Abstract
Most organisms that grow in the presence of oxygen possess catalases and/or peroxidases, which are necessary for scavenging the H2O2 produced by aerobic metabolism. In this work we investigate the pathways that regulate the Caulobacter crescentus katG gene, encoding the only enzyme with catalase-peroxidase function in this bacterium. The transcriptional start site of the katG gene was determined, showing a short 5′ untranslated region. The katG regulatory region was mapped by serial deletions, and the results indicate that there is a single promoter, which is responsible for induction at stationary phase. An oxyR mutant strain was constructed; it showed decreased katG expression, and no KatG protein or catalase-peroxidase activity was detected in stationary-phase cell extracts, implying that OxyR is the main positive regulator of the C. crescentus katG gene. Purified OxyR protein bound to the katG regulatory region between nucleotides −42 and −91 from the transcription start site, as determined by a DNase I footprinting assay, and a canonical OxyR binding site was found in this region. Moreover, OxyR binding was shown to be redox dependent, given that only oxidized proteins bound adjacent to the −35 sequence of the promoter and the katG P1 promoter was activated by OxyR in an H2O2-dependent manner. On the other hand, this work showed that the iron-responsive regulator Fur does not regulate C. crescentus katG, since a fur mutant strain presented wild-type levels of katG transcription and catalase-peroxidase production and activity, and the purified Fur protein was not able to bind to the katG regulatory region.
Aerobic respiration is a powerful source of reactive oxygen species. Among these, hydrogen peroxide can damage enzymes by oxidizing thiol groups and iron-sulfur centers and can produce mutagenic and lethal lesions by conversion to hydroxyl radicals in the presence of metal ions (60). Cells have several protective mechanisms against these toxic compounds. Enzymatic detoxification of hydrogen peroxide in bacteria is performed mainly by catalases, of which three classes have been described: monofunctional catalases, catalase-peroxidases, and manganese catalases (pseudocatalases) (70). The monofunctional catalases and the catalase-peroxidases contain heme as a prosthetic group. In contrast to the ubiquitous distribution of monofunctional catalases from prokaryotes to eukaryotes, most catalase-peroxidases have been found in bacteria; only a minority have been found in fungi and other eukaryotic organisms (16, 43, 48).
Most bacteria appear to express one or more catalases in response to peroxide stress, and the different types of catalases are regulated independently. Escherichia coli produces two catalases: a catalase-peroxidase (hydroperoxidase I [HPI]) and a monofunctional catalase (HPII), encoded by the katG and katE genes, respectively. The expression of HPI is controlled by the positive regulator OxyR in response to H2O2 (61) and by RpoS in an OxyR-independent manner (24). Cold shock proteins (Csps) are also required for maximum HPI expression (49). HPII is part of the RpoS regulon and functions in stationary phase (42). Bacillus subtilis possesses three monofunctional catalases. KatA is induced by H2O2 or metal limitation (6, 9), and its expression is regulated by the Fur family paralogue PerR (7). KatE is induced by salt, heat, and ethanol stress and by glucose starvation in a σB-dependent manner (14), and KatX, the major catalase in dormant spores, is a forespore-specific gene controlled by σF (4). Mycobacteria display diverse distributions of catalases among different species. Mycobacterium tuberculosis produces only one HPI-type catalase-peroxidase (KatG) (20, 73), whereas some species have only an HPII-type catalase, and others produce both types (37, 67).
The oxidative stress response in Caulobacter crescentus has been studied for several years, and recently several of the regulatory pathways involved have been elucidated. C. crescentus has several antioxidant enzymes: a cytosolic iron superoxide dismutase (SodB), a periplasmic copper-zinc superoxide dismutase (SodC), and a catalase-peroxidase (KatG) (55, 58). The genome sequence also indicates the existence of a third superoxide dismutase and of alkyl hydroperoxide reductases (45). Global transcriptional analysis has shown that the extracytoplasmic function (ECF) sigma factors σF, σT, and σE regulate many genes involved in protecting cells against oxidative damage (1, 2, 31). Recently, it was demonstrated that Fur works as an activator and as a repressor, integrating iron metabolism and the oxidative stress response in C. crescentus (11). Fur plays an important role in oxidative stress resistance generated by hydroperoxides, as evidenced by the fact that a C. crescentus fur mutant was highly sensitive to H2O2 and tert-butyl hydroperoxide.
The katG gene has been shown to be solely responsible for catalase and peroxidase activity in C. crescentus and to be induced by H2O2 and in stationary phase (58). A putative transcriptional regulator (SkgA) has been proposed to be involved in katG expression at stationary phase (51). In addition, it was recently demonstrated that the transcription termination factor Rho is also important for catalase-peroxidase activity in C. crescentus (23).
In this work our goal was to investigate the roles of the transcriptional regulators OxyR and Fur in controlling the C. crescentus katG gene. An oxyR-null mutant strain was generated and shown to be highly sensitive to hydrogen peroxide. Determination of the transcription start site showed that the C. crescentus katG gene is transcribed by a single promoter activated by OxyR in stationary phase and in response to H2O2. We also showed that only oxidized OxyR binds to a conserved OxyR binding site in the katG regulatory region. Furthermore, this work provided evidence that the C. crescentus katG gene does not belong to the Fur regulon and that catalase-peroxidase activity is not affected in a fur mutant strain.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids are described in Table 1. C. crescentus was grown at 30°C with agitation in PYE medium (13) supplemented with kanamycin (5 μg/ml), tetracycline (1 μg/ml), streptomycin (5 μg/ml), chloramphenicol (1 μg/ml), or nalidixic acid (25 μg/ml) as necessary. E. coli strains were grown at 37°C in Luria-Bertani medium supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), tetracycline (12.5 μg/ml), or chloramphenicol (30 μg/ml) as required. Plasmids were introduced into Caulobacter strains by conjugation with E. coli strain S17-1 (57). All primers used in this work are listed in Table 2.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | E. coli strain for cloning purposes | 19 |
| S17-1 | E. coli strain for plasmid mobilization | 57 |
| C. crescentus | ||
| NA1000 | Synchronizable derivative of wild-type CB15 | 15 |
| SP3811 | NA1000 ΔoxyR::Ωspec | This study |
| SP3811(pMROxyR) | NA1000 ΔoxyR::Ωspec (pMROxyR) | This study |
| SP3811(pUJOxyR) | NA1000 ΔoxyR::Ωspec (pUJOxyR) | This study |
| SP0057 | NA1000 Δfur | 11 |
| SP0057(pMRFur) | NA1000 Δfur (pMRFur) | 11 |
| Plasmids | ||
| pGEM-T Easy | Cloning vector; Ampr | Promega |
| pProEX HTa | Overexpression vector; Ampr | Invitrogen |
| pNPTS138 | Suicide vector containing oriT and sacB; Kanr | D. Alley |
| pMR20 | Broad-host-range low-copy-number vector; Tetr | 52 |
| pUJ142 | Xylose-inducible promoter; Chlr | 36 |
| pRKlacZ290 | pRK2-derived vector with a promoterless lacZ gene; Tetr | 17 |
| pHP45Ω | Vector containing a Specr cassette | 50 |
TABLE 2.
