Abstract
While foreign-born persons constitute only 11% of the population in the state of Rhode Island, they account for more than 65% of incident tuberculosis (TB) annually. We investigated the molecular-epidemiological differences between foreign-born and U.S.-born TB patients to estimate the degree of recent transmission and identify predictors of clustering. A total of 288 isolates collected from culture-confirmed TB cases in Rhode Island between 1995 and 2004 were fingerprinted by spoligotyping and 12-locus mycobacterial interspersed repetitive units. Of the 288 fingerprinted isolates, 109 (37.8%) belonged to 36 genetic clusters. Our findings demonstrate that U.S.-born patients, Hispanics, Asian/Pacific islanders, and uninsured patients were significantly more likely to be clustered. Recent transmission among the foreign-born population was restricted and occurred mostly locally, within populations originating from the same region. Nevertheless, TB transmission between the foreign-born and U.S.-born population should not be neglected, since 80% of the mixed clusters of foreign- and U.S.-born persons arose from a foreign-born source case. We conclude that timely access to routine screening and treatment for latent TB infection for immigrants is vital for disease elimination in Rhode Island.
INTRODUCTION
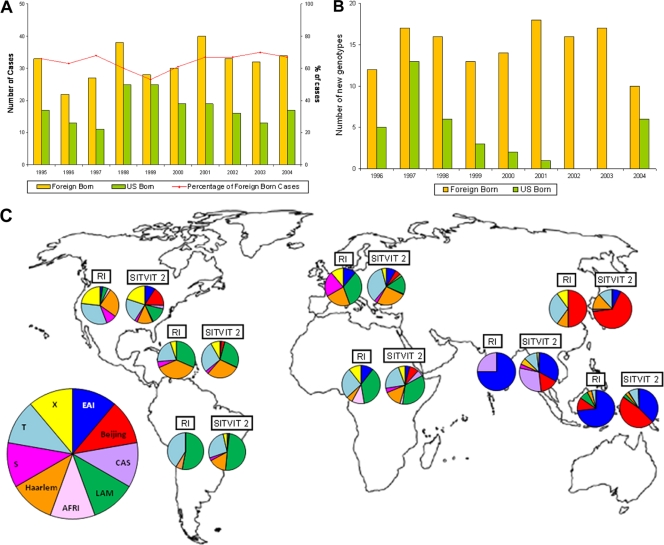
In the past 2 decades, the decline in tuberculosis (TB) cases in the United States occurred predominantly in the U.S.-born population. Consequently, foreign-born persons have accounted for over 50% of all TB cases since 2001 (10, 12). The failure of TB case rates among foreign-born persons to decline as expected since implementation of measures to control resurgent TB in the United States in the early 1990s is likely due to a higher prevalence of latent TB infection (LTBI) and failure to effectively prevent reactivation of the disease (6, 11, 34). Tuberculosis control policy and strategies in the United States since 1989 have been geared toward disease elimination (8, 9, 14, 24), and much progress has been made. However, recent epidemiologic trend of TB in the United States suggests that TB among foreign-born persons still poses a threat to disease elimination (10). In the state of Rhode Island, the influence of foreign-born individuals is reflected in the TB incidence: while foreign-born persons constitute only 11% of the population of Rhode Island (40), they have accounted for about 65% of the incident cases between 1995 and 2004 (Fig. 1A) (35). Moreover, at the time of our study in 2005, incident TB case rate among foreign-born persons (28.5 per 100,000 population) was 15.8 times higher than that among U.S.-born persons (1.8 per 100,000 population) (35).
Fig. 1.
(A) Number and proportion of all reported TB cases in Rhode Island by country of birth, 1995 to 2004. (B) Number and proportion of new isolated genotypes in TB cases in Rhode Island by country of birth, 1996 to 2004. (C) Distribution of major M. tuberculosis lineages according to patient region of origin: comparison between all unique strains isolated in TB patients of Rhode Island (RI) between January 1995 and December 2004, and all strains included in the international genotyping database (SITVIT 2). (The world outline map used in the figure was obtained from WorldAtlas.com and is used with permission.).
As incident TB cases in Rhode Island or the United States retreat into identifiable subpopulations such as foreign-born, there is a need to identify contributing factors that may have implications for targeted control measures. In such a context, the use of molecular tools in combination with conventional epidemiological methods has proven to be indispensable to highlight the TB transmission dynamics and the emergence of new strains due to transcontinental importation (1, 15, 18, 23, 25, 28, 36). In population-based studies, isolates with a unique genotypic profile are considered to reflect reactivation of LTBI as opposed to isolates sharing identical genotypic profiles (clustered isolates) as a result of recently acquired infection (1, 18, 19, 23, 25, 26, 37, 41, 42). The aim of the present study was to investigate the molecular-epidemiological differences between foreign-born and U.S.-born TB patients in Rhode Island. Two different molecular typing methods were used (spoligotyping and 12-locus mycobacterial interspersed repetitive units [MIRUs]) to determine the rate of recently transmitted TB and to identify the circulating genotypic lineages of M. tuberculosis. We particularly focused on defining the transmission patterns and predictors of clustering.
MATERIALS AND METHODS
Eligibility for inclusion in study population.
Patients with culture-confirmed TB diagnosed between January 1995 and December 2004 in Rhode Island, for whom isolates were available, were eligible for the present study. The study was reviewed and approved by the Institutional Review Board for Studies on Human Subjects of both the Rhode Island Department of Health and The Miriam Hospital.
Definitions.
Persons born in any of the 50 states and the District of Columbia were considered U.S.-born. All other persons were classified as foreign-born. Foreign-born persons who presented with active TB within 5 years of residence in the United States were classified as “recent immigrants,” while those in whom TB occurred after 5 years were classified as “remote immigrants.” The duration of U.S. residence was used as a surrogate marker for duration of Rhode Island residence since the time of migration to Rhode Island was not available.
Data collection and DNA fingerprinting.
Using a standardized form, trained persons abstracted data on demographics, TB risk factors, clinical characteristics, and treatment outcome from outpatient medical records. The data were obtained from the Rhode Island Department of Health electronic records for eligible patients for whom outpatient charts had incomplete data. Contact tracing data was reviewed to identify known epidemiological links between the patients.
