Abstract
Accumulation of compatible solutes is a strategy widely employed by bacteria to achieve cellular protection against high osmolarity. These compounds are also used in some microorganisms as thermostress protectants. We found that Bacillus subtilis uses the compatible solute glycine betaine as an effective cold stress protectant. Glycine betaine strongly stimulated growth at 15°C and permitted cell proliferation at the growth-inhibiting temperature of 13°C. Initial uptake of glycine betaine at 15°C was low but led eventually to the buildup of an intracellular pool whose size was double that found in cells grown at 35°C. Each of the three glycine betaine transporters (OpuA, OpuC, and OpuD) contributed to glycine betaine accumulation in the cold. Protection against cold stress was also accomplished when glycine betaine was synthesized from its precursor choline. Growth of a mutant defective in the osmoadaptive biosynthesis for the compatible solute proline was not impaired at low temperature (15°C). In addition to glycine betaine, the compatible solutes and osmoprotectants l-carnitine, crotonobetaine, butyrobetaine, homobetaine, dimethylsulfonioactetate, and proline betaine all served as cold stress protectants as well and were accumulated via known Opu transport systems. In contrast, the compatible solutes and osmoprotectants choline-O-sulfate, ectoine, proline, and glutamate were not cold protective. Our data highlight an underappreciated facet of the acclimatization of B. subtilis to cold environments and allow a comparison of the characteristics of compatible solutes with respect to their osmotic, heat, and cold stress-protective properties for B. subtilis cells.
Bacillus subtilis can be found ubiquitously in the upper layers of the soil. Drying of the soil occurs frequently, and as a consequence, the salinity and osmolarity in this ecological niche increase. Such a rise in the external osmotic potential inevitably triggers water efflux from the B. subtilis cell (11). To prevent a reduction in water content and hence a drop of turgor, the cells initially increase the concentration of ions (primarily K+) through transport processes (35, 65) and subsequently accumulate compatible solutes, such as proline and glycine betaine, for their long-term adjustment to high-osmolarity surroundings (7, 65). The amassing of compatible solutes enables the cell to counteract the outflow of water and to reduce the ionic strength of the cytoplasm though K+ efflux systems without compromising turgor (11, 43).
Compatible solutes are operationally defined as organic compounds that can be amassed to high cytoplasmic levels but do not interfere with protein functions and other cellular activities (14). Microbial cells can accumulate these compounds both by osmotically stimulated de novo synthesis and by osmotically regulated import (11, 43, 68). The cellular content of compatible solutes is determined by the degree of the osmotic stress perceived by the cell, and it can reach molar concentrations under severe stress conditions (11, 68). Sources of these compounds in natural settings are osmotically down-shocked or decaying microbial and eukaryotic cells and excretion products of animals and plants (63). Since they are typically found in very low concentrations (nM or μM) in the environment, high-affinity transport systems for these organic osmolytes are required for them to be scavenged (11, 43, 68). Exposure of B. subtilis to high-salinity environments results in the synthesis of large amounts of proline (65). Proline can also be captured by B. subtilis as an osmoprotectant from environmental sources via the osmotically inducible OpuE transporter (61).
A physiologically very important osmoprotectant for B. subtilis is glycine betaine, whose accumulation via uptake or synthesis from the precursor choline confers a considerable degree of osmotic tolerance (10). Uptake of preformed glycine betaine is mediated by three osmotically inducible transport systems: the ABC transporters OpuA and OpuC and the carrier OpuD, a member of the BCCT family (10, 11, 68). The OpuA, OpuC, and OpuD transporters also serve for the acquisition of a substantial number of osmoprotectants other than glycine betaine (10). With the exception of ectoine (39), each of these compatible solutes is chemically related to either glycine betaine or proline (10).
Recent studies ascribe cell-protective functions to compatible solutes that significantly extend their traditionally studied roles as osmoprotectants (67). In particular, compatible solutes have been shown to participate in adaptation to high or low growth temperatures (2, 16-18, 20, 25, 27, 36, 47, 64, 67). In B. subtilis, glycine betaine has been reported to have growth-promoting effects both at the low (15°C) and the high (52°C) temperature boundaries of growth (12, 36).
In the upper layers of the soil, B. subtilis is subjected to temperature changes both during the course of the day, in connection with the day and night cycle, and over longer time periods as a consequence of seasonal changes. Rapid and severe temperature up- and downshifts elicit genetic and cellular adaptive reactions that are collectively known at the cold shock and heat shock stress responses, respectively (54). Such temperature shifts and sustained growth near the upper and lower temperature boundaries of B. subtilis also trigger the long-lasting induction of the SigB-controlled general stress response defense of B. subtilis (12, 37).
Cold shock imposes severe constraints on the B. subtilis cell with respect to protein synthesis, protein folding and stability, changes in DNA topology and mRNA secondary structures, and the biophysical properties of the cytoplasmic membrane (62). In laboratory settings, a sudden 22°C temperature downshift, from 37°C to 15°C, is typically used to trigger the B. subtilis cold shock response. However, it is unlikely that B. subtilis is very often exposed in natural settings to such a drastic and sudden reduction in growth temperature. Rather, one can foresee a gradual temperature reduction of the soil with lower temperatures maintained for longer time periods. Studies focusing on the adaptive molecular and physiological reactions of B. subtilis to a sustained cold environment are limited. Transcriptional profiling of B. subtilis cells that were continuously grown under chill stress conditions (15°C) revealed profound changes in the physiology of cold-stressed cells in comparison to that of cells grown at 37°C: transcription of about 280 genes is induced, and the transcription of about 300 genes is repressed (15). Strikingly, there is in essence no overlap between the genes whose transcription is induced by cold shock and those whose transcription is induced in cells continuously cultivated at low temperature (5). Hence, cold shock and prolonged growth in the cold apparently require very distinct cellular and genetic adaptation reactions of the B. subtilis cell.
Our focus here is the adaptation of B. subtilis to prolonged growth in cold, which we term the cold stress response to distinguish it from the acute cold shock response. Prompted by the initial observation that glycine betaine has strong protective effects for B. subtilis cells subjected to cold stress (growth at 15°C) and that it can even rescue the cold-sensitive growth phenotype of a sigB mutant (12), we have performed a comprehensive physiological analysis of the role of various compatible solutes as cold stress protectants for B. subtilis. Our data show that the accumulation of selected compatible solutes provides excellent protection against cold stress during cultivation at low temperature (15°C to 13°C). Our findings highlight an underappreciated facet of the acclimatization of B. subtilis to cold environments and compare the characteristics of compatible solutes with respect to their osmotic, heat, and cold stress-protective properties for B. subtilis cells.
MATERIALS AND METHODS
Growth media and cultivation conditions.
Throughout this study, we used the B. subtilis 168 (trpC2) wild-type strain and genetically defined mutants derived from this isolate (Table 1). B. subtilis strains were routinely maintained on Luria-Bertani agar plates. The various B. subtilis strains were cultivated in Spizizen's minimal medium (SMM) (33), with 0.5% (wt/vol) glucose as the carbon source and l-tryptophan (20 mg liter−1) to satisfy the auxotrophic growth requirements of strains derived from B. subtilis 168 (trpC2). A solution of trace elements was added to SMM (33). The osmolality of the growth medium was increased by the addition of NaCl from a 5 M stock solution. Compatible solutes and other additives were sterilized by filtration and added to the growth medium, usually from 100 mM stock solutions. All B. subtilis cultures were inoculated from exponentially growing precultures in prewarmed minimal medium to optical densities at 578 nm (OD578s) of 0.1, and the cultures were then propagated at 37°C. These precultures were allowed to grow at 37°C to an OD578 of 0.5, diluted to an OD578 of 0.1 in 20 ml SMM in a 100-ml Erlenmeyer flask, and subsequently transferred to the lower growth temperatures indicated in the individual experiments. To record growth curves of B. subtilis strains, cultures were grown in 20-ml volumes of SMM in 100-ml Erlenmeyer flasks incubated in a shaking water bath set at 220 rpm; growth of bacterial cultures was monitored by measuring the OD578. The antibiotics kanamycin (5 μg ml−1), erythromycin-lincomycin (0.4 μg ml−1 and 15 μg ml−1, respectively), spectinomycin (100 μg ml−1), and tetracycline (10 μg ml−1) were used for the selection of B. subtilis strains carrying chromosomal copies of in vitro-constructed gene disruption mutations with insertion of an antibiotic resistance cassette.
