Abstract
Hundreds of bacterial species use microcompartments (MCPs) to optimize metabolic pathways that have toxic or volatile intermediates. MCPs consist of a protein shell encapsulating specific metabolic enzymes. In Salmonella, an MCP is used for 1,2-propanediol utilization (Pdu MCP). The shell of this MCP is composed of eight different types of polypeptides, but their specific functions are uncertain. Here, we individually deleted the eight genes encoding the shell proteins of the Pdu MCP. The effects of each mutation on 1,2-PD degradation and MCP structure were determined by electron microscopy and growth studies. Deletion of the pduBB′, pduJ, or pduN gene severely impaired MCP formation, and the observed defects were consistent with roles as facet, edge, or vertex protein, respectively. Metabolite measurements showed that pduA, pduBB′, pduJ, or pduN deletion mutants accumulated propionaldehyde to toxic levels during 1,2-PD catabolism, indicating that the integrity of the shell was disrupted. Deletion of the pduK, pduT, or pduU gene did not substantially affect MCP structure or propionaldehyde accumulation, suggesting they are nonessential to MCP formation. However, the pduU or pduT deletion mutants grew more slowly than the wild type on 1,2-PD at saturating B12, indicating that they are needed for maximal activity of the 1,2-PD degradative enzymes encased within the MCP shell. Considering recent crystallography studies, this suggests that PduT and PduU may mediate the transport of enzyme substrates/cofactors across the MCP shell. Interestingly, a pduK deletion caused MCP aggregation, suggesting a role in the spatial organization of MCP within the cytoplasm or perhaps in segregation at cell division.
Many diverse bacteria use proteinaceous microcompartments (MCPs) as simple organelles for the optimization of metabolic pathways that have toxic or volatile intermediates (4, 11, 14, 61). Bacterial MCPs are polyhedral in shape and 100 to 150 nm in cross-section (about the size of a large virus) and consist of a protein shell that encapsulates metabolic enzymes. They are composed of 10,000 to 20,000 polypeptides of 10 to 20 types, and there is no evidence for lipid components. Based on sequence analysis, it is estimated that MCPs are produced by 20 to 25% of bacteria and function in seven or more different metabolic processes (2, 4, 14). Different types of MCPs have related protein shells but differ in their encapsulated enzymes. In the cases that have been studied, MCPs encase enzymes that catalyze sequential reactions with a toxic or volatile intermediate. The best-studied MCP is the carboxysome, which is used to enhance autotrophic CO2 fixation by confining CO2 in the immediate vicinity of ribulose bisphosphate carboxylase monooxygenase (11, 42). Other MCPs are used to confine toxic/volatile aldehydes formed during the catabolism of ethanolamine and 1,2-propanediol (1,2-PD) or have unknown functions (8, 24, 41, 46, 47, 54). The protein shell of MCPs is thought to act as a diffusion barrier that helps retain the volatile/toxic intermediate and channel it to downstream enzymes (19, 24, 41, 44).
The protein shells of bacterial MCPs are typically composed of 5 to 10 different proteins that have bacterial microcompartment (BMC) domains (60). Recent crystallography of BMC proteins from several organisms has provided insights into the structural basis of shell assembly and function (17, 26, 32, 33, 48, 55, 56). In crystals, single-BMC-domain proteins usually form flat hexamers that tile into molecular sheets proposed to form the facets of the shell (32). Shell proteins with two tandem BMC domains form hexagonal trimers suitably shaped to form mixed sheets with single-BMC-domain proteins, suggesting that MCP shells are a mosaic of different types of BMC domain proteins (26, 33, 48). Another BMC protein (EutS) is a bent hexamer that could form the edges of the shell (57). A striking feature of BMC domain proteins is that they have central pores thought to mediate the transport of enzyme cofactors, substrates, and products between the interior of the MCP and the cytoplasm of the cell (32). Different shell proteins have pores that differ in charge and size, suggesting substrate selectivity (17, 26, 32, 33, 48, 55, 56). Moreover, some BMC domain proteins appear to have gated pores, and in one instance a BMC domain protein has an iron-sulfur center that might be used to conduct electrons between the cell cytoplasm and the interior of the MCP (16, 39). Lastly, a BMC domain fused to a probable DNA-binding protein was reported (57), and one class of shell protein which lacks a recognizable BMC domain (CcmL type) forms pentamers proposed to form the vertices of the shell (55). Overall, structural studies suggest that the shells of diverse MCPs are built from several types of functionally specialized shell proteins needed for assembly of the shell, metabolite transport, and other functions.
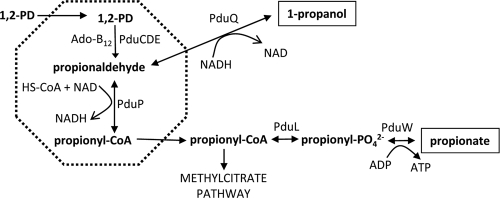
Our prior studies showed that an MCP is used for coenzyme B12-dependent 1,2-PD utilization (Pdu MCP) by Salmonella enterica (4, 6, 7, 17, 23, 24, 34, 35, 50). 1,2-PD is a major product of the anaerobic degradation of the common plant sugars rhamnose and fucose, and this product is thought to be an important carbon and energy source in anoxic environments such as sediments, the depths of soils, and the large intestines of higher animals (38). Moreover, the capacity to degrade 1,2-PD is tentatively linked to pathogenesis in Salmonella and Listeria (9, 15, 25, 31). The first two steps of 1,2-PD degradation take place within the lumen of the Pdu MCP, where 1,2-PD is converted to propionaldehyde and then to propionyl-coenzyme A (CoA) by B12-dependent diol dehydratase (PduCDE) and propionaldehyde dehydrogenase (PduP), respectively (Fig. 1) (23, 24, 34). Propionyl-CoA is thought to exit the MCP into the cytoplasm, where it is converted to propionate or enters central metabolism via the methylcitrate pathway (27, 38). The shell of the Pdu MCP is proposed to confine the propionaldehyde formed in the first step of 1,2-degradation in order to mitigate its toxicity as well as reduce DNA damage and limit diffusive loss through the cell membrane into the environment (6, 23, 24). The genes for 1,2-PD utilization (pdu) are found in a single contiguous cluster (pocR, pduF, pduABB′ CDEGHJKLMNOPQSTUVWX) (5-7, 12, 20, 21, 30, 34, 35, 45). Analyses of purified MCPs and labeling studies indicate that the Pdu MCP is composed of at least 17 polypeptides, all of which are encoded by the pdu operon (the PduABB′CDEGHJKNOPSTUV gene) (13, 23, 40). The shell of the Pdu MCP is thought to include eight different polypeptides (PduABB′JKNTU) (23). PduABB′JKTU have a BMC domain(s), and PduN is homologous to a pentamer that forms the vertices of the carboxysome shell (6). Prior studies with Salmonella showed that PduA is a component of the shell (24) and that pduAB or pduJK double deletion mutants were defective in MCP formation (24, 50). In addition, these double mutants where shown to accumulate propionaldehyde to toxic levels, resulting in a 20-hour period of growth arrest and increased DNA damage, indicating that a primary function of the Pdu MCP is to mitigate propionaldehyde toxicity (24, 50). In more-recent studies, the Citrobacter pdu operon was expressed in Escherichia coli and shown to mediate the formation of MCPs (39). Further studies in this system showed that the pduA or pduBB′ genes were required for MCP formation (39). Very recently, the Citrobacter PduABB′JKN proteins were produced in recombinant E. coli and shown to be necessary and sufficient for formation of what appeared to be empty MCP shells, suggesting that they are structural proteins of the MCP shell (40). Thus, prior studies suggest that the PduABB′JKNTU proteins are shell components. However, their specific roles are uncertain, and the effects of deleting these genes singly (with the exception of pduA and pduBB′) on MCP structure and 1,2-PD degradation have not been reported previously. Therefore, to better understand the specific functional and structural roles of the Pdu MCP shell proteins, we constructed in-frame deletion mutants of each Salmonella pdu shell gene individually (pduA, pduBB′, pduJ, pduK, pduN, pduT, and pduU) and determined their effects on MCP structure and 1,2-PD catabolism using electron microcopy, growth studies, and metabolite measurements.