DNA fingerprinting of prospectively archived M. tuberculosis isolates were performed at the Centers for Disease Control and Prevention (CDC) and the Institut Pasteur de Guadeloupe by spoligotyping (26) and 12-locus MIRU typing (39) using published protocols. Clusters were defined as two or more patients infected with isolates showing identical spoligotyping and 12-locus MIRU typing patterns, in contrast to patients with unique strains. The percentage of TB attributed to recent transmission was calculated according to the “n − 1 method” (37). The source case of a cluster was defined as the earliest case to show TB symptoms.
Database comparison and phylogenetic analysis.
Spoligotypes in binary format and 12-digit MIRU patterns were entered in the SITVIT2 proprietary database of the Institut Pasteur de Guadeloupe, which is an updated version of the previously released SpolDB4 database (5) (available online at http://www.pasteur-guadeloupe.fr:8081/SITVITDemo). At the time of the present study, SITVIT2 contained genotyping information on more than 70,000 M. tuberculosis clinical isolates from 160 countries of origin. In this database, spoligotype international type (SIT) and MIRU international type (MIT) designate spoligotyping and 12-locus MIRU patterns shared by two or more patient isolates. Major spoligotyping-based phylogenetic clades were assigned according to signatures provided in SpolDB4 (5).
Statistical analyses.
All analyses were carried out using Stata statistical software, release 10.0 (StataCorp, College Station, TX). An initial descriptive analysis compared the frequency of different demographic, clinical, and treatment characteristics between foreign-born and U.S.-born patients. A second univariate analysis was performed to identify risk factors for clustering. For categorical variables, differences between groups were assessed by using the chi-square test (or the Fisher exact test where necessary). P values of <0.05 were considered as statistically significant. A stepwise multivariate regression analysis was performed including sex, age group, and all variables associated with clustering according to the univariate analysis (P < 0.20) to identify independent predictors of clustering. Statistical significance was assessed by using likelihood ratio tests for all of the categorical variables. A multiple imputation parameter model (29) was used to estimate the missing values of the variable “medical insurance” (n = 52).
RESULTS
Study population.
Between January 1995 and December 2004, 496 cases of active TB were reported in Rhode Island, of which 327 were culture confirmed. Of the culture-confirmed cases, 288 (88.1%) patients had stored isolates available and were successfully fingerprinted by spoligotyping and 12-locus MIRU typing. A total of 265 (81.0%) patients had data available on demographics, TB risk factors, clinical characteristics, and treatment outcome and were included in the epidemiological analysis.
Country of birth and characteristics of the foreign-born patients.
Of the 265 patients included in the epidemiological analysis, 176 (66.4%) were foreign born from 42 different countries; 68.2% did not speak English. The predominant country of birth of the foreign-born persons was Cambodia (12.5%), followed by Guatemala (10.8%), Dominican Republic (10.2%), Laos (6.3%), the Philippines (5.1%), and Portugal (5.1%). The mean duration of U.S. residence prior to TB presentation was 10.3 years (standard deviation, 12.2; range, 0 to 80 years) among the foreign-born patients. The duration of U.S. residence was greater than 5 years in 56.5% of the patients and within 5 years in 43.5%. Prior to presentation with TB symptoms, only 31 (20.3%) of the foreign-born patients were previously diagnosed with LTBI.
Epidemiological profiles of foreign-born and U.S.-born patients.
Demographic and epidemiological profiles of the patients showed important dissimilarities between the foreign-born and U.S.-born persons (Table 1). The age distribution varied significantly between groups as there were more patients aged <30 years and fewer aged >65 years in the foreign-born patients. All six U.S.-born pediatric patients had foreign-born parents. Foreign-born patients were significantly more likely than U.S.-born patients to be Hispanic or a Asian/Pacific Islander. They were more likely to be employed and yet also more likely to be without health insurance. In addition, foreign-born patients were more likely to have no underlying medical conditions (other than HIV infection) and no history of drug-use. The mean time from the onset of TB symptoms to the first presentation at a health facility was 72 days, and from time of presentation to diagnosis was 36 days among the foreign-born patients, which were not different from those noted among U.S.-born patients (76 and 36 days, respectively).
Table 1.
Sociodemographic, clinical and treatment characteristics of U.S.-born and foreign-born patients, Rhode Island, 1995–2004
| Characteristic | Foreign-born subjects (n = 176) |
Native-born subjects (n = 89) |