Primers used in this study
| Primer | Sequence (5′-3′)a |
|---|---|
| OxyR5 | GAACTGGGCCCCGTGCTCGCAGC |
| OxyR6 | GCAAAGCTTCTGATAGACCTCAGTGGG |
| OxyR7 | CGAAAGCTTCTTCAGATACTGGAGTTG |
| OxyR8 | GAGAATTCCGACAGCAGTCTCATT |
| OxyR9 | GCGAATTCATGCTGTTACCCACGCTCC |
| OxyR10 | GGGAAGCTTCGAAGGTCCGATCTCTGAG |
| OxyR11 | CAGAATTCGGCGACGAAGCACCCCCAG |
| KatG3b,c | CTGGGATCCCCCCTTGCCCCTCGATG |
| KatG14B | TCTAAGCTTCCATTGGTAAAATTGAT |
| KatG15b | ATGGATCCCTTTTCGCGCCGCAGAATG |
| KatG16b | CAGGATCCCCAGGGGTAGGGAGCGG |
| KatG17b | GGAAAGCTTGTGGGTTCGAAATGTCC |
| KatG19b | CCGGATCCGGAATGGTTTCCTCTATC |
| KatGH1b,c,d | AAGCTTCCGTGGCCCATGGGGCATTTG |
Underlined letters indicate restriction enzyme recognition sites, used for cloning purposes.
Also used for EMSA probes.
Also used for amplification of the footprinting probe.
Also used for the primer extension assay.
Determination of the katG transcription start site.
The transcription start site of katG was determined by primer extension assays using a 32P-labeled primer and total RNA from strain NA1000. Total RNA was isolated from mid-log- and stationary-phase cells with the Trizol reagent (Invitrogen) according to the manufacturer's instructions. Primer KatGH1 was end labeled with 30 μCi [γ-32P]ATP using T4 polynucleotide kinase (GE Healthcare Life Sciences) and was extended with ImProm-II reverse transcriptase (Promega). The DNA sequencing ladder was obtained by cycle sequencing with the M13 universal primer and M13 single-stranded DNA (ssDNA) as the template by using the Thermo Sequenase cycle sequencing kit (USB).
Construction of transcriptional lacZ fusions and β-galactosidase assays.
Different fragments corresponding to the katG regulatory region were PCR amplified with appropriately designed primers (Table 2 and Fig. 2) by using a plasmid containing the katG upstream region and part of its coding region as a template (23). These PCR products (comprising fragments of 170 to 430 bp) were cloned into plasmid pGEM-T Easy, sequenced, and then subcloned as BamHI/HindIII fragments into the pRKlacZ290 vector to generate transcriptional fusions to the lacZ gene. C. crescentus cultures containing these reporter plasmids were grown in PYE medium up to the mid-log or stationary (24 h) phase. When hydrogen peroxide was used, the mid-log-phase cells were incubated for 15 min with 60 μM H2O2 and were assayed for β-galactosidase activity as described previously (38).
Construction and complementation of a C. crescentus oxyR mutant.
oxyR was deleted by allelic replacement of the oxyR coding region with a spectinomycin resistance cassette obtained from plasmid pHP45Ω. Two fragments, containing the regions upstream and downstream of the oxyR gene (1,300 bp and 980 bp, respectively), were amplified by PCR with primer pairs OxyR5/OxyR6 and OxyR7/OxyR8 and were cloned sequentially into the pGEM-T Easy vector. The resultant 2,280-bp ApaI/EcoRI fragment was then cloned into the pNTPS138 suicide vector, and the 2.0-kb Ωspec cassette was ligated between the two fragments. The pNPTΔoxyR::Ωspec vector was introduced into C. crescentus NA1000 by conjugation, and one Kans Specr mutant clone generated by double recombination was selected, generating strain SP3811 (ΔoxyR::Ωspec). The gene replacement was confirmed by PCR and Southern blot experiments.
For complementation of the oxyR mutant, a 1,300-bp DNA fragment containing the entire oxyR gene, including the promoter region, was amplified by PCR with primer pair OxyR10/OxyR11 and was cloned into the low-copy-number vector pMR20 to produce pMROxyR. In the case of strains carrying pRKlacZ290, a second vector was constructed containing the oxyR gene (amplified with primers OxyR9 and OxyR10) under the regulation of a xylose-inducible promoter, generating pUJOxyR. This vector was used to complement the oxyR mutant strain, since it carries a chloramphenicol resistance marker instead of a tetracycline resistance marker.
Oxidative-stress survival.
Resistance to hydrogen peroxide was determined by a disk inhibition assay. C. crescentus cultures were grown until mid-log phase or early stationary phase (24 h), and 0.5-ml samples were spread on top of PYE plates. Then 6-mm-diameter paper disks soaked with 3% hydrogen peroxide were applied to the agar surface. Diameters of clearing zones were measured after incubation of the plates for 48 h at 30°C.
Overproduction and purification of the OxyR protein.
The coding region of the C. crescentus NA1000 oxyR gene (CC3811) was amplified by PCR with primer set OxyR9/OxyR10, and the 950-bp EcoRI/HindIII fragment was cloned into vector pProEX HTa (Invitrogen). The resultant plasmid was transformed into E. coli DH5α, and the expression of OxyR as a polyhistidine tail fusion protein (His-OxyR) was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) from mid-log-phase cultures grown in LB medium. Bacteria were then harvested by centrifugation, resuspended in suspension buffer (50 mM Tris-HCl [pH 8.5], 5 mM β-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride [PMSF]), and lysed on ice by sonication. Following clarification by centrifugation, His-OxyR was purified from the soluble extract by nitrilotriacetic acid (NTA)-resin affinity chromatography (Qiagen).
Anti-KatG immunoblotting.
Immunoblotting was performed essentially as described previously (64). Whole-cell lysates of C. crescentus were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were transferred to nitrocellulose membranes and were incubated with anti-KatG antiserum (23) at a 1:2,000 dilution. An anti-Fur polyclonal antiserum in a 1:1,000 dilution (11) was used as a control of the amount of protein loaded.
Catalase activity assay.
Total-cell extracts were obtained from C. crescentus cultures in stationary phase. Cultures grown in PYE medium for 24 h were harvested, and a total-protein extract was obtained by a brief sonication in lysis buffer (25 mM potassium phosphate [pH 7.0], protease inhibitor cocktail [Sigma], and 1 mM PMSF). In situ enzyme activities were assayed as described previously (23), using inhibition of diaminobenzidine oxidation by horseradish peroxidase-H2O2 for catalase activity.
EMSA.
