Abstract
We present a cryo-electron tomographic analysis of the three-dimensional architecture of a strain of the Gram-negative bacterium Bdellovibrio bacteriovorus in which endogenous MreB2 was replaced with monomeric teal fluorescent protein (mTFP)-labeled MreB2. In contrast to wild-type Bdellovibrio cells that predominantly displayed a compact nucleoid region, cells expressing mTFP-labeled MreB2 displayed a twisted spiral organization of the nucleoid. The more open structure of the MreB2-mTFP nucleoids enabled clear in situ visualization of ribosomes decorating the periphery of the nucleoid. Ribosomes also bordered the edges of more compact nucleoids from both wild-type cells and mutant cells. Surprisingly, MreB2-mTFP localized to the interface between the spiral nucleoid and the cytoplasm, suggesting an intimate connection between nucleoid architecture and MreB arrangement. Further, in contrast to wild-type cells, where a single tight chemoreceptor cluster localizes close to the single polar flagellum, MreB2-mTFP cells often displayed extended chemoreceptor arrays present at one or both poles and displayed multiple or inaccurately positioned flagella. Our findings provide direct structural evidence for spiral organization of the bacterial nucleoid and suggest a possible role for MreB in regulation of nucleoid architecture and localization of the chemotaxis apparatus.
Survival of prokaryotic cells requires faithful replication and segregation of chromosomes and control of the compaction state of the genome so that it fits within the confines of the bacterial cytoplasm while enabling access of cellular machines needed for replication, segregation, and transcription. Compared to eukaryotic cells, bacteria have the added complexity of initiating new rounds of DNA replication before the completion of cell division. The bacterial nucleoid is comprised of chromosomal DNA and DNA-associated proteins and occupies most of the cytoplasm. Despite its amorphous appearance, the nucleoid is remarkably well organized, with ordered spatial localization of genetic loci and origins of replication, reminiscent of the more elaborate organization of the eukaryotic nucleus (21). The bacterial nucleoid transitions between open and more compact forms, depending upon whether cells are in growth phase or stationary phase (27), but little is known about its three-dimensional (3D) architecture in intact cells (35).
The role of the bacterial actin homolog MreB in cell shape maintenance, chromosome segregation, and establishment of cell polarity is well established (reviewed in references 22, 47, 52, and 56). Light microscopic studies indicate that MreB forms helical arrays near the inner cell membrane, where it acts with MreC, MreD, and penicillin-binding proteins to mediate peptidoglycan synthesis. MreB spirals are dynamic and can dissociate during the cell cycle, shifting MreB to the cell division site. MreB interacts directly with the Escherichia coli RNA polymerase (29) and the ParC subunit of topoisomerase IV, a DNA-associated enzyme that unlinks replicated daughter chromosomes during chromosome segregation (34). It also associates with the chromosomal region adjacent to the origins of replication in Caulobacter crescentus (20). Whether cell membrane-proximal MreB or MreB associated with central ring structures mediates all of these interactions is unclear. Thus, determination of the 3D structure of the nucleoid and localization of MreB at higher resolution is essential to further explore the potential connection between MreB and the bacterial chromosome.
Cryo-electron tomography is a powerful tool to image in intact bacterial cells structural components such as ribosomes, filaments, chemoreceptor arrays, and dense granules (38, 42), but in situ visualization of nucleoids in intact cells by cryo-electron microscopy has proven to be challenging (35). This is either because most bacterial cells are too thick (>500 nm wide) except at their polar regions, making it difficult to obtain useful structural information in the central portions of the cell, or because in cells that are thin enough to be imaged using cryo-electron tomography, the nucleoid region appears diffuse. Here, we use the predatory Gram-negative bacterium Bdellovibrio bacteriovorus to investigate the 3D structure of the nucleoid since the cells are only ∼300 nm wide, and the nucleoid is both well separated from cytoplasmic membrane and easily distinguished from the rest of the cytoplasm (3). In addition to wild-type cells, we have analyzed a strain expressing the bacterial actin homolog MreB2 that has been tagged with a C-terminal monomeric teal fluorescent protein (mTFP) (17). In other bacteria, alteration of MreB activity has been shown by fluorescence microscopy to alter the positioning and compaction state of the nucleoid (11, 20, 29, 50). MreB2 is one of two MreB actin homologs present in B. bacteriovorus. Replacement of the native MreB1 with MreB1-mTFP results in stalling and developmental arrest of the cells within the invaded E. coli prey, making this line less suitable for structural analysis. However, replacement of the native MreB2 with MreB2-mTFP results in cells that have diverse shapes and cell lengths, including a minority (20%) of spherical cells that are not predatory and a majority (∼80%) which are vibrioid or elongated cells, which septate less often than wild-type cells but still complete predatory development normally to form free-swimming attack-phase cells (17). We show that in the latter population, expression of the tagged MreB2 shifts the nucleoid into a more distinct spiral organization and leads to cell division defects including those involving chromosome segregation and proper positioning of the bacterial machinery for chemotaxis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The host-dependent B. bacteriovorus strains used in this study were the following: wild-type predatory HD100; HD100mreB2-mtfp, a strain containing integrated plasmid pAKF40a, a kanamycin resistance gene replacement vector that replaces wild-type mreB2 with an mreB2 that is fused in frame at its C terminus with the coding sequence of monomeric bright teal fluorescent protein, mTFP; and the control strain HD100Bd2345::Kmr, a cell line that has an integrated pK18mobsacB kanamycin resistance vector to disrupt the ABC transporter gene to act as a plasmid control for the pK18 backbone of pAKF40a (17). HD100 was cocultured at 22 to 25°C with E. coli strain RP3098, a mutant with all flagellar and chemotaxis proteins deleted and grown as described previously (3). The other two strains were cocultured at 29°C on YPSC (yeast extract, peptone, sodium acetate, and l-cysteine) overlay plates with E. coli strain S17-1 that contains plasmid pZMR100 to confer kanamycin resistance (17). Briefly, to obtain a freshly cleared B. bacteriovorus prey lysate, an overnight prey culture of E. coli S17-1(pZMR100) was grown in yeast extract-tryptone (YT) medium supplemented with kanamycin to a final concentration of 50 μg/ml. A total of 150 μl of the overnight prey culture was added to 2 ml of a Ca-HEPES buffer (25 mM HEPES, 2 mM CaCl2, pH 7.6, with NaOH) with additional antibiotic to maintain the final concentration at 50 μg/ml, and the suspension was inoculated with a large, well-isolated plaque of B. bacteriovorus cells from a YPSC overlay plate. The culture was shaken (at 29°C and 250 rpm for ∼48 h) until the lysate cleared as assessed by light microscopy, as described by Borgnia et al. (3).
