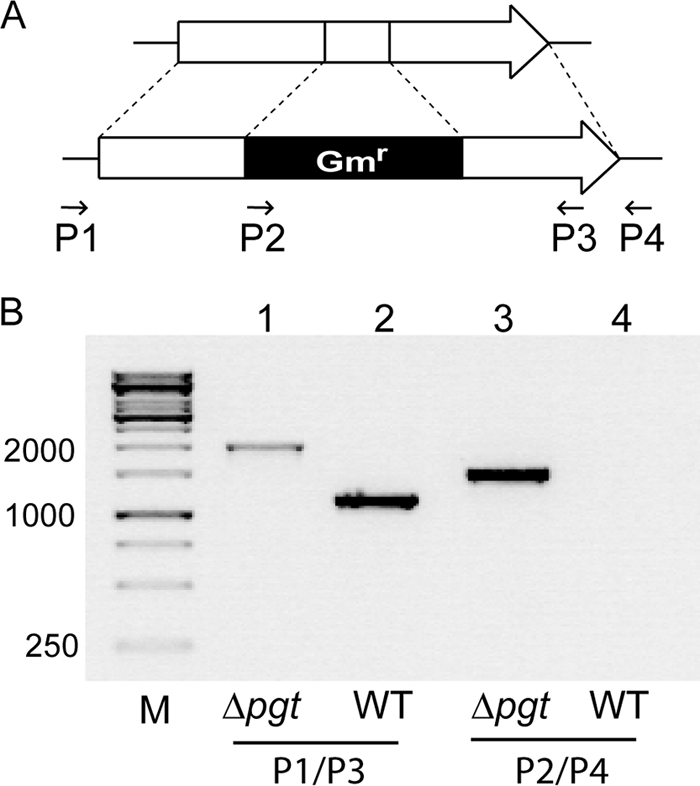
Insertional inactivation of the mlr5650 locus in Mesorhizobium. (A) Schematic representation of the mlr5650 locus disrupted by insertion of a gentamicin resistance cassette (Gmr). The arrows and numbers indicate the direction of the primers (P1, P2, P3, and P4) used for PCR analysis. (B) Confirmation of gene disruption by PCR and DNA gel electrophoresis. PCR was performed with DNA from the Δpgt mutant in lanes 1 and 3 and from the wild type (WT) in lanes 2 and 4. The primer pair P1/P3 was used for amplification of fragments in lanes 1 and 2, and the primer pair P2/P4 was used for amplification of fragments in lanes 3 and 4. The expected sizes of the fragments in lanes 1, 2, and 3 were 2.0, 1.2, and 1.5 bp, respectively. M, 1-kb marker.
Pgt is activated during phosphate deprivation and is involved in the synthesis of different glycoglycerolipids.
During phosphate deprivation, plants and some bacteria replace a fraction of their phosphoglycerolipids with glycoglycerolipids (15). We tested whether, in Mesorhizobium, glycoglycerolipids accumulate after phosphate deprivation by growing wild-type and Δpgt strains in phosphate-replete or -depleted minimal medium. Lipids were separated by one-dimensional TLC, and glycolipids were visualized by staining with α-naphthol. No glycolipids were observed when the cells were grown under full nutrition (Fig. 2). However, a complex pattern of glycolipids was visible in an extract from phosphate-starved wild-type cells. The glycolipid pattern resembles the one previously described after recombinant expression of the Mesorhizobium pgt+ gene (16). Two of the glycolipids accumulating in Mesorhizobium under phosphate deficiency comigrate with DGD and GGD (Fig. 2). Monosaccharide analysis of these two lipids by GC of alditol acetates revealed that the first lipid (DGD) contained 96% galactose and 4% glucose (standard deviation [SD], ±0.8%; n = 3) and the second lipid (GGD) contained 53.8% galactose and 46.2% glucose (SD, ±2.5%; n = 3), which is in good agreement with the calculated data. Additional glycolipids accumulating in Mesorhizobium during phosphate deprivation were separated into three distinct bands, which comigrate with TGlycDs obtained after heterologous expression of Pgt. Therefore, these additional glycolipids of Mesorhizobium were identified as different molecular species of TGlycD (16). A further, unknown glycolipid (U1) (Fig. 2) was detected in Mesorhizobium after phosphate deprivation. This lipid was previously not found after heterologous Pgt expression (16). U1 displays a mobility similar to that of diglucosyldiacylglycerol when separated by one-dimensional TLC (Fig. 2). However, analysis of alditol acetates by GLC revealed that galactose is the only sugar in the head group (92.6% galactose, 7.4% glucose; SD, ±5.8%; n = 3). None of the glycolipids (DGD, GGD, U1, and TGlycDs) was detected in the Δpgt mutant, indicating that Pgt activity is responsible for the accumulation of all of these glycolipids in wild-type Mesorhizobium during phosphate deprivation.
FIG. 2.
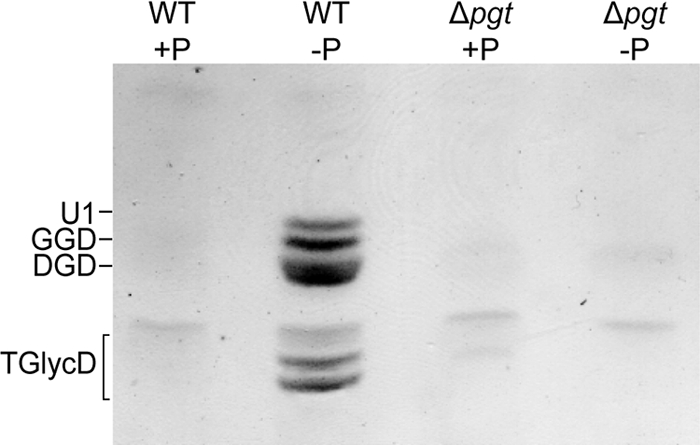
Glycoglycerolipids from Mesorhizobium wild type (WT) and Δpgt mutant grown in phosphate-replete (+P) or -depleted (−P) medium. Lipids were extracted from the cells and separated by TLC with acetone-toluene-water (91:30:8). Glycoglycerolipids were stained with α-naphthol.