Probes representing the regulatory region of the katG gene were obtained by PCR amplification with appropriate primers (Table 2) and were end labeled with [γ-32P]ATP and T4 polynucleotide kinase (Invitrogen). Unincorporated nucleotides were removed with a QIAquick PCR purification kit (Qiagen). DNA binding was performed in a 20-μl reaction volume containing binding buffer (20 mM Tris-HCl [pH 8.0], 40 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol [DTT], 0.1 mg/ml bovine serum albumin, 10% glycerol), salmon sperm DNA (0.1 mg/ml) as a competitor, labeled DNA probes, and increasing amounts of purified His-OxyR protein (0, 0.25, 0.5, 0.75, 1, and 2 μg). To assay for OxyR binding under reducing conditions, 200 mM DTT was added to the binding reaction mixtures (59). In competition assays, a 30-fold molar excess of a cold probe was used to challenge the labeled probe. After incubation at room temperature for 20 min, the samples were loaded onto a native 5% polyacrylamide gel and were electrophoresed in 0.5× Tris-borate-EDTA (TBE) buffer for 3 h at 250 V. Radioactive species were detected by autoradiography. Electrophoretic mobility shift assays (EMSA) using purified Fur protein were carried out exactly as described previously (11).
DNase I footprinting.
DNase I protection experiments were performed with a KatG3/H1 fragment generated from PCR using the corresponding primers. The PCR amplification was performed only with the end-labeled KatGH1 primer in order to obtain a probe with a single 32P-labeled end. The probe was incubated with increasing amounts of purified His-OxyR protein (0, 0.5, 1.0, 2.0, and 7.0 μg) in exactly the same buffer and under exactly the same conditions used for EMSA. Before DNA digestion, 20 μl of a CaCl2-MgCl2 solution (5 mM CaCl2 and 10 mM MgCl2) was added, followed by incubation for 1 min at room temperature. Then RQ1 RNase-Free DNase I (Promega) was added to the reaction mixture, which was incubated for exactly 1 min. The reaction was stopped by the addition of stop solution (200 mM NaCl, 30 mM EDTA, and 1% SDS), and the product was extracted with phenol-chloroform-isoamyl alcohol (25:24:1) and was precipitated with ethanol. Reaction mixtures were run on a 6% polyacrylamide-urea sequencing gel alongside sequencing ladders generated from the katG3/H1 fragment, by using the Thermo Sequenase cycle sequencing kit (USB).
RESULTS AND DISCUSSION
Identification of the katG TSS.
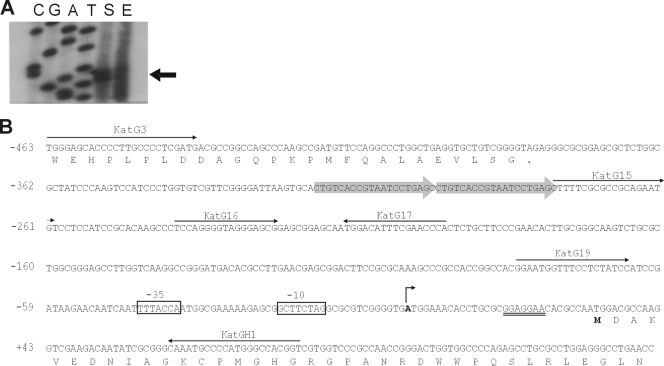
The katG transcription start site (TSS) was determined by primer extension assays using total RNA from strain NA1000 both in mid-log phase and in early stationary phase (24 h) (Fig. 1A). The results showed two close labeled bands, and we considered the stronger band to be the katG transcription start site, localized at an adenine 30 bp upstream of the annotated translational initiation codon (ATG) (Fig. 1). A putative promoter region (named P1) can be found upstream of the katG TSS, which presents a −35 region with high similarity to the proposed sigma 70 consensus sequence for C. crescentus (TTGAC-N16-CCTANA) (35) (Fig. 1B). Furthermore, primer extension analysis confirmed that this promoter is responsible for increased expression of katG in stationary phase, as previously reported (23, 58).
FIG. 1.
(A) Determination of the katG transcriptional start site. Primer extension analysis was carried out using total RNA from strain NA1000 at the exponential-growth (E) or stationary (S) phase as a template. The 32P-labeled katGH1 primer hybridizing to katG mRNA was extended with reverse transcriptase. A DNA-sequencing ladder of phage M13mp18 was used as a molecular size marker. The arrow indicates the band corresponding to the start site observed. (B) Nucleotide sequence of the katG regulatory region. The bent arrow indicates the transcription start site (designated position +1), and the −35 and −10 promoter sequences are boxed. The RBS is double underlined; the proposed translation start site is in boldface; and a direct repeat is indicated by shaded arrows. The arrows indicate the positions of each oligonucleotide used for primer extension, transcriptional fusions, gel mobility shift assays, and footprint assays.
On the basis of the TSS determination and a Clustal W alignment of C. crescentus KatG with the orthologous proteins from five selected alphaproteobacteria (not shown), we are proposing a new initiation codon for C. crescentus KatG (Fig. 1B). According to this new annotation, the KatG coding region starts at an ATG codon preceded by a good Shine-Dalgarno sequence (GGAGGAA), and the deduced protein sequence has 727 amino acids.
Mapping of the katG regulatory region.
Although the katG gene has a 5′ untranslated region (5′ UTR) of only 30 bp, the entire intergenic region upstream of the KatG initiation codon is very long, containing 410 bp (Fig. 1B). In addition to the P1 promoter sequence, there is also a direct repeat 305 bp upstream of the initiation codon (Fig. 1B), raising the possibility of the existence of a second promoter in this region. To determine whether there are other cis regulatory elements controlling katG expression, a series of deletions in the katG upstream region were constructed in transcriptional fusions with the lacZ gene (Fig. 2). These plasmid constructs were introduced into C. crescentus strain NA1000, and their expression was analyzed by β-galactosidase activity assays (Fig. 2).
FIG. 2.
Deletion mapping of the katG regulatory region. The scheme of the katG locus is shown at the top. Boxes indicate open reading frames, and the arrows above them show the direction of transcription. Nucleotide coordinates are relative to the first nucleotide of the katG transcription start site (bent arrow) as determined by primer extension analysis (+1). The −10 and −35 sequences are indicated as P1. The stem and loop indicate a putative Rho-independent transcription terminator. Below the map, each transcriptional fusion construct is diagramed; coordinates indicate the extent of the regulatory katG region cloned in front of the lacZ reporter gene. The plasmids carrying the constructs were introduced into C. crescentus NA1000, and promoter activity was measured by β-galactosidase (β-gal) assays both in exponential-phase (exp) and in stationary-phase (stat) cells. The results are expressed in Miller units (38) and are averages and standard deviations from at least three independent assays.
The lacZ fusion construct containing the complete upstream region from katG, including the direct repeat, the promoter P1, the 5′ UTR, and 55 bp of the coding region, produces about 490 β-galactosidase units in mid-log phase and 1,280 units in stationary phase, confirming that katG transcription is increased in the transition from exponential growth to stationary phase, as reported previously (23, 58). The absence of the region containing the direct repeat between positions −460 and −274 in construct KatG15/H1 did not produce a large decrease in katG expression, suggesting that this region does not have a regulatory role relevant to katG expression. The KatG16/H1 and KatG19/H1 constructs, which contain the complete P1 promoter sequence, the 5′ UTR, and 55 bp of the coding region, showed approximately 3-fold induction in stationary phase, indicating that the P1 promoter is responsible for katG transcriptional induction at this phase.