FIG. 1.
Model for 1,2-propanediol degradation by Salmonella. The dashed line indicates the shell of the MCP, which is composed of eight different polypeptides (PduABB′JKNTU). The first two steps of 1,2-PD degradation occur in the lumen of the compartment and the remaining steps in the cytoplasm. The function of the Pdu MCP is to sequester the propionaldehyde produced by the first reaction of 1,2-PD degradation to minimize its toxicity. For the Pdu MCP to function, enzyme substrates, products, and cofactors must cross the shell and the MCP must segregate properly during cell division. Abbreviations: 1,2-PD, 1,2-propanediol; PduCDE, coenzyme B12-dependent diol dehydratase; PduP, propionaldehyde dehydrogenase; PduL, phosphotransacylase; PduW, propionate kinase; PduQ, 1-propanol dehydrogenase.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains used in this study are listed in Table 1. The rich medium used was Luria-Bertani/Lennox (LB) medium (Difco, Detroit, MI) (36). The minimal medium used was no-carbon-E (NCE) medium (3, 58).
TABLE 1.
Bacterial strains used in this study
| Straina | Genotype | Source or reference |
|---|---|---|
| BE464 | ΔpduA687::frt | This study |
| BE213 | ΔpduBB′675 | This study |
| BE184 | ΔpduJ654 | This study |
| BE770 | ΔpduK680 | This study |
| BE772 | ΔpduN681::frt | This study |
| BE885 | ΔpduT682::frt | This study |
| BE901 | ΔpduU683::frt | This study |
| BE287 | /pLAC22-no insert | 59 |
| TA1464 | ΔpduA687::frt/pLAC22-pduA | This study |
| TA1409 | ΔpduBB′675/pLAC22-pduBB′ | This study |
| TA1411 | ΔpduBB′675/pLAC22-no insert | This study |
| TA1412 | ΔpduJ654/pLAC22-pduJ | This study |
| TA1414 | ΔpduJ654/pLAC22-no insert | This study |
| BE777 | ΔpduK680::frt/pLAC22-no insert | This study |
| BE778 | ΔpduK680::frt/pLAC22-pduK | This study |
| BE779 | ΔpduN681::frt/pLAC22-no insert | This study |
| BE780 | ΔpduN681::frt/pLAC22-pduN | This study |
| BE918 | ΔpduT682::frt/pLAC22-pduT | This study |
| BE917 | ΔpduT682::frt/pLAC22-no insert | This study |
| BE924 | ΔpduU683::frt/pLAC22-pduU | This study |
| BE925 | ΔpduU683::frt/pLAC22-no insert | This study |
All strains are derivatives of Salmonella enterica serovar Typhimurium LT2.
Chemicals and reagents.
Antibiotics were from Sigma Chemical Company (St. Louis, MO). Isopropyl-β-d-thiogalactopyranoside (IPTG) was from Diagnostic Chemicals Limited (Charlotteville PEI, Canada). Restriction enzymes and T4 DNA ligase were from New England BioLabs (Beverly, MA). Other chemicals were from Fisher Scientific (Pittsburgh, PA).
General molecular methods.
Agarose gel electrophoresis, plasmid purification, PCR, restriction digests, ligation reactions, and electroporation were carried out using standard protocols as previously described (35, 49). Plasmid DNA was purified by the alkaline lysis procedure (49) or by using Qiagen products (Qiagen, Chatsworth, CA) according to the manufacturer's instructions. Following restriction digestion or PCR amplification, DNA was purified using Promega Wizard PCR Preps (Madison, WI) or Qiagen gel extraction kits. Restriction digests were carried out using standard protocols (49). For ligation of DNA fragments, T4 DNA ligase was used according to the manufacturer's directions. Electroporation was carried out as previously described (6).
P22 transduction.
Transductional crosses were performed as previously described, using P22 HT105/1 int-210, a mutant phage that has high transducing ability (53). Transductants were tested for phage contamination and sensitivity by streaking on green plates against P22 H5.
Construction of clones for complementation studies.
The coding sequences of pduA, pduBB′, pduJ, pduK, pduN, pduT, and pduU were cloned into pLAC22 via PCR with template pEM55 as previously described (6). Vector pLAC22 allows tight regulation of protein production by IPTG (59). The DNA sequences of all clones were verified.
Construction of pdu deletion mutants.
Two PCR-based methods were used to construct pdu deletion mutants. In each case, deletions removed nearly the entire coding sequence but left predicted translation signals of all pdu genes intact. The pduBB′ and pduJ deletions were made by the method of Miller and Mekalanos with modifications previously described (37). The pduA, pduK, pduN, pduT, and pduU deletions were made by linear transformation of PCR products with modifications as described previously (18, 24). For mutants made by linear transformation, the kanamycin resistance cassette was moved to wild-type Salmonella by P22 transduction and then removed using the flp recombinase as described previously (18). The DNA sequences of Del pduA687 (BE464) and Del pduK680 (BE770) were verified by direct sequencing of chromosomal DNA (1 μg/μl) purified using a Qiagen DNeasy tissue kit and using primers pduA-348FF (GCCCATCATACGGGAGATTCGAGC), pduA-114FR (CTGCCATAGCCGTCTCTCGTATAG), pduK448FF (ACACTCGCTGGTCGTGCATTA), and pduK431FR (AAGCGGCGACATATGGATAT). In addition, PCR was used to verify all deletions as described previously (18).