TABLE 1.
B. subtilis strains used in this study
| Straina | Relevant genotype | Source |
|---|---|---|
| 168 | trpC2 | Laboratory collection |
| JGB23 | Δ(opuA::erm)4 Δ(opuBD::tet)23 opuC20::Tn10 (spc) opuD+ | This study |
| JGB24 | Δ(opuA::erm)4 Δ(opuBD::tet)23 opuC+ Δ(opuD::neo)2 | This study |
| JGB25 | opuA+ Δ(opuBD::tet)23 opuC20::Tn10 (spc) Δ(opuD::neo)2 | This study |
| JGB26 | Δ(opuA::erm)4 opuB+opuC20::Tn10 (spc) Δ(opuD::neo)2 | This study |
| JGB27 | Δ(opuA::erm)4 Δ(opuBD::tet)23 opuC20::Tn10 (spc) Δ(opuD::neo)2 | This study |
| SOB9 | Δ(gbsAB::neo)2 | This study |
| TMB5 | Δ(proHJ::tet)1 | This study |
All mutants are derivatives of the B. subtilis wild-type strain 168.
Chemicals.
Glycine betaine, choline chloride, γ-butyrobetaine, and l-carnitine were purchased from Sigma-Aldrich (Steinheim, Germany), and acetoin (3-hydroxy-3-butanon) was obtained from Merck (Darmstadt, Germany). Proline betaine (stachydrine-hydrochloride) was synthesized by Extrasynthese (Genay, France). Ectoine was purchased from Biomol (Hamburg, Germany). Crotonobetaine was a kind gift from J. Brass (Lonza AG, Visp, Switzerland). Dimethylsulfonioactetate (DMSA) was generously provided by M. Jebbar (University of Rennes, France), and homobetaine and choline-O-sulfate were kind gifts from G. Nau-Wagner (University of Marburg, Germany). Radiolabeled [1-14C]glycine betaine (55 mCi mmol−1) was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). The antibiotics kanamycin, erythromycin, lincomycin, spectinomycin, and tetracycline were purchased form Sigma-Aldrich (Steinheim, Germany).
Construction of B. subtilis mutant strains.
Previous studies focusing on the uptake and synthesis of compatible solutes and their role in osmotic stress resistance (10) and high-temperature stress adaptation (36) employed the widely used B. subtilis laboratory strain JH642 (BGSC 1A96; J. Hoch, La Jolla, CA). However, this strain background carries a previously unrecognized defect in the ilvB gene, thereby causing a reduction in the activity of the acetolactate synthase complex that results in a cold-sensitive growth phenotype (66). To avoid complications in the interpretation of the data originating from our cold stress experiments, we constructed a new isogenic set of mutant strains with defects in systems for compatible-solute synthesis and uptake in the B. subtilis 168 (trpC2) strain background, since this strain is ilvB+ and is not temperature sensitive. Mutants in the opuA, opuB, opuC, opuD, gbsAB, and proHJ loci were constructed by transforming B. subtilis 168 (trpC2) with chromosomal DNA isolated from previously constructed gene disruption mutants marked with an antibiotic resistance cassette in the B. subtilis JH642 strain background and by selecting for the appropriate antibiotic resistance (Table 1). The preparation of chromosomal DNA, DNA transformation of the B. subtilis 168 (trpC2) strain, and the selection for the integration of gene disruptions marked with an antibiotic resistance cassette by a double-homologous recombination event all followed routine procedures. The genotypes of the resulting mutants are summarized in Table 1. It should be noted that in each of these B. subtilis mutant strains, the gene (opuE) for the osmotically inducible proline uptake is intact (61).
Transport assays with [1-14C]glycine betaine and determination of intracellular glycine betaine pools.
To determine the initial glycine betaine uptake in cultures of B. subtilis 168, cells were grown in SMM to an OD578 of 0.5 at the indicated temperatures with or without the addition of 0.4 M NaCl. Uptake of [1-14C]glycine betaine by the cells in 2-ml aliquots at 37°C with a final glycine betaine concentration of 10 μM was measured in the individual assay. The assay conditions followed a previously described protocol (36), and the amount of [1-14C]glycine betaine taken up by the B. subtilis cells was determined by scintillation counting. Intracellular glycine betaine pools in exponentially grown cells of B. subtilis were analyzed. Cells were cultivated in SMM at the indicated temperatures and salinities in the presence of 1 mM glycine betaine, spiked with 0.64 μM [1-14C]glycine betaine to an OD578 of 0.5. The intracellular-glycine-betaine concentrations were measured by scintillation counting and calculated as described previously (36), using a volume of a B. subtilis cell of 0.67 μl per 1 OD578 unit of cell culture (S. Moses, E. P. Bakker, and E. Bremer, unpublished data).
HPLC analysis of proline pools.
The proline content of B. subtilis strain 168 (trpC2) cells (OD578 = 2) grown in SMM either at 37°C, at 37°C with 0.4 M NaCl, or at 15°C was quantitated by high-performance liquid chromatography (HPLC) analysis. Sample preparation, derivatization of amino acids with 9-fluorenyl-methoxy-carbonyl chloride (FMOC), separation of the modified amino acids by HPLC, detection of the fluorescent labeled amino acids, and quantification of proline were all carried out as previously described (36).
Isolation of RNA and Northern blot analysis.
Total RNA was prepared from exponentially growing cells (OD578 = 0.5) of the B. subtilis strain 168. The preparation of single-stranded antisense RNA probes, Northern blot analysis, and the detection of hybridizing mRNA species were all carried out as described previously (36).
RESULTS
Influence of a reduction in temperature on growth rate of B. subtilis.
The growth rate of the B. subtilis 168 (trpC2) strain at 35°C was 0.45 h−1, and the cells had a doubling time of 1.5 h. To assess the influence of temperature on the growth rate, we grew this strain in a minimal medium (SMM), with 0.5% glucose as the carbon source, at various temperatures (from 35°C down to 13°C). The lowest temperature this strain was able to grow at was 15°C, and at this growth temperature, it had a growth rate of 0.03 h−1 and a doubling time of 23 h. At 13°C, the cells were unable to grow in a minimal medium. Hence, as expected, a reduction in temperature has a strong influence on growth rate (Fig. 1) and the growth of the B. subtilis wild-type strain was abolished in a chemically defined medium in a narrow temperature window, between 15°C and 13°C (Fig. 2).
FIG. 1.
Influence of growth temperature on the growth rate of B. subtilis 168. The B. subtilis wild-type strain 168 was grown in SMM at the indicated temperatures. The cultures (20 ml) were propagated in 100-ml Erlenmeyer flasks in a shaking water bath set at 220 rpm, and growth was monitored by measuring the OD578. Growth rates were calculated for the exponential growth phase.
FIG. 2.
Protection of B. subtilis 168 against cold stress by glycine betaine. The cells of the wild-type strain were inoculated (OD578 of about 0.1) from a preculture grown at 37°C in SMM and were then cultivated in a shaking water bath set at 220 rpm at the indicated temperatures in the absence (open circles) or the presence (closed circles) of 1 mM glycine betaine.
Glycine betaine is an efficient cold stress protectant.
