Abstract
The objectives of this study were to monitor the stability of rifampin (RIF) in Löwenstein-Jensen medium (L-J medium) and 7H9 broth, which are the media commonly used for drug susceptibility testing (DST) of Mycobacterium tuberculosis. Rifampin degradation in stock solution, 7H9 broth, and L-J medium and during the inspissation process for L-J medium preparation was serially monitored by high-performance liquid chromatography (HPLC). L-J medium-based DST was conducted to examine the effect of L-J medium storage on the DST outcome. The RIF stock solution was stable for at least 3 months when kept at either 4°C or −20°C; RIF in 7H9 broth and L-J medium was almost 50% decayed after 1 week of storage at 37°C, and rifampin could not be detected in 7H9 or L-J medium after 3 weeks or 6 weeks of storage at 37°C. Approximately half of the drug was decomposed after 4 months of storage at 4°C for both media, and after 6 months of storage at 4°C, RIF in L-J medium was undetectable, while 38% of RIF remained in 7H9 medium. Approximately 21, 24, 29, and 35% RIF degradations were detected when the L-J medium was coagulated at 75°C, 80°C, 85°C, and 90°C, respectively. The DST outcomes when using L-J medium stored for different periods of time were consistent with the RIF concentration monitoring data. Rifampin in stock solution is stable for at least 3 months at a reduced storage temperature. Media containing RIF should be prepared strictly according to validated standard operating procedures. RIF degradation is a possible reason for false resistance categorizations of M. tuberculosis isolates in the clinical laboratory.
INTRODUCTION
Although the global incidence of tuberculosis (TB) is declining, drug resistance is rapidly emerging and spreading (1, 13). Insufficient control measures have led to an increase in the prevalence of drug-resistant strains and, more importantly, the extent of drug resistance (10). In certain high-burden countries, the nature of multidrug-resistant (MDR) tuberculosis has evolved from incidental to epidemic.
Drug susceptibility testing (DST) for mycobacteria based on drug-containing Löwenstein-Jensen (L-J) medium is a method used ubiquitously around the world, especially in resource-limited settings, where it is usually the only laboratory technique for the diagnosis of drug-resistant TB. The proportion method and the absolute concentration method are widely used DST methods based on L-J medium. However, discordance between laboratory diagnosis and clinical treatment outcomes of drug-resistant tuberculosis is not uncommon (3, 9). Clinically, some patients with MDR TB diagnosed on the basis of DST achieve cure with first-line antituberculosis drug regimens. The cause for this inconsistency is worthy of investigation.
Rifampin (RIF) is one of the most commonly used anti-TB drugs and is the backbone of most successful TB treatment regimens. In addition, more than 90% of RIF-resistant Mycobacterium tuberculosis strains are also resistant to isoniazid (INH); hence, RIF resistance is a marker for MDR TB (12).Clearly, an accurate definition of the RIF susceptibility of bacilli is of great importance. Our study sought to serially monitor rifampin concentrations to identify whether drug instability in culture medium plays a role in inaccurate DST results, to determine the expiry date for RIF-containing L-J medium and 7H9 broth, and to define the stability of RIF in stock solution using different solvents.
MATERIALS AND METHODS
Medium preparation.
7H9 broth (Difco) was prepared according to the manufacturer's instruction. Oleic acid-albumin-dextrose-catalase (OADC) (10%; BD, Franklin Lakes, NJ) was added after the broth medium had been sterilized. For the RIF (Sigma, St. Louis, MO)-containing broth preparation, a freshly made RIF–N,N-dimethylformamide (DMF) solution was added to achieve a final concentration of 40 mg/liter.
L-J medium was prepared according to World Health Organization (WHO) guidelines (14), using antibiotic-free hen eggs. Aliquots (6 to 8 ml) were dispensed into 20- by 150-mm sterilized 25-ml screw-cap tubes, or alternatively, 0.5-ml aliquots were dispensed into 2-ml Eppendorf tubes, and lids were securely fastened. The preparation was solidified by placing the tubes into an inspissator prewarmed to 80°C and coagulating the medium for 50 min at 85°C. For the RIF-containing medium preparation, RIF solution was added to the crude L-J liquid mixture before inspissation to achieve a final concentration of 40 mg/liter.