Specimen preparation and procedures for cryo-electron tomography.
B. bacteriovorus cells (5 μl of cleared lysate) were applied to glow-discharged holey carbon grids (Quantifoil MultiA; Micro Tools GmbH, Germany), thinned by blotting, and plunge-frozen in liquid ethane using a manual gravity plunger. Low-dose (1.5 electrons/Å2 per image) tomographic tilt series were collected in steps of 1.5° over an angular range of +65° to −65° using either a Titan Krios transmission electron microscope or a G2 Polara transmission electron microscope, both from FEI Company (Hillsboro, OR), at voltages of 300 kV and 200 KV, respectively, with the specimen stage maintained by liquid nitrogen cooling to ∼−180°C. Images were recorded at nominal magnifications of either ×18,000 or ×22,000 and under focus values ranging from 5 to 7 μm on a Gatan 2,048- by 2,048-pixel charge-coupled device (CCD) camera located at the end of a postcolumn GIF 2000 energy filter (Gatan Inc., Pleasanton, CA). Protein A-gold conjugates (15 nm; BBInternational, Cardiff, United Kingdom) were added directly on the carbon side of the grids (3) as well as mixed with the cell solution in a ratio of 1:4 to provide fiducial markers for alignment of each tilt series.
Image analysis.
3D reconstructions were obtained by weighted back-projection of aligned images using the IMOD software package (28). Individual cells were extracted from the calculated tomograms and denoised by nonlinear anisotropic diffusion as implemented in Bsoft (23). Cells were manually segmented and surface rendered using the Amira software package (Mercury Computer System, Inc.). Nucleoid and cell lengths were estimated using both Amira and IMOD.
Transmission electron microscopy of immunogold-labeled cell sections.
Cells were mixed in a 1:1 ratio with 8% formaldehyde in 50 mM HEPES buffer (pH 7.2 at 22°C) and incubated at 22°C without shaking for times ranging from 10 to 60 min. Subsequently, cells were centrifuged in an Eppendorf centrifuge at 14,000 × g. The pellet was rinsed three times (5 min each) in HEPES buffer and then resuspended in 5% molten gelatin. Cells were immediately spun again, and the tubes were placed on ice to solidify the gelatin. The pellet was cut into cubes that were placed in 2.3 M sucrose containing 15% polyvinylpyrrolidone for 16 h. The cubes were frozen onto Leica specimen stubs and sectioned at −130°C using a Leica Ultracut UCT and FCS (fluorescence correlation spectroscopy) cryo-ultramicrotome. Sections between 80 nm and 120 nm in thickness were picked up with a droplet containing methylcellulose and sucrose as previously described (59) and placed on carbon-coated formvar grids. Sections were sequentially labeled with rabbit anti-green fluorescent protein (GFP) antibody (Abcam 290 antibody) and 10-nm gold-labeled protein A (Utrecht University, Netherlands) and then embedded and contrasted with 2% methylcellulose added to 0.3% uranyl acetate. Excess fluid was withdrawn with filter paper, and grids were allowed to air dry. mTFP labeling patterns were assessed with a Tecnai-T12 transmission electron microscope (FEI, Hillsboro OR).
RESULTS
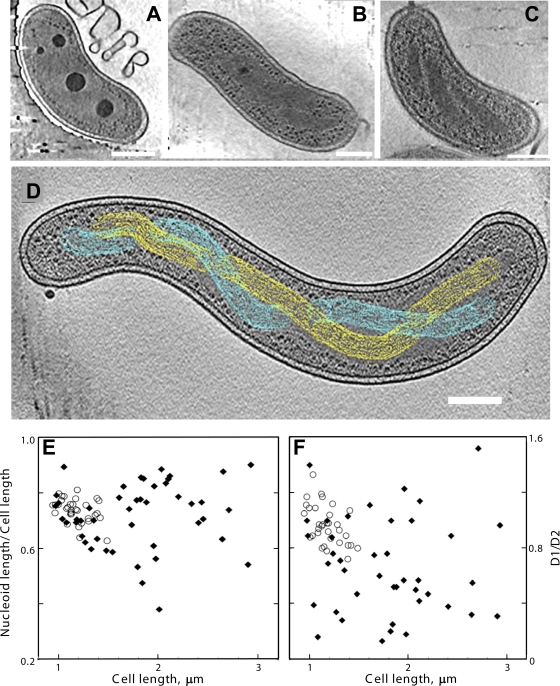
Cryo-electron tomography of plunge-frozen wild-type HD100 B. bacteriovorus cells (n = 60 from four preparations) enables visualization of the main structural elements present within the cells, which are primarily comma shaped (Fig. 1A and B). Of the cells that could be accurately measured, lengths ranged from ∼0.9 to 1.5 μm (1.2 ± 0.1 μm, mean ± standard deviation [SD]; n = 32) (Fig. 1E). As reported previously (3), the inner and outer cell membranes are intact, of similar densities, and evenly spaced by ∼25 nm, except at the anterior pole, where the membranes are spaced slightly farther apart. There are large dense granules resembling those characterized in our earlier study (3) that were shown to be enriched in polyphosphates, required to provide a cellular energy reservoir. Electron-dense ribosomes and other macromolecular complexes are evident within the bacterial cytoplasm. The nucleoid appears as a centrally located, finely textured region that is devoid of ribosomes and other macromolecular complexes (Fig. 1A and B). A majority (∼52%) of the cells display a fully amorphous nucleoid, while ∼34% display an amorphous nucleoid with a hint of a discernible spiral structure in limited regions. Only ∼13% of cells have an open nucleoid arrangement that shows unambiguous spirals, the clearest example of which is presented in Fig. 1B. The pitch of the nucleoid spirals is 260 nm ± 70 nm (n = 19 pitch measurements on spirals from seven cells). The nucleoid often mirrors cell shape (Fig. 1A), and the tips of the nucleoid position at approximately equal distances from the cell poles (Fig. 1F), suggesting that nucleoid extension is paralleled by cell length extension during cell division.