In addition to phosphate starvation, we also tested the effect of nitrate depletion and high-salt and low-oxygen concentrations in the medium on the accumulation of glycolipids in Mesorhizobium. However, growth under these stress conditions did not lead to glycolipid accumulation as revealed by TLC (see Fig. S1 in the supplemental material) or Q-TOF mass spectrometry.
Separation and identification of phosphoglycerolipids and nonphosphorous lipids in Mesorhizobium wild-type and Δpgt strains.
For the analysis of the complex lipid pattern of Mesorhizobium cells grown in the presence or absence of phosphate, two-dimensional TLC was used to obtain full separation of the phosphoglycerolipids and nonphosphorous lipids (3). The lipids were identified by comigration with authentic standards, by staining with specific reagents, or by Q-TOF mass spectrometry. Two-dimensional TLC separation of extracts from Mesorhizobium wild type grown under full nutrition revealed the presence of PC, PG, CL, MMPE, DMPE, and the phosphorus-free lipid OL (Fig. 3A). PE was not detected. OL was identified by staining with ninhydrin, which is specific for free amino groups, and by Q-TOF analysis (see Fig. S2A in the supplemental material). Growth under phosphate limitation resulted in the accumulation of a series of glycoglycerolipids which were absent from the wild type grown under full nutrition. GGD, DGD, and the lipid spots containing TGlycD were recovered from the two-dimensional TLC and identified by comigration in one-dimensional TLC with the respective lipids obtained from recombinant Pgt-expressing cells (Fig. 3B) (16).
FIG. 3.
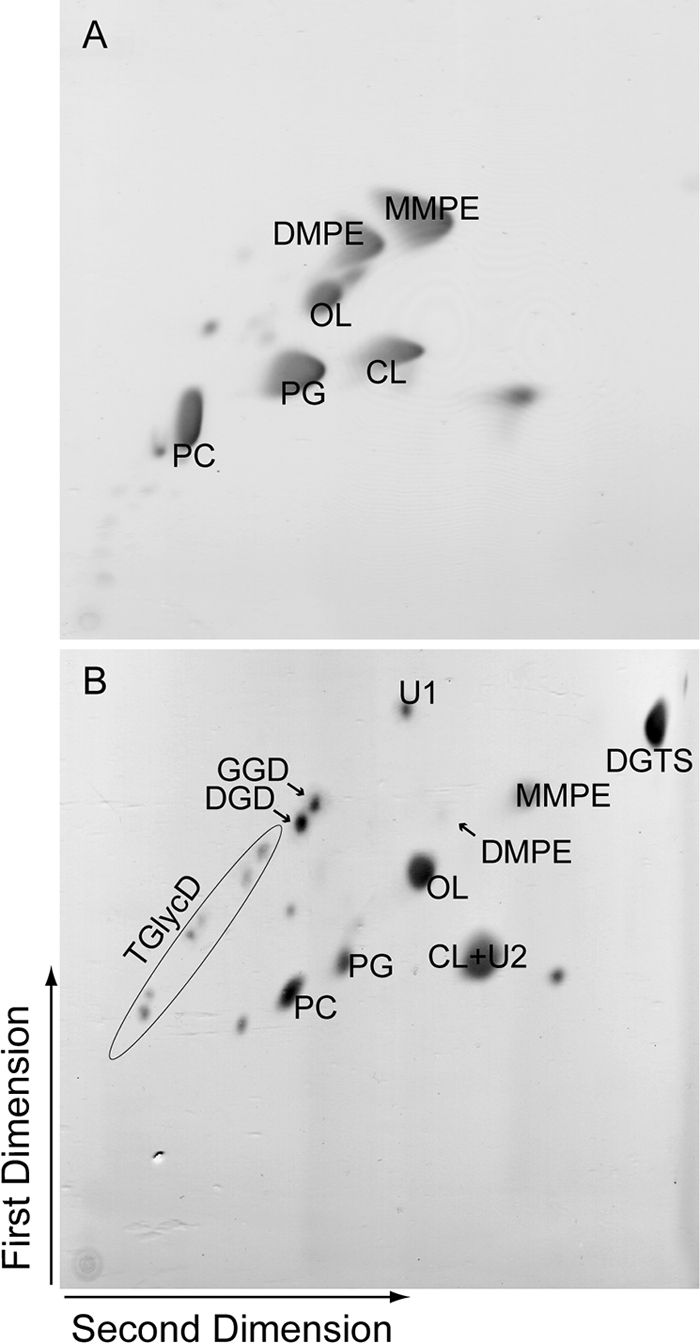
Two-dimensional TLC of lipid extracts from Mesorhizobium wild type grown under phosphate-replete (A) or phosphate-depleted (B) conditions. Lipids were separated in the first dimension in chloroform-methanol-water (65:25:4) and in the second dimension in chloroform-methanol-glacial acetic acid-water (85:15:10:3.5) and then stained with iodine vapor.