Rifampin stability monitored by HPLC. (i) RIF concentration analysis by HPLC.
The L-J medium sample-processing steps for high-performance liquid chromatography (HPLC) analysis were as follows. Freshly made rifapentine (RFP) (Huabang Pharmaceutics Co., Chongqing, China) was added to a 0.5-ml aliquot of medium in a 2-ml Eppendorf tube as an internal standard (IS) (final concentration, 90 mg/liter). The medium was then subjected to tissue homogenization (TissuelyseII; Qiagen, Germany). The mixture was deproteinized twice by adding a 2-fold volume of methanol and then centrifuged at 5,000 × g for 10 min, and the supernatant was collected for HPLC analysis. No processing step was needed for 7H9 broth and RIF stock solution other than the addition of the internal standard RFP. Twenty microliters of processed supernatant or 7H9 broth was assayed directly by HPLC (L-6200 Intelligent pump, L-4200 UV-vis detector, 655A-40 autosampler, 655A-52 column oven, and D-2000 Chromato-Integrator [Hitachi, Japan] and a Chrom C18 column [3.9 by 150 mm, 5 μm; Waters]). The fluid phase consisted of methanol–10 mmol · L1 monopotassium phosphate-disodium hydrogen phosphate (pH 5.8) (66:34). Chromatography was performed at room temperature at a flow rate of 1.0 ml/min with a UV detector at 336 nm.
A calibration curve for L-J medium was constructed by spiking with 5, 10, 20, 30, 40, and 50 mg/liter of RIF in L-J medium without RIF before processing. Quintuplicate samples were processed for each concentration. Calibration curves for the stock solution and 7H9 broth were constructed in a fashion similar to those used with L-J medium but without homogenization/deproteinization. Concentrations were derived from a linear regression analysis of the peak area ratio (analyte/IS)-versus-concentration curves. Calibration curve linearity was verified statistically using correlation coefficient (r) estimations. Extraction recoveries were calculated by comparing the peak areas for the processed spiked samples with those of standard solutions at the same concentrations (10, 30, and 50 mg/liter of rifampin; n = 5), which were injected directly into the HPLC instrument. The limit of detection (LOD) and limit of quantification (LOQ) were estimated mathematically from the standard curve equations. The LOD was equal to 3.3 times the standard deviation (SD) of the y axis intercepts. The LOQ was obtained by multiplying the y axis intercepts by 10 (2).
Precision was determined with quintuple measurements of three RIF concentrations (10, 30, and 50 mg/liter). All samples were spiked with RIF on day 1 and extracted and analyzed on day 1 (intraday; n = 5) and on various days (interday; n = 5). Accuracy was assessed at the same concentrations (n = 5) and expressed as the percent deviation from the theoretical value. The confidence interval of the mean recovery was calculated.
(ii) Two-factor factorial analysis.
To find the causes of RIF instability in L-J medium, two factors were considered, heat and protein binding. Heat relates to the inspissation process for medium preparation with the RIF concentration in L-J medium before and after the inspissation being assessed. Protein binding relates to the interaction between homogenized eggs and RIF in L-J medium; the RIF concentration in the premixture with or without egg was analyzed. Samples were classified into four groups (8). Group 1 (NN) consisted of samples without eggs that did not undergo coagulation, group 2 (AA) consisted of samples with eggs that underwent a coagulation step at 85°C for 50 min, group 3 (NA) consisted of samples without eggs that were exposed to the coagulation step, and group 4 (AN) included samples with eggs that did not undergo heat coagulation. Quintuplicate samples were processed for each tested condition.
(iii) Effect of duration and temperature of inspissation when preparing L-J medium.