FIG. 1.
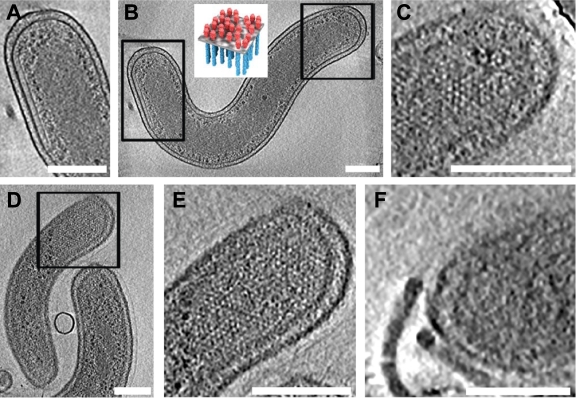
Cryo-electron tomography of HD100 wild-type and MreB2-mTFP B. bacteriovorus cells. (A) Tomographic slice (12.5 nm thick) of a wild-type cell that has a compact nucleoid surrounded by dark granular structures, presumably bacterial ribosomes. (B) Tomographic slice (12.5 nm thick) of a wild-type cell that has its nucleoid organized in two twisted strands. The strand thickness is ∼75 nm, with a helical repeat that varies from 320 nm to 334 nm along the cell length. (C) Tomographic slice (21 nm thick) of an MreB2-mTFP cell that has distinct nucleoid helical banding. (D) Depiction of the path of separated strands (in yellow and cyan) of the spiral nucleoid in an MreB2-mTFP cell where the strands could be resolved along the entire length of the cell. The variation in width of the strand along the spiral can be seen clearly in the cellular tomogram (see Video S3 in the supplemental material); for simplicity, this variation is not highlighted in the colored rendering of the spiral done using the object editor in the program IMOD (28). The volume occupied by one nucleoid strand is indicated using yellow mesh, and blue mesh is used for the volume of the other. (E and F) Nucleoid size plotted as a function of cell length (E) and positioning of the ends of the nucleoids relative to the anterior (distance D1) and posterior (distance D2) cellular poles (F). The ratio of D1/D2 is plotted as a function of cell length. In both plots, distances in wild-type cells are indicated by open circles, and distances in mutant cells are indicated by closed diamonds. Bar, 200 nm.
Cryo-electron tomography of the 3D structures of 70 HD100mreB2-mtfp (MreB2-mTFP) cells from four preparations shows that, like wild-type cells (3), these cells can be comma shaped (Fig. 1C) but are often heterogeneous in shape (also see various examples in Fig. 2 to 4), with extensive flexibility that permits them to even mold around the edges of the carbon film of the electron microscopic grids (see Video S1 in the supplemental material). The flexibility of wild-type B. bacteriovorus membranes may assist entry of attack-phase cells into the bdelloplast (3) as well as egress of the daughter cells from discrete exit pores (16). Although we also observed short spherical MreB2-mTFP cells like those reported earlier (17), the nonspherical MreB2-mTFP cells that we imaged are typically longer (range, 0.9 to 2.9 μm; mean ± SD, 1.8 ± 0.5 μm; n = 46) (Fig. 1E) than wild-type cells and can have multiple, extensive bends (Fig. 2 and 3; see also Videos S1, S2, S4, S6, and S7). Even longer cells (>3.7 μm) exist in the population, but they extend beyond the imaging area and were not included in the length analysis. The most remarkable structural feature of the majority of MreB2-mTFP cells is the striking spiral organization of the nucleoid in both normal-length and elongated MreB2-mTFP cells (Fig. 1C and D, 2, and 3; see also Videos S2 to S4). Of the 70 cells, 64% have nucleoids with obvious spirals, 18% have amorphous nucleoids similar to those seen in most wild-type cells, and 17% contain nucleoids with mixed morphologies. Occasionally, intertwined strands can be traced to visualize the left- and right-handed directions of the twisted spiral (Fig. 1D; see also Videos S3 to S5), as well as the loop formed at the end of the spiral which presents as a ribosome-filled central area surrounded by lighter gray nucleoid regions (Fig. 3H and I). The pitch of the nucleoid spirals was 230 nm ± 60 nm (n = 27 pitch measurements on spirals from 10 cells).
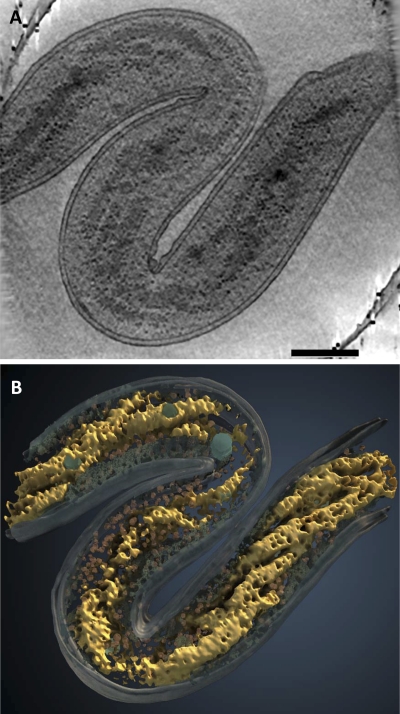
FIG. 2.
Spiral architecture of the nucleoid in MreB2-mTFP cells. (A) Tomographic slice (21 nm thick) through the 3D volume of a cell with division defects. (B) 3D surface rendering of the cell shown in panel A with the spiral nucleoid (yellow), putative ribosomes (red), cytoplasm (blue), and dense granules (greenish blue) highlighted. Bar, 200 nm.