A second, unknown glycolipid (U2) was detected in Mesorhizobium during phosphate deprivation (Fig. 3). Isolation of the U2 spot from two-dimensional TLC plates and analysis using one-dimensional TLC resulted in the separation of two spots, cardiolipin (CL) and a glycolipid U2, the latter one comigrating with DGD and stained with α-naphthol. U2 can be detected in lipid extracts from the phosphate-starved wild-type and Δpgt mutant strains but not in phosphate-replete cultures. Thus, accumulation of U2 is independent of Pgt and, therefore, a second phosphate-deprivation-dependent glycosyltransferase must exist in Mesorhizobium.
An additional nonphosphorous lipid was detected by two-dimensional TLC (Fig. 3B), which was present in the wild type and Δpgt mutant grown under phosphate starvation. This lipid was stained with Dragendorff's reagent, which is specific for quaternary ammonium groups as found in PC and in DGTS. Q-TOF analysis confirmed that this lipid was DGTS (see Fig. S2B in the supplemental material).
Quantification of glycoglycerolipids, OL, and DGTS in Mesorhizobium during phosphate starvation.
In a first approach to study the lipid changes during phosphate deprivation on a quantitative level, total fatty acids of Mesorhizobium were measured by GC-MS of FAMEs. Table 1 shows the fatty acid distribution of Mesorhizobium wild type and Δpgt mutant grown under normal or phosphate-deficient conditions. The predominant fatty acids—18:1, 18:1(11-Me), and 19:0-cyclo—constitute ca. 80% of the total fatty acids. The amount of 16:0 was increased under phosphate deprivation, while the amounts of the other fatty acids in WT and Δpgt grown with or without phosphate were very similar. Two fatty acids, 18:1 and 18:1(11-Me), which are abundant in Mesorhizobium (32; the present study) were absent or hardly detectable in previous studies (7, 17, 32). The fatty acid 18:1 is the precursor for 18:1(11-Me) and 19:0-cyclo (14, 39). Therefore, the contents of these three fatty acids are interconnected and supposedly dependent on the culture conditions and the growth phase. This might explain the discrepancies in the fatty acid profiles in the different studies (7, 17, 32).
TABLE 1.
Fatty acid profile of Mesorhizobium wild-type and Δpgt mutant strains grown under phosphate-replete (+P) and phosphate-depleted (−P) conditions
| Fatty acid(s) | Mean fatty acid profile (mol%)a |
|||
|---|---|---|---|---|
| Wild type |
Δpgt mutant |
|||
| +P | −P | +P | −P | |
| 14:0 | 0.1 | 0.4 | 0.2 | 0.2 |
| 16:0 | 7.4 | 12.9 | 7.7 | 11.1 |
| 16:1 | 0.5 | 1.0 | 0.6 | 0.8 |
| 17:0 | 0.1 | ND | 0.3 | ND |
| 17:0-iso | 0.5 | 1.6 | 0.3 | 3.0 |
| 17:0-cyclo | 0.4 | 0.3 | 0.6 | 0.2 |
| 18:0 | 3.8 | 2.9 | 3.9 | 5.2 |
| 18:1 | 45.3 | 31.7 | 36.6 | 58.1 |
| 18:1(11-Me) | 18.0 | 8.1 | 19.0 | 4.3 |
| 19:0-cyclo | 23.5 | 40.8 | 29.9 | 16.2 |
| 20:0 | 0.5 | 0.5 | 0.9 | 0.8 |
| 18:1, 18:1(11-Me), 19:0-cyclo | 86.8 | 80.6 | 85.5 | 78.6 |
Values represent the mean of three measurements ± the standard deviation of one bacterial culture each. The experiment was repeated, and three additional measurements were performed with very similar results. ND, not detected. The standard deviations were <1.0% for all values.
The accumulation of glycoglycerolipids, DGTS and OL implies a possible role of these lipids as surrogates for phosphoglycerolipids under phosphate starvation. Changes in lipid content were determined after lipid separation via two-dimensional TLC (see Fig. 3) and quantification by GC-MS of fatty acid methyl esters. Because of the low abundance of the different glycoglycerolipids, only two glycoglycerolipid fractions were isolated for quantification, i.e., one fraction containing GGD, DGD and U1, and another fraction containing the TGlycDs (see Fig. 3). Furthermore, CL and the lipid U2 were quantified together because they comigrate in two-dimensional TLC. Table 2 shows the lipid composition of wild type and Δpgt mutant grown in the presence or absence of phosphate. Under high-phosphate conditions, there is no significant difference between the wild type and the Δpgt mutant according to the Student t test (P ≤ 0.05). The phosphoglycerolipids comprise ca. 90% of total polar lipids, with PC, MMPE, and PG being the predominant lipid classes. The only nonphosphorous lipid, OL, represents ca. 10 mol%. Under phosphate starvation, the Pgt-dependent glycolipids (GGD, DGD, U1, and TGlycDs) accumulate in the wild type to ca. 10 mol%. Furthermore, the amount of DGTS increases to more than 20 mol%. OL is slightly increased under phosphate deprivation. There is a strong reduction in the content of the phosphoglycerolipids PC, MMPE, and DMPE in the wild type during phosphate deficiency. The amount of the lipid fraction containing CL and U2 (calculated on the basis of four fatty acids per lipid molecule) was ca. 10 mol% during phosphate deprivation. The loss of GGD, DGD, U1, and TGlycD in the Δpgt mutant was compensated for by the increase of other, sugar-free lipids during phosphate deprivation. DGTS is the predominant lipid in Δpgt under low phosphate with ca. 30 mol%. The OL content is elevated in the mutant. The increase in CL + U2 during phosphate deprivation is comparable to the wild type. During phosphate deprivation, all of the phosphoglycerolipids were decreased in the mutant compared to the wild type. Taken together, phosphate starvation leads to a decrease in the amounts of phosphoglycerolipids from 90 to ca. 50% in the wild type and in the mutant. DGTS, OL, and glycolipids (including GGD, DGD, TGlycDs, U1, and U2) increase during phosphate deprivation and serve as surrogates for phosphoglycerolipids. The loss of Pgt-dependent glycoglycerolipids in the mutant can therefore be compensated for by an increased accumulation of DGTS, OL, and presumably U2.