L-J medium was prepared as described above but with the following parameters altered. After dispensing of the premixture of L-J medium, tubes were kept in a preheated inspissator at 85°C for 50 min, 1.5 h, or 2 h. To examine the effects of temperature, tubes were kept in a preheated inspissator at 75°C, 80°C, 85°C, or 90°C for 50 min. For each conditional test, quintuplicate samples were processed.
(iv) Stability of the RIF stock solution.
A 4 g/liter volume of RIF solution was prepared by using 3 different solvents: methanol, DMF, and dimethyl sulfoxide (DMSO). Solutions and aliquots were all made in glass tubes, which were sealed with Parafilm. The tubes were then stored at 4°C and −20°C. The RIF concentrations in fresh solution and stock solution were detected by high-performance liquid chromatography weekly for 3 months as outlined above [see “Rifampin stability monitored by HPLC. (i) RIF concentration analysis by HPLC”] after being diluted to 40 mg/liter with water.
(v) Monitoring of rifampin stability in L-J medium and 7H9 broth.
The rifampin concentrations in L-J medium and 7H9 broth, which were kept at 4°C and 37°C, were monitored by HPLC as outlined above. Medium stored at 4°C was monitored monthly until the end of the sixth month; L-J medium or 7H9 medium stored at 37°C was monitored every other day or once a week until no RIF peak could be detected by HPLC.
Bacterial growth detection using L-J medium stored for different periods of time. (i) Strain screening by a MABA susceptibility test.
One hundred sixteen clinical isolates of M. tuberculosis were identified as being rifampin-sensitive strains by the L-J medium absolute concentration method (RIF concentrations were 50 and 250 mg/liter). All the strains were subcultured onto L-J medium and incubated for 2 weeks. Bacterial suspensions were prepared by using 5% (vol/vol) Tween 80 in 0.9% sodium chloride, and the turbidity was adjusted to 1 McFarland turbidity standard. Suspensions were further diluted 1:25 with 7H9 broth. Microplate alamarBlue assay (MABA) susceptibility testing was performed according to a method introduced previously by Franzblau et al. (4). Final concentrations of RIF for the MABA were 0, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.0, and 4.0 mg/liter. Strain H37Rv (ATCC 27294) was used as a control.
(ii) Drug susceptibility testing (proportion method) using L-J medium stored for different periods of time.
Ten clinical isolates of M. tuberculosis with known MICs [see Bacterial growth detection using L-J medium stored for different periods of time. (i) Strain screening by a MABA susceptibility test] were selected for DST by the proportion method: 2 strains had MICs of <0.05 mg/liter, 2 strains had MICs between 0.2 and 0.4 mg/liter, 2 strains had MICs between 0.8 and 1.0 mg/liter, 2 strains had MICs between 1.0 and 1.5 mg/liter, and 2 strains had MICs higher than 1.5 mg/liter but lower than 2 mg/liter. The DST proportion method was performed according WHO guidelines with 102 and 104 cells inoculated into 40 mg/liter RIF-containing L-J medium. The reference strain, H37Rv 27294 (RIF MIC, 0.1 to 0.2 mg/liter), was used as a control.
RESULTS
Validation of the method.
When HPLC was performed, the retention time for RIF was around 3.44 min, and that for RFP was around 6.69 min. The intra- and interday accuracy and precision data and the mean recoveries of rifampin are summarized in Table 1. The relative standard deviations for RIF were below 3.78% and 5.61% for intra- and interday precision analyses, respectively. The recovery rate of RIF for all the tested samples was between 97.47 and 103.52%. The calibration curves of RIF in different media showed good linearity over the concentration range of 5 to 50 mg/liter (r was higher than 0.999 in all cases) (Table 1).When rifampin was detected by HPLC, the LOD values were 0.08, 0.11, and 0.15 mg/liter and the LOQ values were 0.23, 0.33, and 0.45 mg/liter for L-J medium, 7H9 broth, and RIF stock solution, respectively.
Table 1.