To confirm the localization of the P1 promoter and to test the existence of a second promoter downstream of the direct repeat, two other transcriptional fusions were constructed. While the KatG3/14B fusion construct contains the direct repeat, this region is absent in KatG15/14B, but part of the katG P1 promoter (−35 region) is missing in both of these constructs. No β-galactosidase activity was detected in these constructs, confirming that katG expression is under the control of a single promoter (P1).
In addition to transcriptional regulation, a posttranscriptional regulatory mechanism for the C. crescentus katG gene has been suggested previously (23). In agreement with that suggestion, a comparison between KatG3/H1 transcriptional and translational fusion constructs of NA1000 at stationary phase showed 2.4-fold more katG induction in the translational fusion construct (1,200 and 2,900 β-galactosidase units in the transcriptional and translational fusion constructs, respectively). In most Mycobacterium species, the katG gene, encoding catalase-peroxidase, is preceded by the furA gene, a member of the Fur (ferric uptake regulator) family (32). In M. tuberculosis and Mycobacterium smegmatis, the furA-katG mRNA is transcribed from the furA promoter and is likely processed by a single-stranded RNase (53). It has been suggested that translation is required for katG mRNA stabilization in the mycobacterial system, since both a polypurine sequence (PPS) and translation initiation contribute to the stability of katG mRNA (53). In C. crescentus, katG mRNA could undergo the same regulation process, given that the katG PPSs of M. tuberculosis (GGAAGGAA) and M. smegmatis (GAAAGGAAA) are very similar to the C. crescentus katG ribosome binding site (RBS) (GGAGGAA) (Fig. 1B). Furthermore, the C. crescentus katG RBS is complementary to the 16S rRNA from C. crescentus (CCUCCUUUCU) (12), suggesting that regulation of mRNA translation could be exerted via the availability of the RBS.
Construction and characterization of the oxyR mutant.
It is well established that catalases from several bacteria are regulated by OxyR upon exposure to hydrogen peroxide (30, 33, 44, 65). H2O2 reversibly activates OxyR at the posttranslational level through the oxidation of two cysteine residues and the formation of an intramolecular disulfide bond (74). The disulfide bond is then reduced by glutaredoxin 1 (GrxA) and glutathione (γ-l-glutamyl-l-cysteinylglycine [GSH]), which is, in turn, reduced by glutathione reductase (Gor), both GrxA and GorA are part of the OxyR regulon in E. coli (3). OxyR belongs to the LysR family of DNA binding transcriptional regulators (LTTRs) (10), the most abundant class of transcriptional regulators in bacteria (39). These proteins have a conserved amino-terminal domain containing a helix-turn-helix DNA-binding motif, and the carboxy-terminal part of the protein constitutes the inducer-binding or regulatory domain (34). Unlike OxyR, most of the LysR family members are activated by binding of coinducer molecules in the carboxy-terminal domain.
The C. crescentus NA1000 genome presents 11 putative LTTRs (45). One of them (CC3811) was identified as an orthologue of E. coli OxyR by the alignment of OxyR amino acid sequences (Fig. 3). Although there is some variation within the helix-turn-helix region that might account for differences in DNA binding specificity, the two redox-active cysteine residues (Cys199 and Cys208) responsible for OxyR activation are conserved in C. crescentus OxyR. To determine whether C. crescentus katG belongs to the OxyR regulon, a strain in which oxyR was deleted (SP3811) was generated by exchanging the oxyR gene for a spectinomycin resistance cassette in the wild-type strain NA1000. The absence of the oxyR coding region in strain SP3811 was confirmed by PCR and Southern blot analysis (not shown).
FIG. 3.
Comparison of the C. crescentus and E. coli OxyR amino acid sequences. The C. crescentus (CC) and E. coli (EC) OxyR amino acid sequences were aligned using ClustalW. The DNA binding helix-turn-helix is indicated by asterisks. The two conserved cysteines (Cys-199 and Cys-208) essential for OxyR activation are shown in boldface and shaded. The conserved amino acids Leu-200, Leu-224, Pro-241, and Arg-266, relevant for the stabilization of reduced OxyR, are shown in boldface.
In bacteria, the inactivation of oxyR results in increased sensitivity to peroxides and other oxidant agents (18, 26, 29, 33, 40, 46). The oxidative stress response of SP3811 was analyzed after exposure to hydrogen peroxide. SP3811 exhibited increased sensitivity to H2O2, as demonstrated by zones of inhibition larger than those of strain NA1000 in a plate assay with both exponential- and stationary-phase cells (Table 3), as expected for an oxyR mutant. This phenotype was complemented by the oxyR gene in trans.
TABLE 3.
Zone-of-inhibition assay for H2O2
| Strain | Diam of zone of clearing (cm)a |
|
|---|---|---|
| Exponential phase | Stationary phase | |
| NA1000(pMR20) | 2.75 ± 0.07 | 2.50 ± 0.08 |
| SP3811(pMR20) | 5.75 ± 0.21 | 5.43 ± 0.13 |
| SP3811(pMROxyR) | 2.80 ± 0.14 | 2.58 ± 0.10 |
Average diameter of the growth inhibition halos from three independent experiments ± standard deviation.
Analysis of the KatG expression pattern in oxyR and fur mutants.
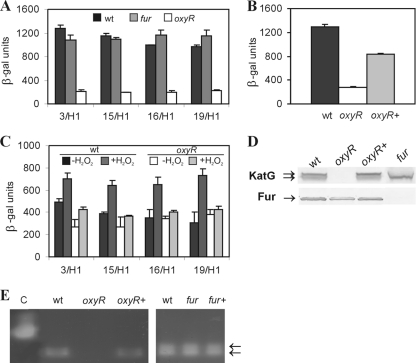
To verify the roles of the Fur and OxyR transcriptional regulators in controlling the C. crescentus katG gene, the KatG expression profile was determined at the transcriptional, translational, and posttranslational levels in oxyR (SP3811) and fur (SP0057) mutant strains.
First, katG transcription in strains SP3811 and SP0057 was evaluated using the same katG-lacZ transcriptional fusions used for NA1000 (Fig. 2 and 4A). The β-galactosidase activities obtained for the KatG3/H1 and KatG15/H1 constructs in the fur mutant in stationary phase were slightly diminished relative to those for strain NA1000. On the other hand, expression driven by the other constructs in the fur mutant showed a small increase. Although we cannot explain this small variation depending on the plasmid constructs used, these results suggest that Fur does not play a role in C. crescentus katG expression. Positive regulation of catalase-encoding genes by Fur has been reported previously. In E. coli, HPI catalase activity is increased in the presence of iron in a Fur-dependent manner, and Fur is responsible for increased levels of HPII catalase activity at stationary phase (5, 21, 69). In Staphylococcus aureus, a fur mutation results in decreased catalase activity through a decrease in katG transcription (22).
FIG. 4.