To study the function of glycine betaine as a cold stress protectant for B. subtilis, we grew the wild-type strain in SMM at various temperatures in the absence or presence of 1 mM glycine betaine. Glycine betaine modestly affected cell growth at 37°C, but the growth of the cells was greatly enhanced by the addition of glycine betaine to the medium at 15°C (Fig. 2). The cells propagated without glycine betaine had a doubling time of 23 h, and those propagated in the presence of glycine betaine had a much shorter doubling time, 12.3 h. An even more striking chill-protective effect of glycine betaine was observed when the B. subtilis cells were cultivated at 13°C. The culture propagated in the absence of glycine betaine did not grow and reached a maximal OD578 of approximately 0.5 after 170 h of incubation. In contrast, the culture supplemented with 1 mM glycine betaine withstood the cold stress; it had a doubling time of 32.2 h and reached an OD578 of approximately 5 after 170 h of incubation (Fig. 2). However, when the growth temperature was lowered by just 2°C, from 13°C down to 11°C, glycine betaine no longer afforded cell growth (Fig. 1).
Compatible solutes are typically found in natural habitats of microorganisms in very low concentrations (63), and microorganisms therefore developed high-affinity uptake systems to scavenge these compounds from scarce environment resources (11, 52, 68). To test the effectiveness of glycine betaine as a chill stress protectant at low external concentrations, we cultured the wild-type B. subtilis strain at 15°C with decreasing concentrations of glycine betaine (from 1 mM down to 25 μM) and then measured the optical density of the cultures after 72 h of incubation (Fig. 3). In the absence of glycine betaine, the growth yield of the culture was very low. However, even the lowest concentration of glycine betaine tested in this experiment (25 μM) had a beneficial effect on the growth yield of B. subtilis (Fig. 3). Full protection of the cells against chill stress was afforded by the addition of approximately 300 μM glycine betaine to the growth medium (Fig. 3).
FIG. 3.
Low concentrations of glycine betaine are sufficient for protection against chill stress. Cells of the B. subtilis strain 168 were grown in SMM at 15°C in a shaking water bath set at 220 rpm in the presence of various concentrations of glycine betaine. The cells were inoculated (OD578 of about 0.1) from a preculture grown at 37°C in SMM, and the optical densities of the cold-stressed cultures were measured after 72 h.
Transport and accumulation of [1-14C]glycine betaine by cold-stressed B. subtilis cells.
It is apparent from the data presented in Fig. 3 that B. subtilis can take advantage of low concentrations of glycine betaine in its environment to achieve protection against cold stress. To investigate this further, we conducted transport assays with radiolabeled [1-14C]glycine betaine and also monitored the size of the intracellular glycine betaine pool of cells grown at low temperature. We measured [1-14C]glycine betaine uptake of chill-stressed B. subtilis cells (at 15°C and at an OD578 of 0.5) at a final substrate concentration of 10 μM. We then compared the glycine betaine uptake activity of these cells with that of cells that were grown at 37°C in the absence or presence of 0.4 M NaCl. Glycine betaine uptake in B. subtilis is osmotically inducible (10), and the transport data presented in Fig. 4 A reflect this fact. [1-14C]glycine betaine uptake in cells grown at 37°C in SMM occurred at a rate of approximately 49 nmol min−1 mg of protein−1, and this rate was increased to 120 nmol min−1 mg of protein−1 when the cells were subjected to a modest osmotic stress with 0.4 M NaCl. [1-14C]glycine betaine uptake in cells grown at 15°C was substantially lower than that observed in cells grown at 37°C and proceeded with a transport rate of approximately 12 nmol min−1 mg of protein−1 (Fig. 4A).
FIG. 4.
Uptake and accumulation of radiolabeled glycine betaine under chill stress conditions. (A) For the determination of the uptake of [1-14C]glycine betaine under different temperature and osmotic conditions, cells of the B. subtilis 168 wild-type strain were propagated at 37°C (open circles), at 37°C with 0.4 M NaCl (closed circles), or at 15°C (closed squares) to an OD578 of approximately 0.5. These cells were then assayed for [1-14C]glycine betaine uptake at a final substrate concentration of 10 μM. The data show a representative transport experiment selected from three replicates. (B) For the determination of the intracellular glycine betaine pool, cells of the B. subtilis 168 wild-type strain were grown at 35°C, 30°C, 25°C, 20°C, and 15°C in the presence of 1 mM glycine betaine (spiked with 0.64 μM [1-14C]glycine betaine). After the cultures reached an OD578 of 0.5, the amount of the accumulated [1-14C]glycine betaine was determined by scintillation counting. The intracellular glycine betaine pools were calculated using a value of 0.67 μl per 1 OD578 unit of B. subtilis cells for the intracellular volume of B. subtilis cells. The values shown represent data (triplicates each) from two independent experiments.
We then assessed whether the intracellular pool of glycine betaine would change with decreasing growth temperature. For these experiments, cells of B. subtilis 168 were inoculated to an OD578 of 0.1 in minimal medium containing 1 mM glycine betaine spiked with 0.64 μM [1-14C]glycine betaine. The cultures were then allowed to grow to an OD578 of 0.5 at growth temperatures of 35°C, 30°C, 25°C, 20°C, and 15°C. After the cells reached the desired optical density, samples were removed from these cultures and used to determine their radiolabeled glycine betaine content. Using a cell volume of 0.67 μl per 1 OD578 unit of cell culture of B. subtilis (S. Moses, E. P. Bakker, and E. Bremer, unpublished results), we calculated a glycine betaine content of 191 ± 3 mM for the cells that were grown at 35°C (Fig. 4B). The glycine betaine content of the cells gradually increased as the growth temperature of the cultures was dropped, and glycine betaine concentrations of 393 ± 1 mM were reached in those cells that were cultivated at 15°C (Fig. 4B). Hence, despite the fact that the initial [1-14C]glycine betaine uptake activity of B. subtilis cells at 15°C is low (Fig. 4A), continuously cold-stressed B. subtilis cells can amass a considerable glycine betaine pool over time. Its size is approximately double that found in B. subtilis cells propagated at 35°C in the absence of osmotic stress (Fig. 4B). The glycine betaine pool of the cold-stressed cells (15°C) matches that of B. subtilis cells grown at 35°C in SMM in the presence of 0.4 M NaCl (435 ± 9 mM) (data not shown), growth conditions that constitute a moderate degree of osmotic stress for B. subtilis.
Each of the three glycine betaine transporters of B. subtilis contributes to protection against cold stress.
Each of the three glycine betaine import systems (OpuA, OpuC, and OpuD) operating in B. subtilis is individually sufficient to attain full protection of cells grown in SMM with 1.2 M NaCl in the presence of 1 mM glycine betaine against osmotic stress (41). Each of them is also sufficient to confer protection of cells grown at 52°C in the presence of 1 mM glycine betaine against heat stress (36). To test the individual contribution of the OpuA, OpuC, and OpuD transporters to protection against cold stress by glycine betaine, we used an isogenic set of strains in which only one of these glycine betaine import systems was functional. We cultivated these mutant strains at 15°C in SMM in the presence of 1 mM glycine betaine and found that the ABC-type transporters OpuA and OpuC and the betaine-choline-carnitine transporter (BCCT)-type transporter OpuD were each involved in glycine betaine uptake at low temperature and that each was sufficient to achieve full protection against cold stress (Fig. 5 A). As expected, a strain in which only the choline importer OpuB was functional or a strain that was simultaneously defective in the OpuA, OpuC, and OpuD glycine betaine uptake systems, there was no protection against cold stress by glycine betaine (Fig. 5A). This finding demonstrates that no unknown glycine betaine importer operates in cold-stressed B. subtilis cells and that glycine betaine must enter the cell in order to confer protection against cold stress.
FIG. 5.
Contributions of the OpuA, OpuC, and OpuD transporters to glycine betaine-mediated protection of B. subtilis against cold stress. (A) Cells of the B. subtilis wild-type strain 168 (wt) and its mutant derivatives JGB25 (OpuA+), JGB26 (OpuB+) JGB24 (OpuC+), JGB23 (OpuD+), and JGB27 (OpuA− OpuB− OpuC− OpuD−) were inoculated from precultures grown at 37°C to an OD578 of about 0.1 in either SMM or in SMM containing 1 mM glycine betaine (GB), and the growth yield of these cultures was determined after 120 h of incubation at 15°C. (B) For the determination of the intracellular glycine betaine pool, cells of the B. subtilis 168 wild-type strain and its mutant derivatives were grown in the presence of 1 mM glycine betaine (spiked with 0.64 μM [1-14C]glycine betaine) at 37°C (black bars), at 37°C in the presence of 0.4 M NaCl (gray bars), or at 15°C (white bars). The values shown represent data from two independent experiments with two replicas each.