TABLE 2.
Polar lipid composition of Mesorhizobium wild-type and Δpgt strains grown under phosphate-replete (+P) and phosphate-depleted (−P) conditions
| Lipid(s) | Mean polar lipid composition (mol%) ± SDa |
|||
|---|---|---|---|---|
| Wild type |
Δpgt mutant |
|||
| +P | −P | +P | −P | |
| GGD, DGD, U1 | ND | 7.3 ± 1.7 | ND | ND |
| TGlycD | ND | 3.0 ± 0.3 | ND | ND |
| DGTS | ND | 23.7 ± 7.2 | ND | 31.9 ± 5.4 |
| OL | 11.3 ± 2.6 | 15.7 ± 1.6 | 9.7 ± 4.7 | 20.0 ± 5.5 |
| CL, U2b | 4.6 ± 0.7 | 10.7 ± 1.3 | 3.9 ± 0.2 | 10.8 ± 1.4 |
| PC | 30.0 ± 5.2 | 15.3 ± 4.6 | 32.1 ± 1.3 | 16.6 ± 6.0 |
| PG | 16.7 ± 5.2 | 14.3 ± 3.1 | 22.5 ± 1.6 | 11.0 ± 5.0 |
| MMPE | 33.5 ± 6.5 | 9.2 ± 0.7 | 28.6 ± 2.1 | 8.0 ± 0.8 |
| DMPE | 4.0 ± 0.5 | 0.8 ± 0.7 | 3.1 ± 0.4 | 1.6 ± 0.2 |
Standard deviations are based on three independent experiments. ND, not detected (<0.5%).
Calculation based on the presence of four acyl groups per lipid; the calculation with only two acyl groups results in values of 19.4 ± 2.2 mol% (wild type, −P) and 19.5 ± 2.3 mol% (Δpgt mutant, −P) (see the text).
Pgt activity is not required for growth under phosphate deprivation.
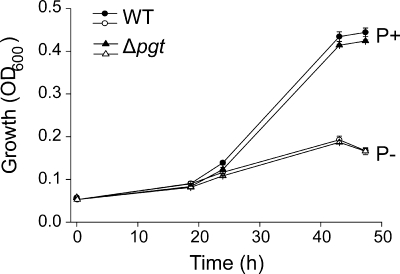
The accumulation of glycolipids, OL, and DGTS suggests that these phosphorus-free lipids are important for growth of Mesorhizobium under phosphate starvation. To investigate the function of the Pgt-dependent glycoglycerolipids, we analyzed the growth of wild-type and Δpgt mutant cells under conditions of high (1.25 mM) and low (0.6 μM) phosphate. The two lines are impaired in growth under phosphate starvation, but there is no difference between wild-type and mutant cells under high- or low-phosphate conditions (Fig. 4). Therefore, Pgt activity is not essential during phosphate starvation.
FIG. 4.
Growth curves of Mesorhizobium wild type (WT) and Δpgt mutant in media with 1.25 mM (filled symbols) or 0.6 μM (open symbols) phosphate. Mean values and error bars were calculated from three independent measurements.
Role of Pgt-dependent glycoglycerolipids in plant-microbe interactions and nitrogen fixation.
Nodulation experiments were performed to investigate the role of glycoglycerolipids for symbiotic interactions of M. loti with its host plant Lotus japonicus. The plants and bacteria were grown with high or low phosphate prior and after the infection. Plants were grown on sand, and the roots were inoculated with Mesorhizobium wild type or the Δpgt mutant. At 4 weeks after infection, nodule formation was studied. The number of nodules detected was dependent on the growth conditions. Under high phosphate, a single plant produced between 150 and 200 nodules, while under phosphate starvation, the number of nodules was reduced to values between 0 and 50 per plant (Fig. 5A). There were no significant differences in nodule numbers between plants treated with Mesorhizobium wild type or Δpgt mutant.
FIG. 5.