Analysis of catalase-peroxidase expression in the oxyR and fur mutants. (A) β-Galactosidase (β-gal) activity of each katG construct in strains NA1000 (wt) (filled bars), SP0057 (fur) (shaded bars), and SP3811 (oxyR) (open bars) at stationary phase. (B) Expression of the KatG3/H1 construct in NA1000 (wt), SP3811 (oxyR), and SP3811 complemented with the gene in trans (oxyR+). (C) β-Galactosidase activity of each katG construct in strains at mid-log phase with or without 60 μM H2O2. Filled bars, NA1000; dark shaded bars, NA1000 with H2O2; open bars, SP3811; light shaded bars, SP3811 with H2O2. The results are expressed in Miller units (38) and are averages from at least three independent assays. (D) Immunoblot analysis of Caulobacter crescentus cell extracts with polyclonal antisera. Equal amounts of protein from total-cell extracts of strains NA1000 (wt), SP3811 (oxyR), SP3811(pMROxyR) (oxyR+), and SP0057 (fur) at stationary phase were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to nitrocellulose membranes. The membranes were probed with either anti-KatG or anti-Fur antisera. Arrows indicate the bands corresponding to KatG and Fur. (E) Extracts (100 μg of total protein) from NA1000 (wt), SP3811 (oxyR), SP3811(pMROxyR) (oxyR+), SP0057 (fur), and SP0057(pMRFur) (fur+) at stationary phase were electrophoresed on a polyacrylamide gel under nondenaturing conditions, and the gel was stained for catalase activity. The arrows indicate the specific enzymatic activity (catalase-peroxidase migrates as a doublet). Catalase from beef liver (0.05 mg; Sigma) was used as a positive control (C).
The absence of the oxyR gene caused a severe reduction in β-galactosidase activity in all katG fusion constructs (Fig. 4A), indicating that C. crescentus katG transcription is dependent on the OxyR regulator at stationary phase. As a control, the β-galactosidase activity of the KatG3/H1 construct was also tested in the OxyR-complemented strain. Figure 4B shows that the presence of OxyR in strain SP3811(pUJOxyR) partially restored katG transcription, confirming the participation of OxyR in katG transcriptional regulation.
The OxyR family is widespread among prokaryotes, and nearly all known oxyR genes share overlapping promoters with other genes (26). The majority of genes located adjacent to oxyR, such as ahpC, dps, and oxyS, are involved in oxidative stress protection and are regulated by OxyR (44). In Rhodobacter sphaeroides and Rhodobacter capsulatus, the katE and katG genes, respectively, are located adjacent to the oxyR gene on the chromosome and share an upstream DNA sequence (71). This kat-oxyR gene organization is found in many alphaproteobacteria—Rhizobium etli (66), Brucella abortus (26), and Agrobacterium tumefaciens (44)—suggesting a general regulatory mechanism for these genes in alphaproteobacteria. Nevertheless, although the C. crescentus katG gene has been shown to be regulated by OxyR, this genetic organization is not maintained in this bacterium. In C. crescentus, the ahpD gene is located adjacent to oxyR on the chromosome, and they share an upstream DNA sequence, indicating that the ahpD gene could also be regulated by OxyR in C. crescentus. Furthermore, it has been shown that in Bradyrhizobium japonicum, another member of the alphaproteobacteria subdivision, katG is responsible for H2O2 degradation in an OxyR-independent manner (47), suggesting that katG regulation and gene organization are not phylogenetically conserved.
For E. coli it has been shown that a 100 nM excess of intracellular H2O2 is enough to activate the OxyR system (56). For C. crescentus it was demonstrated previously that the katG gene is induced by hydrogen peroxide (58). To define which katG regulatory region responds to the presence of H2O2 and whether this induction is dependent on OxyR in C. crescentus, the katG-lacZ fusions were tested in strain SP3811 at mid-log phase with 60 μM H2O2 (Fig. 4C). In all katG constructs, β-galactosidase activity was induced to similar levels in the presence of H2O2. Moreover, H2O2 was not able to induce katG expression in the oxyR mutant strain, suggesting that the P1 promoter is activated by OxyR in an H2O2-dependent manner.
Next, accumulation of the KatG protein was determined by immunoblotting, and catalase activity was assessed by an in situ assay of H2O2 decomposition. Levels of immunoreactive KatG and catalase activity in stationary-phase fur mutant cells were comparable to those in NA1000 (Fig. 4D and E), indicating that the small differences observed in SP0057 katG transcription were not accompanied by equivalent changes in the levels and activity of KatG. Although the results with the fur mutant extract showed that the faster-migrating of the two bands corresponded to the KatG protein (Fig. 4D), the meaning of this observation is not clear, since the two bands presented similar catalase activity (Fig. 4E). These results suggest that Fur does not participate directly or indirectly in catalase-peroxidase expression or activity, even though its activity depends on the binding of a heme group.
On the other hand, as expected from the katG transcription results, no immunoreactive KatG protein or catalase activity was observed in stationary-phase oxyR mutant cells (Fig. 4D and E), confirming the OxyR-dependent expression of C. crescentus katG. Both phenotypes were complemented by the oxyR gene in trans in strain SP3811(pMROxyR).
Analysis of OxyR and Fur binding in the katG regulatory region.
The catalase-peroxidase expression in the oxyR mutant suggests direct binding of OxyR to the katG regulatory region, activating katG transcription in an H2O2-dependent manner. In order to verify this hypothesis and to map the OxyR binding site, DNA binding assays were carried out using radioactively labeled fragments of katG constructs as probes (Fig. 2) and different amounts of purified His-OxyR protein (Fig. 5). The presence of increasing amounts of oxidized OxyR caused retardation of the KatG3/H1 probe migration in a dose-dependent manner (Fig. 5A). The entire amounts of probes KatG3/H1, KatG15/H1, and KatG16/H1 were retarded with 2 μg of His-OxyR protein (Fig. 5B). However, this amount of oxidized His-OxyR was not enough to completely shift the KatG19/H1 probe (Fig. 5B), indicating that the OxyR binding site was probably localized downstream of position −234 and upstream of position −88 (Fig. 1B). To exclude the presence of a second OxyR binding site upstream of position −234, the KatG3/17 probe (a fragment of 267 bp from position −463 to −196) was constructed. The results showed that oxidized His-OxyR did not affect KatG3/17 probe migration (Fig. 1B and 5C), suggesting the existence of a single OxyR binding site downstream of position −234.
FIG. 5.
OxyR and Fur binding to katG regulatory regions, assessed by gel electrophoresis mobility shift assays. DNA fragments were 32P labeled and were incubated either without protein (0) or with increasing concentrations of purified protein. (A) KatG3/H1 fragment incubated with 0, 0.25, 0.5, 0.75, 1, or 2 μg of oxidized OxyR (OxyRox). (B) Comparative shift assay with each katG fragment incubated with (+) or without (−) 2 μg of oxidized OxyR. (C) KatG3/17 fragment incubated with 0, 0.25, 0.5, 0.75, 1, or 2 μg of oxidized OxyR (OxyRox). (D) KatG3/H1 fragment incubated with 0, 0.25, 0.5, 1, or 2 μg of reduced OxyR (OxyRred) in the presence of 200 mM DTT. (E) A binding assay of the KatG3/H1 probe incubated with 2 μg of oxidized OxyR was carried out in the absence of competitor (+) or in the presence of a 30-fold excess of the unlabeled KatG3/H1 fragment (S) or the CC1640 coding region (N) as specific and nonspecific competitors, respectively. 0, no protein. (F) Control experiment using as a probe the CC1640 coding region, incubated with oxidized OxyR at the following concentrations: 0.5, 1.0, 2.0, 5.0, and 10.0 μg of protein. (G) The KatG3/H1 fragment was incubated with no protein (0) or with increasing concentrations of purified Fur protein (50, 200, 500, and 1,000 nM).