Growth studies.
Growth rates were determined using a Synergy HT microplate reader (BioTek, Winooski, VT) as previously described (35). Doubling times were calculated from semilog plots, where doubling time was calculated as 0.693/(2.303)(slope of the linear region of the plot). For determination of propionaldehyde levels, 50-ml cultures were grown in 250-ml Erlenmeyer flasks at 37°C with shaking at 275 rpm in an Innova I2400 incubator shaker (New Brunswick Scientific) (24). These cultures were inoculated to an optical density at 600 nm (OD600) 0.1 with an LB overnight culture that had been centrifuged and resuspended in NCE with 1 mM MgSO4.
Propionaldehyde determination.
Cultures were sampled at timed intervals. Cells were removed by centrifugation followed by filtration with 0.22-μm Millex-GV syringe filters (Millipore Corporation). Propionaldehyde was then determined by high-performance liquid chromatography (HPLC) and by the 3-methyl- 2-benzothiazolinone hydrazone (MBTH) assay as described previously (50).
Electron microscopy.
For electron microscopy, strains were grown in 125-ml Erlenmeyer flasks containing 10 ml of NCE minimal medium supplemented with 1 mM MgSO4, 0.5% succinate, 0.4% 1,2-PD, and 50 μM ferric citrate. Cultures were inoculated to an OD600 of 0.1 with an LB overnight culture that had been centrifuged and resuspended in NCE medium with 100 μM MgSO4. After the cultures reached optical densities between 1 and 1.2 at 600 nm, cells were harvested by centrifugation. Imbedding, sectioning, and electron microscopy were carried out as described previously (6, 24).
SDS-PAGE and Western blots.
Protein concentration was determined using Bio-Rad (Hercules, CA) protein assay reagent, with bovine serum albumin (BSA) as a standard. SDS-PAGE was performed using Bio-Rad 18% Tris-HCl ready gels. For Western blots, proteins were transferred from SDS-PADE gels to nitrocellulose membranes and detected by primary antibody from rabbit and goat anti-rabbit immunoglobulin conjugated to alkaline phosphatase as a secondary antibody (Bio-Rad). Chromogenic developing agents were used in accordance with the manufacturer's instructions (Bio-Rad).
DNA sequencing and analysis.
DNA sequencing was carried out at the Iowa State University DNA Facility using Applied Biosystems, Inc., automated sequencing equipment. The template for DNA sequencing was plasmid DNA purified using Qiagen 100 tips or Qiagen miniprep kits. BLAST software was used for sequence similarity searching (1).
RESULTS
Electron microscopy of MCP shell mutants.
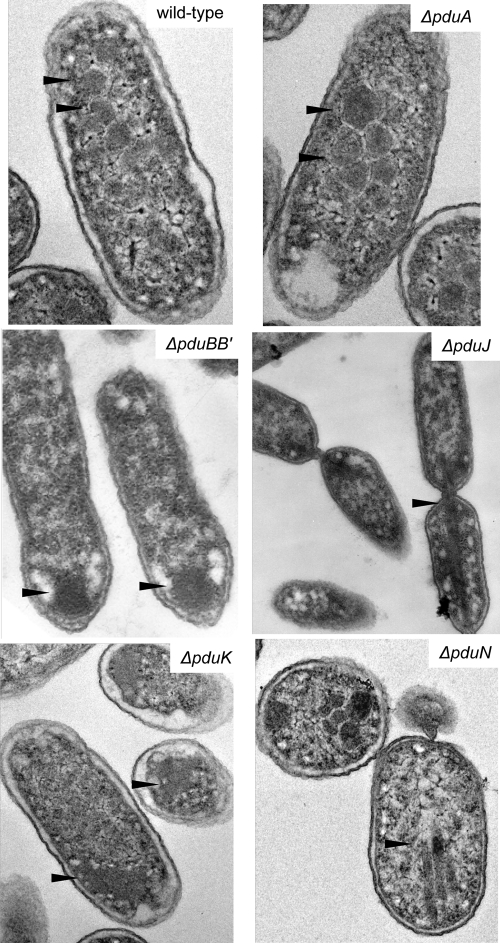
Our prior studies indicated that the pduA, pduB, pduB′, pduJ, pduK, pduN, pduT, and pduU genes of Salmonella encode shell proteins of the Pdu MCP (23, 24, 50); however, the effects of deleting individual Salmonella shell protein genes on MCP structure have not been reported. Previously, a Salmonella pduA deletion mutant was investigated by our laboratory, but we later found that this mutant was constructed based on erroneous DNA sequence data and deleted both the pduA gene and the start of the pduB gene (24). Here, a new pduA deletion mutant was constructed based on a corrected DNA sequence, and electron microscopy was used to examine the effects of the pduA, pduBB′, pduJ, pduK, pduN, pduT, or pduU deletion mutations on the structure of the Pdu MCP of Salmonella. In all cases, several sections and hundreds of cells were examined from cultures grown on at least 2 different days. Representative images are presented in Fig. 2 and Fig. S2 to S9 in the supplemental material. Results showed that a strain with a pduA deletion formed MCPs that were somewhat enlarged compared to the wild type but generally similar in appearance (Fig. 2). For both wild-type Salmonella and the pduA deletion mutant, 40 MCPs were measured (80 total) in thin sections. Each MCP was measured twice, first on the longest axis and then 90 degrees to the first measurement, using ImageJ software (43) or manual measurements. The average of these two measurements was taken to indicate the cross-sectional length of the MCP. The mean cross-sectional length of wild-type MCPs ± 1 standard deviation was 123 ± 30 nm, compared to 155 ± 48 nm for MCPs from the pduA deletion mutant. We also plotted the size distribution of MCPs from wild type and the pduA mutant (Fig. S1). Compared to the wild type, the pduA mutant formed about 9-fold more MCPs that measured ≥180 nm. Thus, the pduA deletion mutant formed enlarged MCPs. This is in contrast to prior studies with the incorrect pduA mutant mentioned above (actually a pduAB double mutant), which did not form MCPs at all (24).
FIG. 2.