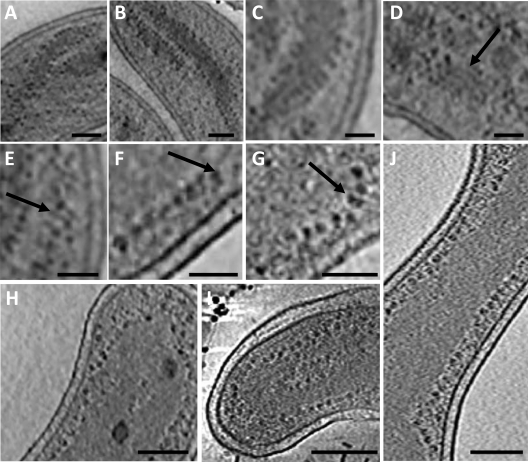
FIG. 3.
Well-separated nucleoid spirals and ribosome distribution at the edge of the nucleoid in MreB2-mTFP (panels A to G, I, and J) and wild-type (H) cells. (A and B) Ribosomes (dark dots) line the edge of the nucleoid (medium-gray density). (C, D, and E) Nucleoid protrusions, 10 nm wide, or parallel ribbons (arrow) are decorated by ribosomes. (F and G) Pairs or strings of ribosomes (arrow) line the nucleoid edge. Nucleoid-associated ribosomes in a wild-type cell (H) and in two other MreB2-mTFP mutant cells (I and J). Bars, 100 nm (panel A), 75 nm (panels B, D, and E), 50 nm (panels C, F, and G), and 200 nm (panels H to J).
Atomic force microscopy (AFM) studies of isolated E. coli nucleoids (45) indicate that they have a hierarchical organization with an ∼10-nm-sized fiber representing the smallest structural unit of nucleoid organization. The open nucleoid spirals evident in long, unsegregated cells, such as the one shown in Fig. 2A and in Video S2 in the supplemental material, provide a unique glimpse of the in situ organization of the nucleoid. Magnified views of this cell indicate a close association of ribosomes with the edges of the nucleoid (Fig. 3A and B). The nucleoid itself can form ∼10-nm-wide protrusions (Fig. 3C) or strands (Fig. 3D and E) with one or more associated ribosomes, possibly associated with increased translational activity in this particular cell. In two other more highly magnified cells, ribosomes at the nucleoid edge are present in arrangements (Fig. 3F and G) like those seen in bacterial translation extracts or in lysates of E. coli spheroplasts that are actively translating mRNA (4). Comparatively few cytoplasmic ribosomes or membrane-associated ribosomes are seen in this cell, where long stretches of undecorated inner membrane are evident (Fig. 3A, C, and D; see also Video S2). Both wild-type cells (Fig. 3H) and mutant cells (Fig. 3I and J), including those with more compact nucleoids, display ribosomes associated with the edge of their nucleoids although this effect is less pronounced when there are high levels of cytoplasmic ribosomes. Ribosomes do not surround intracellular dense granules, which are typically encompassed by the nucleoid, even in cells that have dispersed nucleoids (Fig. 1A and 4 B; see also Videos S1 to S3 and S5 to S7 in the supplemental material).
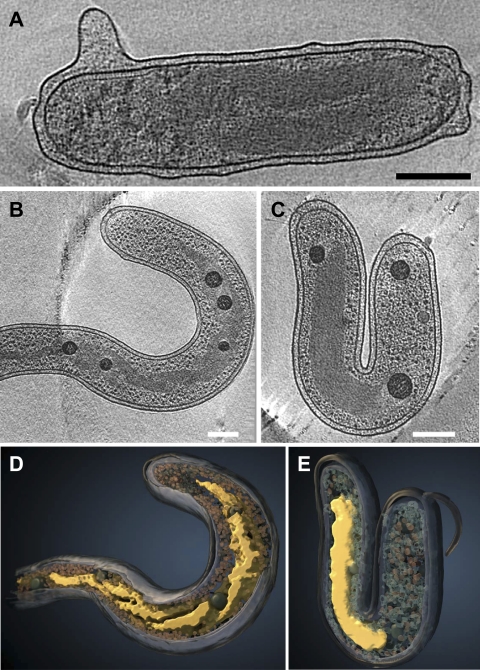
FIG. 4.
Diversity of nucleoid structures and localizations among MreB2-mTFP mutant cells in 21-nm-thick tomographic slices from three cells. (A) Example of a nucleoid with two strands that orient parallel to each other. This nucleoid does not extend to the cellular pole on the left side but extends up to the region of the chemotaxis receptor arrays, seen in a side view at the upper left corner of the cell. (B) This nucleoid mainly has crossed-over helical strands through the cytoplasm but unfolds toward both poles. (C) A compact nucleoid positioned asymmetrically. (D and E) Surface rendering of the cells shown in panels B and C, respectively. The color scheme is the same as that used in Fig. 2. Bar, 200 nm.
The presence of significantly elongated cells in the population (Fig. 1E) suggests that there may be cell division defects occurring in the MreB2-mTFP cells. Certain cells lack apparent cell division septa and have extended nucleoids with a large number of spiral turns (Fig. 2A) while others have extended nucleoids with a disorganized, open domain structure (see Video S6 in the supplemental material). The range of the ratios of nucleoid length to cell length varies more widely in the mutant (Fig. 1E), as does the positioning of the nucleoid ends relative to the two cellular poles (Fig. 1F). The MreB2-mTFP cells that are longer than wild-type cells are three times more likely to have asymmetric nucleoid placement than mutant cells that have lengths comparable to those of wild-type cells (Fig. 1F). Asymmetric nucleoid positioning is illustrated in the cells shown in Fig. 4A and C, with the former showing a more open structure in which two strands of the nucleoid are evident. In some of the longest cells, nucleoids are present as double-stranded spirals that taper into a long thin density (Fig. 4B and D; see Video S5). Thus, in addition to the prevalent spiral nucleoid arrangement in most MreB2-mTFP cells, considerable variation in the nucleoid structure, length, and positioning is evident within cells of the population.