To assess the glycine betaine pool present in exponentially growing cells (OD578 of 0.5) of B. subtilis mutants possessing only one functional glycine betaine import system, we grew our isogenic set of opu mutant strains at 15°C in the presence of 1 mM glycine betaine (spiked with 0.64 μM [1-14C]glycine betaine). Each of these strains built up a glycine betaine pool that was considerably larger than that found in cells grown at 37°C (Fig. 5B). The glycine betaine content of the cold-stressed cells resembled that of the corresponding mutants grown at 37°C in the presence of 0.4 M NaCl (Fig. 5B). Hence, each of the glycine betaine transporters of B. subtilis contributes substantially to glycine betaine accumulation in response to cold stress. The glycine betaine pool present in either cold- or salt-stressed cells of the strain with the intact OpuA system matches that of the wild-type strain in which OpuA, OpuC, and OpuD are simultaneously functional (Fig. 5B). This can be understood in view of the fact that the transport capacity (Vmax) of the OpuA transporter is much higher than that of the combined OpuC and OpuD transport systems in salt-stressed B. subtilis cells (41).
Synthesis of glycine betaine from its precursor choline confers protection against cold stress.
B. subtilis not only uses preformed glycine betaine as an effective osmostress protectant but also can synthesize it from the precursor choline and can thereby achieve a considerable degree of osmotic tolerance. Choline is taken up with high affinity via the OpuB and OpuC ABC transporters and is then oxidized by the GbsB and GbsA enzymes to glycine betaine, with glycine betaine aldehyde as the intermediate (9, 42). Choline has no osmoprotective capacity per se, since it does not serve as an osmoprotectant for a B. subtilis mutant strain (gbsAB) that cannot enzymatically convert choline into glycine betaine (8). We found that choline was an effective cold stress protectant for B. subtilis (Fig. 6) but that it lost this chill stress-protective property in the gbsAB mutant strain SOB9, whereas this strain was still well protected from the cold by an exogenous supply of glycine betaine since SOB9 possesses three intact glycine betaine uptake systems (Fig. 6). The same stress-protective pattern was previously observed when choline and glycine betaine were used as heat stress protectants for a mutant with a defect in glycine betaine synthesis (36).
FIG. 6.

Contributions of glycine betaine synthesis to protection of B. subtilis against cold stress. The B. subtilis cells were inoculated (OD578 of about 0.1) from a preculture grown at 37°C in SMM and were then cultivated in a shaking water bath set at 220 rpm at 15°C. The wild-type B. subtilis strain 168 (open symbols) and its isogenic gbsAB mutant derivative SOB9 [Δ(gbsAB::neo)2] (closed symbols) were grown in the absence or glycine betaine and choline (circles) or the presence of 1 mM glycine betaine (squares) or 1 mM choline (triangles).
Synthesis of the compatible solute proline does not confer protection against cold stress.
In the absence of exogenously provided compatible solutes, salt-stressed B. subtilis cells synthesize large quantities of proline to fend off the detrimental effects of high osmolality on cell physiology (65). B. subtilis possesses two routes for proline biosynthesis (6): (i) the ProB-ProA-ProI enzymes produce proline for anabolic purposes (13), and (ii) the synthesis of proline as an osmostress protectant depends on the ProJ-ProA-ProH enzymes. A proHJ mutant cannot produce proline as an osmostress protectant (J. Brill and E. Bremer, unpublished results) but is no proline auxotroph (6).
We determined the proline content of B. subtilis 168 cells cultivated at 15°C by HPLC analysis and found a greatly elevated proline pool in these cells in comparison to that in cells that were cultivated at 37°C (Fig. 7 A). The proline content of the cold-stressed B. subtilis cells approaches that of cells that were cultivated under moderate-osmotic-stress conditions with 0.4 M NaCl (Fig. 7A). Transcription of the proHJ operon is upregulated under osmotic stress conditions (10, 31, 56). We used Northern blot analysis to assess the transcription of the proHJ gene cluster in cells grown at 15°C and found that (i) transcription was upregulated in comparison to the level for cells cultivated at 37°C and that (ii) the presence of 1 mM glycine betaine in the growth medium of cold-stressed cells reduced the expression of the proHJ operon (Fig. 7B). Taken together, these observations suggested that proline biosynthesis might contribute to cold acclimatization of B. subtilis cells. To test this hypothesis genetically, we grew the Δ(proHJ::tet)1 mutant strain TMB5 at 15°C and compared its growth properties with those of the B. subtilis wild-type strain 168. Surprisingly, no difference in the growth properties of these two strains was observed (Fig. 7C), demonstrating that the observed production of proline does not confer protection against cold stress in growing cells.
FIG. 7.
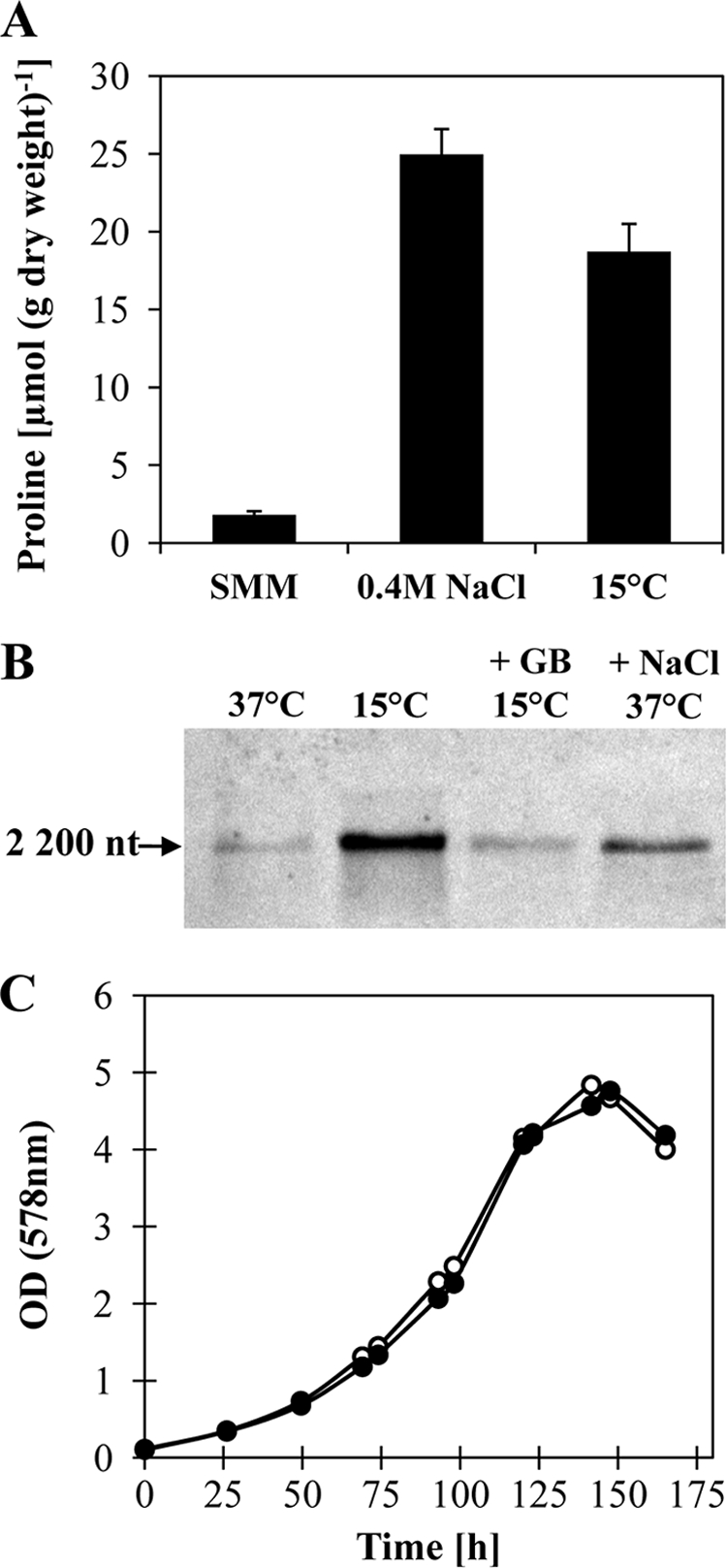
Proline synthesis is induced by growth of B. subtilis in the cold but does not contribute to protection against cold stress. (A) Cultures of the B. subtilis 168 strain were grown in SMM at 37°C, at 37°C with 0.4 M NaCl, and at 15°C to an OD578 of about 2. Their proline content was determined by HPLC analysis. The data shown represent measurements of 3 independently grown cultures with two replicas each. (B) Total RNA was isolated from exponentially growing B. subtilis cells (OD578 = 0.5 to 1) cultivated in SMM either at 37°C, at 15°C, at 15°C with 1 mM glycine betaine (GB), or at 37°C with 0.4 M NaCl. Equal amounts of the obtained RNA (10 μg) were denatured and separated according to size on a agarose gel and blotted onto a nylon membrane, and the RNA was then subjected to Northern blot analysis using a single-stranded antisense RNA probe specific for proHJ; the measured length of the proHJ transcript is about 2,200 nucleotides (nt), and the calculated length of the transcript is 2,087 nucleotides. (C) Growth of the wild-type strain (open circles) in SMM at 15°C is compared with that of its isogenic proHJ mutant derivative TMB5 [Δ(proHJ::tet)1] (closed circles).