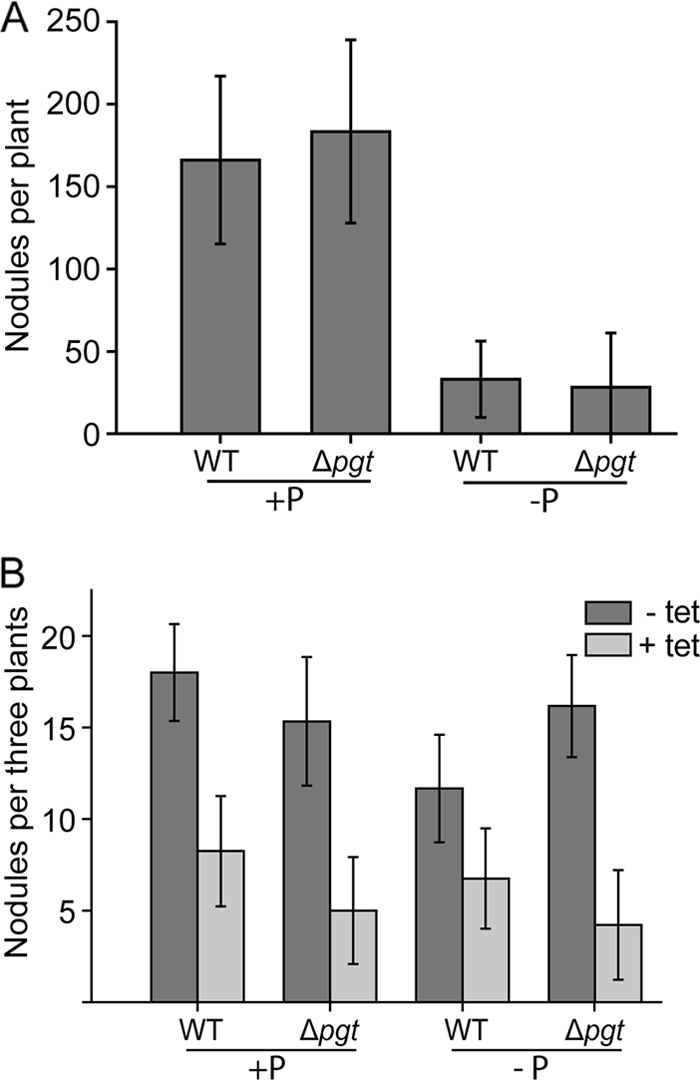
Number of nodules formed on Lotus roots inoculated with Mesorhizobium wild type (WT) or Δpgt mutant. Plants were grown under phosphate-replete or -depleted conditions, and bacteria were grown in medium with (+P) or without (−P) phosphate. (A) Plants grown on sand. Mean values and standard deviations are derived from 15 plants each. (B) Plants were grown under axenic conditions on synthetic medium. At 48 h after infection, the roots were treated with tetracycline (+ tet) or with sterile water (− tet). Mean values and standard deviations are derived from 20 to 30 plants; the results for one of three replicate experiments with similar results are shown.
To study whether glycolipids play a more subtle role during nodulation, Lotus plants were inoculated with Mesorhizobium, and the infection process was stopped after a defined time period by adding antibiotics to the roots according to Olivares et al. (23). This experimental setup was designed to compare only the initial infection rates rather the degree of nodulation after a prolonged exposure to rhizobial bacteria. Therefore, Lotus plants were grown under axenic conditions on agar plates. At 48 h after infection with Mesorhizobium wild type or Δpgt mutant, the root surface of the plants was treated with tetracycline to stop bacterial growth. Nodule formation was recorded 3 weeks after infection. No significant difference in the number of nodules on Lotus roots infected with Mesorhizobium wild type or Δpgt mutant was observed, independent of phosphate supply (Fig. 5B). Therefore, deficiency in Pgt-dependent glycolipid production in Mesorhizobium per se is not essential for nodulation when the plant and the bacteria are grown in the presence or absence of phosphate.
The surface of functionally active nodules is characterized by a red color indicative for the presence of leghemoglobin (22). No differences in nodule color were observed in plants infected with Mesorhizobium wild type or Δpgt mutant (see Fig. S3 in the supplemental material). Furthermore, plant growth and leaf color were very similar when we compared Lotus plants infected with Mesorhizobium wild type or Δpgt mutant grown in the presence or absence of phosphate (see Fig. S3 in the supplemental material). Therefore, the plants infected with Δpgt mutant did not suffer from nitrogen starvation. Taken together, the Mesorhizobium Δpgt mutant is capable of nitrogen fixation and can supply plants with nitrogen under high- and low-phosphate conditions in a way similar to that of wild-type cells.
DISCUSSION
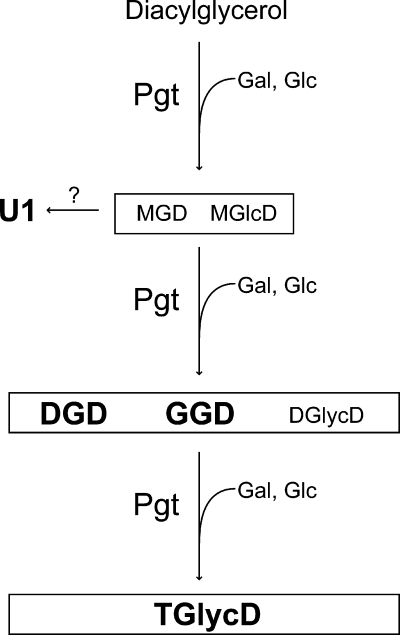
Replacement of phosphoglycerolipids with nonphosphorous lipids represents a physiological adaptation mechanism of many bacteria, algae, and plants to survive and compete in natural environments with low phosphate. M. loti contains a gene coding for a processive glycosyltransferase, which synthesizes a series of glycolipids after heterologous expression (16). To investigate the endogenous function of the pgt+ gene, a deletion mutant of Mesorhizobium was generated, and the cells were grown under conditions with high and low phosphate. There were no differences in the growth rate of wild-type and Δpgt strains, demonstrating that the pgt+ gene is not essential. However, Mesorhizobium wild type accumulated different glycolipids under phosphate starvation that were absent from the mutant. Therefore, Pgt represents a phosphate-starvation-dependent glycosyltransferase involved in glycoglycerolipid synthesis. Figure 6 shows a model for the processive synthesis of the different glycoglycerolipids by Pgt in Mesorhizobium.
FIG. 6.
Processive synthesis of different glycolipids by the Mesorhizobium glycosyltransferase Pgt. Starting with diacylglycerol as primary acceptor, Pgt produces MGD and monoglucosyl diacylglycerol (MGlcD) by transfer of galactose (Gal) or glucose (Glc), respectively, from the corresponding UDP-sugars (16). The two glycolipids are further glycosylated, resulting in the accumulation of DGD, GGD, and other diglycosyl diacylglycerols (DGlycD). A further glycosylation step leads to the synthesis of different TGlycDs with galactose or glucose in the head group (16; the present study). The unknown, Pgt-dependent glycolipid U1 might represent a DGD form with a divergent linkage between the sugars, or it might result from derivatization of MGD by an unknown enzyme. Boldface letters indicate lipids that are found in Mesorhizobium. MGD, MGlcD, and DGlycD represent minor components, which are detected only after heterologous Pgt expression (16).