Foreign-born vs U.S.-born |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | OR (95% CI) | Pa | |
| Sex | ||||||
| Female | 71 | 40.3 | 30 | 33.7 | 1.00 (reference) | 0.29 |
| Male | 105 | 59.7 | 59 | 66.3 | 0.75 (0.42–1.32) | |
| Age group (yr) | ||||||
| 0–29 | 52 | 29.5 | 11 | 12.4 | 1.00 | <0.01 |
| 30–44 | 45 | 25.6 | 10 | 11.2 | 0.95 (0.37–2.45) | |
| 45–64 | 52 | 29.5 | 27 | 30.3 | 0.41 (0.18–0.91) | |
| >65 | 27 | 15.3 | 41 | 46.1 | 0.14 (0.06–0.31) | |
| Race/ethnicity | ||||||
| Black | 29 | 16.5 | 15 | 16.9 | 1.00 | <0.01 |
| White | 22 | 12.5 | 65 | 73.0 | 0.18 (0.08–0.39) | |
| Hispanic | 73 | 42.1 | 1 | 1.1 | 37.76 (4.77–299.07) | |
| Asian/Pacific Islander | 52 | 29.0 | 7 | 7.9 | 3.84 (1.41–10.50) | |
| Medical insurance | ||||||
| No | 79 | 44.9 | 13 | 14.6 | 1.00 | <0.01 |
| Yes | 70 | 39.8 | 50 | 56.2 | 0.23 (0.11–0.48) | |
| Unknown* | 27 | 15.3 | 26 | 29.2 | ||
| Employment | ||||||
| No | 49 | 27.8 | 44 | 49.4 | 1.00 | <0.01 |
| Yes | 84 | 47.7 | 27 | 30.3 | 2.79 (1.48–5.30) | |
| Unknown* | 43 | 24.4 | 18 | 20.2 | ||
| HIV status | ||||||
| Negative | 122 | 69.3 | 49 | 55.1 | 1.00 | 0.54 |
| Positive | 17 | 9.7 | 9 | 10.1 | 0.76 (0.30–2.07) | |
| Unknown* | 37 | 21.0 | 31 | 34.8 | ||
| History of latent TB | ||||||
| No | 123 | 69.9 | 57 | 64.0 | 1.00 | 0.24 |
| Yes | 31 | 17.6 | 9 | 10.1 | 1.60 (0.68–4.06) | |
| Unknown* | 22 | 12.5 | 23 | 25.8 | ||
| Prior history of active TB | ||||||
| No | 162 | 92.0 | 78 | 87.6 | 1.00 | 0.43 |
| Yes | 8 | 4.5 | 6 | 6.7 | 0.64 (0.19–2.33) | |
| Unknown* | 6 | 3.4 | 5 | 5.6 | ||
| Sputum AFB smear result | ||||||
| Negative | 87 | 49.4 | 41 | 46.1 | 1.00 | 0.95 |
| Positive | 62 | 35.2 | 31 | 34.8 | 0.98 (0.54–1.80) | |
| Unknown* | 27 | 15.3 | 17 | 19.1 | ||
| Site of disease | ||||||
| Extrapulmonary | 39 | 22.2 | 20 | 22.5 | 1.00 | 0.95 |
| Pulmonary | 137 | 77.8 | 69 | 77.5 | 1.01 (0.55–1.88) | |
| History of drug use | ||||||
| No | 170 | 96.6 | 77 | 86.5 | 1.00 | <0.01b |
| Yes | 2 | 1.1 | 8 | 9.0 | 0.11 (0.01–0.59) | |
| Unknown* | 4 | 2.3 | 4 | 4.5 | ||
| Excessive alcohol use | ||||||
| No | 133 | 75.6 | 56 | 62.9 | 1.00 | 0.27 |
| Yes | 37 | 21.0 | 22 | 24.7 | 0.71 (0.37–1.38) | |
| Unknown* | 6 | 3.4 | 11 | 12.4 | ||
| Underlying medical condition | ||||||
| No | 137 | 77.8 | 55 | 61.8 | 1.00 | <0.01 |
| Yes | 39 | 22.2 | 34 | 38.2 | 0.46 (0.25–0.84) | |
| Drug resistance | ||||||
| No | 139 | 79.0 | 83 | 93.3 | 1.00 | <0.01 |
| Yes | 37 | 21.0 | 6 | 6.7 | 3.68 (1.45–11.09) | |
| Treatment outcome | ||||||
| Completed treatment | 145 | 82.4 | 68 | 76.4 | 1.00 | <0.01 |
| Died before treatment completed | 21 | 11.9 | 0 | 0.0 | ||
| Lost to follow up | 5 | 2.8 | 14 | 15.7 | 0.17 (0.06–0.48) | |
| Unknown* | 5 | 2.8 | 7 | 7.9 | ||
*, Not included in the χ2 and OR calculation.
Fisher exact P value.
The proportions of drug-resistant cases were significantly higher among foreign-born patients than in U.S.-born patients (21.0% versus 6.7%; P < 0.01). Among the foreign-born patients, 37 (21.0%) were resistant to any first-line drug, 18 (10.2%) were resistant to isoniazid, 9 (5.1%) were resistant to streptomycin, and 8 (4.5%) were resistant to both isoniazid and streptomycin. The proportions of multidrug resistance (i.e., resistant to both rifampin and isoniazid) was similar among foreign-born and U.S.-born populations (2.3% versus 0.6%; P = 0.22).
Fingerprinting and cluster analysis.
Spoligotyping and 12-locus MIRU typing were used to assess the genetic diversity among the isolated M. tuberculosis strains and to define genetic clusters. Among the 288 fingerprinted clinical isolates, 215 distinct SIT-MIT combinations could be identified, 131 (60.9%) of which preexisted in the SITVIT 2 database. Figure 1B shows the occurrence of new genotypes, i.e., SIT-MIT combinations that were not present in the study population in the preceding year(s), in the U.S.-born and foreign-born patients. Overall, 78.7% of the 169 genotypes newly identified after 1995 occurred in foreign-born patients, with the highest number of new genotypes introduced between 2001 and 2003. In some years, all of the new genotypes that were identified occurred in only foreign-born patients (Fig. 1B).
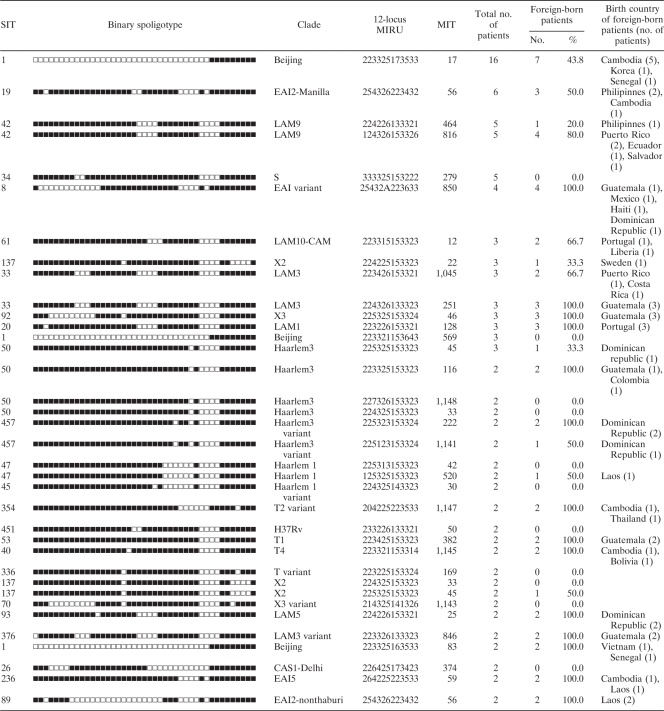
A total of 109 clustered isolates (37.8%) were found to be distributed in 36 clusters containing from 2 to 16 isolates (Table 2), the remaining 179 isolates were considered unique (62.2%). Of the 97 clustered TB patients for which country of origin was known, 44 (45.4%) were in 10 clusters with mixed U.S.-born and foreign-born patients, 22 (22.7%) were in 12 clusters containing only U.S.-born patients and 31 (32.0%) were in 14 clusters containing only foreign-born patients. In the 10 mixed clusters, the source case was foreign-born in 8 clusters (80%). Assuming that each cluster contains one source case and that all other cases in the cluster were due to recent transmission, we estimated the proportion of TB cases attributed to recent transmission be 25.3%. Among the cases involved in recent transmission, 54.1% were foreign-born and 45.9% were U.S.-born.