Recovery efficiency and precision of RIF concentration monitoring by HPLCa
| Sample | Calibration curve (r value) | Original RIF concn (mg/liter) | Mean concn detected by HPLC (mg/liter) ± SD | Mean recovery rate (%) ± SD | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|---|
| L-J medium | y = 0.0074x − 0.0052 (0.9993) | 10 | 10.18 ± 0.32 | 101.76 ± 0.03 | 3.10 | 3.68 |
| 30 | 30.95 ± 0.93 | 103.16 ± 0.03 | 3.00 | 4.87 | ||
| 50 | 50.30 ± 0.54 | 100.60 ± 0.01 | 1.07 | 2.73 | ||
| 7H9 medium | y = 0.0114x − 0.0027 (0.9995) | 10 | 9.88 ± 0.37 | 98.78 ± 3.74 | 3.78 | 5.61 |
| 30 | 29.47 ± 0.70 | 98.22 ± 3.17 | 2.36 | 3.48 | ||
| 50 | 51.76 ± 0.57 | 103.52 ± 2.55 | 1.10 | 5.24 | ||
| Stock solution | y = 0.0073x + 0.0023 (0.9997) | 10 | 10.56 ± 0.35 | 105.61 ± 0.04 | 3.54 | 4.29 |
| 30 | 29.35 ± 0.23 | 99.00 ± 0.98 | 0.79 | 2.89 | ||
| 50 | 50.71 ± 0.27 | 101.00 ± 1.00 | 0.53 | 0.89 |
n = 5 −x ± s. RSD, relative standard deviation.
Two-factor factorial analysis.
The analysis showed that coagulation at 85°C for 50 min during the preparation of L-J medium led to about a 28% RIF degradation. The variance analyses indicated that both heat and protein could lead to RIF degradation. The RIF concentration was significantly lower in protein-containing L-J medium (AA = 28.88 mg/liter; AN = 30.66 mg/liter) than in media without protein (NN = 39.47 mg/liter; NA = 36.49 mg/liter) (F = 10.324; P = 0.005; n = 10). Similarly, RIF concentrations were significantly lower in the heated group (AA = 28.88 mg/liter; NA = 36.49 mg/liter) than in medium not exposed to heat in preparation (NN = 39.47 mg/liter; AN = 30.66 mg/liter) (F = 114.775; P < 0.001; n = 10). The effect of heat on the stability of RIF was more obvious than that of protein binding, and there was no interaction between protein binding and heat (F = 0.561; P = 0.465; n = 10).
Effect of inspissation time duration and inspissation temperature on RIF stability.
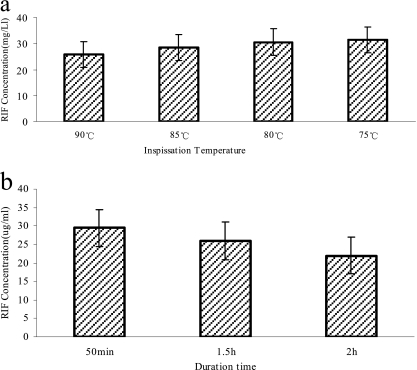
For the premixture with a RIF concentration of 40 mg/liter, following inspissation at 75°C, 80°C, 85°C, and 90°C for 50 min, the detected RIF concentrations were 31.43 ± 0.56, 30.55 ± 0.66, 28.42 ± 1.29, and 25.85 ± 1.06 mg/liter, respectively. Proportionally, 21.43%, 23.63%, 28.95%, and 35.38% RIF degradations occurred, respectively, when inspissation was performed at different temperatures. After the inspissation of the premixture at 85°C for 50 min, 1.5 h, and 2 h, the RIF concentrations detected were 29.46 ± 1.5, 25.99 ± 0.83, and 22.02 ± 1.05 mg/liter, respectively. The results indicated that 26.34%, 35.03%, and 44.95% RIF degradations occurred when the mixture was coagulated at 85°C for 50 min, 1.5 h, and 2 h, respectively (Fig. 1).
Fig. 1.
Effect of temperature and duration of inspissation on the rifampin concentration of the prepared L-J medium. (a) RIF concentration in freshly made L-J medium when coagulated for 50 min at 75°C, 80°C, 85°C, and 90°C separately. (b) RIF concentration in freshly made L-J medium when coagulated at 85°C for 50 min, 1.5 h, and 2 h separately.