Heterologous expression of Pgt led to the synthesis of GGD, DGD, and several molecular species of TGlycD with different combinations of glucose and galactose in their head groups (16). The glycolipid patterns after heterologous Pgt expression and that of Mesorhizobium wild type grown under phosphate starvation are highly similar. A further unknown Pgt-dependent glycolipid (U1) accumulated that contained galactose in the head group but was not observed after heterologous Pgt expression (16). Since it shows a similar mobility during TLC to that of diglucosyldiacylglycerol, U1 presumably contains two sugars in its head group. However, the TLC mobility of U1 is different from bacterial DGD with the typical (1→6)-linkage between the two galactose moieties. Previously, heterologous expression of a homologous glycosyltransferase from Agrobacterium led to the detection of a diglucosyldiacylglycerol with a (1→3)-linkage between the two sugars (16). This lipid is characterized by a higher mobility compared to the major diglucosyldiacylglycerol with (1→6)-linkage, synthesized by the agrobacterial glycosyltransferase. Therefore, the higher TLC mobility of U1 might be explained by a difference in linkage between the two galactoses. It is also possible that U1 represents a derivative of one of the other Pgt-dependent glycolipids after enzymatic modification. For example, acylation of the sugar would decrease the polarity and therefore increase the mobility of this lipid. Acyl derivatives of glycolipids exist in different bacteria (15). Additional experiments are required for final resolution of the U1 head group structure.
Choma et al. (7) analyzed the lipid compositions of different Mesorhizobium strains grown under full nutrition. The main phosphoglycerolipids were PE, MMPE, DMPE, PG, CL, and OL as the only nonphosphorous lipid. In this analysis, separation by two-dimensional TLC system, specific staining with different reagents, comigration with standards, and Q-TOF mass spectrometry allowed the identification of almost all lipids. Thus, we could for the first time identify the nonphosphorous lipid DGTS in Mesorhizobium. R. sphaeroides, and Sinorhizobium meliloti, which are related to Mesorhizobium, also synthesize DGTS during phosphate starvation (3, 13). DGTS synthesis depends on the two genes btaA and btaB (18, 20, 29). Orthologs of these two genes are found in some bacteria, particularly in members of the Alphaproteobacteria group, including Mesorhizobium (12, 21, 29). Furthermore, we detected another putative glycolipid (U2) which does not depend on Pgt activity. It was stained with α-naphthol, suggesting the presence of one or more sugars in its head group. Additional work is required to resolve the structure of the lipid U2 and identify the enzyme(s) involved in its synthesis.
Under normal conditions, membrane lipids in Mesorhizobium consist of ca. 90% of phosphoglycerolipids, the remainder being OL. Under phosphate deprivation, the wild type accumulates large amounts of different glycoglycerolipids and DGTS, and the content of OL increases as well. This increase in nonphosphorous lipids leads to a decrease in the proportion of phosphoglycerolipids to 50% of total membrane lipids. Similar responses are known from the related species Rhodobacter and Sinorhizobium. During phosphate deprivation, these two organisms accumulate DGTS, OL, and SQD. Furthermore, the glucosylgalactosyldiacylglycerol αGlc(1→4)βGal-diacylglycerol accumulates in Rhodobacter (Rhodobacterales), which is structurally different from GGD from Mesorhizobium. Another related species, the anoxygenic photosynthetic bacterium Blastochloris viridis (Rhizobiales), contains MGD, DGD, and glucuronosyl diacylglycerol, but in this organism glycolipids are not increased during phosphate starvation (19). Therefore, GGD, DGD, and the different TGlycDs identified in Mesorhizobium represent the first neutral glycoglycerolipids shown to accumulate during phosphate deprivation in the Rhizobiales. In the Mesorhizobium Δpgt mutant grown under phosphate depletion, the phosphoglycerolipids are decreased to a level similar to that observed for the wild type. The lack of Pgt-dependent glycolipids in Δpgt mutant is compensated for by the accumulation of DGTS, U2, and OL. These nonphosphorous lipids presumably mutually replace each other during phosphate-deficient growth conditions. This can explain why Pgt-dependent glycoglycerolipids are not required for growth of Δpgt during phosphate starvation. The mutual replacement of different nonphosphorous lipids was also observed in knock out mutants of Rhodobacter and Sinorhizobium when grown under phosphate starvation. The loss of SQD or DGTS in knockout mutants of Rhodobacter resulted in a partial compensation by increased amounts of OL or neutral glycolipids, respectively (2, 18). The most important surrogate lipid for phosphoglycerolipids in Sinorhizobium is DGTS, which accumulates to >60% of total lipids during phosphate starvation (20, 34). The loss of DGTS in a Sinorhizobium DGTS knockout mutant was almost completely compensated for by a strong accumulation of OL. Deletion mutants of DGTS, OL, or SQD, as well as double mutants of SQD/DGTS or SQD/OL, are not impaired in growth. Only the double-knockout mutant of DGTS/OL or the triple-mutant DGTS/OL/SQD is impaired in growth during phosphate starvation, presumably due to the complete loss of surrogate lipids (20). The fact that a large set of different nonphosphorous lipids is increased during phosphate deficiency suggests that these lipids can functionally replace each other during growth and nodule formation under phosphate deficiency. This can explain why the loss of Pgt-dependent glycolipids in Mesorhizobium or of other nonphosphorous lipids in Sinorhizobium or Rhizobium has no effect on nodulation (20, 34, 35). Furthermore, it is possible that the phosphate supply to the bacteroids in the nodules is much higher than in the surrounding soil. Therefore, the ability to accumulate different nonphosphorous lipids might be of minor importance for the symbionts and for nitrogen fixation (11, 20). Taken together, these results indicate a redundancy between the nonphosphorous lipids, which contributes to the flexibility of these organisms to adapt their membrane lipid compositions to changing environments.