A broad spectrum of compatible solutes confers protection against chill stress to B. subtilis.
In addition to glycine betaine, a broad spectrum of compatible solutes can provide effective osmoprotection to B. subtilis (10). We tested whether any of the 12 known osmoprotectants would also stimulate growth in the cold. With the notable exceptions of proline, choline-O-sulfate, and ectoine, all these compatible solutes enhanced growth under chill stress conditions (Fig. 8 A). Exogenously provided glutamate has osmoprotective effects for B. subtilis that stem from its ProHJ-dependent enzymatic conversion into the compatible solute proline (M. Bleisteiner and E. Bremer, unpublished results), but it is a heat stress protectant per se (36). We therefore tested a possible cold stress-protective function of glutamate but found that it did not possess such a property (Fig. 8A).
FIG. 8.
Protection of B. subtilis against chill stress by various compatible solutes. (A) Cultures of the B. subtilis wild-type strain 168 were inoculated in SMM to an OD578 of about 0.1 from a preculture grown at 37°C and grown in the presence of 1 mM (each) the various compounds tested for chill protection for 72 h at 15°C. (B) The cold protectants for the B. subtilis wild-type strain 168 (A) were then tested for protection against chill stress with its quadruple mutant derivative, strain JGB27 (opuA, opuB, opuC, and opuD). For this experiment, cultures were inoculated in SMM to an OD578 of about 0.1 from a preculture grown at 37°C and cultivated in the presence of 1 mM (each) various compounds for 120 h at 15°C. We tested in the same experiment the cold stress-protective effect of glycine betaine for the wild-type B. subtilis 168 strain (denoted “wt”).
In their genome-wide transcriptional profiling study of cold-shock-induced B. subtilis genes, Beckering et al. (2002) discovered that the gene cluster encoding an ABC transporter for acetoin was upregulated by a rapid temperature downshift from 37°C to 15°C (5). This finding led these authors to speculate that acetoin or metabolic derivatives of this compound might function as cryoprotectants under cold shock conditions (5). This prompted us to test a possible chill-protective function of acetoin for B. subtilis cells that were continuously cultured in the cold (15°C). However, we detected no noticeable cold stress-protective property of acetoin when the growth medium of cold-stressed B. subtilis cells contained 1 mM this compound (Fig. 8A).
All cold stress protectants are accumulated via the known Opu transport systems.
The uptake systems for compatible solutes in B. subtilis are well characterized, and the substrate specificities of the various transporters involved in osmoprotectant uptake (Opu) have been studied in considerable detail (10). Noticeably, these systems also mediate the uptake of those compatible solutes that function as heat stress protectants for B. subtilis (36). We therefore tested in growth experiments whether the uptake of the eight chill stress protectants identified by us (Fig. 8A) were also imported into cold-stressed B. subtilis cells via the OpuA, OpuB, OpuC, and OpuD transport systems. We used for these experiments an isogenic set of mutant strains in which only one of the aforementioned compatible-solute uptake systems was functional. There was no protection against chill stress by any of the tested compatible solutes in quadruple mutant strain JGB27, in which the OpuA, OpuB, OpuC, and OpuD transport systems were simultaneously defective (Fig. 8B). The pattern for the acquisition of the eight chill stress protectants exactly matched that observed when these solutes are taken up by B. subtilis for osmoprotective purposes (Table 2) (10).
TABLE 2.
Stress-protective properties of compatible solutes in B. subtilis
| Compatible solute | Uptake via: |
Stress protectiona |
||||||
|---|---|---|---|---|---|---|---|---|
| OpuA | OpuB | OpuC | OpuD | OpuE | Osmostress (1.2 M NaCl) | Heat stress (52°C) | Cold stress (15°C) | |
| Glycine betaine | + | − | + | + | − | + | + | + |
| Choline | − | + | + | − | − | + | + | + |
| Homobetaine | + | − | + | − | − | + | + | + |
| Carnitine | − | − | + | − | − | + | + | + |
| γ-Butyrobetaine | − | − | + | − | − | + | + | + |
| Crotonobetaine | − | − | + | − | − | + | + | + |
| DMSA | + | − | + | + | − | + | + | + |
| Proline | − | − | − | − | + | + | + | − |
| Proline betaine | + | − | + | − | − | + | − | + |
| Choline-O-sulfate | − | − | + | − | − | + | − | − |
| Ectoine | − | − | + | − | − | + | − | − |
| Glutamateb | − | − | − | − | − | + | + | − |
The osmotic stress protection of the various compatible solutes was tested for B. subtilis cells that were grown in SMM with 1.2 M NaCl; heat stress protection was evaluated for cells grown in SMM at 52°C, and chill stress protection was assessed for cells grown in SMM at 15°C. The concentration of the compatible solutes in the growth assays was 1 mM each. The data on the protection of B. subtilis from osmotic and heat stress by compatible solutes were compiled from previously reported data (10, 36). The listed data for protection of B. subtilis from cold stress by compatible solutes are from this study.
Glutamate is taken up under the various stress conditions via the GltT transporter (M. Bleisteiner and E. Bremer, unpublished data).
Upregulation of the structural gene for the BCCT-type glycine betaine transporter OpuD by cold stress.
The expression of the opuA, opuB, opuC, and opuD loci is upregulated in B. subtilis cells that are osmotically challenged (31, 56), and there is also a modest upregulation in the transcription of these loci in cells challenged by continuous heat stress (52°C) (36). To test if such an upregulation also occurred in cells cultivated in the cold (15°C), we used Northern blot analysis but found no evidence for enhanced transcription of the opuA, opuB, and opuC operons (data not shown). This finding is consistent with previous data from genome-wide transcriptional profiling experiments (15). We detected, however, an upregulation of opuD transcription in cold-stressed B. subtilis cells (Fig. 9). The OpuD glycine betaine transporter is a member of the BCCT family (41, 68). Transcription of opuD is osmotically inducible and occurs from both a SigA-type promoter and a SigB-dependent promoter (A. Schlüfter, F. Spiegelhalter, and E. Bremer, unpublished data). Since the SigB regulon is induced in cold-stressed B. subtilis cells (12, 15, 38), we attribute the cold stress induction of opuD expression to the SigB-dependent promoter.
FIG. 9.