Stability of RIF stock solution.
Analysis of the freshly made 40-mg/liter RIF stock solution by HPLC detected RIF concentrations in DMF, DMSO, and methanol solvent of 40.03 ± 0.67, 39.42 ± 0.74, and 31.08 ± 1.05 mg/liter, respectively. The use of methanol for RIF preparation resulted in 22.3% RIF degradation compared with that using DMF and DMSO as the solvents. No obvious change was detected for the RIF stock solution when stored at either 4°C or −20°C over the 3 months examined.
Stability of RIF in 7H9 broth and L-J medium.
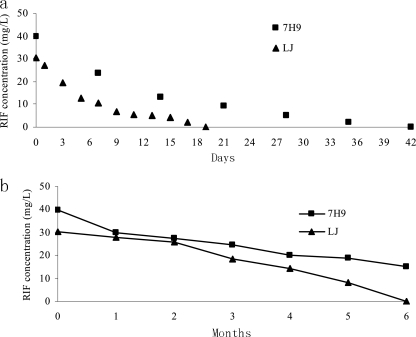
RIF in 7H9 broth and L-J medium was nearly 50% degraded after 1 week of storage at 37°C, and no RIF curve could be detected for L-J medium after 3 weeks of storage at 37°C and for 7H9 brother after 6 weeks. For media kept at 4°C, about 10% RIF degradation was detected for both 7H9 and L-J media after 1 month of storage, and nearly half was decayed for both media after 4 months of storage. RIF in L-J medium was undetectable after 6 months of storage, while only 38% remained for 7H9 broth. The continuously monitored outcome of the stability of RIF in 7H9 and L-J media is demonstrated in Fig. 2.
Fig. 2.
Serial monitoring outcomes of RIF stability in L-J medium and 7H9 broth when kept at 37°C and 4°C. (a) Stability of RIF in 7H9 and L-J media when kept at 37°C. (b) Stability of RIF in 7H9 and L-J media when kept at 4°C.
Outcomes of MABA tests.
Among 116 RIF-susceptible strains determined by the L-J medium absolute concentration method, the MIC distribution of the strains was as follows: 74 strains had MICs < 0.05 mg/liter, 15 strains had MICs between 0.05 and 0.1 mg/liter, 5 strains had MICs between 0.1 and 0.2 mg/liter, 6 strains had MICs between 0.2 and 0.4 mg/liter, 3 strains had MICs between 0.8 and 1.0 mg/liter, 2 strains had MICs between 1.0 and 1.5 mg/liter, and 11 strains had MICs between 1.5 and 2.0 mg/liter.
Partial DST outcomes for strains with MICs close to or below the RIF-resistant cutoff value are presented in Table 2. For H37Rv and clinical isolates with MICs lower than 0.4 mg/liter, the DST outcomes were consistent when using medium kept at 4°C for 0 to 6 months. For isolates with MICs higher than 0.8 mg/liter, discrepancies were apparent. The longer the L-J medium was stored, the sooner that visible growth was detected.
Table 2.