In many Gram-negative bacteria, transport and metabolism of Pi is regulated at the transcriptional level. The regulated genes harbor an 18-bp consensus sequence upstream of their start codon, designated the “Pho box.” Expression of these genes is controlled by a transcriptional regulator, PhoB, which binds to the Pho box after phosphorylation. A list of Pho box-containing promoters of genes in Sinorhizobium and other members of Proteobacteria, including Mesorhizobium, was recently compiled by Yuan et al. (36). Interestingly, the mlr1574 gene of Mesorhizobium, which presumably is involved in DGTS synthesis under phosphate deprivation, was shown to harbor a Pho box motif in the promoter region. However, the pgt+ gene involved in glycoglycerolipid synthesis was not included in the list and therefore presumably is devoid of a Pho box. Detailed investigation of the sequence upstream of the pgt+ gene by alignment with the Pho box consensus sequence described by Yuan et al. (36) resulted in the identification of only one sequence with low similarity to the consensus (identity of 11 bases out of 18). Therefore, in contrast to the DGTS gene mlr1574, the pgt+ gene presumably does not contain a Pho box. These results indicate that Pgt activity might not be regulated via the transcriptional regulator PhoB, but other regulative mechanisms might be responsible for glycoglycerolipid accumulation under phosphate deprivation in Mesorhizobium. For example, enzyme activity might be posttranslationally regulated. Recently, phospholipase C activity was shown to be required for lipid changes during phosphate deprivation in Sinorhizobium (37). Therefore, the content of diacylglycerol might be involved in the regulation of glycoglycerolipid synthesis under phosphate deprivation.
Supplementary Material
Acknowledgments
This study was supported by a grant (Ho3870/1-2) from the Deutsche Forschungsgemeinschaft to G.H.
Footnotes
Published ahead of print on 14 January 2011.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Beiss, U. 1964. Zur Papierchromatographischen Auftrennung Von Pflanzenlipiden. J. Chromatogr. 13:104-110. [DOI] [PubMed] [Google Scholar]
- 2.Benning, C., J. T. Beatty, R. C. Prince, and C. R. Somerville. 1993. The sulfolipid sulfoquinovosyldiacylglycerol is not required for photosynthetic electron transport in Rhodobacter sphaeroides but enhances growth under phosphate limitation. Proc. Natl. Acad. Sci. U. S. A. 90:1561-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benning, C., Z. H. Huang, and D. A. Gage. 1995. Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 317:103-111. [DOI] [PubMed] [Google Scholar]
- 4.Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 5.Browse, J., P. McCourt, and C. R. Somerville. 1985. A mutant of arabidopsis lacking a chloroplast-specific lipid. Science 227:763-765. [DOI] [PubMed] [Google Scholar]
- 6.Cedergren, R. A., and R. I. Hollingsworth. 1994. Occurrence of sulfoquinovosyl diacylglycerol in some members of the family Rhizobiaceae. J. Lipid Res. 35:1452-1461. [PubMed] [Google Scholar]
- 7.Choma, A., and I. Komaniecka. 2002. Analysis of phospholipids and ornithine-containing lipids from Mesorhizobium spp. Syst. Appl. Microbiol. 25:326-331. [DOI] [PubMed] [Google Scholar]
- 8.Choma, A., and I. Komaniecka. 2003. The polar lipid composition of Mesorhizobium ciceri. Biochim. Biophys. Acta 1631:188-196. [DOI] [PubMed] [Google Scholar]
- 9.Gage, D. A., Z. H. Huang, and C. Benning. 1992. Comparison of sulfoquinovosyl diacylglycerol from spinach and the purple bacterium Rhodobacter sphaeroides by fast atom bombardment tandem mass spectrometry. Lipids 27:632-636. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Gaude, N., et al. 2004. The galactolipid digalactosyldiacylglycerol accumulates in the peribacteroid membrane of nitrogen-fixing nodules of soybean and Lotus. J. Biol. Chem. 279:34624-34630. [DOI] [PubMed] [Google Scholar]
- 12.Geiger, O., N. González-Silva, I. M. López-Lara, and C. Sohlenkamp. 2010. Amino acid-containing membrane lipids in bacteria. Prog. Lipid Res. 49:46-60. [DOI] [PubMed] [Google Scholar]
- 13.Geiger, O., V. Rohrs, B. Weissenmayer, T. M. Finan, and J. E. Thomas-Oates. 1999. The regulator gene phoB mediates phosphate stress-controlled synthesis of the membrane lipid diacylglyceryl-N,N,N-trimethylhomoserine in Rhizobium (Sinorhizobium) meliloti. Mol. Microbiol. 32:63-73. [DOI] [PubMed] [Google Scholar]
- 14.Grogan, D. W., and J. E. Cronan, Jr. 1997. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 61:429-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hölzl, G., and P. Dörmann. 2007. Structure and function of glycoglycerolipids in plants and bacteria. Prog. Lipid Res. 46:225-243. [DOI] [PubMed] [Google Scholar]
- 16.Hölzl, G., et al. 2005. Processive lipid galactosyl/glucosyltransferases from Agrobacterium tumefaciens and Mesorhizobium loti display multiple specificities. Glycobiology 15:874-886. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis, B. D. W., S. Sivakumaran, S. W. Tighe, and M. Gillis. 1996. Identification of Agrobacterium and Rhizobium species based on cellular fatty acid composition. Plant Soil 184:143-158. [Google Scholar]