Electron microscopy of wild-type Salmonella and selected deletion mutants. The bar is 200 nm. For more images, see Fig. S2 to S9 in the supplemental material.
pduB and pduB′ are overlapping genes (23, 39) and hence were deleted together. In a pduBB′ deletion mutant, MCP formation was eliminated. No MCPs were observed in several hundred cells examined, and a single polar body was seen in about 20% of the cells (Fig. 2; see also Fig. S2 to S4 in the supplemental material). In addition, <1% contained unusual structures other than the polar bodies. Given their low numbers, these structures were most likely electron microscopy artifacts. In contrast, no polar bodies were observed in wild-type Salmonella, MCPs were present in >90% of cells, and unusual protein structures were seen in <1% of cells. Thus, the electron microscopy indicates that the pduBB′ deletion mutant was unable to from MCPs.
In a strain with a pduJ deletion, highly elongated structures were observed in 22% of cells (Fig. 2). Further, ∼20% of the examined cells contained amorphous inclusion bodies similar in appearance to those seen for the pduBB′ deletion mutant. Most cells (57%) lacked MCPs or other unusual structures. Fewer than 1% of cells contained unusual protein structures of uncertain origin. This is in stark contrast to what was found for the wild type, where ∼90% of cells contained normal-appearing MCPs. Thus, a pduJ deletion either prevents MCP formation or leads to the formation of elongated structures. This tentatively suggests that assembly might arrest at two different steps in a pduJ mutants, raising the possibility of more than one assembly pathway.
A pduK deletion resulted in the formation of what appeared to be aggregates of MCPs (Fig. 2; see also Fig. S5 to S8 in the supplemental material) in ∼52% of the cells examined. About 1% of cells contained unusual structures of uncertain origin. In ∼47% of the cells examined, neither normal nor aggregated MCPs were detected. In contrast, no aggregates similar in appearance to those formed by the pduK deletion mutant were observed in wild-type cells, and ∼90% of cells contained normal-appearing MCPs. The aggregates formed in pduK mutants differed from the polar bodies found in pduBB′ mutants in that they appeared to be composed of MCPs delineated by shells, whereas the perimeters of the polar bodies were amorphous and lacked any observable indication of a shell. Moreover, growth studies and metabolite measurements indicated that the MCP aggregates formed in a pduK mutant function normally, supporting an intact shell (see below). Thus, we infer that a pduK deletion primarily results in the formation of aggregates of normally functioning MCPs.
A pduN mutant formed MCPs with a variety of morphologies, including elongated, enlarged, and aggregated MCPs and some with rounded cross-sections (Fig. 2; see also Fig. S9 in the supplemental material). The majority (∼90%) of cells observed contained MCPs with unusual shapes. Based on visual appearance (size, shape, and staining density), we judge that few to none of the MCPs were of normal appearance. The variety of MCP sizes and shapes seen in a pduN deletion mutant is not easily described by size measurements; we therefore refer the reader to Fig. 2 and Fig. S9.
We also examined the effects of a pduT or a pduU deletion on MCP structure. These mutations had no obvious effects on MCP formation, based on electron microscopy observations (not shown). For the pduU and pduT mutants, ∼90% of cells contained normal-appearing MCPs. By visual inspection, the size, shape, and staining density of MCPs from these mutants were similar to those of the wild type. The cross-sectional sizes of MCPs formed by pduT and pduU deletion mutants were determined as described above. The mean values ± 1 standard deviation were 121 ± 20 nm for the ΔpduT mutant and 113 ± 23 nm for the ΔpduU mutant compared to the value for the wild type, which was 123 ± 30 nm. In addition, the size distributions of MCPs found in the wild type and in pduT and pduU mutants were similar (see Fig. S1 in the supplemental material). Thus, pduT and pduU mutants form normal-appearing MCPs.
PduN is a component of the Salmonella Pdu MCP.
PduN is homologous to CcmL and CsoS4A, which are non-BMC, pentameric proteins proposed to form the vertices of the carboxysomes (55). However, in prior studies, PduN was not identified as a component of purified Salmonella Pdu MCPs (23). This may have been due to low abundance. CsoS4A was not initially detected in purified carboxysomes (although it was detected in later studies by Western blot analysis), and individual icosahedra have only 12 vertex proteins among the hundreds of hexamers needed to form the triangular facets that comprise the bulk of the shell (10, 55). In addition, green fluorescent protein (GFP)-labeled PduN was shown to associate with what appear to be empty MCP shells when the PduABB′JKN proteins from Citrobacter are expressed from a plasmid in E. coli (40). Thus, PduN is thought to be a low-abundance component of the Pdu MCP. To directly test whether PduN is a component of intact Salmonella Pdu MCPs, Western blot anslyses were performed (see Fig. S10 in the supplemental material). A band near 12 kDa was detected in MCPs purified from wild-type S. enterica, while no PduN band was detected in MCPs purified from the pduN deletion mutant. Furthermore, when similar amounts of protein were analyzed, Western blot analysis readily detected PduN in purified MCPs but not in crude cell extracts (Fig. S10). This indicated that purified MCPs were enriched in PduN, strongly indicating that PduN protein is a component of intact Pdu MCPs from Salmonella.
Growth of MCP shell mutants on 1,2-PD with saturating or limiting B12.
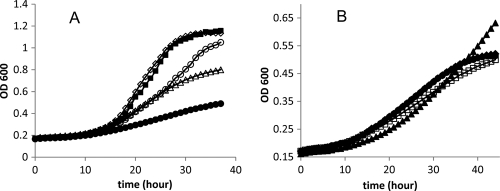
Prior studies showed that strains of Salmonella having a pduAB or pduJK double deletion were unable to form MCPs and accumulated propionaldehyde to levels that induced 20 to 30 h of growth arrest and increased DNA damage, supporting the idea that the Pdu MCP functions to minimize propionaldehyde toxicity (50). For this report, we conducted growth studies on strains with nonpolar deletions in pduA, pduBB′, pduJ, pduK, pduN, pduT, or pduU individually and on wild-type Salmonella. At saturating B12, the wild type grew normally and accumulated propionaldehyde to about 2 mM (Fig. 3). Under similar growth conditions, two classes of mutants were found. For pduA, pduBB′, pduJ, and pduN deletion mutants, propionaldehyde accumulated to 12 to 20 mM and induced a period of growth arrest that lasted 15 to 20 h. The results obtained with a pduBB′ deletion mutant are shown in Fig. 3. For this mutant, growth arrest lasted 20 h and propionaldehyde peaked at 18.2 mM. For pduA, pduJ, and pduN deletion mutants, the following values were obtained for growth arrest and maximum propionaldehyde level: for ΔpduA, 12 h and 12 mM; for ΔpduJ, 16 h and 14 mM; and for ΔpduN, 20 h and 16 mM. The growth rates for pduA, pduBB′, pduJ, and pduN deletion mutants were about the same as those for the wild type (1.2, 1.0, 1.2, and 0.93 times the wild-type rate, respectively) at saturating B12 (Table 2). In contrast, for pduK, pduT, and pduU deletion mutants, propionaldehyde levels stayed below 2 mM (similar to the wild-type level), and growth rates with 1,2-PD and saturating B12 were similar to the wild-type rate for the ΔpduK mutant (0.96 relative to the wild-type rate) and moderately impaired for the ΔpduT and ΔpduU mutants (0.67 and 0.66 times the wild-type rate, respectively) (Fig. 4 and Table 2).