Table 2.
Genotypes and country of birth of clustered patients, Rhode Island, 1995-2004
Of the clustered cases, 17.4% had identifiable local epidemiological links, and one cluster with three family members had both foreign-born and U.S.-born persons within the cluster. There was another family cluster of two U.S.-born children, but the suspected source case (foreign-born relative) was not part of our study population. Clustered cases for which no evident local epidemiological link could be identified often shared the same country or continent of birth (Table 2). For example, the Beijing-type, the largest cluster of 16 patients occurred predominantly in patients born in Asia and the United States. Similar continental patterns were observed for the Haarlem 3, Latino-American, and Mediterranean (LAM) 9, S, and X2 clades. Moreover, the distribution of M. tuberculosis clades among TB cases assumed to result from reactivation of latent infection (unique cases) differs according to the patient's continents of origin and reflects the global distribution of major M. tuberculosis clades reported in the global spoligotyping database (Fig. 1C; based on unpublished data from Institut Pasteur de Guadeloupe).
Risk factors for recent transmission of tuberculosis.
Overall, the odds of being a clustered case did not change significantly between 1995 and 2004 (odds ratio [OR] per additional year = 1.09; 95% confidence interval [CI] = 0.99 to 1.20; P = 0.07). The variables found to represent significant predictive risk for membership in a cluster were birth in the United States (adjusted OR = 5.80; 95% CI = 2.37 to 14.17); Hispanic race (adjusted OR = 8.56; 95% CI = 2.96 to 24.71) and Asian/Pacific Islander race (adjusted OR = 6.05; 95% CI = 2.00 to 18.26) compared to the black race; and having no medical insurance (adjusted OR = 0.41; 95% CI = 0.19 to 0.86) (Table 3). Further analysis showed that both recent immigrants and remote immigrants with TB were less likely than U.S.-born patients to have clustered strains (OR = 0.51 [95% CI = 0.27 to 0.99] and OR = 0.52 [95% CI = 0.28 to 0.96], respectively). However, follow-up of the association revealed that increasing duration of U.S. residence prior to TB presentation was not associated with an increasing risk of being in a case cluster (OR = 1.00; 95% CI = 0.99 to 1.01).
Table 3.
Univariate and multivariate analysis of risk factors for clustering, Rhode Island, 1995-2004
| Characteristica | Unique and clustered strains |
Clustered vs unique |
|||||
|---|---|---|---|---|---|---|---|
| Unique (n = 168) |
Clustered (n = 97) |
Crude OR (95% CI) | Adjusted OR (95% CI) | P | |||
| No. | % | No. | % | ||||
| Sex | |||||||
| Female | 68 | 40.5 | 33 | 34.0 | 1.00 (reference) | 1.00 | 0.53 |
| Male | 100 | 59.5 | 64 | 66.0 | 1.32 (0.76–2.30) | 1.20 (0.68–2.14) | |
| Age group (yr) | |||||||
| 0–29 | 36 | 21.4 | 27 | 27.8 | 1.00 | 1.00 | 0.64 |
| 30–44 | 37 | 22.0 | 18 | 18.6 | 0.65 (0.31–1.38) | 0.76 (0.32–1.80) | |
| 45–64 | 49 | 29.2 | 30 | 30.9 | 0.82 (0.42–1.60) | 0.85 (0.39–1.82) | |
| >65 | 46 | 27.4 | 22 | 22.7 | 0.64 (0.31–1.30) | 0.60 (0.24–1.49) | |
| Country of origin | |||||||
| Foreign born | 121 | 72.0 | 55 | 56.7 | 1.00 | 1.00 | <0.01 |
| US born | 47 | 28.0 | 42 | 43.3 | 1.97 (1.12–3.43) | 5.54 (2.25–13.65) | |
| Race/ethnicity | |||||||
| Black | 37 | 22.0 | 7 | 7.2 | 1.00 | 1.00 | <0.01 |
| White | 50 | 29.8 | 37 | 38.1 | 3.91 (1.57–9.74) | 3.35 (1.20–9.33) | |
| Hispanic | 41 | 24.4 | 33 | 34.0 | 4.25 (1.68–10.77) | 8.58 (2.97–24.75) | |
| Asian/Pacific Islander | 39 | 23.2 | 20 | 20.6 | 2.71 (1.03–7.16) | 6.00 (1.98–18.18) | |
| Medical insurance | |||||||
| No | 51 | 30.4 | 41 | 42.3 | 1.00 | 1.00 | 0.03 |
| Yes | 82 | 48.8 | 38 | 39.2 | 0.58 (0.32–1.05) | 0.41 (0.18–0.93) | |
| Unknown* | 35 | 20.8 | 18 | 18.6 | |||
| Employment | |||||||
| No | 50 | 29.8 | 43 | 44.3 | 1.00 | ||
| Yes | 75 | 44.6 | 36 | 37.1 | 0.56 (0.30–1.03) | ||
| Unknown* | 43 | 25.6 | 18 | 18.6 | |||
| HIV status | |||||||
| Negative | 111 | 66.1 | 60 | 61.9 | 1.00 | ||
| Positive | 19 | 11.3 | 7 | 7.2 | 0.68 (0.23–1.82) | ||
| Unknown* | 38 | 22.6 | 30 | 30.9 | |||
| History of latent TB | |||||||
| No | 112 | 66.7 | 68 | 70.1 | 1.00 | ||
| Yes | 27 | 16.1 | 13 | 13.4 | 0.79 (0.35–1.72) | ||
| Unknown* | 29 | 17.3 | 16 | 16.5 | |||
| Prior history of active TB | |||||||
| No | 150 | 89.3 | 90 | 92.8 | 1.00 | ||
| Yes | 10 | 6.0 | 4 | 4.1 | 0.67 (0.15–2.40) | ||
| Unknown* | 8 | 4.8 | 3 | 3.1 | |||
| Sputum AFB smear result | |||||||
| Negative | 80 | 47.6 | 52 | 53.6 | 1.00 | ||
| Positive | 61 | 36.3 | 33 | 34.0 | 0.83 (0.46–1.49) | ||
| Unknown* | 27 | 16.1 | 12 | 12.4 | |||
| Site of disease | |||||||
| Extrapulmonary | 37 | 22.0 | 22 | 22.7 | 1.00 | ||
| Pulmonary | 131 | 78.0 | 75 | 77.3 | 0.96 (0.51–1.85) | ||
| History of drug use | |||||||
| No | 158 | 94.0 | 89 | 91.8 | 1.00 | ||
| Yes | 5 | 3.0 | 5 | 5.2 | 1.78 (0.40–7.92) | ||
| Unknown* | 5 | 3.0 | 3 | 3.1 | |||
| Excessive alcohol use | |||||||
| No | 126 | 75.0 | 63 | 64.9 | 1.00 | ||
| Yes | 33 | 19.6 | 26 | 26.8 | 1.58 (0.83–2.98) | ||
| Unknown* | 9 | 5.4 | 8 | 8.2 | |||
| Underlying medical condition | |||||||
| No | 123 | 73.2 | 69 | 71.1 | 1.00 | ||
| Yes | 45 | 26.8 | 28 | 28.9 | 1.11 (0.61–2.00) | ||
| Drug resistance | |||||||
| No | 134 | 79.8 | 87 | 89.7 | 1.00 | ||