- 18.Klug, R. M., and C. Benning. 2001. Two enzymes of diacylglyceryl-O-4′-(N,N,N-trimethyl)homoserine biosynthesis are encoded by btaA and btaB in the purple bacterium Rhodobacter sphaeroides. Proc. Natl. Acad. Sci. U. S. A. 98:5910-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linscheid, M., B. W. Diehl, M. Övermöhle, I. Riedl, and E. Heinz. 1997. Membrane lipids of Rhodopseudomonas viridis. Biochim. Biophys. Acta 1347:151-163. [DOI] [PubMed] [Google Scholar]
- 20.López-Lara, I. M., et al. 2005. Phosphorus-free membrane lipids of Sinorhizobium meliloti are not required for the symbiosis with alfalfa but contribute to increased cell yields under phosphorus-limiting conditions of growth. Mol. Plant-Microbe Interact. 18:973-982. [DOI] [PubMed] [Google Scholar]
- 21.López-Lara, I. M., C. Sohlenkamp, and O. Geiger. 2003. Membrane lipids in plant-associated bacteria: their biosyntheses and possible functions. Mol. Plant-Microbe Interact. 16:567-579. [DOI] [PubMed] [Google Scholar]
- 22.Minder, A. C., et al. 2001. Phosphatidylcholine levels in Bradyrhizobium japonicum membranes are critical for an efficient symbiosis with the soybean host plant. Mol. Microbiol. 39:1186-1198. [PubMed] [Google Scholar]
- 23.Olivares, J., J. Casadesus, and E. J. Bedmar. 1980. Method for testing degree of infectivity of Rhizobium meliloti strains. Appl. Environ. Microbiol. 39:967-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orgambide, G. G., R. I. Hollingsworth, and F. B. Dazzo. 1992. Structural characterization of a novel diglycosyl diacylglyceride glycolipid from Rhizobium trifolii ANU843. Carbohydr. Res. 233:151-159. [DOI] [PubMed] [Google Scholar]
- 25.Orgambide, G. G., S. Philip-Hollingsworth, R. I. Hollingsworth, and F. B. Dazzo. 1994. Flavone-enhanced accumulation and symbiosis-related biological activity of a diglycosyl diacylglycerol membrane glycolipid from Rhizobium leguminosarum biovar trifolii. J. Bacteriol. 176:4338-4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orgambide, G. G., R. N. Reusch, and F. B. Dazzo. 1993. Methoxylated fatty acids reported in Rhizobium isolates arise from chemical alterations of common fatty acids upon acid-catalyzed transesterification procedures. J. Bacteriol. 175:4922-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacovsky, R. S., and G. Fuller. 1988. Mineral and lipid composition of Glycine-Glomus-Bradyrhizobium symbioses. Physiologia Plantarum 72:733-746. [Google Scholar]
- 28.Reiter, W. D., C. Chapple, and C. R. Somerville. 1997. Mutants of Arabidopsis thaliana with altered cell wall polysaccharide composition. Plant J. 12:335-345. [DOI] [PubMed] [Google Scholar]
- 29.Riekhof, W. R., C. Andre, and C. Benning. 2005. Two enzymes, BtaA and BtaB, are sufficient for betaine lipid biosynthesis in bacteria. Arch. Biochem. Biophys. 441:96-105. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Eisenlohr, H., et al. 1999. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J. Bacteriol. 181:7485-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohlenkamp, C., I. M. López-Lara, and O. Geiger. 2003. Biosynthesis of phosphatidylcholine in bacteria. Prog. Lipid. Res. 42:115-162. [DOI] [PubMed] [Google Scholar]
- 32.Tighe, S. W., et al. 2000. Analysis of cellular fatty acids and phenotypic relationships of Agrobacterium, Bradyrhizobium, Mesorhizobium, Rhizobium, and Sinorhizobium species using the Sherlock Microbial Identification System. Int. J. Syst. Evol. Microbiol. 50(Pt. 2):787-801. [DOI] [PubMed] [Google Scholar]
- 33.Warnecke, D., and E. Heinz. 2003. Recently, discovered functions of glucosylceramides in plants and fungi. Cell. Mol. Life Sci. 60:919-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weissenmayer, B., J. L. Gao, I. M. Lopez-Lara, and O. Geiger. 2002. Identification of a gene required for the biosynthesis of ornithine-derived lipids. Mol. Microbiol. 45:721-733. [DOI] [PubMed] [Google Scholar]
- 35.Weissenmayer, B., O. Geiger, and C. Benning. 2000. Disruption of a gene essential for sulfoquinovosyldiacylglycerol biosynthesis in Sinorhizobium meliloti has no detectable effect on root nodule symbiosis. Mol. Plant-Microbe Interact. 13:666-672. [DOI] [PubMed] [Google Scholar]
- 36.Yuan, Z. C., R. Zaheer, R. Morton, and T. M. Finan. 2006. Genome prediction of PhoB regulated promoters in Sinorhizobium meliloti and twelve proteobacteria. Nucleic Acids Res. 34:2686-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zavaleta-Pastor, M., et al. 2010. Sinorhizobium meliloti phospholipase C required for lipid remodeling during phosphorus limitation. Proc. Natl. Acad. Sci. U. S. A. 107:302-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, X., S. M. Ferguson-Miller, and G. E. Reid. 2009. Characterization of ornithine and glutamine lipids extracted from cell membranes of Rhodobacter sphaeroides. J. Am. Soc. Mass Spectrom. 20:198-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, Y. M., and C. O. Rock. 2008. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 6:222-233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.