Partial outcomes for drug susceptibility testing of strains with different MICs
| Strain | MIC (mg/liter) | Medium durationb (mo) | Culture outcome of inoculated CFU counts (104/102)a at wk: |
|||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| H37Rv | 0.1–0.2 | Control | −/− | −/− | 2+/− | 3+/17 | 4+/20 | 4+/20 |
| 0 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 1 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 2 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 3 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 4 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 5 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 6 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 6039 | <0.05 | Control | −/− | −/− | C/Cc | 4+/31 | 4+/40 | 4+/40 |
| 0 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 1 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 2 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 3 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 4 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 5 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 6 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 6917 | 0.8–1.0 | Control | −/− | −/− | 3+/+ | 4+/+ | 4+/20 | 4+/40 |
| 0 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 1 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 2 | −/− | −/− | −/− | 2+/C | 3+/C | 4+/40 | ||
| 3 | −/− | −/− | −/− | 3+/+ | 4+/20 | 4+/40 | ||
| 4 | −/− | −/− | −/− | 3+/20 | 4+/50 | 4+/40 | ||
| 5 | −/− | −/− | −/− | 3+/− | 4+/10 | 4+/50 | ||
| 6 | −/− | −/− | +/− | 4+/20 | 4+/30 | 4+/60 | ||
| 6982 | 1.0–1.5 | Control | −/− | −/− | 2+/− | 3+/− | 4+/20 | 4+/50 |
| 0 | −/− | −/− | −/− | −/− | −/− | −/− | ||
| 1 | −/− | −/− | −/− | −/− | −/− | 12/− | ||
| 2 | −/− | −/− | −/− | −/− | −/− | 2+/3 | ||
| 3 | −/− | −/− | −/− | −/− | −/− | 2+/15 | ||
| 4 | −/− | −/− | −/− | −/− | −/− | 3+/15 | ||
| 5 | −/− | −/− | −/− | 2+/− | 4+/C | 4+/20 | ||
| 6 | −/− | −/− | C/− | 3+/− | 4+/C | 4+/11 | ||
| 6919 | 1.5–2.0 | Control | −/− | −/− | C/− | 3+/− | 3+/10 | 4+/20 |
| 0 | −/− | −/− | −/− | C/C | C/C | 3+/10 | ||
| 1 | −/− | −/− | −/− | C/C | C/C | 3+/15 | ||
| 2 | −/− | −/− | −/− | C/C | C/C | 4+/40 | ||
| 3 | −/− | −/− | −/− | C/C | C/C | 4+/20 | ||
| 4 | −/− | −/− | −/− | C/C | C/C | 4+/10 | ||
| 5 | −/− | −/− | C/− | 2+/C | 3+/C | 4+/8 | ||
| 6 | −/− | −/− | C/− | 3+/C | 3+/C | 4+/10 | ||
L-J medium culture grading criteria according to WHO guidelines.
Control indicates L-J medium without RIF.
“C” is the abbreviation for “conversion,” which indicates that very small colonies were observed but not enumerable.
DISCUSSION
With the increasing pandemic of drug-resistant tuberculosis around the world, DST techniques based on L-J medium will be used more and more frequently. Compared with liquid culture DST, the use of L-J medium has some obvious advantages. Above all, it is cheap, only costing about one-third of the cost of liquid culture DST. Second, no expensive apparatus is needed, which makes it very popular in countries with high TB prevalences and low incomes. Nonetheless, some disadvantages also exist. It is well known that DST using solid medium is time-consuming, has inconsistent outcomes, and demonstrates low reproducibility. Another disadvantage is that the technique has not been standardized completely (7). Although a standard operating procedure (SOP) was recommended by the WHO, many uncertain factors like specimen characteristics, bacterial load for inoculation, and inconsistencies in the quality of L-J medium prepared by different laboratories make an external quality assurance system for solid medium DST difficult, so data comparisons among laboratories might be inappropriate.
When preparing drug-containing L-J medium, the following questions might be frequently asked. Can leftover drug solution be stored? At what temperature and how long can the solution be kept? This is especially important for an expensive drug like rifampin, or for a drug like isoniazid, since although very tiny amounts of powder are actually required, to make the weighing by balance more accurate, much more than what is needed should be taken each time. Can we save the stock solution for future use? It is already known that during the inspissation process of L-J medium preparation, heating at 85°C for 50 min will cause drug degradation to some degree, but how extensive is this change? How long should the expiry date be set up for drug-containing medium? For products sold commercially, an expiry date of 1 to ∼6 months is set by the manufacturer without any supporting data or information. To answer all these questions, we conducted experiments to serially monitor rifampin stability in L-J medium and in 7H9 broth by high-performance liquid chromatography.