OxyR binds to its target sites in its oxidized form and, in most cases, activates gene expression by contacting the alpha subunit of RNA polymerase (25, 75). In some cases, however, repression and/or activation of gene expression by reduced OxyR has been observed (27, 72, 75). In Neisseria gonorrhoeae, OxyR represses catalase expression (65), and in Burkholderia pseudomallei and Sinorhizobium meliloti, OxyR acts as a dual regulator, repressing katG and katA expression, respectively, during normal growth and activating the expression of these genes during exposure to oxidative stress (30, 33).
To analyze the redox dependence of OxyR DNA-binding properties in C. crescentus, EMSA was carried out in the presence of 200 mM DTT (Fig. 5D). In the presence of DTT, His-OxyR was not able to shift KatG3/H1 migration, indicating that only in an oxidized state does OxyR bind to the katG regulatory region. This could also explain why large amounts of His-OxyR were needed for the complete shift of katG probes, since fully oxidized His-OxyR proteins may not be obtained during the purification process (63, 65).
The specificity of oxidized His-OxyR binding was demonstrated, since a complete loss of the shift was observed when an excess of unlabeled DNA probe was used as a specific competitor (Fig. 5E, lane S) but not with an unlabeled nonspecific DNA (Fig. 5E, lane N). As a negative control, it was demonstrated that the OxyR protein did not bind to a DNA probe of a similar size corresponding to the coding region of another gene, even if 10 μg His-OxyR was used (Fig. 5F).
The results obtained for catalase-peroxidase accumulation and activity in the fur mutant suggested that the katG gene is not directly regulated by Fur. In order to verify whether Fur interacts with the katG regulatory region, gel retardation experiments were carried out with the KatG3/H1 probe and different amounts of purified His-Fur protein, as shown in Fig. 5G. It has been shown previously that as low a concentration as 50 nM Fur was enough to shift specific probes (11). No retarded band was observed after electrophoresis, confirming that Fur is not able to bind to the katG regulatory region and probably does not act as a direct repressor of katG expression. In fact, a predicted Fur binding site was not detected in the C. crescentus katG regulatory region (11). Despite this fact, the fur mutant strain is highly sensitive to peroxide-generated oxidative stress (11), suggesting that the lack of control of iron metabolism in C. crescentus causes a level of oxidative stress greater than the ability of the cell to respond. Interestingly, to our knowledge, this is the first report of Fur not participating directly or indirectly in katG regulation in bacteria.
Determination of the OxyR binding site.
To determine the specific sequence of OxyR that binds to the C. crescentus katG regulatory region, purified His-OxyR was used in a DNase I footprinting assay (Fig. 6A). A probe consisting of the entire katG regulatory region (KatG3/H1 fragment) was radiolabeled at its 3′ extremity (KatGH1), incubated with increasing amounts of OxyR protein, and digested with DNase I. Several bands between positions −42 and −91 with respect to the transcription start site were protected from digestion in the presence of His-OxyR protein. OxyR binding sites are unusually long (>45 bp) and have limited sequence similarity. In E. coli the OxyR consensus binding motif follows the sequence ATAGntnnnanCTATnnnnnnnATAGntnnnanCTAT (62, 63). Examination of the putative oxidized OxyR binding site (AATGntnnnnnCTATnnnnnnnATAAnnnnnanCAAT) upstream of the C. crescentus katG P1 promoter showed that 14 of 20 bases match the consensus for the E. coli OxyR binding site (Fig. 6B). In addition, the OxyR binding site of C. crescentus katG also contained the highly conserved LysR-binding motif T-N11-A (54).
FIG. 6.
(A) DNase I footprinting assay of OxyR in the katG regulatory region. The KatG3/H1 probe was end labeled and was incubated in the presence or absence of increasing concentrations of oxidized OxyR (0, 0.5, 1.0, 2.0, and 7.0 μg). The DNA-protein complexes were treated with DNase I as described in Materials and Methods. The protected regions are boxed alongside the gel and in the sequence to the left. The minus sign indicates no protein. The asterisk marks a hypersensitive site. (B) Nucleotide sequence of the katG regulatory region. The positions of the katG promoter (−10 and −35 regions) are shaded, and the RBS and translation initiation codon (ATG) are shown in boldface. Other features are indicated as follows: dashed underline, the whole region protected by OxyR; filled circles, conserved motifs for the OxyR binding site; filled inverted triangle, transcription start site. The A-N11-T motif conserved in the binding sites of LysR regulators is indicated below the sequence.
It was previously reported that both the oxidized and reduced forms of OxyR bind DNA, but OxyR uses two different modes of binding to enable it to act as both an activator and a repressor (63). Reduced OxyR binds at the promoter region of OxyR-regulated genes and represses transcription by overlapping the −35 element (8, 28, 41). In C. crescentus, the OxyR binding motif was located directly upstream of the katG −35 promoter element, in agreement with reports of the binding motifs of other OxyR-activated genes (28, 46, 63), corroborating the correct binding mode for gene activation.
The facts that stationary-phase induction of katG is dependent on OxyR and that this activation requires OxyR to be in the oxidized state indicate that the cell turns into a highly oxidized environment at the onset of stationary phase. This is probably true for both the cytosol and the periplasm, since KatG and SodC have been found in both compartments of the cell (55). At stationary phase Caulobacter cells undergo a very significant change in physiology, and ultimately in morphology, when adapting to nutrient deprivation, by which they become increasingly more fit to respond to several stresses (68). In this context, OxyR appears to play a major role in turning on the expression of a set of genes in response to an increasing amount of reactive oxygen species at this phase.
Taken together, these data shed new light on previous descriptions of the regulation of catalase activity in several bacteria. Our results showed that in C. crescentus, the fur gene is not involved in regulating the expression or the activity of catalase-peroxidase. It was observed that C. crescentus OxyR directly activates katG in an H2O2-dependent manner and that only an oxidized form of the protein was able to bind to the katG regulatory region. Interestingly, there is a basal level of katG transcription in the oxyR mutant that is not accompanied by detectable levels of KatG protein or activity, suggesting that posttranscriptional regulation of the C. crescentus katG gene also occurs. Posttranslational regulation of katG has also been proposed in previous work (23), indicating that this gene is subject to many levels of regulation, probably in response to different cellular signals. Nevertheless, these steps of katG regulation remain to be established.
Acknowledgments
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). During the course of this work, V.C.S.I., J.F.D.S.N., and V.S.B. were supported by postdoctoral fellowships from FAPESP. M.V.M. was partially supported by CNPq.
We thank Patrícia Barco and Letícia Bisson for helping with the construction of the oxyR mutant strain.
Footnotes
Published ahead of print on 21 January 2011.