Some of the MreB2-mTFP cells display dramatically increased levels of polar chemoreceptor assemblies. Unlike wild-type B. bacteriovorus cells, where a single partially hexagonally ordered polar chemoreceptor array forms close to the base of the single flagellum (3), MreB2-mTFP cells frequently have large and extended arrays that are often present at both poles (Fig. 5A to C; see Video S9 in the supplemental material). These chemoreceptor assemblies can occupy up to a third of the length of the cell (Fig. 5D and E; see Video S10); certain cells also display two complete polar flagellar assemblies (Fig. 5F; see Videos S1 and S8). The partially ordered hexagonal packing arrangement of the chemoreceptor arrays, with a lattice spacing of 11 nm, in the mutant cells is similar to that demonstrated for other Gram-negative bacteria (5, 26).
FIG. 5.
Flagellar assembly and localization of the receptor arrays at the poles of MreB2-mTFP cells in 21-nm-thick tomographic slices. Expanded views of polar chemoreceptor arrays shown from the side (A) or the top (C) encompass the rectangular boxed areas that are indicated on the cell shown in panel B. The top view is of a tomographic section that contains the surface region of the bacterium; the receptors are visualized as white dots while the receptor array of the side view forms a thin line under the inner cell membrane. (Inset) Schematic model of chemoreceptor arrays to illustrate top and side views, reprinted from the work of Khursigara et al. (26). Another cell contains a large chemotaxis receptor cluster, which is shown in a top view in panel D and magnified in panel E. (F) Two closely spaced, intact flagella are evident at the pole of a third cell, shown in a 10.4-nm tomographic slice. Bar, 200 nm.
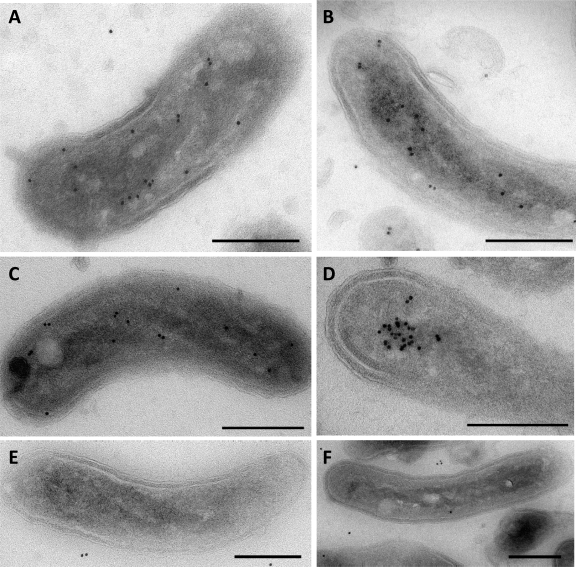
We performed transmission electron microscopy of immunogold-labeled MreB2-mTFP cell sections to covisualize MreB2 and the unlabeled nucleoid at electron microscopic resolution. Analysis of images from 30 sections containing >300 gold labels shows that ∼85% of gold particles border the nucleoid spiral, ∼10% localize close to the inner membrane, and ∼5% are in random locations (Fig. 6A to C). Strikingly, certain sections (Fig. 6D) contain a cluster of labeled MreB2-mTFP at the nucleoid. We also analyzed a kanamycin-resistant control strain that expresses wild-type MreB2, but not mTFP, and that has nearly wild-type levels of predation activity (17). Both cryo-electron tomography of whole cells and electron microscopy of thin sections from 30 cells confirm that size, cell morphology, and nucleoid localization in this strain are like those of wild-type cells with no discernible gold labeling on the nucleoid (Fig. 6E and F).
FIG. 6.
Immuno-localization of MreB2-mTFP. Projection electron microscopic images from representative sections of MreB2-mTFP-expressing cells (A to D) or control HD100Bd2345::Kmr cells that express wild-type MreB2 but lack mTFP (E and F). Sections were treated with anti-GFP antibody and then with 10-nm gold-labeled protein A to label surface antigens of the sections for imaging by transmission electron microscopy. Bar, 200 nm.
DISCUSSION
Visualizing the 3D structure of the bacterial nucleoid under near-native conditions is essential to map dynamic changes in nucleoid structure that occur during cell growth and division. DNA compaction is achieved by multiple means, including exclusion of cytoplasmic proteins, low diffusion of DNA, and a system of DNA-binding proteins that structure the chromosome into distinct topological domains (51). Bacterial chromosomes have been proposed to form ring-like structures or coiled spiral arrangements (53) although cryo-electron microscopic projection imaging studies typically reveal amorphous nucleoid regions delineated by ribosome-free areas of the cell (15). Cryo-electron tomographic imaging of the bacterial nucleoid of MreB2-mTFP cells and a small proportion of wild-type cells clearly indicates spiral chromosomes well separated from the cell periphery. This architecture is consistent with analyses of nucleoids of E. coli using soft X-ray tomography (31) and of Staphylococcus aureus using AFM (41) that also display structured nucleoids that are separated from the cell membrane. Moreover, fluorescent DNA labeling in Bacillus subtilis indicates spiral patterns of incorporated fluorescent DNA base analogues during replication of the bacterial chromosome although the contributions of nucleoid-binding proteins could not be visualized (1). Our reconstructions of intact frozen hydrated cells, imaged without stain to attain near-native conditions, show nucleoids with clearly delineated substructures (Fig. 2A and 3A and I) that most closely resemble the coiled spiral model. We expect that compact amorphous nucleoids, most prevalent in wild-type cells, contain chromosomes similarly arranged, albeit with more tightly coiled spirals. The presence of ribosomes closely apposed to the bulk nucleoid surface (Fig. 3A, B, and H to J) or on 10-nm-wide nucleoid structures within or at the edge of the nucleoid (Fig. 3C to E), with occasional clusters of ribosomes (Fig. 3F and G), reveals the likelihood of close transcriptional and translational coupling, which has been difficult to discern using fluorescence microscopic methods (32, 36, 40). Notably, tight spatial compartmentalization of gene-specific transcription has also recently been visualized along bacterial chromosomes (40).