A number of papers examining HPLC methods for the determination of RIF concentrations have been published (6, 11), but none have evaluated the stability of RIF in L-J medium and 7H9 broth. In our assay, a rapid, accurate, and reproducible HPLC method was developed and validated to measure rifampin concentrations in L-J medium and 7H9 broth medium. This method is suitable for studies of RIF concentrations in culture media, so it may be beneficial for quality control strategies for medium production.
In our assay, an interesting phenomenon was observed when methanol was used as a solvent to prepare the RIF solution. We found that methanol led to more than 20% RIF degradation compared with DMF and DMSO as the initial solvent, for uncertain reasons. Ethanol was also tried as a solvent for RIF, but it was observed that RIF was insoluble in ethanol. These findings indicate that inappropriate solvent use for RIF might result in incorrect DST results for rifampin, as lower RIF concentrations in the corresponding L-J medium will surely lead to some RIF-sensitive strains being wrongly classified as being RIF resistant. For the RIF stock solution, our data indicate that RIF stock solution kept either at 4°C or at −20°C is eligible for reuse at least within 3 months. Those outcomes are consistent with those reported previously by Griffith and Bodily (5). Even though the RIF stock solution is relatively stable, long-term storage is not recommended, since an evaporation of organic solvent will occur even for solutions kept at −20°C. During our serial monitoring process, a concentration increase was seen because of evaporation (data not shown), so observations were ceased after 3 months.
The WHO has guidelines for L-J medium preparation in which the inspissation process is conducted at 85°C for 50 min. In our assay, we found that even this standardized process can cause about 26 to 29% RIF degradation so that the 40 mg/liter of RIF in the premixture typically would decrease to a final RIF concentration of about 28 to 29 mg/liter. Our work also proved that the quality of RIF-containing L-J medium deteriorates when coagulation is done at too high a temperature or for too long. For the 4 chosen inspissation temperatures, 75°C, 80°C, 85°C, and 90°C, the higher the temperature, the lower the RIF concentration that was detected by HPLC. Concerning the inspissation process duration time, the longer the inspissation process, the more notable the RIF degradation. Overheating could lead to a disastrous DST outcome, as inspissation for 2 h at 85°C caused about 45% RIF degradation. The coagulation of the premixture at 90°C also incurred significant (35%) RIF degradation. Although a lower temperature like 75°C resulted in higher RIF concentrations, the medium became fragile and not suitable for DST. As the inspissation temperature and duration time are very critical for L-J medium quality, the SOP of RIF-containing medium preparation should be strictly adhered to.
Rifampin was not stable in either L-J medium or 7H9 broth. As we found when it was kept at 37°C, RIF in 7H9 broth medium and L-J medium was nearly 50% degraded after 1 week, and no RIF curve could be detected for L-J medium after 3 weeks of storage at 37°C or after 6 weeks of storage for 7H9 liquid medium at the same temperature. DST based on L-J medium requires 6 weeks to report the final results, yet our data indicate that RIF in L-J medium has already totally degraded by that time. The Bactec MGIT 960 system (Becton Dickinson and Company, NJ) using 7H9 broth as a medium might have similar issues. Further evaluation of both solid and liquid culture systems is needed.
RIF in both media kept at 4°C was relatively stable compared with that at 37°C, but the RIF degradation was significant as well (Fig. 2). Theoretically, if media stored for different periods of time have different concentrations of RIF, then the use of media kept for different durations of time would necessarily produce some discrepant DST outcomes. To prove this hypothesis, we first screened clinical tuberculosis isolates with MICs close to the RIF resistance cutoff value (which is 1 mg/liter for liquid medium DST), as those strains would be most likely to be affected by small variations in drug concentrations when DST is performed. We selected those strains from the clinical isolates which were defined as being rifampin susceptible by the absolute concentration method, as the absolute concentration method applies a more stringent threshold to define drug resistance (the RIF concentration for the absolute concentration method is 50 mg/liter, and that for the proportion method is 40 mg/liter). The bacteriological outcomes of DST proved our hypothesis (Table 2). Due to the complexity of DST, some isolates with similar MICs produced discrepant DST outcomes, and for the same strain, repeat testing also presented discrepant results; however, the overall trend of DST outcomes was clear: the longer the L-J medium was kept, the sooner visible colonies were observed. There is no doubt that there is a danger of misdiagnosis when old medium is used to perform DST. Our data also demonstrated that the effect of RIF degradation on the DST outcome was not as drastic as we had expected. Even for the medium kept for 6 months, despite very little RIF being detected, bacterial inhibition potency still persisted to some extent. The reason for this might be that RIF degradation products have some inhibition potency as well.