| Yes | 34 | 20.2 | 9 | 9.3 | 0.40 (0.16–0.91) | ||
| Unknown* | 0 | 0.0 | 1 | 1.0 | |||
| Treatment outcome | |||||||
| Completed treatment | 134 | 79.8 | 79 | 81.4 | 1.00 | ||
| Died before treatment completed | 17 | 10.1 | 4 | 4.1 | 0.40 (0.13–1.23) | ||
| Lost to follow-up | 11 | 6.5 | 8 | 8.2 | 1.23 (0.48–3.20) | ||
| Unknown* | 6 | 3.6 | 6 | 6.2 | |||
*, Not included in χ2 and OR calculations.
Similar analyses were performed to identify risk factors for membership to a mixed cluster of foreign-born and U.S.-born patients, reflecting ongoing transmission between these two populations. Only membership to a large cluster (a cluster of >3 patients) remained significant after adjustment for possible confounders (Table 4). In addition, specific analysis of foreign-born clustered cases showed that remote immigrants were significantly more likely than recent immigrants to be a part of a mixed cluster of foreign-born and U.S.-born patients (OR = 3.97; 95% CI = 1.12 to 14.0).
Table 4.
Univariate and multivariate analysis of risk factors for membership to a mixed cluster of foreign-born and U.S.-born patients, Rhode Island, 1995-2004
| Characteristica | Clustered strains |
Mixed vs unmixed |
|||||
|---|---|---|---|---|---|---|---|
| Unmixed (n = 53) |
Mixed (n = 44) |
Crude OR (95% CI) | Adjusted OR (95% CI) | P | |||
| No. | % | No. | % | ||||
| Sex | |||||||
| Female | 16 | 30.2 | 17 | 38.6 | 1.00 (reference) | 1.00 | 0.87 |
| Male | 37 | 69.8 | 27 | 61.4 | 0.69 (0.27–1.74) | 0.92 (0.34–2.52) | |
| Age group (yr) | |||||||
| 0–29 | 19 | 35.8 | 8 | 18.2 | 1.00 | 1.00 | 0.09 |
| 30–44 | 11 | 20.8 | 7 | 15.9 | 1.51 (0.43–5.31) | 1.92 (0.44–8.32) | |
| 45–64 | 14 | 26.4 | 16 | 36.4 | 2.71 (0.91–8.11) | 3.19 (0.88–11.49) | |
| >65 | 10 | 18.9 | 13 | 29.5 | 3.43 (1.04–11.22) | 5.18 (1.29–20.87) | |
| Country of origin | |||||||
| Foreign-born | 31 | 58.5 | 24 | 54.5 | 1.00 | ||
| Native-born | 22 | 41.5 | 20 | 45.5 | 1.17 (0.48–2.84) | ||
| Race/ethnicity | |||||||
| Black | 9 | 17.0 | 11 | 25.0 | 1.00 | ||
| White | 5 | 9.4 | 2 | 4.5 | 2.94 (0.50–17.14) | ||
| Hispanic | 22 | 41.5 | 11 | 25.0 | 1.25 (0.21–7.51) | ||
| Asian/Pacific Islander | 17 | 32.1 | 20 | 45.5 | 3.05 (0.47–19.66) | ||
| Medical insurance | |||||||
| No | 29 | 54.7 | 12 | 27.3 | 1.00 | ||
| Yes | 17 | 32.1 | 21 | 47.7 | 2.99 (1.07–8.41) | ||
| Unknown* | 7 | 13.2 | 11 | 25.0 | |||
| Employment | |||||||
| No | 20 | 37.7 | 23 | 52.3 | 1.00 | ||
| Yes | 24 | 45.3 | 12 | 27.3 | 0.43 (0.16–1.19) | ||
| Unknown* | 9 | 17.0 | 9 | 20.5 | |||
| HIV status | |||||||
| No | 41 | 77.4 | 19 | 43.2 | 1.00 | ||
| Yes | 2 | 3.8 | 5 | 11.4 | 5.39 (0.77–59.82) | ||
| Unknown* | 10 | 18.9 | 20 | 45.5 | |||
| History of latent TB | |||||||
| No | 39 | 73.6 | 29 | 65.9 | 1.00 | ||
| Yes | 7 | 13.2 | 6 | 13.6 | 1.15 (0.29–4.49) | ||
| Unknown* | 7 | 13.2 | 9 | 20.5 | |||
| Prior history of active TB | |||||||
| No | 53 | 100.0 | 37 | 84.1 | 1.00 | ||
| Yes | 0 | 0.0 | 4 | 9.1 | |||
| Unknown* | 0 | 0.0 | 3 | 6.8 | |||
| Sputum AFB smear result | |||||||
| No | 25 | 47.2 | 27 | 61.4 | 1.00 | ||
| Yes | 21 | 39.6 | 12 | 27.3 | 0.53 (0.20–1.41) | ||
| Unknown | 7 | 13.2 | 5 | 11.4 | |||
| Site of disease | |||||||
| Extrapulmonary | 12 | 22.6 | 10 | 22.7 | 1.00 | ||
| Pulmonary | 41 | 77.4 | 34 | 77.3 | 1.00 (0.38–2.58) | ||
| History of drug use | |||||||
| No | 50 | 94.3 | 39 | 88.6 | 1.00 | ||
| Yes | 2 | 3.8 | 3 | 6.8 | 1.92 (0.21–23.91) | ||
| Unknown* | 1 | 1.9 | 2 | 4.5 | |||
| Excessive alcohol use | |||||||
| No | 33 | 62.3 | 30 | 68.2 | 1.00 | ||
| Yes | 15 | 28.3 | 11 | 25.0 | 0.81(0.29–2.23) | ||
| Unknown* | 5 | 9.4 | 3 | 6.8 | |||
| Underlying medical condition | |||||||
| No | 39 | 73.6 | 30 | 68.2 | 1.00 | ||
| Yes | 14 | 26.4 | 14 | 31.8 | 1.30 (0.49–3.44) | ||
| Drug resistance | |||||||
| No | 46 | 86.8 | 42 | 95.5 | 1.00 | ||
| Yes | 7 | 13.2 | 2 | 4.5 | 0.31 (0.03–1.79) | ||
| Treatment outcome | |||||||
| Completed treatment | 48 | 90.6 | 31 | 70.5 | 1.00 | ||
| Died before treatment completed | 2 | 3.8 | 2 | 4.5 | 1.55 (0.21–11.57) | ||
| Lost to follow up | 1 | 1.9 | 7 | 15.9 | |||
| Unknown* | 2 | 3.8 | 4 | 9.1 | |||
| Cluster size | |||||||
| Small cluster (≤3 patients) | 44 | 83.0 | 16 | 36.4 | 1.00 | 1.00 | <0.01 |
| Large cluster (>3 patients) | 9 | 17.0 | 28 | 63.6 | 9.89 (3.55–27.57) | 2.67 (1.64–4.34) | |
*, Not included in χ2 and OR calculations.
DISCUSSION
During the past decade in Rhode Island, the relatively small population of foreign-born persons disproportionately bore the burden of incident TB disease. Our data not only support the hypothesis that reactivation of M. tuberculosis infection likely acquired prior to migration to Rhode Island was a major contributor to incident cases but also demonstrates that prolonged residence in Rhode Island prior to TB presentation for foreign-born patients was associated with recent transmission between foreign-born and U.S.-born persons. The high proportion of new genotypes found in foreign-born persons, as well as the phylogeographical distribution of M. tuberculosis clades among the foreign-born unique cases, is consistent with our hypothesis that most of foreign-born patients developed reactivation disease from infection acquired prior to residing in Rhode Island.