FIG. 3.
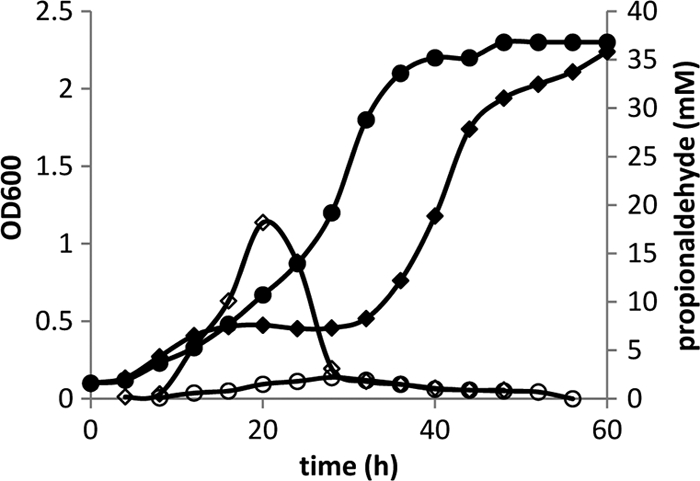
Growth and propionaldehyde production by wild-type Salmonella and a pduBB′ deletion mutant during growth on 1,2-PD minimal medium at saturating B12 (150 nm). Wild type: growth (closed circles) and propionaldehyde production (open circles). pduBB′ deletion mutant: growth (closed diamonds) and propionaldehyde production (open diamonds). The pduBB′ deletion mutant underwent a period of growth arrest between 12 and 32 h that corresponded with a spike in propionaldehyde levels. Generally similar results were obtained with pduA, pduBB′ pduJ, and pduN deletion mutants (see text). Cultures were grown in 250-ml flasks, and samples were removed at timed intervals to determine optical density and propionaldehyde levels in the culture medium.
TABLE 2.
MCP phenotypes of pdu deletion mutants
| Relevant genotype (strain) | MCP formation | Growth arrest | Doubling time with B12 (h) |
Growth lag on 1,2-PD minimal medium (h) | |
|---|---|---|---|---|---|
| Limiting | Saturating | ||||
| Wild-type S. enterica LT2 | Normal | No | 16.9 ± 0.83 | 5.4 ± 0.11 | 17.4 ± 0.3 |
| ΔpduA (BE464) | Enlarged | Yes | 7.8 ± 0.44 | 4.5 ± 0.25 | 17.5 ± 0.3 |
| ΔpduBB′ (BE213) | Absent, with polar body present | Yes | 4.3 ± 0.10 | 5.2 ± 0.07 | 13.4 ± 0.1 |
| ΔpduJ (BE184) | Elongated or amorphous | Yes | 7.1 ± 0.52 | 4.5 ± 0.11 | 16.1 ± 0.5 |
| ΔpduK (BE770) | Aggregated | No | 16.6 ± 0.37 | 5.6 ± 0.17 | 17.6 ± 0.1 |
| ΔpduN (BE772) | Elongated and varied shapes | Yes | 4.1 ± 0.41 | 5.8 ± 0.16 | 15.9 ± 0.3 |
| ΔpduT (BE885) | Normal | No | 18.0 ± 1.13 | 8.1 ± 0.15 | 18.2 ± 0.3 |
| ΔpduU (BE901) | Normal | No | 16.5 ± 0.60 | 8.2 ± 0.28 | 20.0 ± 0.2 |
FIG. 4.
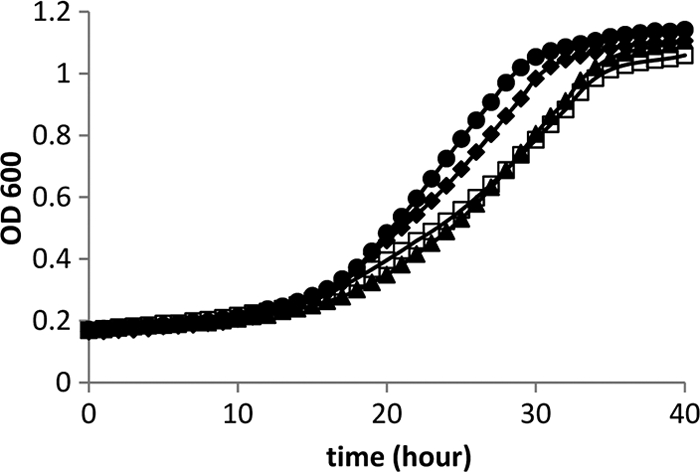
Growth of pduK, pduT, and pduU deletion mutants on 1,2-propanediol with saturating B12 (150 nm). Wild-type Salmonella (closed circles) and ΔpduK (closed diamonds), ΔpduT (open squares), and ΔpduU (closed triangles) mutants. Growth curves were obtained with a Biotek Synergy microplate reader as described in Materials and Methods.
Prior studies also showed that a pduAB double deletion mutant grew faster than the wild type during growth on 1,2-PD with limiting B12 (<50 nm) (24). This suggests that the shell of the Pdu MCP acts as a barrier to B12, which is a required cofactor for the diol dehydratase enzyme (PduCDE) encased within the MCP (24). Here, we found that strains with mutations in pduA, pduBB′, pduJ, or pduN also grew substantially faster than wild-type S. enterica at 20 nm B12 (2.2, 3.9, 2.4, or 4.1 times the wild-type rate, respectively) (Fig. 5A and Table 2). In contrast, however, strains with pduK, pduT, or pduU mutations grew at about the same rate as wild-type at limiting B12 (1.02, 0.94, or 1.02 times the wild-type rate, respectively) (Fig. 5B and Table 2). The pduU deletion mutant was somewhat unusual in that it showed an increased lag time compared to the wild type and grew to a higher cell density. For the above-mentioned studies, growth rates (Table 2) were determined with a microplate reader using 6 replicates for each strain. Propionaldehyde was determined from cultures grown in shake flasks. This was done to provide sufficient amounts of sample for metabolite analyses and for consistency with prior studies (24) since culture conditions could affect the production of propionaldehyde and other metabolites. The observed growth phenotypes on 1,2-PD medium with saturating or limiting B12 indicate that PduA, PduBB′, PduJ, and PduN are required for the structural integrity of the MCP shell but that PduK, PduT, and PduU are not and hence may have specialized functional roles.