In summary, our assay provided an analytical method for the determination of rifampin concentrations in L-J medium and 7H9 broth, which may prove useful for monitoring the quality of drug-containing media. Serial RIF concentration monitoring suggests that RIF stock solution could be used for medium preparation for at least 3 months when stored at a reduced temperature; drug-containing medium should be prepared strictly according to standard operation procedures; for optimal DST outcomes, RIF-containing L-J medium should not be older than 1 month; and RIF degradation in testing medium may be a possible reason for false resistance detection in the clinical laboratory.
ACKNOWLEDGMENT
The work was supported by research funding from the Infectious Diseases Special Project, Ministry of Health of China (grant 2008ZX10003-005).
Footnotes
Published ahead of print on 22 December 2010.
REFERENCES
- 1. Chan E. D., Iseman M. D. 2008. Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr. Opin. Infect. Dis. 21:587–595 [DOI] [PubMed] [Google Scholar]
- 2. Cheneviera P., Massiasa L., Gueylarda D., Farinotti R. 1998. Determination of ethambutol in plasma by high-performance liquid chromatography after pre-column derivatization. J. Chromatogr. B Biomed. Sci. Appl. 708:310–315 [DOI] [PubMed] [Google Scholar]
- 3. Espinal M. A., et al. 2000. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcome in 6 countries. JAMA 283:2537–2545 [DOI] [PubMed] [Google Scholar]
- 4. Franzblau S. G., et al. 1998. Rapid, low-technology MIC determination with clinical M. tuberculosis isolates by using the Microplate Alamar Blue assay. J. Clin. Microbiol. 36:362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffith M. E., Bodily H. L. 1992. Stability of antimycobacterial drugs in susceptibility testing. Antimicrob. Agents Chemother. 36:2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartkoorn R. C., et al. 2007. A rapid and sensitive HPLC-MS method for the detection of plasma and cellular rifampicin. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 857:76–82 [DOI] [PubMed] [Google Scholar]
- 7. Homorodean D., Diaconescu C., Henegariu S. E., Creçtu S. D., Zamora C. D. 1997. The comparative results of the antibiograms for Mycobacterium tuberculosis performed in 2 different laboratories. Pneumoftiziologia 46:21–22 [PubMed] [Google Scholar]
- 8. Mandich M. B., Ritchie S. K., Mullett M. 1996. Transition times to oral feeding in premature infants with and without apnea. J. Obstet. Gynecol. Neonatal Nurs. 25:771–776 [DOI] [PubMed] [Google Scholar]
- 9. Migliori G. B., Espinal M., Danilova I. D., Punga V. V., Grzemska M., Raviglione M. C. 2002. Frequency of recurrence among MDR-TB cases ‘successfully’ treated with standardised short-course chemotherapy. Int. J. Tuberc. Lung Dis. 6:858–864 [PubMed] [Google Scholar]
- 10. Mukherjee J. S., et al. 2004. Programmes and principles for management of multidrug-resistant tuberculosis. Lancet 363:474–481 [DOI] [PubMed] [Google Scholar]
- 11. Quenelle D., et al. 2004. Sustained release characteristics of rifampin-loaded microsphere formulations in nonhuman primates. Drug Deliv. 11:239–246 [DOI] [PubMed] [Google Scholar]
- 12. Ramaswamy S., Musser J. M. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79:3–29 [DOI] [PubMed] [Google Scholar]
- 13. Sharma S. K., Mohan A. 2006. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest 130:261–272 [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization 1998. Laboratory services in tuberculosis control. Part III: culture. World Health Organization, Geneva, Switzerland [Google Scholar]