Targeted screening and treatment of LTBI remains the primary means of controlling TB in the foreign-born population once they reside in the United States (2, 13), but widespread implementation of this strategy remains a major challenge. Political involvement, managerial capability, cost-effectiveness, effective information delivery, and access to high-risk groups are as important constraints as medical challenges to the implementation of targeted testing for LTBI (24, 27, 32). The analysis of the sociodemographic and clinical characteristics of the foreign-born TB patients revealed several possible barriers, including language difficulties, lack of medical insurance, young age, and/or absence of other medical conditions that will cause patient to seek medical attention. Therefore, expanded TB screening services that actively move into at-risk communities outside of traditional health clinic sites will be important in reaching these persons during the window period of latent infection. Involving community-based organizations has proved effective in increasing cure rates, detecting new cases, and decreasing costs compared to traditional practices (22, 32, 33). In addition, application of current screening guidelines that emphasize screening of recent immigrants within 5 years of immigration would miss a large proportion of the foreign-born persons who developed TB, since more than 56% of them had resided in the United States for >5 years before developing disease, and most of these remote migrants (69.4%) acquired disease through reactivation of latent infection. This is consistent with the finding that among immigrants from high-incidence countries, the risk of developing active tuberculosis from latent infection can persist at least for some decades following entry (43). Thus, targeted LTBI screening guidelines should be updated to classify all foreign-born persons from high-incidence countries as a high-risk population irrespective of time since entry into the United States and should be tested for LTBI as proposed by other researchers in a recent guideline (2), since TB case rates remain higher in foreign-born than in U.S.-born persons even more that 20 years after arrival (7).
In the U.S.-born population, the lack of perceived risk of TB might have contributed to delayed LTBI diagnosis. Primary care clinicians receive little ongoing education services, and a large proportion of them demonstrated a lack of knowledge in TB screening and treatment guidelines (30, 38). Keeping frontline clinicians informed of the problem and current screening and treatment guidelines through continuing medical education in the face of declining case rates is necessary to maintain TB expertise.
The proportion of Rhode Island TB patients attributed to recent transmission was estimated at 25%, which is smaller than the transmission rates observed in similar studies conducted in the United States (in the range of 28 to 52%) (1, 17, 18, 36). However, we did not observe a decrease of the number of clustered cases with time, in contrast to previous long-term population-based studies (18, 25). Recent transmission should therefore not be neglected, and more effective measures should be taken to break the TB transmission chains. Since early recognition of disease symptoms and institution of effective treatment is a key factor in prevention of transmission (3, 20, 21), particular efforts should be made to reduce the delays in diagnosis observed in both foreign-born and U.S.-born patients. Education of health care professionals may decrease the time to diagnosis once the patient has presented for care, but patient delays in seeking care will require more complex interventions addressing social factors contributing to health disparity access.
Our findings demonstrate high clustering of foreign-born patients by continent of birth with little clustering between patients from different continents. This could indicate that recent transmission in the U.S.-born and foreign-born populations occurs locally, within populations originating from the same region. Moreover, the tendency of TB transmission exclusively within specific subgroups is more pronounced in recent immigrants, as suggested by their predominant membership to clusters of foreign-born patients only. A similar trend was observed in other studies and is probably due to the fact that new arrivals are more likely to live with other foreign-born persons in their own ethnic communities (4, 41). In the foreign-born population of Rhode Island, people originating from Central America, the Caribbean, and Southeast Asia seem to be the most involved in local transmission chains. In addition, recent transmission seems to occur within specific ethnic groups, particularly Hispanics and Asians/Pacific Islanders, as suggested by the significant ethnic disparities between clustered and unique cases. Several contributory factors such as stigma, illegal immigration status, fear of inability to legalize immigration status, language difficulties, and poverty were observed in our study and could explain the delays on the part of these high-risk groups to seek TB services (16, 44). Some of these concerns need to be addressed through focused patient education programs or campaigns using selected lay print media, radio, and television advertisements, as well as linguistically appropriate informational handouts.