Fluorescence microscopic studies of other bacteria indicate that MreB forms highly dynamic, helical structures that are close to the inner bacterial membrane and span nearly the entire cellular length (19, 30, 50, 55, 56). This localization is consistent with its role in cell shape determination (9, 17, 18, 37), where it functions with penicillin-binding proteins, MreC, and MreD to orchestrate peptidoglycan synthesis (11, 13, 39, 54) and to directly impart rigidity to the cell wall (58). However, even the highest-resolution fluorescence studies leave unclear the spatial arrangement of the cell membrane relative to the nucleoid and the MreB spirals (2). This complicates the interpretation of how MreB influences chromosome segregation as it does not appear to be the force-generating motor to separate the nascent chromosomes although in E. coli, MreB interacts with a subunit of topoisomerase IV, ParC, that is required for the decatenation of chromosomes during bacterial septation (34). While our findings on the spiral nucleoid and MreB2 immunoelectron microscopic localization could be influenced by the mTFP tag, they raise the interesting possibility that MreB2 can associate with bacterial chromosomes, rather than localizing at the cell periphery. The localization of MreB2 is distinct from that of proteins that associate with or insert into the inner cell membrane, including RNase E, a component of the degradosome multiprotein complex (33), MinD, a protein that is required for mid-cell division placement in other bacteria (10), DnaA, the initiator of chromosomal replication (43), and polar chemoreceptor assemblies (59). The presence of MreB2 in or near the nucleoid could arise from interactions with RNA polymerases, the transcription terminator Rho, topoisomerases, and/or the bacterial elongation factor EF-Tu, which are highly conserved proteins that associate directly with MreB (6, 12, 29, 34) and which have homologous counterparts in B. bacteriovorus (46). There is considerable resemblance between the localization pattern of MreB2-mTFP and that of RNA polymerase, which has been shown to form scattered bright foci within nucleoids and a weaker, broader distribution throughout the nucleoid detectable by fluorescence microscopy (25, 32). Electron microscopic observations localizing gyrases and topoisomerases support this hypothesis (14, 24), as does the presence of ribosomes that are arrayed at the edge of the nucleoid (Fig. 3), since eukaryotic and prokaryotic actins are known to interact with ribosomal elongation factors.
Nucleoid localization of MreB proteins is consistent with the occurrence of highly elongated cells that lack division septa (see Videos S2, and S4 to S6 in the supplemental material), asymmetric chromosome localization (Fig. 4A and C), or bilobed chromosomes (Fig. 4A), which are reminiscent of cell division and chromosome segregation defects observed in other cells (30, 50, 57). MreB2 also influenced the positioning of polar chemotactic arrays, giving rise to multiple flagella (Fig. 5F), and large arrays of mislocalized chemoreceptors that were present sometimes at both poles (Fig. 5A to E). These results support the role of MreB in chemotaxis receptor positioning derived from light microscopic studies (19, 44, 48). Whether reduced activity or inappropriate levels of MreB2 or indirect effects of decoupling of nucleoid architecture to cell length extension cause abnormal polar protein distribution remains to be determined. The paucity of information about how other bacterial MreB proteins influence localization of polar assemblies has been noted (47). Nucleoid-associated MreB2 is likely less important for cell shape determination since, in contrast to earlier results (50), asymmetric nucleoid distribution did not lead to cell thinning in the DNA-free regions (Fig. 4B and C), and the majority of attack-phase MreB2-mTFP cells show fairly normal cellular morphologies (17). In contrast, MreB1 may be more important in this capacity since MreB1-mTFP cells stall early in growth phase development, forming relatively few filamentous cells within the bdelloplast (17).
Regulation of nucleoid architecture during the bacterial cell cycle is a multifaceted process involving DNA-binding proteins, topoisomerases and gyrases, and, during active transcription, RNA polymerases (7). How might the addition of mTFP to MreB2 both cause impaired chromosome segregation and increase the proportion of nucleoids that have a more open architecture? Similar to results seen with MreB counterparts in other bacteria (8, 49), insertional inactivation of MreB1 or MreB2 in B. bacteriovorus leads to cell lethality, but MreB1-mTFP and MreB2-mTFP cells survive with distinct phenotypic defects, suggesting that MreB function is impaired in both strains but not absent (17). Altered MreB2 could lead to abnormal chromosome decatenation or supercoiling through misregulation of the B. bacteriovorus topoisomerase II, GyrA, which is highly homologous (35% identity and 56% similarity) to ParC of E. coli. RNA polymerase is another potential target. Functional disruption of MreB or of RNA polymerase using temperature-sensitive alleles or rifampin treatment leads to similar defects in chromosome segregation (29, 30, 50). Interactions between MreB and RNA polymerase in vivo and in vitro in E. coli (29) are predicted to occur in 125 prokaryotes, including deltaproteobacteria (6). Finally, RNA polymerase works in concert with Fis, a nucleoid-binding protein that interacts with RNA polymerase that is primed for stationary-phase transcription upon replacement of growth phase sigma factor 70 with sigma factor 38. This complex regulates transcription of the DNA-binding protein from starved cells (Dps), which is critical for nucleoid compaction as cells enter stationary phase (27). The presence in B. bacteriovorus of homologs for RNA polymerase subunits, Fis, Dps, and at least seven RNA polymerase sigma factors (46) suggests that a similar mechanism may regulate nucleoid architecture in this organism during the transition from growth and division to attack-phase cells. Continuing studies of bacterial cells using correlative light/electron microscopy, chemical imaging, and comparative genomics will undoubtedly yield further important insights into the regulation of nucleoid structure and function and the fascinating role of MreB within the nucleoid.
Supplementary Material
Acknowledgments
This work was supported by funding to S.S. and to J.L.S.M. from the Center for Cancer Research at the National Cancer Institute, NIH, Bethesda, MD.
We thank Martin Kessel and Mario Borgnia for helpful discussions throughout the course of this work.