REFERENCES
- 1.Alvarez-Martinez, C. E., R. F. Lourenço, R. L. Baldini, M. T. Laub, and S. L. Gomes. 2007. The ECF sigma factor σT is involved in osmotic and oxidative stress responses in Caulobacter crescentus. Mol. Microbiol. 66:1240-1255. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Martinez, C. E., R. L. Baldini, and S. L. Gomes. 2006. A Caulobacter crescentus extracytoplasmic function sigma factor mediating the response to oxidative stress in stationary phase. J. Bacteriol. 188:1835-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslund, F., M. Zheng, J. Beckwith, and G. Storz. 1999. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl. Acad. Sci. U. S. A. 25:6161-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagyan, I., L. Casillas-Martinez, and P. Setlow. 1998. The katX gene, which codes for the catalase in spores of Bacillus subtilis, is a forespore-specific gene controlled by σF, and KatX is essential for hydrogen peroxide resistance of the germinating spore. J. Bacteriol. 180:2057-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benov, L., and F. Sequeira. 2003. Escherichia coli Δfur mutant displays low HPII catalase activity in stationary phase. Redox Rep. 8:379-383. [DOI] [PubMed] [Google Scholar]
- 6.Bol, D. K., and R. E. Yasbin. 1991. The isolation, cloning and identification of a vegetative catalase gene from Bacillus subtilis. Gene 109:31-37. [DOI] [PubMed] [Google Scholar]
- 7.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 8.Charoenlap, N., et al. 2005. OxyR mediated compensatory expression between ahpC and katA and the significance of ahpC in protection from hydrogen peroxide in Xanthomonas campestris. FEMS Microbiol. Lett. 249:73-78. [DOI] [PubMed] [Google Scholar]
- 9.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. U. S. A. 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christman, M. F., G. Storz, and B. N. Ames. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc. Natl. Acad. Sci. U. S. A. 86:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silva Neto, J. F., V. S. Braz, V. C. S. Italiani, and M. V. Marques. 2009. Fur controls iron homeostasis and oxidative stress defense in the oligotrophic alpha-proteobacterium Caulobacter crescentus. Nucleic Acids Res. 37:4812-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ely, B. 1992. DNA sequence of the 3′ end of the Caulobacter crescentus 16S rRNA gene. Nucleic Acids Res. 20:1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ely, B. 1991. Genetics of Caulobacter crescentus. Methods Enzymol. 204:372-384. [DOI] [PubMed] [Google Scholar]
- 14.Engelmann, S., C. Lindner, and M. Hecker. 1995. Cloning, nucleotide sequence, and regulation of katE encoding a σB-dependent catalase in Bacillus subtilis. J. Bacteriol. 177:5598-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evinger, M., and N. Agabian. 1977. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol. 132:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraaije, M. W., H. P. Roubroeks, W. R. Hagen, and W. J. Van Berkel. 1996. Purification and characterization of an intracellular catalase-peroxidase from Penicillium simplicissimum. Eur. J. Biochem. 235:192-198. [DOI] [PubMed] [Google Scholar]
- 17.Gober, J. W., and L. Shapiro. 1992. A developmentally regulated Caulobacter flagellar promoter is activated by 3′ enhancer and IHF binding elements. Mol. Biol. Cell 3:913-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn, J. S., S. Y. Oh, and J. H. Roe. 2002. Role of OxyR as a peroxide-sensing positive regulator in Streptomyces coelicolor A3(2). J. Bacteriol. 184:5214-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557. [DOI] [PubMed] [Google Scholar]
- 20.Heym, B., Y. Zhang, S. Poulet, D. Young, and S. T. Cole. 1993. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J. Bacteriol. 175:4255-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoerter, J. D., et al. 2005. Reduced hydroperoxidase (HPI and HPII) activity in the Δfur mutant contributes to increased sensitivity to UVA radiation in Escherichia coli. J. Photochem. Photobiol. B 79:151-157. [DOI] [PubMed] [Google Scholar]
- 22.Horsburgh, M. J., E. Ingham, and S. J. Foster. 2001. In Staphylococcus aureus, fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 183:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Italiani, V. C. S., V. S. Braz, H. Xiao, H. M. Steinman, and M. V. Marques. 2010. Catalase-peroxidase activity is decreased in a Caulobacter crescentus rho mutant. FEMS Microbiol. Lett. 303:48-54. [DOI] [PubMed] [Google Scholar]
- 24.Ivanova, A., C. Miller, G. Glinsky, and A. Eisenstark. 1994. Role of rpoS (katF) in oxyR-independent regulation of hydroperoxidase I in Escherichia coli. Mol. Microbiol. 12:571-578. [DOI] [PubMed] [Google Scholar]
- 25.Kiley, P. J., and H. Beinert. 2003. The role of Fe-S proteins in sensing and regulation in bacteria. Curr. Opin. Microbiol. 6:181-185. [DOI] [PubMed] [Google Scholar]
- 26.Kim, J. A., and J. Mayfield. 2000. Identification of Brucella abortus OxyR and its role in control of catalase expression. J. Bacteriol. 182:5631-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeBlanc, J. J., A. K. Brassinga, F. Ewann, R. J. Davidson, and P. S. Hoffman. 2008. An ortholog of OxyR in Legionella pneumophila is expressed postexponentially and negatively regulates the alkyl hydroperoxide reductase (ahpC2D) operon. J. Bacteriol. 190:3444-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loprasert, S., M. Fuangthong, W. Whangsuk, S. Atichartpongkul, and S. Mongkolsuk. 2000. Molecular and physiological analysis of an OxyR-regulated ahpC promoter in Xanthomonas campestris pv. phaseoli. Mol. Microbiol. 37:1504-1514. [DOI] [PubMed] [Google Scholar]
- 29.Loprasert, S., R. Sallabhan, W. Whangsuk, and S. Mongkolsuk. 2002. The Burkholderia pseudomallei oxyR gene: expression analysis and mutant characterization. Gene 296:161-169. [DOI] [PubMed] [Google Scholar]
- 30.Loprasert, S., R. Sallabhan, W. Whangsuk, and S. Mongkolsuk. 2003. Regulation of the katG-dpsA operon and the importance of KatG in survival of Burkholderia psedomallei exposed to oxidative stress. FEBS Lett. 542:17-21. [DOI] [PubMed] [Google Scholar]
- 31.Lourenço, R. F., and S. L. Gomes. 2009. The transcriptional response to cadmium, organic hydroperoxide, singlet oxygen and UV-A mediated by the σE-ChrR system in Caulobacter crescentus. Mol. Microbiol. 72:1159-1170. [DOI] [PubMed] [Google Scholar]
- 32.Lucarelli, D., M. L. Vasil, W. Meyer-Klaucke, and E. Pohl. 2008. The metal-dependent regulators FurA and FurB from Mycobacterium tuberculosis. Int. J. Mol. Sci. 9:1548-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo, L., et al. 2005. Role of oxyR from Sinorhizobium meliloti in regulating the expression of catalases. Acta Biochim. Biophys. Sin. 37:421-428. [DOI] [PubMed] [Google Scholar]
- 34.Maddocks, S. E., and P. C. Oyston. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154:3609-3623. [DOI] [PubMed] [Google Scholar]