Nevertheless, nearly one-third of the clusters involved both U.S.-born and foreign-born patients, suggesting ongoing transmission between these populations. Transmission occurred mainly from the foreign-born to the U.S.-born population, since foreign-born patients were identified as the source case in 80% of these clusters. Moreover, these mixed clusters were significantly larger than unmixed clusters and involved widespread TB strain types. These clusters could have arisen from particularly transmissible or virulent strains, therefore not restrained to specific subpopulations, as suggested by other studies (19).
Some limitations should be taken into account when interpreting the results of the present study. First, the restriction of the study to culture-confirmed cases diagnosed in a defined time period and failure to type all cases during that period may have missed some members of potential clusters and misclassified some strains as unique or missed persons with epidemiological links. In addition, the use of spoligotyping with classical 12-locus MIRU as typing methods likely overestimated the proportion of clustered cases belonging to the Beijing clade, since it has been shown that the combination of these techniques is not sufficient to fully discriminate Beijing isolates (31). Second, the assumption that isolates in clusters result from recent transmission from a single source should be taken with care, since TB in clustered patients may have occurred through coincidental reactivation from LTBI during the observation period. In addition, clusters of exclusively foreign-born persons may have arisen from importation of prevalent circulating strains in their birth country, leading to an overestimation of the rate of recent transmission. Although this cohort looks at patients diagnosed between 1995 and 2004, the issues of foreign-born contribution to the U.S. TB burden remains relevant today; 2009 incident data for the United States continues to demonstrate that 60.2% of all cases are in the foreign-born (10). To reach elimination ongoing characterization of transmission is required to design and maintain support for targeted testing and treatment programs.
Our findings demonstrate that incident TB among foreign-born persons who accounted for the majority cases in Rhode Island is a manifestation of reactivation of infection likely acquired before arrival in the state. Furthermore, there was a prolonged period of time between the time of U.S. immigration and the development of disease that provides an opportunity for implementation of preventive measures. The profile of the foreign-born patients suggests that there are potential barriers to a passive screening for LTBI. Bold expansion of screening and treatment for LTBI to effectively reach this population will be necessary for TB elimination. Recent transmission occurred within specific ethnic communities, as well as between the foreign-born and U.S.-born population, suggesting the need to improve public health interventions in both populations.
ACKNOWLEDGMENTS
This study was supported by a grant from the Rhode Island Foundation (grant 20030199) awarded to A.K. and grants awarded by the European Regional Development Fund, European Commission (ERDF/FEDER, A34-05), and the Regional Council of Guadeloupe (research grant CR/08-1612) to N.R. Other sources of support include NIH K23 developmental grant K23 AI071760 (A.K.) and the Centers for AIDS Research (F.S.G.).
We thank coworkers at the Institut Pasteur of Guadeloupe for various aspects regarding genotyping analysis, Karine Brudey and Christophe Sola for an initial comparison of preliminary spoligotyping results using the SpolDB4 database, Julie Millet and Thierry Zozio for helping with 12-locus MIRU typing, and Thomas Burguière and Véronique Hill for the final SITVIT2 database analysis. We also thank the nurses and staff of the RISE TB Clinic, Miriam Hospital, for assistance with locating records and Anthony Dellagrotta of the Rhode Island Department of Health Mycobacteriology Laboratory, Division of Laboratories, for assistance with TB cultures.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1. Alland D., et al. 1994. Transmission of tuberculosis in New York City: an analysis by DNA fingerprinting and conventional epidemiologic methods. N. Engl. J. Med. 330:1710–1716 [DOI] [PubMed] [Google Scholar]
- 2. American Thoracic Society 2005. Controlling tuberculosis in the United States. Am. J. Respir. Crit. Care Med. 172:1169–1227 [DOI] [PubMed] [Google Scholar]
- 3. Asch S., Leake B., Anderson R., Gelberg L. 1998. Why do symptomatic patients delay obtaining care for tuberculosis? Am. J. Respir. Crit. Care Med. 157:1244–1248 [DOI] [PubMed] [Google Scholar]
- 4. Borrell S., et al. 2010. Tuberculosis transmission patterns among Spanish-born and foreign-born populations in the city of Barcelona. Clin. Microbiol. Infect. 16:568–574 [DOI] [PubMed] [Google Scholar]
- 5. Brudey K., et al. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cain K. P., et al. 2007. Tuberculosis among foreign-born persons in the United States: achieving tuberculosis elimination. Am. J. Respir. Crit. Care Med. 175:75–79 [DOI] [PubMed] [Google Scholar]
- 7. Cain K. P., Benoit S. R., Winston C. A., Mac Kenzie W. R. 2008. Tuberculosis among foreign-born persons in the United States. JAMA 300:405–412 [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention 1989. A strategic plan for elimination of tuberculosis in the United States. MMWR Morb. Mortal. Wkly. Rep. 38:269–272 [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention 2005. Controlling tuberculosis in the United States. Am. J. Respir. Crit. Care Med. 172:1169–1227 [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention 2010. Decrease in reported tuberculosis cases–United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 59:289–294 [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention 1998. Recommendations for prevention and control of tuberculosis among foreign-born persons: report of the working group on tuberculosis among foreign-born persons. MMWR Morb. Mortal. Wkly. Rep. 47:1–319450721 [Google Scholar]
- 12. Centers for Disease Control and Prevention 2009. Reported tuberculosis in the United States, 2008. U.S. Department of Health and Human Services, Atlanta, GA: http://www.cdc.gov/tb/statistics/reports/2008/default.htm#report [Google Scholar]