Cold stress induction of opuD expression. (A) Genetic organization of the ytfP-opuD chromosomal region. The location of the single-stranded probe used to detect the opuD transcript is indicated, and the positions of the SigA- and SigB-responsive promoters are shown. (B) Northern analysis of the opuD transcript. RNA isolation from B. subtilis cells grown either at 37°C or at 15°C and Northern blot analysis were carried out as detailed in legend to Fig. 7. The opuD-specific transcripts were detected using a single-stranded antisense RNA probe; the measured length of the opuD transcript is about 1,800 nucleotides, and the calculated length of the transcript is 1,619 nucleotides. A longer transcript (about 2,900 nucleotides) was also detected with the used opuD probe (A) that comprises an ytfP-opuD mRNA species (calculated length, 2,682 nucleotides). This was ascertained by using an ytfP-specific hybridization probe (A. Schlüfter and E. Bremer, unpublished data).
DISCUSSION
Most of the Earth's biosphere is cold (53), and a large number of Bacteria and Archaea have specifically adapted the functioning of their proteins, their systems for protein folding, their biosynthetic machinery, their genetic apparatus, and their entire physiology to a life in permanently cold environments (26, 55, 58). B. subtilis does not belong to this group of psychrophilic microorganisms; it is a typical mesophile that is temporarily exposed, either suddenly (5) or on a more continued basis (15), to low temperature surroundings in its various habitats. The data reported here ascribe an important physiological function to the accumulation of compatible solutes for the adaptation of B. subtilis to permanently cold surroundings and highlight an underappreciated facet of the physiological repertoire of the B. subtilis cell for adjustment to cold environments.
Much attention has been focused in the past on the protective effects of cold shock proteins, the adjustment in membrane fluidity, the induction of the SigB-controlled general stress regulon, and the reprogramming of gene transcription on a genome-wide scale in either cold-shocked or permanently cold-stressed B. subtilis cells (5, 12, 15, 23, 38, 54, 62). All these cold stress response systems are certainly of importance for the physiological adjustment process of B. subtilis cells exposed either suddenly or on a sustained basis to cold environments. However, the data documented in Fig. 2 clearly show that none of these well-studied cold-adaptive measures can ensure cell growth at 13°C, a temperature that is certainly frequently encountered by B. subtilis in the soil. In contrast, the uptake of glycine betaine readily permitted growth of B. subtilis at 13°C. Protection against cold stress was also afforded by seven other compatible solutes that are structurally related to glycine betaine (Fig. 8A), attesting to the effectiveness of compatible-solute acquisition as a measure for achieving protection of B. subtilis against cold stress.
We note, however, that protection against cold stress by glycine betaine occurs in a narrow temperature window; a drop in growth temperature by just 2°C, from 13°C down to 11°C, no longer allowed glycine betaine to rescue cell growth (Fig. 2). The cold-protective effects of glycine betaine resemble in this aspect those observed when it acts as a heat stress protectant for B. subtilis, since glycine betaine is a thermostress protectant at 52°C but cannot extend the upper growth boundary of B. subtilis beyond this temperature (36).
Our data show that among the 12 osmoprotectants for B. subtilis known to date (10), 8 serve as cold stress protectants as well (Table 2). Each of these solutes is acquired by the cell via the same Opu transport systems used for their uptake under osmotic stress conditions (Fig. 7B and Table 2). Our studies with the mutants defective in the various Opu transport systems clarify two important issues: (i) there is no unrecognized uptake system for compatible solute operational in cold-stressed B. subtilis cells, and (ii) any models in which the cold-protective effects of compatible solutes would be exerted from outside the B. subtilis cell are clearly ruled out. In this respect, B. subtilis resembles Listeria monocytogenes and Yersinia enterolytica, which acquire chill stress-protective compatible solutes such as glycine betaine and l-carnitine through the same transporters as those used for the uptake of these solutes under osmotic stress conditions (1, 2, 64). Since the Opu systems of B. subtilis also function for compatible-solute uptake as heat stress protectants (36), the physiological role of these transporters for protection against stress is certainly much broader than originally anticipated when they were discovered in the context of the adaptation of B. subtilis to high-osmolarity environments (10).
The initial rate of glycine betaine uptake by cold-stressed B. subtilis cells expressing all three glycine betaine transporters (OpuA, OpuC, and OpuD) is very low (Fig. 4A). Nevertheless, over time, the cold-stressed cells can build up a glycine betaine pool that is double the level of that present in cells that are not subjected to temperature stress (Fig. 4B). The glycine betaine pool of the cold-stressed cells matches that found in B. subtilis cells subjected to a modest salt stress elicited by the addition of 0.4 M NaCl to the growth medium (Fig. 5B). Biochemical studies with the OpuA-related glycine betaine transporter Gbu and the OpuC-related glycine betaine and l-carnitine uptake system OpuC from L. monocytogenes (1, 28) revealed that the transport activity of these ABC transporters is activated by cold. This specific activation of the transport activity of these compatible-solute uptake systems counteracts the drop in transport activity that is generally observed when the temperature is reduced (51). Nothing is currently known with respect to a possible cold activation of the transport activity of the B. subtilis OpuA, OpuB, OpuC, and OpuD transporters. In cells subjected to high salinity, there is a significant induction of transcription of the various opu genes (31, 56), but the influence of sustained low temperature (15°C) on the transcription of these genes is minor (15). Only the transcription of the opuD gene is induced in cold-stressed cells (Fig. 9), and this enhanced transcription results in all likelihood from the SigB-dependent promoter of opuD (A. Schlüfter, F. Spiegelhalter, and E. Bremer, unpublished data) because sustained cold stress triggers the induction of the entire SigB general stress regulon (12, 15, 38).
Escherichia coli synthesizes the compatible solute trehalose under high-osmolarity stress conditions (59), and trehalose synthesis was found to be essential for cell viability at low temperature as well (40). We therefore asked if the compatible solute, proline, synthesized by B. subtilis under high-osmolarity stress conditions would also confer protection against cold stress. However, this was not the case. Surprisingly, we found that the transcription of the operon (proHJ) encoding enzymes for the osmoregulatory proline biosynthesis was induced under cold stress conditions (Fig. 7B) and that chill-stressed cells produced considerable amounts of proline (Fig. 7A), but we observed that a proHJ mutant has no growth disadvantage at 15°C (Fig. 7C). The absence of a cold-protective function of newly synthesized proline is consistent with the lack of protection of B. subtilis against cold stress by an exogenous supply of proline (Fig. 7A). However, it is quite puzzling to us why B. subtilis triggers in the cold the production of considerable amounts of proline (Fig. 7A) and then apparently cannot take advantage of this energy-consuming biosynthetic process (6, 13) for its cellular cold stress acclimatization reactions (Fig. 7C). We note that a similar phenomenon was observed when B. subtilis cells were continuously cultured at 52°C. This elevated growth temperature triggered proHJ expression but mysteriously did not lead to proline production, and a proHJ mutant was also not at a growth disadvantage at 52°C (36). We currently have no satisfying physiological explanations for these observations.
Although protection of different compatible solutes against chill stress for various microorganisms has already been noted (1, 2, 4, 24, 40, 45, 64), the molecular, biochemical, and biophysical mechanisms through which cellular cold protection is attained are currently far from clear. The accumulation of glycine betaine by osmotically stressed E. coli cells enhances the volume of free cytoplasmic water (19). Thus, it is possible that the increased glycine betaine content found in cold-stressed B. subtilis (Fig. 4B and 5B) and L. monocytogenes (1) cells aids in the adjustment of the hydration level of the cytoplasm of low-temperature-grown cells. However, it is equally possible that the chill-protective properties of compatible solutes are completely unrelated to their osmotic and water-attracting effects, which are of vital importance for their role as osmoprotectants (11, 43). Hints pointing in this direction come from studies in which the expression of genes encoding various microbial glycine betaine biosynthetic enzymes were used to engineer enhanced cold tolerance, but the attained glycine betaine levels in the recombinant cells were generally so low that significant effects on the water content of the cells are unlikely (22, 34). Cold stress has considerable effects on protein structure (26, 50), and compatible solutes thus might aid in the kinetics of protein folding, maintaining structural stability, and the formation and maintenance of protein complexes. These solutes are therefore sometimes referred to as “chemical chaperones” (21, 25). It is noteworthy in this connection that a combined transcriptome and proteome study of continuously chill-stressed B. subtilis cells revealed a major contribution of posttranscriptional regulatory phenomena in shaping the proteome in the cold (15). Compatible solutes might also help make translation more efficient since the initiation and efficiency of protein biosynthesis and folding are a major hurdle in cold-stressed microbial cells (58, 60, 62).