- 35.McGrath, P. T., et al. 2007. High-throughput identification of transcription start sites, conserved promoter motifs and predicted regulons. Nat. Biotechnol. 25:584-592. [DOI] [PubMed] [Google Scholar]
- 36.Meisenzahl, A. C., L. Shapiro, and U. Jenal. 1997. Isolation and characterization of a xylose-dependent promoter from Caulobacter crescentus. J. Bacteriol. 179:592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milano, A., et al. 1996. The katE gene, which encodes the catalase HPII of Mycobacterium avium. Mol. Microbiol. 19:113-123. [DOI] [PubMed] [Google Scholar]
- 38.Miller, J. H. 1972. Experiments in molecular genetics: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 39.Minezaki, Y., K. Homma, and K. Nishikawa. 2005. Genome-wide survey of transcription factors in prokaryotes reveals many bacteria-specific families not found in archaea. DNA Res. 12:269-280. [DOI] [PubMed] [Google Scholar]
- 40.Mongkolsuk, S., R. Sukchawalit, S. Loprasert, W. Praituan, and A. Upaichit. 1998. Construction and physiological analysis of a Xanthomonas mutant to examine the role of the oxyR gene in oxidant-induced protection against peroxide killing. J. Bacteriol. 180:3988-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mongkolsuk, S., W. Whangsuk, P. Vattanaviboon, S. Loprasert, and M. J. Fuangthong. 2000. A Xanthomonas alkyl hydroperoxide reductase subunit C (ahpC) mutant showed an altered peroxide stress response and complex regulation of the compensatory response of peroxide detoxification enzymes. J. Bacteriol. 182:6845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulvey, M. R., J. Switala, A. Borys, and P. C. Loewen. 1990. Regulation of transcription of katE and katF in Escherichia coli. J. Bacteriol. 172:6713-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadler, V., I. Goldberg, and A. Hochman. 1986. Comparative study of bacterial catalases. Biochim. Biophys. Acta 882:234-241. [Google Scholar]
- 44.Nakjarung, K., S. Mongkolsuk, and P. Vattanaviboon. 2003. The oxyR from Agrobacterium tumefaciens: evaluation of its role in the regulation of catalase and peroxidase responses. Biochem. Biophys. Res. Commun. 304:41-47. [DOI] [PubMed] [Google Scholar]
- 45.Nierman, W. C., et al. 2001. Complete genome sequence of Caulobacter crescentus. Proc. Natl. Acad. Sci. U. S. A. 98:4136-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ochsner, U. A., M. L. Vasil, E. Alsabbagh, K. Parvatiyar, and D. J. Hassett. 2000. Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB, and ahpC-ahpF. J. Bacteriol. 182:4533-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panek, H. R., and M. R. O'Brian. 2004. KatG is the primary detoxifier of hydrogen peroxide produced by aerobic metabolism in Bradyrhizobium japonicum. J. Bacteriol. 186:7874-7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passardi, F., et al. 2007. Phylogenetic distribution of catalase-peroxidases: are there patches of order in chaos? Gene 397:101-113. [DOI] [PubMed] [Google Scholar]
- 49.Phadtare, S., V. Tadigotla, W. H. Shin, A. Senguupta, and K. Severinov. 2006. Analysis of Escherichia coli global gene expression profiles in response to overexpression and deletion of CspC and CspE. J. Bacteriol. 188:2521-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 51.Rava, P. S., L. Somma, and H. M. Steinman. 1999. Identification of a regulator that controls stationary-phase expression of catalase-peroxidase in Caulobacter crescentus. J. Bacteriol. 181:6152-6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts, R. C., et al. 1996. Identification of a Caulobacter crescentus operon encoding hrcA, involved in negatively regulating heat-inducible transcription and the chaperone gene grpE. J. Bacteriol. 178:1829-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sala, C., F. Forti, F. Magnoni, and D. Ghisotti. 2008. The katG mRNA of Mycobacterium tuberculosis and Mycobacterium smegmatis is processed at its 5′ end and is stabilized by both a polypurine sequence and translation initiation. BMC Mol. Biol. 9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 55.Schnell, S., and H. M. Steinman. 1995. Function and stationary-phase induction of periplasmic copper-zinc superoxide dismutase and catalase/peroxidase in Caulobacter crescentus. J. Bacteriol. 177:5924-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1:784-791. [Google Scholar]
- 58.Steinman, H. M., F. Fareed, and L. Weinstein. 1997. Catalase-peroxidase of Caulobacter crescentus: function and role in stationary phase survival. J. Bacteriol. 179:6831-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Storz, G., and S. Altuvia. 1994. OxyR regulon. Methods Enzymol. 234:217-223. [DOI] [PubMed] [Google Scholar]
- 60.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 61.Storz, G., L. A. Tartaglia, and B. N. Ames. 1990. The OxyR regulon. Antonie Van Leeuwenhoek 58:157-161. [DOI] [PubMed] [Google Scholar]
- 62.Tartaglia, L. A., C. J. Gimeno, G. Storz, and B. N. Ames. 1992. Multidegenerate DNA recognition by the OxyR transcriptional regulator. J. Biol. Chem. 267:2038-2045. [PubMed] [Google Scholar]
- 63.Toledano, M. B., et al. 1994. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78:897-909. [DOI] [PubMed] [Google Scholar]
- 64.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tseng, H.-J., A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2003. OxyR acts as a repressor of catalase expression in Neisseria gonorrhoeae. Infect. Immun. 71:550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vargas, M. D. C., et al. 2003. Only one catalase, KatG, is detectable in Rhizobium etli, and is encoded along with the regulator OxyR on a plasmid replicon. Microbiology 149:1165-1176. [DOI] [PubMed] [Google Scholar]
- 67.Wayne, L. G., and G. A. Diaz. 1982. Serological, taxonomic, and kinetic studies of the T and M classes of mycobacterial catalase. Int. J. Syst. Bacteriol. 32:296-304. [Google Scholar]
- 68.Wortinger, M. A., E. M. Quardokus, and Y. V. Brun. 1998. Morphological adaptation and inhibition of cell division during stationary phase in Caulobacter crescentus. Mol. Microbiol. 29:963-973. [DOI] [PubMed] [Google Scholar]
- 69.Zaid, T., T. S. Srikumar, and L. Benov. 2003. Growth of Escherichia coli in iron-enriched medium increases HPI catalase activity. J. Biochem. Mol. Biol. 36:608-610. [DOI] [PubMed] [Google Scholar]
- 70.Zámocký, M., and F. Koller. 1999. Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol. 72:19-66. [DOI] [PubMed] [Google Scholar]
- 71.Zeller, T., and G. Klug. 2004. Detoxification of hydrogen peroxide and expression of catalase genes in Rhodobacter. Microbiology 150:3451-3462. [DOI] [PubMed] [Google Scholar]
- 72.Zeller, T., et al. 2007. Regulation of hydrogen peroxide-dependent gene expression in Rhodobacter sphaeroides: regulatory functions of OxyR. J. Bacteriol. 189:3784-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]
- 74.Zheng, M., F. Aslund, and G. Storz. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718-1721. [DOI] [PubMed] [Google Scholar]
- 75.Zheng, M., et al. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 183:4562-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]