- 13. Centers of Disease Control and Prevention 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 161:221–247 [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention 1999. Tuberculosis elimination revisited: obstacles, opportunities and a renewed commitment. MMWR Morb. Mortal. Wkly. Rep. 48:1–13 [PubMed] [Google Scholar]
- 15. Chin D. P., et al. 1998. Differences in contributing factors to tuberculosis incidence in U.S.-born and foreign-born persons. Am. J. Respir. Crit. Care Med. 158:1797–1803 [DOI] [PubMed] [Google Scholar]
- 16. Coreil J., Lauzardo M., Heurtelou M. 2004. Cultural feasibility assessment of tuberculosis prevention among persons of Haitian origin in South Florida. J. Immigr. Health 6:63–69 [DOI] [PubMed] [Google Scholar]
- 17. El Sahly H. M., et al. 2001. Epidemiologic differences between United States- and foreign-born tuberculosis patients in Houston, Texas. J. Infect. Dis. 183:461–468 [DOI] [PubMed] [Google Scholar]
- 18. Geng E., et al. 2002. Changes in the transmission of tuberculosis in New York City from 1990 to 1999. N. Engl. J. Med. 346:1453–1458 [DOI] [PubMed] [Google Scholar]
- 19. Glynn J. R., et al. 2008. Determinants of cluster size in large, population-based molecular epidemiology study of tuberculosis, northern Malawi. Emerg. Infect. Dis. 14:1060–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Golub J. E., et al. 2006. Delayed tuberculosis diagnosis and tuberculosis transmission. Int. J. Tuberc. Lung Dis. 10:24–30 [PubMed] [Google Scholar]
- 21. Golub J. E., et al. 2005. Patients and health care system delays in pulmonary tuberculosis diagnosis in a low-incidence state. Int. J. Tuberc. Lung Dis. 9:992–998 [PubMed] [Google Scholar]
- 22. Gupta R., Espinal M. A., Raviglione M. C. 2004. Tuberculosis as a major global health problem in the 21st century: a WHO perspective. Semin. Respir. Crit. Care Med. 25:245–253 [DOI] [PubMed] [Google Scholar]
- 23. Iñigo J., et al. 2007. Analysis of changes in recent tuberculosis transmission patterns after a sharp increase in immigration. J. Clin. Microbiol. 45:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Institute of Medicine 2000. Ending neglect: the elimination of tuberculosis in the United States. National Academy Press, Washington, DC: [PubMed] [Google Scholar]
- 25. Jasmer R. M., et al. 1999. A molecular epidemiologic analysis of tuberculosis trends in San Francisco, 1991–1997. Ann. Intern. Med. 130:971–978 [DOI] [PubMed] [Google Scholar]
- 26. Kamerbeek J., et al. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klaudt K. 2000. Mobilizing society against tuberculosis, p. 843–863 In Reichman L. B., Hershfield E. S. (ed.), Tuberculosis: a comprehensive international approach, vol. 144 Marcel Dekker, New York, NY [Google Scholar]
- 28. Lillebaek T., et al. 2001. Risk of Mycobacterium tuberculosis transmission in a low-incidence country due to immigration from high-incidence areas. J. Clin. Microbiol. 39:855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Little R., Rubin D. 2002. Statistical analysis with missing data, 2nd ed John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 30. LoBue P. A., Moser K., Catanzaro A. 2001. Management of tuberculosis in San Diego County: a survey of physicians' knowledge, attitudes and practices. Int. J. Tuberc. Lung Dis. 5:933–938 [PubMed] [Google Scholar]
- 31. Millet J., Miyagi-Shiohira C., Yamane N., Sola C. C., Rastogi N. 2007. Assessment of mycobacterial interspersed repetitive unit-QUB markers to further discriminate the Beijing genotype in a population-based study of the genetic diversity of Mycobacterium tuberculosis clinical isolates from Okinawa, Ryukyu Islands, Japan. J. Clin. Microbiol. 45:3606–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moonan P. K., et al. 2006. What is the outcome of targeted tuberculosis screening based on universal genotyping and location? Am. J. Respir. Crit. Care Med. 174:599–604 [DOI] [PubMed] [Google Scholar]
- 33. Nolan C. M. 1999. Community-wide implementation of targeted testing for and treatment of latent tuberculosis infection. Clin. Infect. Dis. 29:880–887 Rev [DOI] [PubMed] [Google Scholar]
- 34. Patel S., et al. 2007. Risk of progression to active tuberculosis among foreign-born persons with latent tuberculosis. Chest 131:1811–1816 [DOI] [PubMed] [Google Scholar]
- 35. Rhode Island Department of Health Tuberculosis Program 2007. Tuberculosis epidemiology report. Rhode Island Department of Health, Providence, RI [Google Scholar]
- 36. Sharnprapai S., et al. 2002. Genotyping analyses of tuberculosis cases in U.S.- and foreign-born Massachusetts residents. Emerg. Infect. Dis. 8:1239–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Small P. M., et al. 1994. The epidemiology of tuberculosis in San Francisco: a population-based study using conventional and molecular methods. N. Engl. J. Med. 330:1703–1709 [DOI] [PubMed] [Google Scholar]
- 38. Stout J. E., Østbye T., Walter E. B., Hamilton C. D. 2006. Tuberculosis knowledge and attitudes among physicians who treat young children in North Carolina, U.S.A. Int. J. Tuberc. Lung. Dis. 10:783–788 [PubMed] [Google Scholar]
- 39. Supply P., et al. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. U.S. Census Bureau 2010. United States census 2000. U.S. Census Bureau, Washington, DC: http://quickfacts.census.gov/qfd/states/44000.html [Google Scholar]
- 41. van Soolingen D., et al. 1999. Molecular epidemiology of tuberculosis in the Netherlands: a nationwide study from 1993 through 1997. J. Infect. Dis. 180:726–736 [DOI] [PubMed] [Google Scholar]
- 42. van Soolingen D., de Haas P. E., Hermans P. W., Groenen P. M., van Embden J. D. 1993. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J. Clin. Microbiol. 31:1987–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zuber P. L., McKenna M. T., Binkin N. J., Onorato I. M., Castro K. G. 1997. Long-term risk of tuberculosis among foreign-born persons in the United States. JAMA 278:304–307 [PubMed] [Google Scholar]
- 44. Yamada S., Caballero J., Matsunaga D. S., Agustin G., Magana M. 1999. Attitudes regarding tuberculosis in immigrants from the Philippines to the United States. Fam. Med. 31:477–482 [PubMed] [Google Scholar]