FIG. 5.
Growth of Salmonella pdu shell gene deletion mutants on 1,2-propanediol with limiting B12 (20 nM). (A) Wild-type (closed circles) and ΔpduA (open triangles), ΔpduBB′ (open diamonds), ΔpduJ (open circles), and ΔpduN (closed squares) mutants. (B) Wild-type Salmonella (closed circles) and ΔpduK (closed diamonds), ΔpduU (closed triangles), and ΔpduT (open squares) mutants. The growth curves for the wild type and the ΔpduK mutant are difficult to distinguish because they largely overlap. The growth medium was NCE minimal medium with 0.4% 1,2-PD and 20 nM vitamin B12.
Complementation studies.
Studies were performed to determine whether the growth phenotypes of the pduA, pduBB′, pduJ, pduK, pduN, pduT, or pduU mutants could be complemented by the corresponding clone expressed from a plasmid. For the pduA, pduBB′, pduJ, and pduN deletion mutants, limiting B12 was used, and for the pduK, pduT, and pduU deletion mutants, saturating B12 was used. The vector used for complementation was pLAC22, which allows tight regulation of gene expression by IPTG (59). For the pduBB′, pduJ, pduK, pduN, pduT, and pduU deletion mutants, complementation was observed between 0.01 and 0.5 mM IPTG, indicating that the observed growth phenotypes were the result of the mutation under investigation and not due to polarity or an unknown mutation. In the case of the pduA deletion mutant, partial complementation was observed when the inoculum was grown in the presence of 1 mM IPTG to preinduce expression of pduA. No complementation was observed without preinduction (in contrast to what was found for the other deletion mutants tested). Three different pduA deletion mutants were constructed by linear transformation of PCR products, which is designed to generate nonpolar mutations (18, 37), and all three showed partial complementation. The DNA sequence of the pduA clone used for complementation and the pduA deletion present in BE464 were determined, and both were as expected. It is also clear that all three pduA deletion mutants were nonpolar, since each mutant grew well on 1,2-PD, which requires expression of multiple genes downstream of pduA. It seems most likely to us that the lack of full complementation was due to difficulty in establishing the precise gene dosage. Therefore, despite the lack of full complementation, it is likely the phenotypes observed for the pduA deletion mutant resulting from the pduA deletion.
DISCUSSION
In this study, we constructed precise deletion mutants of pduA, pduBB′, pduJ, pduK, pduN, pduT, and pduU individually and examined their phenotypes using electron microscopy, growth tests, and propionaldehyde measurements. The results showed that pduA, pduBB′, pduJ, and pduN were essential for MCP formation and mitigation of propionaldehyde toxicity. In addition, interpretation of the electron microscopy in terms of carboxysome structure (which is better understood) provides some additional insights. The carboxysome is icosahedral, with 20 triangular facets composed of hexagonal BMC domain proteins (28, 29, 52, 55). The triangular facets are thought to be joined by edge proteins (bent hexamers) and vertex proteins that impart the curvature needed to form a closed structure (55, 57). In this report, we showed that pduJ or pduN deletion mutants formed highly elongated MCPs, suggesting that these mutants are impaired for the formation of closed structures (Fig. 2). Given that PduJ comprises a substantial part of the total MCP protein (11%), PduJ is a good candidate for an edge protein that joins facets at the proper angle needed for closure. PduN is homologous to CcmL and CsoS4A, which are pentameric proteins proposed to form the vertices of the carboxysomes (55). Thus, results suggest that PduN is a vertex protein. However, EutN (which is also related to PduN in sequence) forms hexamers in crystals (22), and recent studies with Halothiobacillus have shown that strains carrying deletions of csoS4-A and csoS4-B form a minority of aberrant structures, suggesting that curvature also can be achieved without the presence of the CsoS4A and CsoS4B proteins (10). Thus, some unanswered questions remain regarding the role of PduN and the mechanism by which curvature is imparted to MCPs. We also examined a pduBB′ deletion mutant which eliminates two overlapping genes. The pduBB′ deletion prevented MCP formation and resulted in the formation of large amorphous inclusion bodies presumably composed of misassembled MCP proteins. Prior studies showed that the PduB and PduB′ proteins comprise nearly one-quarter of the total MCP protein, 12.8% and 12.1%, respectively (23). These findings are consistent with a role for PduBB′ as the major proteins that make up the facets of the shell interacting with both edge and vertex proteins and probably lumen enzymes as well. Accordingly, loss of PduBB′ resulted in the complete disassembly of the Pdu MCP. A pduA deletion mutant was also examined, and the results showed that it formed MCPs that were enlarged compared to those of the wild type but generally similar in appearance (Fig. 2). This is in contrast to the results reported for Citrobacter, where absence of pduA prevented MCP formation (39). It was somewhat surprising that deletion of pduA (which comprises about 7.5% of total MCP protein) (23, 24) had a relatively modest effect on MCP structure. This suggests that although PduA is a major component and essential for the mitigation of propionaldehyde toxicity, this protein may have a somewhat minor role in MCP assembly.
The phenotypes of pduT and pduU deletion mutants were also examined. The results indicated that these genes were nonessential for MCP formation and function (mitigation of propionaldehyde toxicity). In addition, prior studies showed that PduT and PduU are minor components of the Pdu MCP (23). Hence, studies suggest that PduT and PduU have specialized functional roles. These could include transport of enzyme substrates, products, cofactors, or electrons across the shell or the repair of Fe-S centers in lumen enzymes. These ideas are supported by the fact that crystal structures show that different shell proteins have Fe-S centers as well as pores that differ in size and charge, suggesting that these proteins might selectively transport particular metabolites (16, 17, 26, 32, 33, 48, 55, 56). In addition, growth studies showed that pduT and pduU deletion mutants grew at about 67% of the rate of wild-type Salmonella at saturating B12 but at a rate similar to that of the wild type at limiting B12. This suggests that PduT and PduU are needed to support maximal catalytic activity of the Pdu MCP for 1,2-PD degradation. This is consistent with the idea that PduT and PduU are used to improve the catalytic efficiency of the Pdu MCP perhaps by facilitating the movement of specific enzyme substrates, cofactors, or products across the MCP shell or by facilitating redox reactions. Prior structural studies showed that PduU has a circularly permuted BMC domain with a pore that is capped by a beta barrel that may serve as a gate during metabolite transport (17). Previous studies of PduT found that it has an iron-sulfur center located at its central pore, which might support electron transfer, redox regulation, or Fe-S repair (16, 39).