Footnotes
Published ahead of print on 10 December 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Berlatzky, I. A., A. Rouvinski, and S. Ben-Yehuda. 2008. Spatial organization of a replicating bacterial chromosome. Proc. Natl. Acad. Sci. U. S. A. 105:14136-14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biteen, J. S., et al. 2008. Super-resolution imaging in live Caulobacter crescentus cells using photoswitchable EYFP. Nat. Methods 5:947-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borgnia, M. J., S. Subramaniam, and J. L. S. Milne. 2008. Three-dimensional imaging of the highly bent architecture of Bdellovibrio bacteriovorus by using cryo-electron tomography. J. Bacteriol. 190:2588-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt, F., et al. 2009. The native 3D organization of bacterial polysomes. Cell 136:261-271. [DOI] [PubMed] [Google Scholar]
- 5.Briegel, A., et al. 2009. Universal architecture of bacterial chemoreceptor arrays. Proc. Natl. Acad. Sci. U. S. A. 106:17181-17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butland, G., et al. 2005. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433:531-537. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera, J. E., C. Cagliero, S. Quan, C. L. Squires, and D. J. Jin. 2009. Active transcription of rRNA operons condenses the nucleoid in Escherichia coli: examining the effect of transcription on nucleoid structure in the absence of transertion. J. Bacteriol. 191:4180-4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu, S.-W., S.-Y. Chen, and H.-C. Wong. 2008. Dynamic localization of MreB in Vibrio parahaemolyticus and in the ectopic host bacterium Escherichia coli. Appl. Environ. Microbiol. 74:6739-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel, R. A., and J. Errington. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113:767-776. [DOI] [PubMed] [Google Scholar]
- 10.de Boer, P. A. J., R. E. Crossley, A. R. Hand, and L. I. Rothfield. 1991. The MinD protein is a membrane ATPase required for the correct placement of the Escherichia coli division site. EMBO J. 10:4371-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Defeu Soufo, H. J., and P. L. Graumann. 2004. Dynamic movement of actin-like proteins within bacterial cells. EMBO Rep. 5:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defeu Soufo, H. J., C. Reimold, U. Linne, T. Knust, J. Gescher, and P. L. Graumann. 2010. Bacterial translation elongation factor EF-Tu interacts and colocalizes with actin-like MreB protein. Proc. Natl. Acad. Sci. U. S. A. 107:3163-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divakaruni, A. V., C. Baida, C. L. White, and J. W. Gober. 2007. The cell shape proteins MreB and MreC control cell morphogenesis by positioning cell wall synthetic complexes. Mol. Microbiol. 66:174-188. [DOI] [PubMed] [Google Scholar]
- 14.Dürrenberger, M., M. A. Bjornsti, T. Uetz, J. A. Hobot, and E. Kellenberger. 1988. Intracellular location of the histonelike protein HU in Escherichia coli. J. Bacteriol. 170:4757-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eltsov, M., and B. Zuber. 2006. Transmission electron microscopy of the bacterial nucleoid. J. Struct. Biol. 156:246-254. [DOI] [PubMed] [Google Scholar]
- 16.Fenton, A. K., M. Kanna, R. D. Woods, S.-I. Aizawa, and R. E. Sockett. 2010. Shadowing the actions of a predator: backlit fluorescent microscopy reveals synchronous nonbinary septation of predatory Bdellovibrio inside prey and exit through discrete bdelloplast pores. J. Bacteriol. 192:6329-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenton, A. K., C. Lambert, P. C. Wagstaff, and R. E. Sockett. 2010. Manipulating each MreB of Bdellovibrio bacteriovorus gives diverse morphological and predatory phenotypes. J. Bacteriol. 192:1299-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figge, R. M., A. V. Divakaruni, and J. W. Gober. 2004. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol. Microbiol. 51:1321-1332. [DOI] [PubMed] [Google Scholar]
- 19.Gitai, Z., N. Dye, and L. Shapiro. 2004. An actin-like gene can determine cell polarity in bacteria. Proc. Natl. Acad. Sci. U. S. A. 101:8643-8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gitai, Z., N. A. Dye, A. Reisenauer, M. Wachi, and L. Shapiro. 2005. MreB actin-mediated segregation of a specific region of a bacterial chromosome. Cell 120:329-341. [DOI] [PubMed] [Google Scholar]
- 21.Goley, E. D., E. Toro, H. H. McAdams, and L. Shapiro. 2009. Dynamic chromosome organization and protein localization coordinate the regulatory circuitry that drives the bacterial cell cycle. Cold Spring Harb. Symp. Quant. Biol. 74:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graumann, P. L. 2007. Cytoskeletal elements in bacteria. Annu. Rev. Microbiol. 61:589-618. [DOI] [PubMed] [Google Scholar]
- 23.Heymann, J. B., and D. M. Belnap. 2007. Bsoft: image processing and molecular modeling for electron microscopy. J. Struct. Biol. 157:3-18. [DOI] [PubMed] [Google Scholar]
- 24.Hobot, J. A., M. A. Bjornsti, and E. Kellenberger. 1987. Use of on-section immunolabeling and cryosubstitution for studies of bacterial DNA distribution. J. Bacteriol. 169:2055-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, D. J., and J. E. Cabrera. 2006. Coupling the distribution of RNA polymerase to global gene regulation and the dynamic structure of the bacterial nucleoid in Escherichia coli. J. Struct. Biol. 156:284-291. [DOI] [PubMed] [Google Scholar]
- 26.Khursigara, C. M., X. Wu, and S. Subramaniam. 2008. Chemoreceptors in Caulobacter crescentus: trimers of receptor dimers in a partially ordered hexagonally packed array. J. Bacteriol. 190:6805-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, J., et al. 2004. Fundamental structural units of the Escherichia coli nucleoid revealed by atomic force microscopy. Nucleic Acids Res. 32:1982-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kremer, J. R., D. N. Mastronarde, and J. R. McIntosh. 1996. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116:71-76. [DOI] [PubMed] [Google Scholar]
- 29.Kruse, T., et al. 2006. Actin homolog MreB and RNA polymerase interact and are both required for chromosome segregation in Escherichia coli. Genes Dev. 20:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruse, T., J. Møller-Jensen, A. Løbner-Olesen, and K. Gerdes. 2003. Dysfunctional MreB inhibits chromosome segregation in Escherichia coli. EMBO J. 22:5283-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Gros, M. A., G. McDermott, M. Uchida, C. G. Knoechel, and C. A. Larabell. 2009. High-aperture cryogenic light microscopy. J. Microsc. 235:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis, P. J., S. D. Thaker, and J. Errington. 2000. Compartmentalization of transcription and translation in Bacillus subtilis. EMBO J. 19:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liou, G.-G., W.-N. Jane, S. N. Cohen, N.-S. Lin, and S. Lin-Chao. 2001. RNA degradosomes exist in vivo in Escherichia coli as multicomponent complexes associated with the cytoplasmic membrane via the N-terminal region of ribonuclease E. Proc. Natl. Acad. Sci. U. S. A. 98:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madabhushi, R., and K. J. Marians. 2009. Actin homolog MreB affects chromosome segregation by regulating topoisomerase IV in Escherichia coli. Mol. Cell 33:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margolin, W. 2010. Imaging the bacterial nucleoid, p. 13-30. In R. T. Dame and C. J. Dorman, Bacterial chromatin. Springer, New York, NY.