B. subtilis devotes considerable efforts to preventing the rigidification of the cytoplasmic membrane under cold stress conditions (44, 49, 62, 66) since this would severely restrict transporter-mediated uptake of ions and nutrients into the cell. Chill stress could potentially be ameliorated by the effects of compatible solutes on either the lipid composition or the biophysical properties of the cytoplasmic membrane (32). Several compatible solutes also have direct effects on the stability of the secondary structure of nucleic acids (48) and thus could reduce translation-inhibiting secondary structures in mRNAs (30, 62). The accumulation of compatible solutes in cold-stressed cells could also influence DNA topology and thereby counteract potential effects on gene transcription that might occur from increases in the degree of negative supercoiling that is observed in cold-stressed B. subtilis cells (29, 46).
It is obvious that we do not yet sufficiently understand the chemical and biophysical properties of compatible solutes (3, 57) that would allow a clear-cut interpretation of the observed effects of these solutes for living microbial cells with respect to their role in protection against temperature stress. Why are certain compatible solutes good osmoprotectants but ineffective against temperature stress (e.g., choline-O-sulfate), and why do others protect against cold stress but not heat stress (e.g., proline betaine) and still others protect against heat stress but not cold stress (e.g., proline) (Table 2)?
An important finding of our studies on the use of compatible solutes by B. subtilis as stress protectants is thus the realization that not all compatible solutes are “created equal” with respect to their physiological functions. To the best of our knowledge, the data assembled by our group on the protective properties of a large number of compatible solutes against osmotic (10), heat (36), and cold (this study) stress and their routes of uptake (Table 2) constitute the only available data set that currently allows a comparison of the contributions of these solutes to the development of osmotic and temperature stress resistance within a given microbial species. Whatever the special chemical and/or biophysical properties of these solutes might be that cause their different stress-protective effects in living cells, our findings on the osmostress- and thermostress-protective functions of compatible solutes in B. subtilis (Table 2) have obvious ramifications for the ecology of other microorganisms in natural settings (63) because uptake systems for compatible solutes are widespread in bacteria (11, 43, 68).
Acknowledgments
We are grateful to J. Gade and J. Sohn for their expert technical assistance throughout this study. We thank J. Brass, M. Jebbar, and G. Nau-Wagner for their kind gifts of various compatible solutes. We are grateful to Vickie Koogle for her kind help in editing the manuscript.
Financial support for this study was provided by the Deutsche Forschungsgemeinschaft, by the Fonds der Chemischen Industrie, and by the LOEWE program of the State of Hessen via the Centre for Synthetic Microbiology (SynMicro; Marburg, Germany).
Footnotes
Published ahead of print on 4 February 2011.
REFERENCES
- 1.Angelidis, A. S., and G. M. Smith. 2003. Role of the glycine betaine and carnitine transporters in adaptation of Listeria monocytogenes to chill stress in defined medium. Appl. Environ. Microbiol. 69:7492-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annamalai, T., and K. Venkitanarayanan. 2009. Role of proP and proU in betaine uptake by Yersinia enterocolitica under cold and osmotic stress conditions. Appl. Environ. Microbiol. 75:1471-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auton, M., D. W. Bolen, and J. Rosgen. 2008. Structural thermodynamics of protein preferential solvation: osmolyte solvation of proteins, aminoacids, and peptides. Proteins 73:802-813. [DOI] [PubMed] [Google Scholar]
- 4.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 5.Beckering, C. L., L. Steil, M. H. Weber, U. Volker, and M. A. Marahiel. 2002. Genomewide transcriptional analysis of the cold shock response in Bacillus subtilis. J. Bacteriol. 184:6395-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belitsky, B. R., J. Brill, E. Bremer, and A. L. Sonenshein. 2001. Multiple genes for the last step of proline biosynthesis in Bacillus subtilis. J. Bacteriol. 183:4389-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boch, J., B. Kempf, and E. Bremer. 1994. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 176:5364-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boch, J., B. Kempf, R. Schmid, and E. Bremer. 1996. Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: characterization of the gbsAB genes. J. Bacteriol. 178:5121-5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boch, J., G. Nau-Wagner, S. Kneip, and E. Bremer. 1997. Glycine betaine aldehyde dehydrogenase from Bacillus subtilis: characterization of an enzyme required for the synthesis of the osmoprotectant glycine betaine. Arch. Microbiol. 168:282-289. [DOI] [PubMed] [Google Scholar]
- 10.Bremer, E. 2002. Adaptation to changing osmolarity, p. 385-391. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, DC.
- 11.Bremer, E., and R. Krämer. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria, p. 79-97. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, DC.
- 12.Brigulla, M., et al. 2003. Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation. J. Bacteriol. 185:4305-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brill, J., T. Hoffmann, H. Putzer, and E. Bremer. T-box-mediated control of the anabolic proline biosynthetic genes of Bacillus subtilis. Microbiology, in press. doi: 10.1099/mic.0.047357-0. [DOI] [PubMed]
- 14.Brown, A. D. 1976. Microbial water stress. Bacteriol. Rev. 40:803-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budde, I., L. Steil, C. Scharf, U. Volker, and E. Bremer. 2006. Adaptation of Bacillus subtilis to growth at low temperature: a combined transcriptomic and proteomic appraisal. Microbiology 152:831-853. [DOI] [PubMed] [Google Scholar]
- 16.Bursy, J., et al. 2008. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl. Environ. Microbiol. 74:7286-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldas, T., N. Demont-Caulet, A. Ghazi, and G. Richarme. 1999. Thermoprotection by glycine betaine and choline. Microbiology 145:2543-2548. [DOI] [PubMed] [Google Scholar]
- 18.Canovas, D., S. A. Fletcher, M. Hayashi, and L. N. Csonka. 2001. Role of trehalose in growth at high temperature of Salmonella enterica serovar Typhimurium. J. Bacteriol. 183:3365-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cayley, S., and M. T. Record, Jr. 2003. Roles of cytoplasmic osmolytes, water, and crowding in the response of Escherichia coli to osmotic stress: biophysical basis of osmoprotection by glycine betaine. Biochemistry 42:12596-12609. [DOI] [PubMed] [Google Scholar]
- 20.Chan, Y. C., and M. Wiedmann. 2009. Physiology and genetics of Listeria monocytogenes survival and growth at cold temperatures. Crit. Rev. Food. Sci. Nutr. 49:237-253. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay, M. K., et al. 2004. The chemical chaperone proline relieves the thermosensitivity of a dnaK deletion mutant at 42 degrees C. J. Bacteriol. 186:8149-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, T. H., and N. Murata. 2002. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 5:250-257. [DOI] [PubMed] [Google Scholar]
- 23.Cybulski, L. E., M. Martin, M. C. Mansilla, A. Fernandez, and D. de Mendoza. 2010. Membrane thickness cue for cold sensing in a bacterium. Curr. Biol. 20:1539-1544. [DOI] [PubMed] [Google Scholar]
- 24.Deshnium, P., Z. Gombos, Y. Nishiyama, and N. Murata. 1997. The action in vivo of glycine betaine in enhancement of tolerance of Synechococcus sp. strain PCC 7942 to low temperature. J. Bacteriol. 179:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamant, S., N. Eliahu, D. Rosenthal, and P. Goloubinoff. 2001. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 276:39586-39591. [DOI] [PubMed] [Google Scholar]
- 26.Feller, G., and C. Gerday. 2003. Psychrophilic enzymes: hot topics in cold adaptation. Nat. Rev. Microbiol. 1:200-208. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Estepa, R., et al. 2006. The ectD gene, which is involved in the synthesis of the compatible solute hydroxyectoine, is essential for thermoprotection of the halophilic bacterium Chromohalobacter salexigens. J. Bacteriol. 188:3774-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerhardt, P. N., L. Tombras Smith, and G. M. Smith. 2000. Osmotic and chill activation of glycine betaine porter II in Listeria monocytogenes membrane vesicles. J. Bacteriol. 182:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grau, R., D. Gardiol, G. C. Glikin, and D. de Mendoza. 1994. DNA supercoiling and thermal regulation of unsaturated fatty acid synthesis in Bacillus subtilis. Mol. Microbiol. 11:933-941. [DOI] [PubMed] [Google Scholar]
- 30.Graumann, P., T. M. Wendrich, M. H. Weber, K. Schröder, and M. A. Marahiel. 1997. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol. Microbiol. 25:741-756. [DOI] [PubMed] [Google Scholar]
- 31.Hahne, H., et al. 2010. A comprehensive proteomics and transcriptomics analysis of Bacillus subtilis salt stress adaptation. J. Bacteriol. 192:870-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harishchandra, R. K., S. Wulff, G. Lentzen, T. Neuhaus, and H. J. Galla. 2010. The effect of compatible solute ectoines on the structural organization of lipid monolayer and bilayer membranes. Biophys. Chem. 150:37-46. [DOI] [PubMed] [Google Scholar]
- 33.Harwood, C. R., and A. R. Archibald. 1990. Growth, maintenance and general techniques, p. 1-26. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Inc., Chichester, United Kingdom.