Lastly, we also investigated a pduK deletion mutant, with some unexpected results. The pduK deletion had no significant effect on propionaldehyde toxicity or on growth of Salmonella on 1,2-PD (Table 2). However, it did cause the MCPs to aggregate into clusters (Fig. 2; see also Fig. S5 to S8 in the supplemental material). Aggregation, with no apparent functional defect, suggests that the PduK protein may be important for spatial organization of the MCP within the cell or might have a role in segregation at cell division. Recent studies indicate that carboxysome spacing and segregation require ParA and MreB (51), but the manner in which these proteins interact with the carboxysome is unknown. In the case of the Pdu MCP, a tentative possibility is that PduK interacts with the cytoskeletal proteins such as ParA to promote proper spacing and segregation during division.
Supplementary Material
Acknowledgments
This work was supported by grants MCB0956451 from the National Science Foundation and AI081146 from the National Institutes of Health.
We thank the ISU DNA Sequencing and Synthesis Facility for assistance with DNA analyses and the ISU Microscopy and Nanoimaging Facility of the Office of Biotechnology for help with the electron microscopy.
Footnotes
Published ahead of print on 14 January 2011.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Altschul, S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beeby, M., T. A. Bobik, and T. O. Yeates. 2009. Exploiting genomic patterns to discover new supramolecular protein assemblies. Protein Sci. 18:69-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkowitz, D., J. M. Hushon, H. J. Whitfield, Jr., J. Roth, and B. N. Ames. 1968. Procedure for identifying nonsense mutations. J. Bacteriol. 96:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobik, T. A. 2006. Polyhedral organelles compartmenting bacterial metabolic processes. Appl. Microbiol. Biotechnol. 70:517-525. [DOI] [PubMed] [Google Scholar]
- 5.Bobik, T. A., M. E. Ailion, and J. R. Roth. 1992. A single regulatory gene integrates control of vitamin B12 synthesis and propanediol degradation. J. Bacteriol. 174:2253-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobik, T. A., G. D. Havemann, R. J. Busch, D. S. Williams, and H. C. Aldrich. 1999. The propanediol utilization (pdu) operon of Salmonella enterica serovar Typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B12-dependent 1,2-propanediol degradation. J. Bacteriol. 181:5967-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobik, T. A., Y. Xu, R. M. Jeter, K. E. Otto, and J. R. Roth. 1997. Propanediol utilization genes (pdu) of Salmonella typhimurium: three genes for the propanediol dehydratase. J. Bacteriol. 179:6633-6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinsmade, S. R., T. Paldon, and J. C. Escalante-Semerena. 2005. Minimal functions and physiological conditions required for growth of Salmonella enterica on ethanolamine in the absence of the metabolosome. J. Bacteriol. 187:8039-8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchrieser, C., C. Rusniok, F. Kunst, P. Cossart, and P. Glaser. 2003. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: clues for evolution and pathogenicity. FEMS. Immunol. Med. Microbiol. 35:207-213. [DOI] [PubMed] [Google Scholar]
- 10.Cai, F., et al. 2009. The pentameric vertex proteins are necessary for the icosahedral carboxysome shell to function as a CO2 leakage barrier. PLoS One 4:e7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon, G. C., et al. 2001. Microcompartments in prokaryotes: carboxysomes and related polyhedra. Appl. Environ. Microbiol. 67:5351-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, P., D. I. Andersson, and J. R. Roth. 1994. The control region of the pdu/cob regulon in Salmonella typhimurium. J. Bacteriol. 176:5474-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng, S., and T. A. Bobik. 2010. Characterization of the PduS cobalamin reductase of Salmonella enterica and its role in the Pdu microcompartment. J. Bacteriol. 192:5071-5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng, S., Y. Liu, C. S. Crowley, T. O. Yeates, and T. A. Bobik. 2008. Bacterial microcompartments: their properties and paradoxes. Bioessays 30:1084-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conner, C. P., D. M. Heithoff, S. M. Julio, R. L. Sinsheimer, and M. J. Mahan. 1998. Differential patterns of acquired virulence genes distinguish Salmonella strains. Proc. Natl. Acad. Sci. U. S. A. 95:4641-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley, C. S., et al. 2010. Structural insights into the mechanisms of transport across the Salmonella enterica Pdu microcompartment shell. J. Biol. Chem. 285:37838-37846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowley, C. S., M. R. Sawaya, T. A. Bobik, and T. O. Yeates. 2008. Structure of the PduU shell protein from the Pdu microcompartment of Salmonella. Structure 16:1324-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dou, Z., et al. 2008. CO2 fixation kinetics of Halothiobacillus neapolitanus mutant carboxysomes lacking carbonic anhydrase suggest the shell acts as a diffusional barrier for CO2. J. Biol. Chem. 283:10377-10384. [DOI] [PubMed] [Google Scholar]
- 20.Fan, C., and T. A. Bobik. 2008. The PduX enzyme of Salmonella enterica is an L-threonine kinase used for coenzyme B12 synthesis. J. Biol. Chem. 283:11322-11329. [DOI] [PubMed] [Google Scholar]
- 21.Fan, C., H. J. Fromm, and T. A. Bobik. 2009. Kinetic and functional analysis of L-threonine kinase, the PduX enzyme of Salmonella enterica. J. Biol. Chem. 284:20240-20248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forouhar, F., et al. 2007. Functional insights from structural genomics. J. Struct. Funct. Genomics 8:37-44. [DOI] [PubMed] [Google Scholar]
- 23.Havemann, G. D., and T. A. Bobik. 2003. Protein content of polyhedral organelles involved in coenzyme B12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 185:5086-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havemann, G. D., E. M. Sampson, and T. A. Bobik. 2002. PduA is a shell protein of polyhedral organelles involved in coenzyme B12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 184:1253-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heithoff, D. M., et al. 1999. Coordinate intracellular expression of Salmonella genes induced during infection. J. Bacteriol. 181:799-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heldt, D., et al. 2009. Structure of a trimeric bacterial microcompartment shell protein, EtuB, associated with ethanol utilisation in Clostridium kluyveri. Biochem. J. 423:199-207. [DOI] [PubMed] [Google Scholar]