- 36.Mascarenhas, J., M. H. W. Weber, and P. L. Graumann. 2001. Specific polar localization of ribosomes in Bacillus subtilis depends on active transcription. EMBO Rep. 2:685-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michie, K. A., and J. Löwe. 2006. Dynamic filaments of the bacterial cytoskeleton. Annu. Rev. Biochem. 75:467-492. [DOI] [PubMed] [Google Scholar]
- 38.Milne, J. L. S., and S. Subramaniam. 2009. Cryo-electron tomography of bacteria: progress, challenges and future prospects. Nat. Rev. Microbiol. 7:666-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammadi, T., et al. 2007. The essential peptidoglycan glycosyltransferase MurG forms a complex with proteins involved in lateral envelope growth as well as with proteins involved in cell division in Escherichia coli. Mol. Microbiol. 65:1106-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montero Llopis, P., et al. 2010. Spatial organization of the flow of genetic information in bacteria. Nature 466:77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morikawa, K., et al. 2006. Bacterial nucleoid dynamics: oxidative stress response in Staphylococcus aureus. Genes Cells 11:409-423. [DOI] [PubMed] [Google Scholar]
- 42.Morris, D. M., and G. J. Jensen. 2008. Toward a biomechanical understanding of whole bacterial cells. Annu. Rev. Biochem. 77:583-613. [DOI] [PubMed] [Google Scholar]
- 43.Newman, G., and E. Crooke. 2000. DnaA, the initiator of Escherichia coli chromosomal replication, is located at the cell membrane. J. Bacteriol. 182:2604-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsen, T., A. W. Yan, G. Gale, and M. B. Goldberg. 2005. Presence of multiple sites containing polar material in spherical Escherichia coli cells that lack MreB. J. Bacteriol. 187:6187-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohniwa, R. L., et al. 2007. Transcription-coupled nucleoid architecture in bacteria. Genes Cells 12:1141-1152. [DOI] [PubMed] [Google Scholar]
- 46.Rendulic, S., et al. 2004. A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303:689-692. [DOI] [PubMed] [Google Scholar]
- 47.Shaevitz, J. W., and Z. Gitai. 2010. The structure and function of bacterial actin homologs. Cold Spring Harb. Perspect. Biol. 2:a000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shih, Y.-L., I. Kawagishi, and L. Rothfield. 2005. The MreB and Min cytoskeletal-like systems play independent roles in prokaryotic polar differentiation. Mol. Microbiol. 58:917-928. [DOI] [PubMed] [Google Scholar]
- 49.Slovak, P. M., G. H. Wadhams, and J. P. Armitage. 2005. Localization of MreB in Rhodobacter sphaeroides under conditions causing changes in cell shape and membrane structure. J. Bacteriol. 187:54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivastava, P., G. Demarre, T. S. Karpova, J. McNally, and D. K. Chattoraj. 2007. Changes in nucleoid morphology and origin localization upon inhibition or alteration of the actin homolog, MreB, of Vibrio cholerae. J. Bacteriol. 189:7450-7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stavans, J., and A. Oppenheim. 2006. DNA-protein interactions and bacterial chromosome architecture. Phys. Biol. 3:R1-10. [DOI] [PubMed] [Google Scholar]
- 52.Thanbichler, M., and L. Shapiro. 2008. Getting organized—how bacterial cells move proteins and DNA. Nat. Rev. Microbiol. 6:28-40. [DOI] [PubMed] [Google Scholar]
- 53.Toro, E., and L. Shapiro. 2010. Bacterial chromosome organization and segregation. Cold Spring Harb. Perspect. Biol. 2:a000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Ent, F., et al. 2006. Dimeric structure of the cell shape protein MreC and its functional implications. Mol. Microbiol. 62:1631-1642. [DOI] [PubMed] [Google Scholar]
- 55.Vats, P., and L. Rothfield. 2007. Duplication and segregation of the actin (MreB) cytoskeleton during the prokaryotic cell cycle. Proc. Natl. Acad. Sci. U. S. A. 104:17795-17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vats, P., J. Yu, and L. Rothfield. 2009. The dynamic nature of the bacterial cytoskeleton. Cell Mol. Life Sci. 66:3353-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waidner, B., et al. 2009. A novel system of cytoskeletal elements in the human pathogen Helicobacter pylori. PLoS Pathog. 5:e1000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, S., H. Arellano-Santoyo, P. A. Combs, and J. W. Shaevitz. 2010. Actin-like cytoskeleton filaments contribute to cell mechanics in bacteria. Proc. Natl. Acad. Sci. U. S. A. 107:9182-9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, P., C. M. Khursigara, L. M. Hartnell, and S. Subramaniam. 2007. Direct visualization of Escherichia coli chemotaxis receptor arrays using cryo-electron microscopy. Proc. Natl. Acad. Sci. U. S. A. 104:3777-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.