- 34.Holmstrom, K. O., S. Somersalo, A. Mandal, T. E. Palva, and B. Welin. 2000. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 51:177-185. [DOI] [PubMed] [Google Scholar]
- 35.Holtmann, G., E. P. Bakker, N. Uozumi, and E. Bremer. 2003. KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J. Bacteriol. 185:1289-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtmann, G., and E. Bremer. 2004. Thermoprotection of Bacillus subtilis by exogenously provided glycine betaine and structurally related compatible solutes: involvement of Opu transporters. J. Bacteriol. 186:1683-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtmann, G., et al. 2004. RsbV-independent induction of the SigB-dependent general stress regulon of Bacillus subtilis during growth at high temperature. J. Bacteriol. 186:6150-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Höper, D., U. Völker, and M. Hecker. 2005. Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis. J. Bacteriol. 187:2810-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jebbar, M., C. von Blohn, and E. Bremer. 1997. Ectoine functions as an osmoprotectant in Bacillus subtilis and is accumulated via the ABC-transport system OpuC. FEMS Microbiol. Lett. 154:325-330. [Google Scholar]
- 40.Kandror, O., A. DeLeon, and A. L. Goldberg. 2002. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. U. S. A. 99:9727-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 178:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kappes, R. M., et al. 1999. Two evolutionarily closely related ABC-transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol. Microbiol. 32:203-216. [DOI] [PubMed] [Google Scholar]
- 43.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 44.Klein, W., M. H. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krispin, O., and R. Allmansberger. 1995. Changes in DNA supertwist as a response of Bacillus subtilis towards different kinds of stress. FEMS Microbiol. Lett. 134:129-135. [DOI] [PubMed] [Google Scholar]
- 47.Kuhlmann, A. U., J. Bursy, S. Gimpel, T. Hoffmann, and E. Bremer. 2008. Synthesis of the compatible solute ectoine in Virgibacillus pantothenticus is triggered by high salinity and low growth temperature. Appl. Environ. Microbiol. 74:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurz, M. 2008. Compatible solute influence on nucleic acids: many questions but few answers. Saline Systems 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mansilla, M. C., L. E. Cybulski, D. Albanesi, and D. de Mendoza. 2004. Control of membrane lipid fluidity by molecular thermosensors. J. Bacteriol. 186:6681-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishiguchi, M. K., and G. N. Somero. 1992. Temperature- and concentration-dependence of compatibility of the organic osmolyte beta-dimethylsulfoniopropionate. Cryobiology 29:118-124. [DOI] [PubMed] [Google Scholar]
- 51.Özcan, N., R. Krämer, and S. Morbach. 2005. Chill activation of compatible solute transporters in Corynebacterium glutamicum at the level of transport activity. J. Bacteriol. 187:4752-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poolman, B., J. J. Spitzer, and J. M. Wood. 2004. Bacterial osmosensing: roles of membrane structure and electrostatics in lipid-protein and protein-protein interactions. Biochim. Biophys. Acta 1666:88-104. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues, D. F., and J. M. Tiedje. 2008. Coping with our cold planet. Appl. Environ. Microbiol. 74:1677-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schumann, W. 2009. Temperature sensors of eubacteria. Adv. Appl. Microbiol. 67:213-256. [DOI] [PubMed] [Google Scholar]
- 55.Shivaji, S., and J. S. Prakash. 2010. How do bacteria sense and respond to low temperature? Arch. Microbiol. 192:85-95. [DOI] [PubMed] [Google Scholar]
- 56.Steil, L., T. Hoffmann, I. Budde, U. Völker, and E. Bremer. 2003. Genome-wide transcriptional profiling analysis of adaptation of Bacillus subtilis to high salinity. J. Bacteriol. 185:6358-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Street, T. O., D. W. Bolen, and G. D. Rose. 2006. A molecular mechanism for osmolyte-induced protein stability. Proc. Natl. Acad. Sci. U. S. A. 103:13997-14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strocchi, M., M. Ferrer, K. N. Timmis, and P. N. Golyshin. 2006. Low temperature-induced systems failure in Escherichia coli: insights from rescue by cold-adapted chaperones. Proteomics 6:193-206. [DOI] [PubMed] [Google Scholar]
- 59.Strom, A. R., and I. Kaasen. 1993. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol. Microbiol. 8:205-210. [DOI] [PubMed] [Google Scholar]
- 60.Ting, L., et al. 2010. Cold adaptation in the marine bacterium, Sphingopyxis alaskensis, assessed using quantitative proteomics. Environ. Microbiol. 12:2658-2676. [DOI] [PubMed] [Google Scholar]
- 61.von Blohn, C., B. Kempf, R. M. Kappes, and E. Bremer. 1997. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol. Microbiol. 25:175-187. [DOI] [PubMed] [Google Scholar]
- 62.Weber, M. H., and M. A. Marahiel. 2002. Coping with the cold: the cold shock response in the Gram-positive soil bacterium Bacillus subtilis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welsh, D. T. 2000. Ecological significance of compatible solute accumulation by micro-organisms: from single cells to global climate. FEMS Microbiol. Rev. 24:263-290. [DOI] [PubMed] [Google Scholar]
- 64.Wemekamp-Kamphuis, H. H., R. D. Sleator, J. A. Wouters, C. Hill, and T. Abee. 2004. Molecular and physiological analysis of the role of osmolyte transporters BetL, Gbu, and OpuC in growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 70:2912-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whatmore, A. M., J. A. Chudek, and R. H. Reed. 1990. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J. Gen. Microbiol. 136:2527-2535. [DOI] [PubMed] [Google Scholar]
- 66.Wiegeshoff, F., and M. A. Marahiel. 2007. Characterization of a mutation in the acetolactate synthase of Bacillus subtilis that causes a cold-sensitive phenotype. FEMS Microbiol. Lett. 272:30-34. [DOI] [PubMed] [Google Scholar]
- 67.Yancey, P. H. 2004. Compatible and counteracting solutes: protecting cells from the Dead Sea to the deep sea. Sci. Prog. 87:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziegler, C., E. Bremer, and R. Krämer. 2010. The BCCT family of carriers: from physiology to crystal structure. Mol. Microbiol. 78:13-34. [DOI] [PubMed] [Google Scholar]