- 27.Horswill, A. R., and J. C. Escalante-Semerena. 1999. Salmonella typhimurium LT2 catabolizes propionate via the 2-methylcitric acid cycle. J. Bacteriol. 181:5615-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iancu, C. V., et al. 2007. The structure of isolated Synechococcus strain WH8102 carboxysomes as revealed by electron cryotomography. J. Mol. Biol. 372:764-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iancu, C. V., et al. 2010. Organization, structure, and assembly of a-carboxysomes determined by electron cryotomography of intact cells. J. Mol. Biol. 396:105-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, C. L. V. J., et al. 2001. Functional genomic, biochemical, and genetic characterization of the Salmonella pduO gene, an ATP:cob(I)alamin adenosyltransferase gene. J. Bacteriol. 183:1577-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joseph, B., et al. 2006. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J. Bacteriol. 188:556-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerfeld, C. A., et al. 2005. Protein structures forming the shell of primitive bacterial organelles. Science 309:936-938. [DOI] [PubMed] [Google Scholar]
- 33.Klein, M. G., et al. 2009. Identification and structural analysis of a novel carboxysome shell protein with implications for metabolite transport. J. Mol. Biol. 392:319-333. [DOI] [PubMed] [Google Scholar]
- 34.Leal, N. A., G. D. Havemann, and T. A. Bobik. 2003. PduP is a coenzyme A-acylating propionaldehyde dehydrogenase associated with the polyhedral bodies involved in B12-dependent 1,2-propanediol degradation by Salmonella enterica serovar Typhimurium LT2. Arch. Microbiol. 180:353-361. [DOI] [PubMed] [Google Scholar]
- 35.Liu, Y., et al. 2007. PduL is an evolutionarily distinct phosphotransacylase involved in B12-dependent 1,2-propanediol degradation by Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 189:1589-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 37.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obradors, N., J. Badia, L. Baldoma, and J. Aguilar. 1988. Anaerobic metabolism of the L-rhamnose fermentation product 1,2-propanediol in Salmonella typhimurium. J. Bacteriol. 170:2159-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsons, J. B., et al. 2008. Biochemical and structural insights into bacterial organelle form and biogenesis. J. Biol. Chem. 283:14366-14375. [DOI] [PubMed] [Google Scholar]
- 40.Parsons, J. B., et al. 2010. Synthesis of empty bacterial microcompartments, directed organelle protein incorporation, and evidence of filament-associated organelle movement. Mol. Cell 38:305-315. [DOI] [PubMed] [Google Scholar]
- 41.Penrod, J. T., and J. R. Roth. 2006. Conserving a volatile metabolite: a role for carboxysome-like organelles in Salmonella enterica. J. Bacteriol. 188:2865-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price, G. D., M. R. Badger, F. J. Woodger, and B. M. Long. 2008. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 59:1441-1461. [DOI] [PubMed] [Google Scholar]
- 43.Rasband, W. 1997. ImageJ. U.S. National Institutes of Health, Bethesda, MD. http://rsb.info.nih.gov/ij/.
- 44.Reinhold, L., R. Kosloff, and A. Kaplan. 1991. A model for inorganic carbon fluxes and photosynthesis in cyanobacterial carboxysomes. Can. J. Bot. 69:984-988. [Google Scholar]
- 45.Rondon, M. R., and J. Escalante-Semerena. 1992. The poc locus is required for 1,2-propanediol-dependent transcription of the cobalamin biosynthetic (cob) and propanediol utilization (pdu) genes of Salmonella typhimurium. J. Bacteriol. 174:2267-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rondon, M. R., A. R. Horswill, and J. C. Escalante-Semerena. 1995. DNA polymerase I function is required for the utilization of ethanolamine, 1,2-propanediol, and propionate by Salmonella typhimurium LT2. J. Bacteriol. 177:7119-7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rondon, M. R., R. Kazmierczak, and J. C. Escalante-Semerena. 1995. Glutathione is required for maximal transcription of the cobalamin biosynthetic and 1,2-propanediol utilization (cob/pdu) regulon and for the catabolism of ethanolamine, 1,2-propanediol, and propionate in Salmonella typhimurium LT2. J. Bacteriol. 177:5434-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sagermann, M., A. Ohtaki, and K. Nikolakakis. 2009. Crystal structure of the EutL shell protein of the ethanolamine ammonia lyase microcompartment. Proc. Natl. Acad. Sci. U. S. A. 106:8883-8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 50.Sampson, E. M., and T. A. Bobik. 2008. Microcompartments for B12-dependent 1,2-propanediol degradation provide protection from DNA and cellular damage by a reactive metabolic intermediate. J. Bacteriol. 190:2966-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savage, D. F., B. Afonso, A. H. Chen, and P. A. Silver. 2010. Spatially ordered dynamics of the bacterial carbon fixation machinery. Science 327:1258-1261. [DOI] [PubMed] [Google Scholar]
- 52.Schmid, M. F., et al. 2006. Structure of Halothiobacillus neapolitanus carboxysomes by cryo-electron tomography. J. Mol. Biol. 364:526-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmieger, H. 1971. A method for detection of phage mutants with altered transducing ability. Mol. Gen. Genet. 110:378-381. [DOI] [PubMed] [Google Scholar]
- 54.Stojiljkovic, I., A. J. Baeumler, and F. Heffron. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 177:1357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka, S., et al. 2008. Atomic-level models of the bacterial carboxysome shell. Science 319:1083-1086. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka, S., M. R. Sawaya, M. Phillips, and T. O. Yeates. 2009. Insights from multiple structures of the shell proteins from the beta-carboxysome. Protein Sci. 18:108-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka, S., M. R. Sawaya, and T. O. Yeates. 2010. Structure and mechanisms of a protein-based organelle in Escherichia coli. Science 327:81-84. [DOI] [PubMed] [Google Scholar]
- 58.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 59.Warren, J. W., J. R. Walker, J. R. Roth, and E. Altman. 2000. Construction and characterization of a highly regulable expression vector, pLAC11, and its multipurpose derivatives, pLAC22 and pLAC33. Plasmid 44:131-151. [DOI] [PubMed] [Google Scholar]
- 60.Yeates, T. O., C. S. Crowley, and S. Tanaka. 2010. Bacterial microcompartment organelles: protein shell structure and evolution. Annu. Rev. Biophys. 39:185-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeates, T. O., C. A. Kerfeld, S. Heinhorst, G. C. Cannon, and J. M. Shively. 2008. Protein-based organelles in bacteria: carboxysomes and related microcompartments. Nat. Rev. Microbiol. 6:681-691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.